Abstract
Background
Clinical assessment and prognostic stratification of primary varicose veins have remained controversial and the molecular pathogenesis is unknown. Previous data have suggested a contribution of the MTHFR (methylenetetrahydrofolate reductase) polymorphism c.677C>T.
Methods
We collected blood and vein specimens from 159 consecutive patients undergoing varicose vein surgery, or autologous vein reconstruction for arterial occlusive disease as controls. We compared the frequencies of c.677C>T and another polymorphism of MTHFR, c.1298A>C, with morphology and types of complicated disease. Morphology was recorded as a trunk or perforator type and peripheral congestive complication was defined as chronic venous insufficiency (CEAP C3–6) associated with edema and skin manifestations.
Findings
Multivariate analysis of genotypes for c.677C>T and c.1298A>C indicated that c.677C>T was associated significantly with the trunk phenotype (43/53 patients, 81%, p < 0.01), while c.1298A>C was associated significantly with the perforator phenotype (18/24 patients, 75%, p < 0.01) of primary varicose veins. Accordingly, when both c.677C>T and c.1298A>C displayed a heterozygous genotype, the patients were more likely to present with both phenotypes. Additionally, c.1298A>C was found to be strongly linked to the congestive complication (34/51 patients, 67%, p < 0.01).
Interpretation
Both polymorphisms of MTHFR may be involved in the morphological specification of primary varicose veins and contribute to the development of complicated disease.
Funding
None.
Highlights
-
•
MTHFR polymorphism c.677C>T characterizes axial trunk and c.1298A>C perforator type morphology in primary varicose veins.
-
•
Mutant genotypes are associated with complicated phenotypes of the disease.
-
•
Genetic hint for distinct perforator type morphology associating further with congestive (CEAP C3–6) disease is provided.
1. Introduction
Primary varicose vein disease constitutes one of the most frequent inherited disorders worldwide and entails a broad spectrum of cosmetic, psychological, medical, and socio-economic implications (Evans et al., 1994, Carpentier et al., 2004). Family based investigations suggested an autosomal dominant inheritance (Cornu-Thenard et al., 1994, Hach, 1967), but the origin of variant clinical courses and morphological presentations is still unknown. This has resulted in a long lasting and sometimes confusing debate on how to deal and treat best (Pannier and Rabe, 2012, Gloviczki and Gloviczki, 2012).
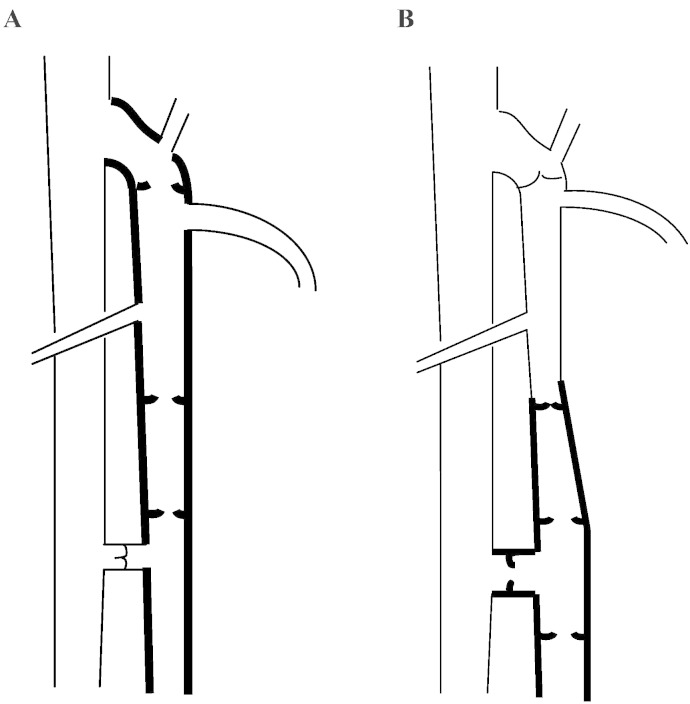
Trunk and perforator types (Fig. 1a and b) of complicated and uncomplicated disease suggest different subsets of genetic origin. Thrombotic disease has been closely related to a common homozygous mutant genotype (TT) at c.677C>T, the most studied polymorphism of the MTHFR (methylenetetrahydrofolate reductase) gene (Milio et al., 2008, Wilmanns et al., 2011), coding for a key enzyme of one-carbon metabolism. The prevalence of c.677C>T accurately overlaps with geographic variations in the incidence of primary varicose veins (Wilmanns et al., 2011, Wilcken et al., 2003, Raffetto, 2011) and has been previously linked to the occurrence of varicose veins (Sverdlova et al., 1998).
Fig. 1.
Two different types of valvular incompetence occur in primary varicose veins associated with two different types of clinical presentation. A, trunk type axial incompetence, begins at the junctional valve and progressively advances towards the medial malleolus in case of the greater or lateral malleolus in case of the smaller saphenous vein. B, perforator type incompetence at one or more typical locations is frequently associated with segmental axial incompetence of draining superfascial tributaries or trunk veins.
Primary varicose veins result from an asymmetric remodeling of extracellular matrix and smooth muscle cells (Lim and Davies, 2009, Naoum and Hunter, 2007, Somers and Knaapen, 2006) contributing to wall thickening, valve incompetence and their typical corkscrew appearance. Pathognomonic tortuosities provide undistinguishable features of palpation and inspection and thus support genetic considerations.
In this study, we report an association between the c.677C>T and the second most studied polymorphism of the MTHFR gene, c.1298A>C, both of which are linked to reduced enzyme activity, with morphological phenotypes and varying clinical presentations of primary varicose veins. The heterozygous or homozygous c.677C>T genotype was associated with the trunk phenotype and the heterozygous or homozygous c.1298A>C genotype with the perforator phenotype. We further linked the double heterozygous c.677C>T/c.1298A>C genotype (CTAC) with the combined trunk and perforator phenotype of primary varicose veins. In addition, we associated the homozygous mutant CC genotype at MTHFR c.1298A>C with the occurrence of congestive disease. Thus, we provide a clue to the impact of MTHFR polymorphisms c.677C>T and c.1298A>C as hereditary components of primary varicose vein disease.
2. Patients and Methods
Blood and vein tissue specimens were collected from 159 consecutive surgical patients, including 116 with primary varicose veins and 37 controls with arterial occlusive disease, at the Maria-Hilf-Krankenhaus in 54550 Daun, Germany, and at the Johanniter-Krankenhaus in 47228 Duisburg, Germany, during a time period from April 2011 to August 2013. Venous tissue was obtained from removed and otherwise discharged varicose veins or left-over from venous reconstruction of arterial occlusive disease. The study was approved by the Ethic Committees of the Medical Associations of Rhineland-Palatinate [No. 837.302.11 (7844)] and North Rhine (No. 2012083), and all probands gave written and informed consent to participate in the study in accordance with the declaration of Helsinki's latest version. Probands with arterial occlusive disease and autologous vein reconstruction served as controls.
Clinical and duplex examination assessed tortuosities, reflux, venous dilation, thrombosis, swelling, pigmentation, eczema, scar and ulcer, inflammation, and painful, cord-like lesions to determine the morphological phenotype or the occurrence of complicated, progressive disease (Suppl. Table 1). Morphology was recorded as the trunk (Fig. 1a), perforator (Fig. 1b, Suppl. Table 2), or combined, indicating the origin of reflux from the deep venous system, or uncertain phenotypes. Incompetent perforators were recorded in upright sitting or standing position as dilated and draining into epifascial tributaries or segments of incompetent trunk veins. Clinical symptoms resulting from venous hypertension and mal-circulation were summarized as “congestive complication”, “congestive disease”, or C3–6 according to the revised CEAP (Clinical–Etiology–Anatomy–Pathophysiology) classification for chronic venous disorders (Suppl. Table 3) (Eklöf et al., 2004), and thus distinguished from asymptomatic presentation, CEAP C2. Patients were attributed to varicothrombosis in case of clinically apparent thrombotic lesions of varicose veins or to recurrent disease in case of new lesions subsequent to previous surgery in accordance with the REVAS definition (Perrin et al., 2000).
Trunk type origin was from the greater saphenous vein in 67 of 116 patients (58%), from the smaller saphenous vein in 7 (6%), and from both in two (2%). Perforator type origin was from the calf in 36 (31%), the thigh in 6 (5%), and from both in 5 (4%). In 16 patients (14%) the phenotype origin from one or the other or both morphologies remained unclear (Suppl. Table 4). Six patients were excluded due to diagnostic overlaps or secondary etiology (Suppl. Table 5). Exploratory pairwise associations (via Chi-squared or Fisher's exact test, as appropriate) between the combined genotypes and the morphologies and complications were recorded (Table 1, Table 2, Suppl. Fig. 1).
Table 1.
Study group characteristics.
| Age (mean ± SD) [years] |
Controls |
Varicose veins |
||
|---|---|---|---|---|
| 68.7 ± 9.7 |
60.9 ± 14.5 |
|||
| Sexa | m |
26 |
44 |
|
| f | 11 | 72 | ||
| Morphology (n = 116) | Trunk type | – | 53 | |
| Perforator type | – | 24 | ||
| Trunk and perforator type | – | 23 | ||
| Trunk type or trunk and perforator type | gsv | – | 67 | |
| ssv | – | 7 | ||
| gsv + ssv | – | 2 | ||
| Perforator type or trunk and perforator type | Thigh | – | 36 | |
| Calf | – | 6 | ||
| Thigh and calf | – | 5 | ||
| Unclear | – | 16 | ||
| Uncomplicated diseases | CEAP C2 | – | 34 | |
| Complicated disease (n = 82) | Congestive diseaseb (n = 51) | CEAP C3 | – | 24 |
| CEAP C4 | – | 12 | ||
| CEAP C5 | – | 1 | ||
| CEAP C6 | – | 14 | ||
| Recurrencesb (n = 21) | Junctional type | – | 5 | |
| Perforator type | – | 11 | ||
| Junctional and perforator type | – | 3 | ||
| Residual veins | – | 2 | ||
| Varicothrombosisb | – | 31 | ||
Number of patients.
Every primary varicose vein patient belongs to one type of morphology, however some are mentioned more than once in case of complicated disease phenotypes.; m, male; f, female; gsv, greater saphenous vein; ssv, smaller saphenous vein.
Table 2.
Combined genotypes of MTHFR c.677C>T and c.1298A>C and number of patients with different courses and morphologies in varicose vein disease. p-Values are the result of a Chi-squared or Fisher's exact test of association between indicated phenotype and combined genotype.
| CCAA | CTAA | CTAC | TTAA | CCAC | CCCC | n | p-Val | ||
|---|---|---|---|---|---|---|---|---|---|
| Morphology | Trunk type | 8 | 20 | 5 | 18 | 2 | – | 53 | < 0.01 |
| Perforator type | 2 | 3 | 2 | 1 | 8 | 8 | 24 | < 0.01 | |
| Trunk and perforator type | 1 | 1 | 18 | 2 | 1 | – | 23 | < 0.01 | |
| Unclear | 3 | 1 | – | – | 8 | 4 | 16 | < 0.01 | |
| Complication | Morphology recorded | 14 | 25 | 25 | 21 | 19 | 12 | 116 | |
| Uncomplicated disease | 7 | 9 | 8 | 4 | 5 | 1 | 34 | 0.22 | |
| Complicated disease | 7 | 16 | 17 | 17 | 14 | 11 | 82 | ||
| Varicothrombosisa | 5 | 8 | 7 | 6 | 3 | 2 | 31 | 0.75 | |
| CEAP C3–6a | 3 | 6 | 12 | 8 | 11 | 11 | 51 | < 0.01 | |
| Recurrencesa | – | 5 | 3 | 5 | 4 | 4 | 21 | 0.24 | |
| Complication recorded | 14 | 25 | 25 | 21 | 19 | 12 | 116 | ||
| Controls | 3 | 13 | 7 | 3 | 8 | 3 | 37 | 0.46 | |
| Excluded | 1 | 1 | – | 2 | 1 | 1 | 6 | – | |
| Total participants | 18 | 39 | 32 | 26 | 28 | 16 | 159 |
Every primary varicose vein patient belongs to one type of morphology (n = 116), however some are mentioned more than once in case of complicated disease phenotypes. For c.677C>T, the minor allelic frequencies were 40% in patients and 35% in controls, for c.1298A>C 29% in patients and 28% in controls.
Four patients (3%) were assessed as perforator type morphology despite the occurrence of axial trunk incompetence due to proximal perforator location (Suppl. Table 6). In some patients with congestive (CEAP C3–6) or thrombotic disease or particularly in those with thigh perforator incompetence, however, duplex examination was unable to sufficiently discriminate between recirculation pathways of trunk or perforator type origin. 16 patients, therefore, were classified as unclear phenotypes (Table 1, Table 2, Suppl. Table 4). Six patients were excluded from evaluation, five for primary varices in case of controls, and one for systemic thrombophlebitis without evidence of primary varicose veins (Table 2, Suppl. Table 5).
Blood specimens were collected from all patients during sampling for routine examination. Vein tissue specimens were obtained from all patients during surgery by dissecting an appropriate piece of tissue (at least 1 cm2) from affected or unaffected (controls) veins and transferred into a freezing vial. Blood and vein tissue specimens were kept on ice for a maximum of 6 h until storage at − 80 °C. DNA extraction from frozen blood samples was performed using the Puregene Kit (Qiagen, Hilden, Germany). For DNA extraction from vein tissue specimens, a standard salting out procedure was used. Genotyping for the MTHFR c.677C>T and c.1298A>C polymorphisms was carried out from blood samples of all patients using polymerase chain reaction (PCR) followed by pyrosequencing on a Pyromark Q96 ID Instrument (Qiagen). Primer sequences and PCR conditions are available on request. Of 37 patients that displayed heterozygous genotypes at MTHFR c.677C>T and/or MTHFR c.1298A>C, parallel tests of blood and tissue specimens were performed and identical genotypes were obtained.
2.1. Statistical Methods
To test the association between two categorical variables, a Chi-squared test, or Fisher's exact test was performed as appropriate. To test the association between three or more categorical variables, a log-linear analysis assuming a multinomial distribution was performed. Forward stepwise variable selection was used to determine the final fitted model where the initial model contained all main effects. The appropriateness of the final model was assessed via the deviance. Two models – specifically the deviances of the final fitted model and the fitted model with the individual term of interest removed – were compared to test the significance of individual terms in the log-linear model. Forward selection was preferred over backward selection due to sample size limitations. A p-value less than 0.05 indicated that the individual term significantly contributed to the final fitted model. All statistical analysis was performed in R (v. 3.1.1).
3. Results
There was no association between c.677C>T genotype and control/patient status as well as c.1298A>C and control/patient status (c.677C>T: Fisher's exact test, p = 0.29; c.1298A>C: Fisher's exact test, p = 1). Thus, we only considered those patients who have the condition in the subsequent analysis.
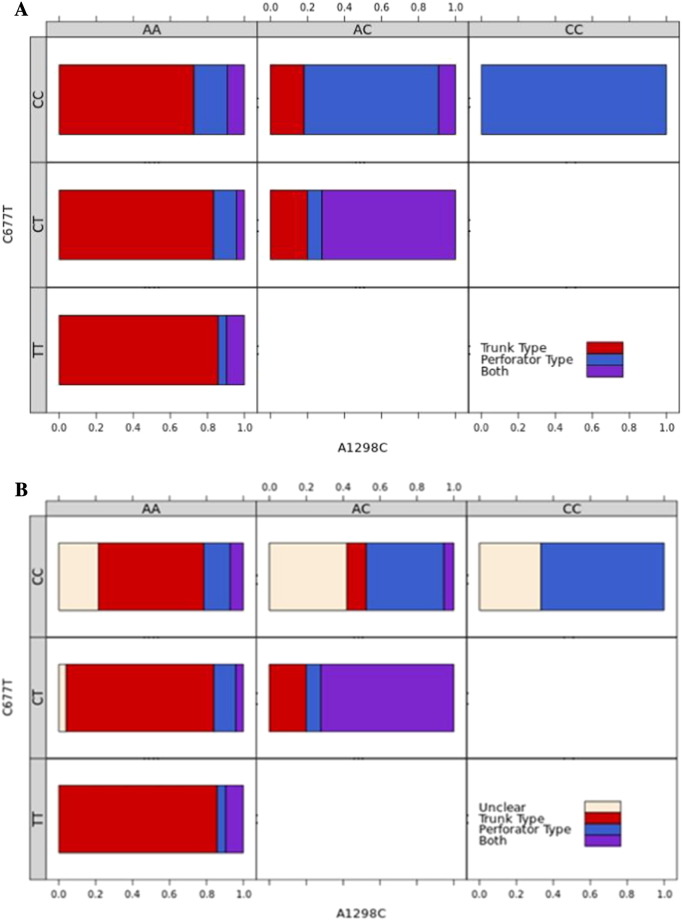
A log-linear analysis, using a log-linear regression model with stepwise selection at a level of 0.05, indicates that heterozygosity or homozygosity for c.677C>T was significantly associated with the trunk phenotype (43/53 patients, 81%, p < 0.01) and heterozygosity or homozygosity for c.1298A>C was significantly associated with the perforator phenotype (18/24 patients, 75%, p < 0.01). Furthermore, as by Fisher´s exact test, double heterozygosity for c.677C>T and c.1298A>C was significantly associated with the combined trunk and perforator phenotype (18/23 patients, 78%, p < 0.01) (Table 2, Fig. 2a). There was also a weak interaction between c.1298A>C and the trunk phenotype, however this did not reach significance (p = 0.051).
Fig. 2.
a) Association between morphological phenotypes and combined genotypes. The proportion of each combined genotype with either trunk phenotype (red), perforator phenotype (blue) or both (purple). Trunk phenotype increases from CC to TT genotypes at c. 677C>T, while perforator phenotype increases from AA to CC genotypes at c.1298A>C. The weak association between homozygous wildtype (AA) genotype at c.1298A>C and trunk phenotype can be seen in the combined genotype CCAA. We would expect to see more subjects with both phenotypes, however, there are fewer subjects with the perforator phenotype, and more with the trunk phenotype than we would expect. Similarly, for the TTAA genotype there are more incidences of both phenotypes than we would expect. When both genotypes are heterozygous, the patient is more likely to present with both phenotypes.
b) Association between unclear phenotypes and combined genotypes. The proportion of each combined genotype with either trunk phenotype (red), perforator phenotype (blue), combined (both) phenotype (purple) or an unclear phenotype (ivory). The unclear phenotype is associated with a homozygous wildtype genotype (CC) at c.677C>T.
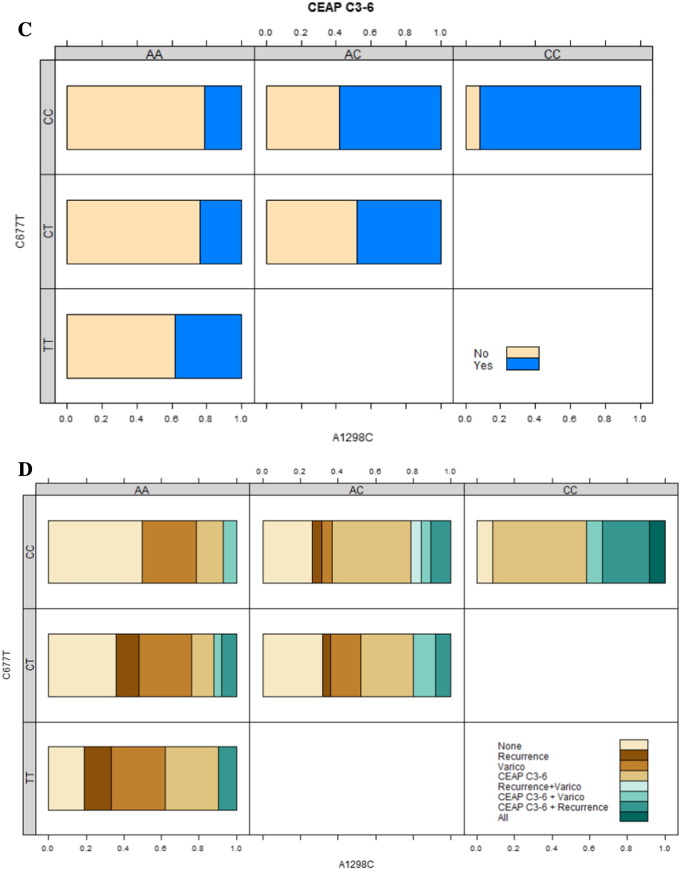
c) Association between CEAP C3–6 complication and combined genotype. Only the homozygous mutant genotype (CC) at c.1298A>C is significantly associated with CEAP C3–6. The risk of a CEAP C3–6 complication increases from AA to CC genotype at c.1298A>C. This would additionally suggest that the perforator phenotype is associated with CEAP C3–6.
d) Association of CEAP C3–6 complication, recurrence, or varicothrombosis with combined genotypes. Green shades indicate at least two complications. Brown shades indicate a single complication, while cream indicates no complication.
A subsequent log-linear analysis including genotype and the unclear phenotype indicates that the unclear phenotype was significantly associated with a homozygous wild type (CC) genotype at c.677C>T (p < 0.01). The unclear phenotype was the highest for the homozygous wild type genotype (CC) regardless of the c.1298A>C genotype, decreased for the heterozygous genotype (CT) and was not present for the homozygous mutant genotype (TT) (Fig. 2b).
Log-linear analysis between genotype and complications indicates that only c.1298A>C was significantly associated with congestive disease (CEAP C3–6, Fig. 2c, Suppl. Tables 3 and 7, p < 0.01). No other significant associations between genotype and complications were recorded although visual inspection suggests a general relationship between mutant genotypes at both polymorphisms and complicated disease and in particular with recurrent disease (Fig. 2d, Suppl. Figs. 2–4). Notably, more patients with junctional type recurrence, mimicking trunk type morphology, presented with c.677C>T mutant genotype compared to those with perforator type recurrence predominantly presenting with mutant c.1298A>C (Suppl. Table 8). A further interaction between varicothrombosis and wildtype genotype, heterozygosity or homozygosity at c.677C>T was suggested (Fig. 2d, Suppl. Fig. 5).
Finally, there appeared to be a significant association between recurrence and varicothrombosis (p = 0.033), as well as between congestive disease and varicothrombosis (p = 0.015) irrespective of genotype.
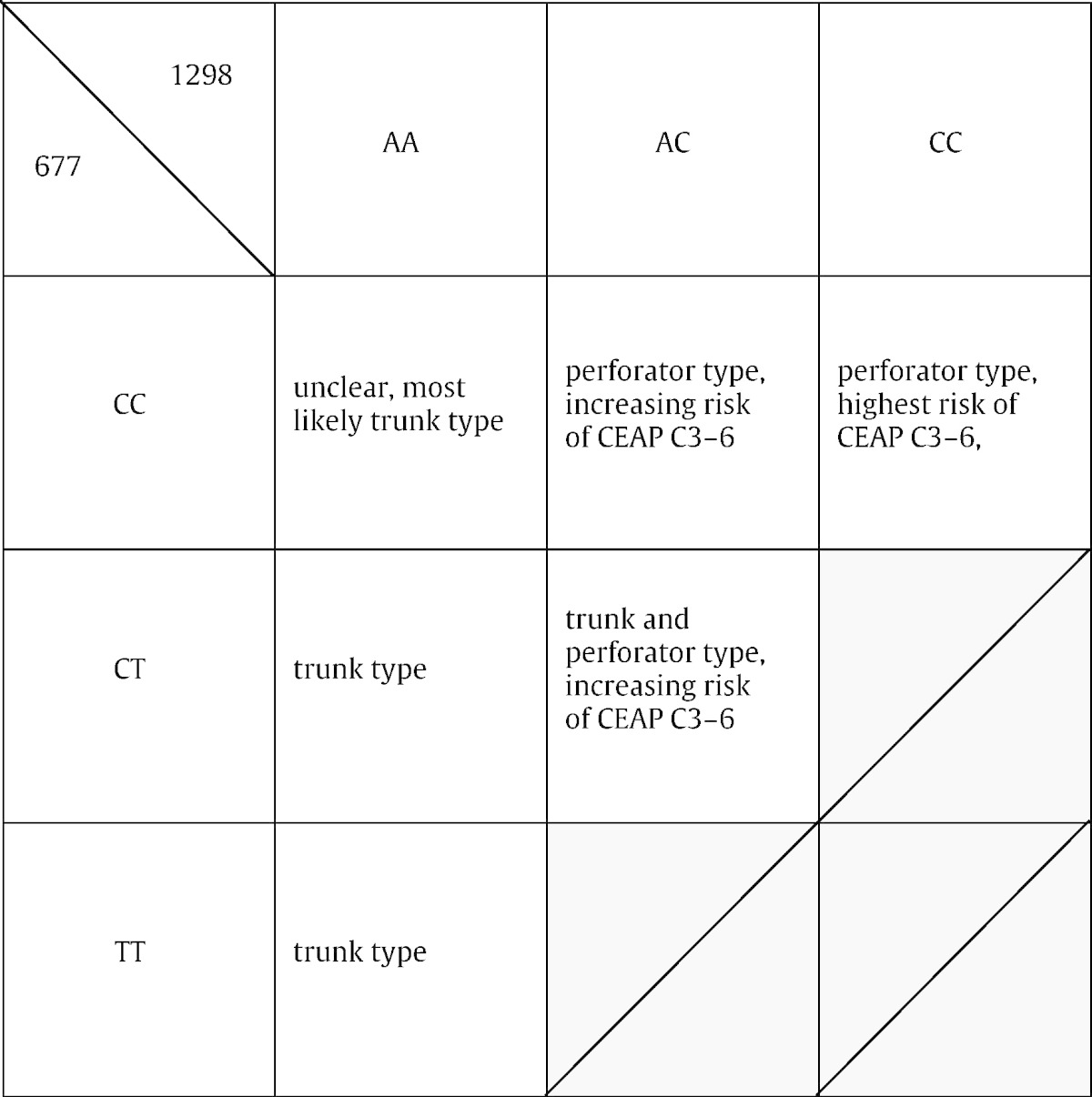
The relationships and genotypes are summarized in Table 3.
Table 3.
Associations of morphological phenotypes and clinical courses with MTHFR c.677C>T and c.1298A>C combined genotypes.
4. Discussion
In this study, we differentially attributed varying morphological presentations and clinical phenotypes of primary varicose vein disorder to the genotypes at MTHFR polymorphisms c.677C>T and c.1298A>C. The axial trunk type morphology was almost exclusively associated with heterozygosity or homozygosity at c.677C>T, the perforator type morphology with heterozygosity or homozygosity at c.1298A>C and in particular double heterozygosity CTAC at c.677C>T and c.1298A>C with the combined trunk and perforator phenotypes.
The association of mutant genotype at c.1298A>C (Table 2, Fig. 2c) with congestive disease was statistically highly significant. The visual association of junctional and perforator type recurrence with mutant genotypes at c.677C>T and c.1298A>C (Fig. 2d, Suppl. Fig. 3, Suppl. Table 8), respectively (although not statistically significant due to small sample size and combined consideration), further indicates a distinct genetic nature in recurrent disease. Finally, a comparable distribution of trunk varices among wildtype, heterozygous or homozygous genotypes at c.677C>T (Fig. 2d, Suppl. Fig. 5) suggests a stochastic origin of varicothrombosis in patients with trunk type morphology. The origin of complicated disease may thus be further linked to specific mutant genotypes at MTHFR (Suppl. Fig. 4; accordingly, however, not statistically significant too at combined consideration), but the detection of further statistically significant differences was precluded due to small sample size and constraints of the statistical methods used.
The assessment of primary varicose veins is routinely performed by clinical and duplex examination. Perforator incompetence contributes to retrograde axial incompetence of the greater or smaller saphenous veins. The more distal incompetent perforators are localized, the easier it is to discriminate between trunk, perforator, or combined trunk and perforator type morphology by duplex examination. This explains the associations between unclear phenotypes and homozygous wildtype (CC) genotype at c.677C>T as well as perforator phenotype and mutant c.1298A>C.
The c.677C>T polymorphism leads to an alanine to valine substitution at codon position 222 (p.Ala222Val) in the presumed catalytic domain of the MTHFR protein, whereas the c.1298A>C polymorphism results in a glutamate to alanine in substitution at codon position 429 (p.Glu429Ala) within the predicted C-terminal regulatory domain of the MTHFR protein (Van der Put et al., 1998). Associations with the MTHFR polymorphisms c.677C>T and c.1298A>C have been reported in arterial occlusive disease (Ilhan et al., 2008), neural tube defects (Van der Put et al., 1998) and late occurrence of colon cancer (Fernández-Peralta et al., 2010). The molecular origins of these associations are unknown and possible associations with complicated phenotypes have not been described. The reduced enzyme activity of the MTHFR polymorphisms c.677C>T and c.1298A>C has been further linked to decreased DNA methylation (Castro et al., 2004) and hyperhomocysteinemia (Sam et al., 2003). Decreased DNA methylation may involve aberrant expression of structural and matrix proteins or reduced DNA integrity resulting in premature aging of venous tissue as essential step within the pathogenesis of primary varicose veins. The complete absence of individuals with a double homozygous TTCC genotype, however, supports a complementary role of both polymorphisms, c.677C>T and c.1298A>C, of MTHFR. Yet, we expect other genetic events to be involved in the pathogenesis of primary varicose veins.
Our results provide a fundamental insight into the nature of primary varicose vein disease. A strong influence of genotypes at MTHFR c.677C>T and c.1298A > C on the morphological specification of primary varicose veins and progression towards complicated disease is suggested. The results provide clues towards the primary origin of congestive (Eklöf et al., 2004, Mozes and Gloviczki, 2004, Whiteley and O'Donnell, 2014) as well as the natural understanding of recurrent (Perrin et al., 2000, Stonebridge et al., 1995) disease and favor an early decision towards surgical therapy in case of homozygous or double heterozygous MTHFR c.677C>T and c.1298A>C mutant genotypes. The described connections indicate possible genetic and epigenetic pathways involved and may help to assess the natural impact of both MTHFR polymorphisms.
Disclosures
The authors declare no conflicts of interest.
Author Contributions
C.W. was the principal investigator, collected blood and tissue samples, and wrote the manuscript, A.C. was involved in statistics and performed parts of genotyping, L.W. did the statistics, prepared the statistical figures and legends, and helped in writing the Methods and Results sections, S.K. collected blood and tissue samples and was involved in study execution, N.G. did parts of genotyping, A.M. was involved in blood and tissue collection and in-hospital management (Duisburg), O.B. contributed to principal planning, discussion of results and the manuscript, H.B. did the statistical planning, P.K.W. was involved in principal planning and execution of the study and collected blood and tissue samples at the Daun hospital, and U.Z. was involved in principal planning and execution of the study, coordinated the genotyping, and participated in writing the manuscript.
Acknowledgments
We thank C. D. Gerharz, Bethesda Krankenhaus Duisburg, for cryopreservation of blood and tissue specimens.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.01.006.
Appendix A. Supplementary data
Supplementary material.
References
- Carpentier P.H., Maricq H.R., Biro C., Poncot-Makinen C.O., Franco A. Prevalence, risk factors, and clinical patterns of chronic venous disorders of lower limbs: a population-based study in France. J. Vasc. Surg. 2004;40:650–659. doi: 10.1016/j.jvs.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Castro R., Rivera I., Ravasco P. 5, 10-methylenetetrahydrofolate reductase (MTHFR) 677C → T and 1298A → C mutations are associated with DNA hypomethylation. J. Med. Genet. 2004;41:454–458. doi: 10.1136/jmg.2003.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu-Thenard A., Boivin P., Baud J.-M., De Vincenzi I., Carpentier P.H. Importance of the familial factor in varicose disease. J. Dermatol. Surg. Oncol. 1994;20:318–326. doi: 10.1111/j.1524-4725.1994.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Eklöf B., Rutherford R.B., Bergan J.J. Revision of the CEAP classification for chronic venous disorders: consensus statement. J. Vasc. Surg. 2004;40:1248–1252. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Evans C.J., Fowkes F.G., Hajivassiliou C.A., Harper D.R., Ruckley C.V. Epidemiology of varicose veins. A review. Int. Angiol. 1994;13:263–270. [PubMed] [Google Scholar]
- Fernández-Peralta A.M., Daimiel L., Nejda N., Iglesias D., Arana V.M., González-Aguilera J.J. Association of polymorphisms MTHFR C677T and A1298C with risk of colorectal cancer, genetic and epigenetic characteristic of tumor, and response to chemotherapy. Int. J. Colorectal Dis. 2010;25:141–151. doi: 10.1007/s00384-009-0779-y. [DOI] [PubMed] [Google Scholar]
- Gloviczki P., Gloviczki M.L. Guidelines for the management of varicose veins. Phlebology. 2012;27(Suppl. 1):2–9. doi: 10.1258/phleb.2012.012s28. [DOI] [PubMed] [Google Scholar]
- Hach W. Ätiologie und Pathogenese der primären Varikose. Dtsch. Med. Wochenschr. 1967;92:1400–1404. [Google Scholar]
- Ilhan N., Kucuksu M., Kaman D., Ilhan N., Ozbay Y. The 677 C/T MTHFR polymorphism is associated with essential hypertension, coronary artery disease, and higher homocysteine levels. Arch. Med. Res. 2008;39:125–130. doi: 10.1016/j.arcmed.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Lim C.S., Davies A.H. Pathogenesis of primary varicose veins. Br. J. Surg. 2009;96:1231–1242. doi: 10.1002/bjs.6798. [DOI] [PubMed] [Google Scholar]
- Milio G., Siragusa S., Minà C. Superficial venous thrombosis: prevalence of common genetic risk factors and their role on spreading to deep veins. Thromb. Res. 2008;123:194–199. doi: 10.1016/j.thromres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Mozes G., Gloviczki P. New discoveries in anatomy and new terminology of leg veins: clinical implications. Vasc. Endovascular Surg. 2004;38:367–374. doi: 10.1177/153857440403800410. [DOI] [PubMed] [Google Scholar]
- Naoum J.J., Hunter G.C. Pathogenesis of varicose veins and implications for clinical management. Vascular. 2007;15:242–249. doi: 10.2310/6670.2007.00069. [DOI] [PubMed] [Google Scholar]
- Pannier F., Rabe E. The relevance of the natural history of varicose veins and refunded care. Phlebology. 2012;27(Suppl. 1):23–26. doi: 10.1258/phleb.2012.012s23. [DOI] [PubMed] [Google Scholar]
- Perrin M.R., Guex J.J., Ruckley C.V. Recurrent varices after surgery (REVAS), a consensus document. Cardiovasc. Surg. 2000;8:233–245. [PubMed] [Google Scholar]
- Raffetto J.D. Superficial thrombophlebitis in varicose vein disease — the particular role of methylenetetrahydrofolate reductase. Phlebology. 2011;26:133–134. doi: 10.1258/phleb.2011.011e02. Editorial. [DOI] [PubMed] [Google Scholar]
- Sam R.C., Burns P.J., Hobbs S.D. The prevalence of hyperhomocysteinemia, methylene tetrahydrofolate reductase C677T mutation, and vitamin B12 and folate deficiency in patients with chronic venous insufficiency. J. Vasc. Surg. 2003;38:904–908. doi: 10.1016/s0741-5214(03)00923-6. [DOI] [PubMed] [Google Scholar]
- Somers P., Knaapen M. The histopathology of varicose vein disease. Angiology. 2006;57:546–555. doi: 10.1177/0003319706293115. [DOI] [PubMed] [Google Scholar]
- Stonebridge P.A., Chalmers N., Beggs I., Bradbury A.W., Ruckley C.V. Recurrent varicose veins: a varicographic analysis leading to a new practical classification. Br. J. Surg. 1995;82:60–62. doi: 10.1002/bjs.1800820121. [DOI] [PubMed] [Google Scholar]
- Sverdlova A.M., Bubnova N.A., Baranovskaya S.S., Vasina V.I., Avitisjan A.O., Schwartz E.I. Prevalence of the methylenetetrahydrofolate reductase (MTHFR) C677T mutation in patients with varicose veins of lower limbs. Mol. Genet. Metab. 1998;63:35–36. doi: 10.1006/mgme.1997.2638. [DOI] [PubMed] [Google Scholar]
- Van der Put, Gabreëls F., Stevens E.M. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am. J. Hum. Genet. 1998;62:1044–1051. doi: 10.1086/301825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M.S., O'Donnell T.F. Debate: whether venous perforator surgery reduces recurrences. J. Vasc. Surg. 2014;60:796–803. doi: 10.1016/j.jvs.2014.06.102. [DOI] [PubMed] [Google Scholar]
- Wilcken B., Bamforth F., Li Z. Geographical and ethnic variation of the 677C > T allele of 5, 10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. J. Med. Genet. 2003;40:619–625. doi: 10.1136/jmg.40.8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmanns C., Casey A., Schinzel H., Walter P.K. Superficial thrombophlebitis in varicose vein disease: the particular role of methylenetetrahydrofolate reductase. Phlebology. 2011;26:135–139. doi: 10.1258/phleb.2009.009075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.