Abstract
The current study evaluated age differences in conditioned pain modulation using a test stimulus that provided the opportunity to evaluate changes in heat pain sensitivity, sensitization, and desensitization within the same paradigm. During this psychophysical test, pain intensity clamping uses REsponse Dependent STIMulation (REDSTIM) methodology to automatically adjust stimulus intensity to maintain a desired pain rating set-point. Specifically, stimulus intensity increases until a pre-defined pain rating (the setpoint) is exceeded, and then decreases until pain ratings fall below the setpoint, with continued increases and decreases dictated by ratings. The subjects are blinded in terms of the setpoint and stimulus intensities. Younger and older subjects completed two test sessions of two REDSTIM trials, with presentation of conditioning cold stimulation between the trials of one session but not the other. The results indicated that conditioning cold stimulation similarly decreased the overall sensitivity of younger and older subjects, as measured by the average temperature that maintained a setpoint rating of 20 (on a scale of 0–100). The conditioning stimulus also significantly enhanced sensitization following ascending stimulus progressions and desensitization following descending stimulus progressions in older subjects relative to younger subjects. Thus, older subjects experienced greater swings in sensitivity in response to varying levels of painful stimulation. These results are discussed in terms of control over pain intensity by descending central modulatory systems. These findings potentially shed new light on the central control over descending inhibition and facilitation of pain.
Keywords: Older adults, Pain inhibition, Pain modulation, Response dependent stimulation (REDSTIM), Pain sensitivity, Aging
1. Introduction
Psychophysical studies of healthy adults have shown that a noxious conditioning stimulus reduces pain from a remote second stimulus [1–3]. The central systems activated by this paradigm, known as Conditioned Pain Modulation (CPM), can reduce experimentally-induced pain evoked by various types of stimulation, including thermal stimulation [4]. The mechanisms underlying desensitization by CPM are thought to involve spinal–medullary–spinal pathways and widespread inhibition of nociceptive wide dynamic range (WDR) neurons [5]. More recent research has revealed both excitatory and inhibitory influences on spinal nociceptive coding by projection systems descending from the brainstem [6,7]. Importantly, these brainstem systems are controlled independently, so that an apparent loss of inhibition could result from enhanced sensitization, and reduced sensitization could appear to be enhanced inhibition. Thus, when attempting to elucidate mechanisms for altered pain sensitivity and/or abnormal pain, it is advantageous to evaluate both sensitization and desensitization.
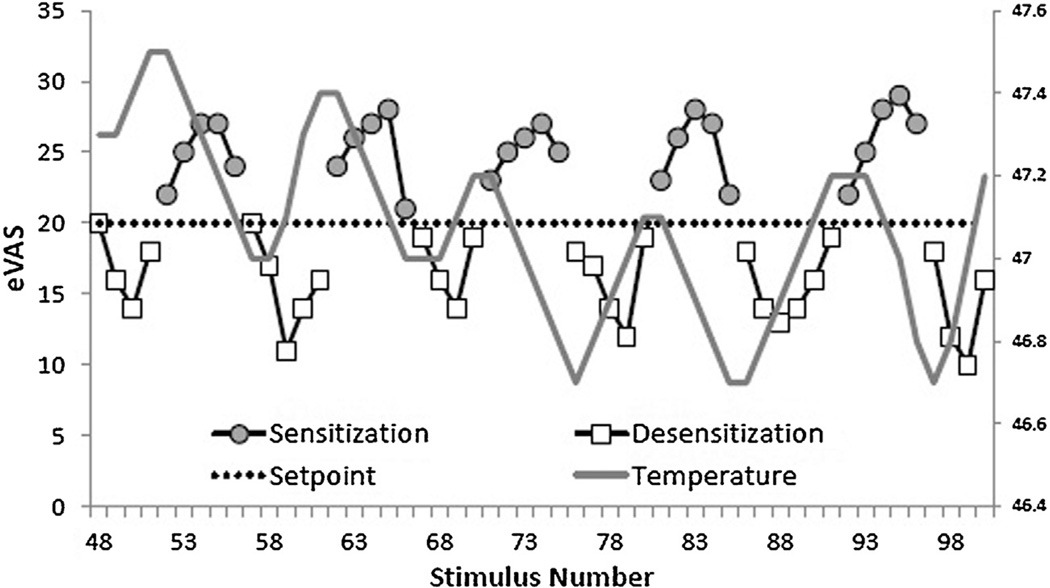
A novel psychophysical procedure involving dynamically changing levels of stimulation designed to clamp (i.e., maintain) pain intensity near a desired set-point provides an opportunity to evaluate trends of sensitization and desensitization within the same paradigm [8–10]. This test, termed REsponse Dependent STIMulation (REDSTIM), involves computer-controlled adjustments of stimulus intensity (i.e., thermode temperature) to maintain a desired pain rating (setpoint). Pain intensity is monitored continuously with a real time electronic visual analogue scale (eVAS). Pain ratings oscillate around the setpoint in response to ascending and descending temperature changes (Fig. 1). For example, when pain ratings are below the setpoint (20 on a 0–100 scale), the stimulus temperature will increase until the pain ratings reach or exceed the setpoint. Conversely, when pain ratings exceed the setpoint, the stimulus temperature will decrease until the ratings reach or fall below the setpoint. Prior work has revealed that REDSTIM methodology establishes an average temperature that maintains the setpoint (overall sensitivity) and also reveals trends of sensitization following ascending series of stimulus intensities and desensitization following descending series of intensities. When pain ratings first exceed the setpoint and the stimulus temperature begins to decrease, the ratings continue to increase before descending (i.e., sensitization). Similarly, when pain ratings first drop below the setpoint and the stimulus temperature begins to rise, the ratings continue to descend before increasing (i.e., desensitization). These trends may reflect excitatory and inhibitory mechanisms of pain modulation [8].
Fig. 1.
Successive eVAS ratings of thermal pain are shown for a single subject during a REDSTIM trial, starting in the middle of the test session and continuing through 6 cycles of desensitization and 5 cycles of sensitization. Ratings above 20 are shown as shaded circles and are labeled as periods of sensitization. The temperature at the beginning of each period of sensitization was equal to or higher than subsequent temperatures which decreased until a rating below the setpoint occurred. Initial ratings below 20 began new periods labeled as desensitization (open squares). The temperature at the beginning of each desensitization period was equal to or lower than subsequent temperatures which increased until a rating above the setpoint occurred, beginning a new period of sensitization.
The REDSTIM procedure may be considered a modification of staircase methods such as stimulus titration, which has been utilized for some time to track thresholds for pain detection [11]. The REDSTIM method is a modification of these procedures with an important advantage. During threshold tracking, subjects are aware that transitions between ascending and descending series are dictated by pain threshold. This presents the opportunity to prevent presentation of painful stimuli by responding early in ascending series, driving thresholds toward stimulus detection rather than pain detection. During REDSTIM trials, the subjects are not aware of transitions between ascending and descending series, as is apparent from ascending and descending trends (see Fig. 1).
The primary aim of this study was to evaluate the capability of the central inhibitory mechanisms activated during CPM to modulate overall pain sensitivity and trend-induced sensitization and desensitization during prolonged REDSTIM in healthy older and younger adults. We hypothesized that conditioning stimulation would reduce overall pain sensitivity during REDSTIM in younger adults (i.e., increase the average temperature at which the setpoint was maintained), coupled with enhanced desensitization and/or reduced sensitization. However, previous studies show that older adults can exhibit no effect of CPM [12–14] or increased thermal pain [4,16–18] during or following exposure to a painful conditioning stimulus, in contrast to pain reduction for younger subjects. Therefore, we hypothesized that older adults would exhibit no change in overall pain sensitivity during REDSTIM following conditioning stimulation, coupled with increased sensitization and/or reduced desensitization. A secondary aim of this study was to evaluate age differences in pain sensitivity, sensitization, and desensitization during REDSTIM at baseline. Previous investigations have indicated that sensitivity to suprathreshold painful stimulation does not change with age [4,19–21]; therefore, we hypothesized no age differences in overall sensitivity, sensitization or desensitization at baseline.
2. Methods
2.1. Participants
Twenty-four healthy younger adults (age = 23.62 years, SD = 3.96; range: 20–34; 8 Female) and nineteen healthy older adults (age = 64.04 years, SD = 7.13; range: 55–77; 13 Female) participated in this study. A recent study showed that adults 55 years and older have reduced CPM [4]; therefore, we are including adults 55 years and older in our older adult group. The racial composition of the younger group was 6 Caucasian, 6 Hispanic, 8 Asian Americans, 2 African Americans, and 2 other. The older adult group included 18 Caucasians and 1 African American. Participants were recruited in the local community by posted advertisements. The study procedures were approved by the University’s Institutional Review Board, and all participants gave written informed consent. The exclusion criteria for being a participant in the study were: (1) inability to reliably rate pain (e.g., pain ratings do not scale in proportion to changes in temperature across varying contact times), (2) current use of narcotics or any tobacco products; chronic use of analgesics, (3) serious systemic disease (e.g., diabetes and thyroid problems, (4) uncontrolled hypertension, (5) cardiovascular or pulmonary disease, (6) neurological problems with significant changes in somatosensory and pain perception at the intended stimulation sites, (7) serious psychiatric conditions (e.g., schizophrenia and bipolar disorder), and (8) chronic pain or any ongoing pain problem (headaches, injury-related pain, etc.). Because potential participants were informed of exclusion criteria prior to the study, no participants were excluded after signing the informed consent form. Participants were also instructed to refrain from use of coffee or any pain medications prior to the experimental sessions on those days.
2.2. Study procedures
2.2.1. Orientation and training session
The orientation and training session lasted approximately 2 h and occurred on a separate day than the experimental sessions. Persons who expressed interest in the study were provided information about the procedures and signed an Informed Consent Form. To determine eligibility, subjects completed a health history questionnaire, supplemented by interview. Subjects then completed a training session to ensure comfort during the testing protocol and to teach them the continuous pain rating system. During this session, subjects also completed the Short-Form Health Survey (SF-36) to assess physical and mental health status [22].
2.2.2. Testing sessions
Participants completed two experimental sessions on non-consecutive days following the orientation and training session. The average number of days between sessions was 4.1 ± 3.7 days for younger adults and 3.8 ± 3.4 days for older adults. One session tested for CPM and the other session served as a control session. The order of the two sessions was counterbalanced among participants. Upon arriving at the laboratory, participants sat on a comfortable chair and relaxed for several minutes. Next, participants were asked about their health and medication use and were shown a video that described the experimental procedures.
During the control session, participants completed two 150 s REDSTIM trials administered to the thenar eminence of the left palm. Ten minutes separated the two trials during which the participant sat quietly.
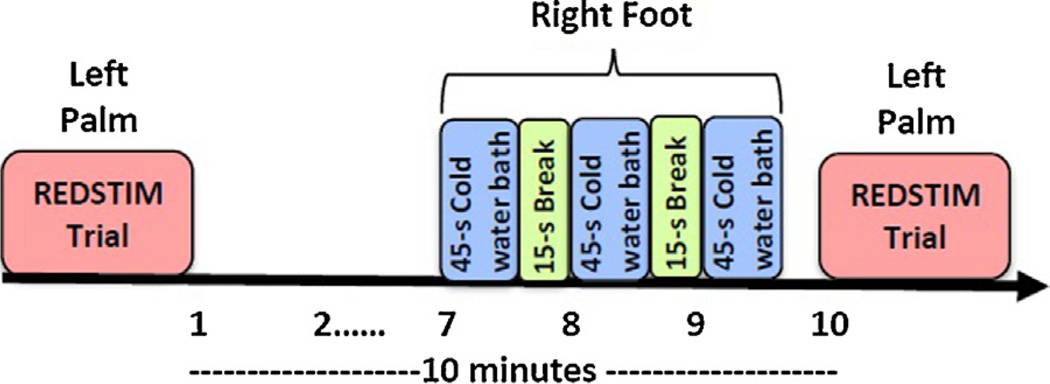
CPM was tested by comparing two 150 s REDSTIM trials (test stimulus) administered to the thenar eminence of the left palm. Ten minutes separated the two trials, with the 3 min conditioning stimulus applied to the right foot just prior to the second trial (Fig. 2).
Fig. 2.
Timeline of a CPM session.
2.2.3. Test stimulus: Response dependent stimulation (REDSTIM)
The response dependent stimulation paradigm presented thermal stimuli with a flat 23 mm × 23 mm copper contact thermode. The thermode was electronically held at the desired temperature by a Peltier thermoelectric device. At the beginning of a trial, the thermode was brought into light skin contact of the thenar eminence of the left palm of reproducible force by solenoid activation. Experimental pain was monitored continuously throughout each trial with an electronic version of a visual analog scale (eVAS) [23]. The eVAS ratings were derived from the output of a low-friction sliding potentiometer with 100 mm of travel. The left endpoint of the scale was identified as “no pain”, while the right endpoint was defined as “intolerable pain”. The position of the slider was electronically converted into a pain rating between 0 and 100. The sliding potentiometer was mounted into the surface of a small inclined desk positioned to facilitate precise operation with minimal fatigue. A pain intensity setpoint was defined as 20 on a 100-point scale, and an algorithm in the control software calculated deviations of the participants’ actual pain ratings from the setpoint as well as the derivative of each error. These data were the bases for automatic adjustments of the stimulus temperature to maintain an average pain rating that equaled the setpoint. The setpoint of 20 was chosen because it allows the experience of mild to moderate levels of pain during continuous stimulation with no risk of intolerable pain.
REDSTIM is composed of an induction and maintenance phase, as participants continuously rate pain intensity. During the induction phase, the thermode temperature increased from 35 °C with temperature steps decreasing in size as pain ratings approached the setpoint of 20. Once pain intensity ratings reached the setpoint, a 150 s maintenance phase began. The pain intensity signal from the eVAS was sampled 1.5 s following each change in thermode temperature, which was automatically adjusted at 1.7 s intervals. When pain intensity was rated below the setpoint, the next temperature increased, and this continued until the ratings reached or exceeded the setpoint. Conversely, when pain ratings exceeded the setpoint the temperature decreased until the ratings reached or fell below the setpoint. If pain ratings equaled the set point, then the temperature did not change. The size of the temperature step increments was (1) proportional to the deviation from the setpoint, (2) a function of the rate of change of pain intensity and, (3) dependent on whether the ratings were increasing, decreasing or not changing. The temperature step increments ranged from 0.1 °C to 0.3 °C, with smaller steps as the ratings approached the setpoint. A temperature limit was set to prevent thermal injury of participants that were pain insensitive (51.0 °C). The custom-built testing system integrated all inputs (eVAS signal) and outputs (stimulus temperature and timing).
2.2.4. Conditioning stimulus: Cold pressor pain (CPP)
Participants were instructed to immerse their right foot to the ankle into a cold water bath. A refrigerated water circulator (Neslab, Portsmouth, NH) cooled a 10″ × 18″ insulated water bath. Water was continuously recirculated to prevent local warming around the foot and was maintained at a constant temperature (10 °C for men; 12 °C for women). In order to have a conditioning stimulus that induced similar levels of pain in men and women, two different temperatures were used during the cold pressor test. Generally, men show less pain sensitivity to cold water baths [24]; thus, men received a lower test temperature. The water bath manipulation included three 45 s immersion trials, separated by 15 s [4]. Participants rated their pain every 15 s of immersion, using a 0–100 scale. Participants were instructed that they could remove their foot if the water became too cold.
2.3. Data reduction
Descriptive statistics were calculated for age, the SF-36 Mental Health scale, and the SF-36-Physical Health scale. The REDSTIM paradigm provides the opportunity to assess overall pain sensitivity and sensitizing and desensitizing trends. Below is a description of the measures used to assess these variables.
2.3.1. Overall heat pain sensitivity
The average temperature needed to maintain the setpoint represented the level of pain sensitivity throughout the 150 s maintenance period of REDSTIM trials. Positive and negative oscillations in ratings around the setpoint were analyzed separately as sensitizing and desensitizing trends.
2.3.2. Sensitizing trends
Pain ratings above the setpoint progressed in positive half-cycles within descending temperature progressions that began when a stimulus at the end of an ascending temperature progression was rated at or just above the setpoint. Ratings progressed to a peak as the temperature decreased, and then they returned to a value at or below the setpoint to begin a negative half cycle. The positive half-cycles defined sensitizing trends to the extent that ratings above the setpoint persisted as the probe temperature declined from the first to the last stimulus in the half-cycle (Fig. 1). Positive half-cycles were identified for each subject, test condition and trial. Then for each half-cycle, deviations of pain ratings from the setpoint were summed over time to produce an area under the curve (AUC) value. Finally, the average AUC of positive half-cycle ratings was calculated for each subject, test condition and trial. From this point forward, this dependent variable will be referred to as “positive-AUC”.
2.3.3. Desensitizing trends
Ratings below the setpoint in negative half-cycles began with the first temperature in ascending progressions that was rated beyond the setpoint, continued to a low peak rating and then returned to the setpoint. These negative half-cycles defined desensitizing trends to the extent that ratings below the setpoint persisted as the probe temperature increased from the first to the last stimulus in the half-cycle (Fig. 1). Negative half-cycles were identified for each subject, test condition and trial. Then for each half-cycle, deviations of pain ratings from the setpoint were summed over time to produce an AUC value. Finally, the average area under the curve (AUC) of negative half-cycles was calculated for each subject, test condition and trial. From this point forward, this dependent variable will be referred to as “negative-AUC”. AUC was chosen as the primary dependent variable for sensitization and desensitization because inspection of the data revealed that both the duration and the amplitude of ascending and descending pain fluctuations increased with age following conditioning modulation. AUC is an effective way to simply express this combined effect and has been used in past research as an outcome variable for continuous heat pain ratings [4].
2.4. Data analysis
First, age differences in overall pain sensitivity, sensitization, and desensitization during REDSTIM were examined. For each dependent variable, scores for trial 1 of each session were averaged to create a baseline score. Each baseline score was analyzed with a 1-way ANCOVA, with age group as the between subjects factor and sex added as a covariate. Average temperature was added as a covariate for all other variables.
Secondly, we determined whether CPM altered the thermode temperature needed to maintain the setpoint from trial 1 to trial 2. The average temperature was analyzed with a 3-way mixed model ANCOVA, with age group as the between subjects factor and session (CPM, Control) and trial (1, 2) as the within subject factors. Sex and average CPP ratings were added as covariates. If the sphericity assumption was violated, then Greenhouse-Geisser degrees of freedom corrections were applied to obtain the critical p-value. Post-hoc comparisons were made with Tukey’s HSD procedure. To determine the magnitude of pain inhibition, effect sizes for each session were calculated using Cohen’s d, defined as the mean for trial 1 minus the mean for trial 2, divided by the pooled within group standard deviation (d = [Xtrial1 − Xtrial2]/pooled standard deviation). Due to the within subjects design, the effect sizes were adjusted as recommended by Portney and Watkins [25]. Effect sizes were calculated for each age group and gender separately within each age group. Reductions in pain sensitivity are reflected by positive effect sizes. Cold water pain ratings were also analyzed with a 1-way ANCOVA with age as the factor and sex added as a covariate.
The third goal was to determine whether the effects of the conditioning stimulus on sensitization (positive-AUC) and desensitization (negative-AUC) differed by age. Change scores for positive-AUC and negative-AUC were calculated for each session (i.e., control and CPM) by subtracting the AUC value for trial 1 from the AUC value for trial 2. Then, the control change score was subtracted from the CPM change score. These adjusted change scores provide a controlled measure of the degree to which sensitizing and desensitizing trends changed as a function of the conditioning stimulus. To determine age differences in the magnitude of CPM effects on sensitization and desensitization, each adjusted change score was analyzed with a 1-way ANCOVA, with age group as the between subjects factor and sex and average CPP rating added as covariates. A level of p ≤ .05 was used for all statistical analyses. For sensitization, a greater positive-AUC change score indicates that sensitization increased following the conditioning stimulus. For desensitization, a greater negative-AUC change score indicates that desensitization increased following the conditioning stimulus. To determine the magnitude of the age differences in sensitization and desensitization, effect sizes were also calculated using Cohen’s d. Cohen’s d was defined as the mean for the young adults minus the mean for the older adults, divided by the pooled within group standard deviation (d = [Xyoung − Xold]/pooled standard deviation). Effect sizes were calculated for men and women separately. A negative effect size reflects greater sensitization/desensitization by the older adults.
A Chi-Square test was conducted to determine if sex distribution differed between age groups. Shapiro–Wilk’s test of normality indicated that the data from the questionnaires were not normally distributed; thus Mann–Whitney U tests were conducted to determine if the SF-36 mental and physical health scales differed by age.
3. Results
3.1. Subject characteristics
A chi-square test indicated that sex distribution differed between age groups, p = .033. No significant differences existed between older and younger subjects on Mental Health status on the SF-36 (younger adults = 84.32 ± 12.95, older adults = 81.47 ± 10.93). However, younger subjects self-reported significantly better physical health status on the SF-36 compared to older subjects (younger adults = 90.17 ± 10.48, older adults = 80.73 ± 18.85). In comparison with national Normative Data of the SF-36, these data indicate that our older adult sample reported extremely better physical and mental health compared with the average adult ranging in age from 55 to 64 years (Physical Health Score Norm = 47.44, Mental Health Score Norm = 51.71).
3.2. Baseline differences in pain sensitivity between age groups
Table 1 presents the average baseline values for each dependent variable for older and younger participants. The univariate analyses revealed no significant baseline differences between older and younger adults on any of the variables. Also, no significant differences existed between the age groups on cold water bath pain ratings (p = .398, Older group: M = 46.43, SD = 31.10; Younger group: M = 54.63, SD = 24.06). Seven subjects removed their foot because the cold water became too painful (3 young females, 1 older female, 2 young males, and 1 older male).
Table 1.
Descriptive characteristics for the baseline REDSTIM variables for younger and older adults.
| Variable | Older adults | Younger adults | p-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Average temperature during REDSTIM (°C) | 46.03 ± 2.03 | 45.60 ± 2.37 | 0.39 |
| Positive wave AUC, pain units | 127.61 ± 56.97 | 126.13 ± 80.06 | 0.98 |
| Negative wave AUC, pain units | 114.56 ± 33.99 | 98.99 ± 24.86 | 0.12 |
3.3. CPM effect on overall sensitivity during trials
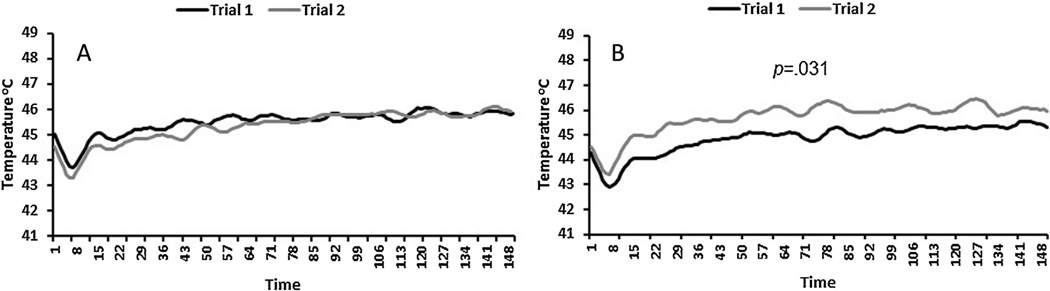
Group, session, and trial main effects were not significant. However, CPM differentially affected the average temperature that maintained a setpoint of 20 for all subjects. The session × trial interaction term was significant, F(1,39) = 4.28, p = .045. A follow-up test indicated that the average temperature needed to maintain a setpoint of 20 during REDSTIM significantly increased from trial 1 (M = 45.54, SE = .36) to trial 2 (M = 46.24, SE = .34) during the CPM session, p = .031. Thus, pain sensitivity decreased for all participants following the cold water conditioning stimulus (Fig. 3). The magnitude of the pain inhibition during the CPM session for the entire sample was moderate, d = 0.42 (Younger adults: male d = 0.31, female d = 0.76; Older adults: male d = 0.55, female d = 0.35).
Fig. 3.
Average real-time thermode temperatures for all subjects during the 150 s maintenance phase of REDSTIM for Trials 1 and 2 during the control session (A) and the CPM session (B). After the conditioning stimulus in a CPM session, between trials 1 and 2, the average temperature needed to maintain a setpoint of 20 increased for all subjects.
3.4. CPM effect on sensitization as a function of age
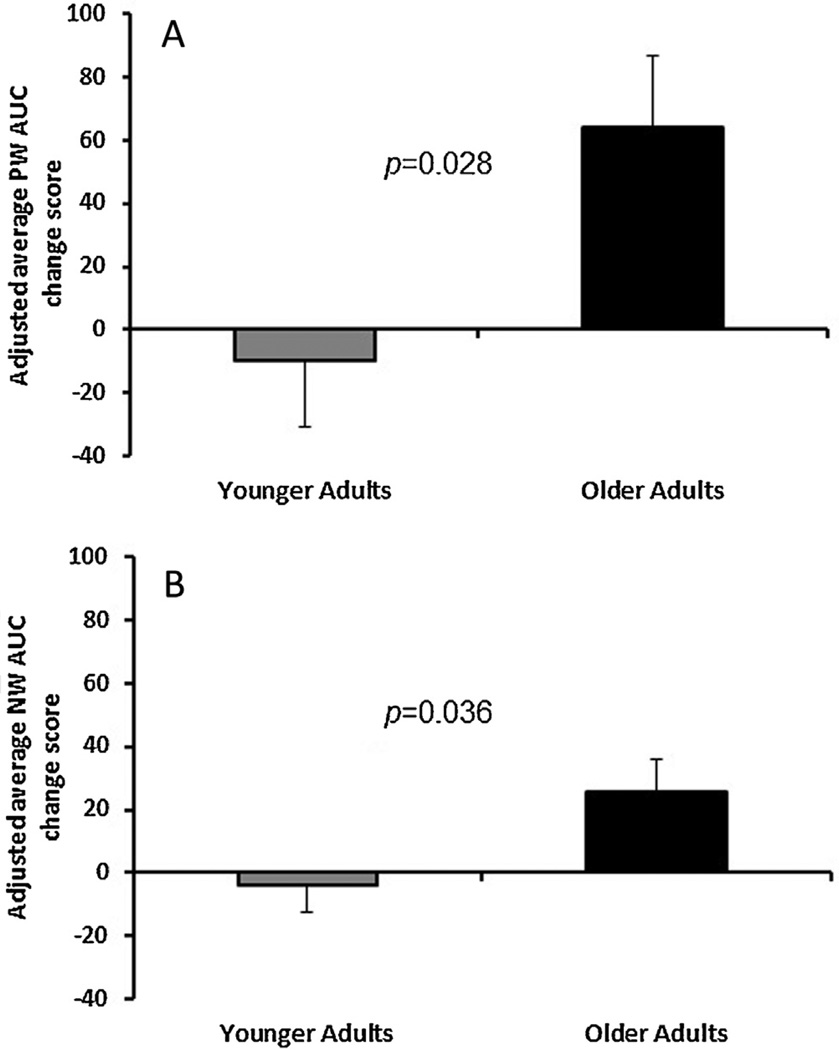
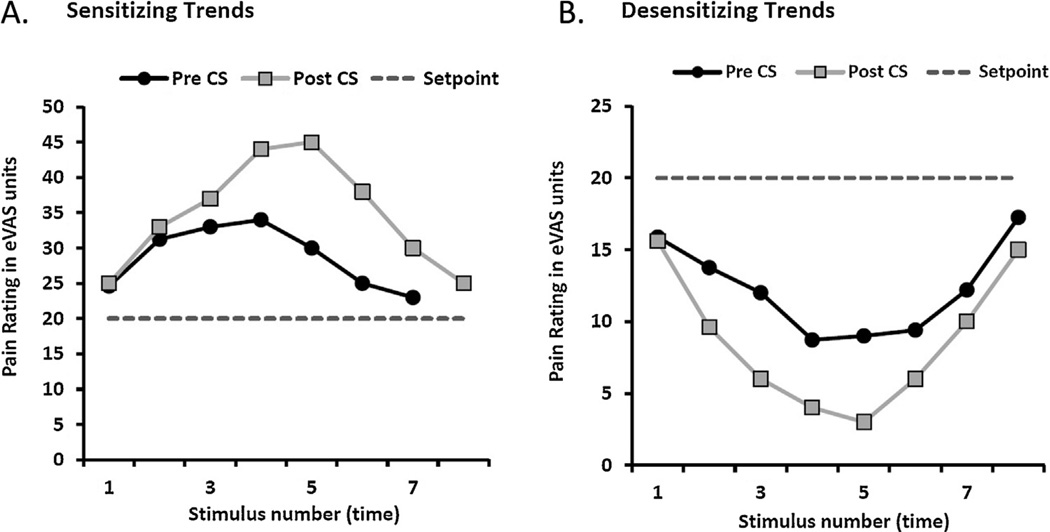
The ANOVA revealed a significant effect of CPM on positive-AUC scores as a function of age, F(1,39) = 5.23, p = .028. The positive-AUC change score was greater for older adults compared to younger adults. The older adults exhibited a substantial increase in AUC for the positive half-cycles following the conditioning stimulus, whereas younger adults showed a small decrease in AUC following conditioning stimulation (Fig. 4A). Thus, trends of sensitization were increased during the CPM paradigm for older compared to wave younger adults (Effect sizes: males d = −0.43; females d = −0.54). Fig. 5A shows the eVAS ratings of pain during the average positive half-cycles pre and post exposure to the conditioning stimulus for a single older subject.
Fig. 4.
Age differences in the adjusted average AUC for positive half-cycles (A) and negative half-cycles (B) during REDSTIM, following the conditioning stimulus. The adjusted positive and negative AUC change scores were significantly greater for older compared to younger adults. PW, positive wave; NW, negative wave.
Fig. 5.
eVAS ratings of pain above the setpoint of 20 (A: Sensitizing trends) and below the setpoint (B: Desensitizing trends), pre- and post-exposure to the conditioning stimulus for a single older subject. CS, conditioning stimulus.
3.5. CPM effect on desensitization as a function of age
For negative-AUC change scores, the ANOVA revealed a significant effect of CPM as a function of age: F(1,39) = 4.73, p = .036. The negative-AUC change score was greater for older adults compared to younger adults. The older adults exhibited a significant increase in AUC for the negative half-cycles following the conditioning stimulus, whereas younger adults showed a minimal decrease in AUC following conditioning stimulation (Fig. 4B). Thus, trends of desensitization also were enhanced during the CPM paradigm for older compared to younger adults (Effect sizes: males d = −0.58; females d = −0.66). Fig. 5B shows the eVAS ratings of pain during the average negative half-cycles pre and post exposure to the conditioning stimulus for a single older subject.
4. Discussion
This study evaluated CPM and age-related effects with a novel and sophisticated psychophysical procedure that provides the opportunity to evaluate trends of desensitization and sensitization within the same paradigm. Pain intensity clamping with the use of REDSTIM methodology to evaluate suprathreshold pain sensitivity offers advantages compared to traditional psychophysical tests. Specifically, participants are blinded to stimulus parameters (i.e., thermode temperature) and are free from direct participant–experimenter interactions, overcoming confounds that can be otherwise unavoidable. Additionally, standardizing a setpoint rating permits safe administration of prolonged suprathreshold stimulation that does not escalate to unacceptable levels. Finally, the REDSTIM paradigm has features which mimic clinical pain states that can be prolonged and fluctuate in intensity. Three important findings emerged from these data. First, no age differences existed in overall pain sensitivity, or sensitizing and desensitizing trends during baseline REDSTIM testing. Secondly, younger and older adults exhibited similar decreases in overall pain sensitivity during REDSTIM following the painful conditioning stimulus. Third, CPM enhanced trends of sensitization and desensitization for older adults relative to younger adults.
4.1. Age differences in REDSTIM at baseline
Numerous studies of age differences in pain sensitivity have involved threshold tests of sensitivity to brief nociceptive stimulation, with older adults demonstrating a higher pain threshold or no difference from younger subjects [18,26]. Identification of threshold pain requires subjects to distinguish between a painful and a non-painful sensation (e.g., warmth and mild heat pain). A number of factors can affect pain threshold responses, including primary afferent dysfunction [27,28] and attentional deficits [7]; both of which are known to increase with age and lead to slower reaction times [29]. Therefore, interpreting threshold elevations for older compared to younger subjects as a reduction in sensitivity to comparable nociceptive inputs is questionable. In contrast, suprathreshold pain powerfully captures a subject’s attention [30,31], and ratings of suprathreshold pain do not rely on reaction times. Studies testing for age differences in suprathreshold pain ratings have yielded mixed results with no apparent unifying effect [20,21,24]. Of particular relevance to the present study, ratings of pain during moderate intensity heat stimulation are not related to age [4,18,20]. Thus, the lack of differences between older and younger adults in overall heat pain sensitivity at baseline for a REDSTIM setpoint of 20 is not surprising.
We also failed to find age-differences in trends of sensitization and desensitization during the baseline REDSTIM trials. Several studies have tested for age differences in facilitation of pain or central sensitization using temporal summation protocols, with older adults exhibiting enhanced summation particularly at lower frequencies of stimulation [18,20,33]. These protocols involve the delivery of brief repetitive noxious stimuli held at a constant intensity. Facilitation of pain by temporal summation reflects a nonlinear integration of C-fiber nociceptive inputs by the CNS, coupled with suppression of A-delta type-2 nociceptor input [34]. In contrast, the REDSTIM protocol involves dynamic step changes during continuous stimulation that likely involves increasing inputs from A-delta and C-fibers in response to each stepped increase in stimulus intensity [35]. Thus, pain sensations induced by the REDSTIM protocol are likely mediated by a unique set of mechanisms relative to temporal summation of pain. Direct comparisons of REDSTIM-induced and temporal summation-induced sensitization are needed to determine whether these phenomena share common mechanisms.
4.2. Effects of CPM on pain sensitivity of young and older subjects
When primed by cold conditioning stimulation, the overall pain sensitivity of younger subjects was reduced during REDSTIM, measured as an increased average temperature that maintained a setpoint rating of 20. This result confirms other demonstrations of an inhibitory effect of CPM on pain [1–4,36]. However, the reduction in pain sensitivity following CPM was comparable for younger and older subjects in the present study, whereas previous studies have described less inhibition for older subjects [4,12–15] or inhibition for young and facilitation for older subjects during CPM paradigms [16,20]. An important factor in these differences may be the oscillations in stimulation intensity during long duration REDSTIM that could mask or interfere with other sources of inhibition or facilitation. Additionally, our questionnaire data indicate that the older adult group was very healthy compared to the average older adult, which could partially explain the lack of age differences in the effect of CPM on pain sensitivity.
4.3. Effects of CPM on sensitizing and desensitizing trends of young and older subjects
Importantly, if REDSTIM methodology did not separately evaluate sensitization and desensitization, we would have interpreted the absence of an age effect of CPM on overall pain sensitivity in terms of no difference in inhibition or facilitation of pain for older subjects. However, our results revealed that pain modulation, as reflected by both sensitizing and desensitizing trends, was enhanced for older compared to younger subjects following a cold water challenge (i.e., CPM). As hypothesized for older adults, elevated pain ratings during the positive half cycles (i.e., AUC above the set point) persisted to a greater degree following the cold water bath, whereas a small decrease in AUC of positive half-cycles was observed for younger adults. Unexpectedly however, lower pain ratings during the negative half cycles (i.e., AUC below the set point) also persisted to a greater degree following the cold water bath for older adults, while a small decrease was observed for younger adults. The capacity to assess both facilitatory and inhibitory influences during REDSTIM assists in the understanding of why CPM reduced average pain ratings equally for younger and older subjects. The balance between trends of sensitization and desensitization was not different for younger and older subjects, with or without CPM. CPM slightly reduced both sensitizing and desensitizing trends of younger subjects, and it increased sensitizing and desensitizing trends comparably for older subjects. The sensitizing and desensitizing trends that dominated the REDSTIM ratings of older subjects appeared to have masked the reduced inhibition by CPM that has been observed for single stimuli for older subjects.
One limitation of the current study is the imbalance of males and females in the older and younger groups. To control for this difference, sex was added as a covariate in the statistical model. Furthermore, the effect sizes indicated that the magnitude of the differences between older and younger adults was in the moderate range for both males and females. It should also be noted that the racial composition between the older and younger groups differed. Specifically, the younger group included more Asian Americans and Hispanics than the older group. However, prior work has shown that Asian Americans do not significantly differ from non-Hispanic Whites in CPM [37]. Additionally, preliminary analyses showed similar trends in sensitization and desensitization between the three racial groups.
Patients who report ongoing clinical pain are characterized typically as having chronic pain, because it is present over months or years. However, close evaluation of many pain conditions reveals an episodic time-course of ongoing pain; it waxes and wanes over time (e.g., [9]). The oscillating nature of “chronic” pain could reflect enhancement by a conditioning pain stimulus of endogenous descending control mechanisms that enhance pain as it increases and decrease pain when it recedes [8,9]. For older subjects in the present study, prior conditioning by a painfully cold stimulus enhanced oscillations in sensitivity to ascending and descending series of painful heat stimulation. Conditioning pain stimulation produced more extreme swings in pain intensity in response to additional nociceptive input that was not constant. While a possible linkage of behavioral trends of pain oscillation with endogenous mechanisms of pain inhibition and facilitation needs to be investigated, the data reveal that both facilitatory and inhibitory modulation of pain can be exaggerated with age.
We have suggested a physiological explanation for trend-dependent pain modulation; however, REDSTIM is a behavioral paradigm. Thus, the fluctuations in pain ratings, while likely indicative of sensitization and desensitization, may also be influenced by other factors such as the subject’s attention when rating pain, the speed of decision making, and motor control ability. For example, a subject who is inattentive or makes slow and rigid behavioral decisions may lag behind during the rating process leading to greater fluctuations in pain ratings. Consequently, in order to avoid attention effects, we structured the CPM paradigm so that the test stimuli were administered pre and post the conditioning stimulus versus during the conditioning stimulus.
4.4. Summary and future directions
Modulation of pain following CPM likely involves well-described central systems of top-down control over nociceptive transmission. Brain stem systems with descending projections modulate nociceptive activation of spinal neurons and receive nociceptive projections from the spinal cord [38]. Also, nociceptive modulation from the brain stem is subject to executive control from cerebral structures, such as prefrontal and cingulate cortex [26,39] which receive nociceptive input [7]. Feedback control of pain by pain is built into these systems that are implicated in attention and anticipation [27]. The REDSTIM paradigm revealed two effects of conditioning pain that likely depend upon these descending control systems. Overall (average) heat pain was reduced by CPM for young and old subjects, revealing an inhibitory effect of cold pain on heat pain. Independent of this overall effect of CPM on heat pain sensitivity, variations in stimulation intensity during REDSTIM increased both sensitizing and desensitizing trends for older subjects by CPM. Thus, central systems that control pain sensitivity according to feedback concerning ascending and descending progressions of pain were especially activated by a conditioning stimulus for older subjects who reported larger and more prolonged swings in pain intensity with variations in stimulus intensity. It is important to note that an exclusion criterion for the present study was an ongoing pain condition. Given the effects of CPM on sensitization and desensitization, it could be instructive to compare older and younger subjects under conditions of ongoing clinical pain.
HIGHLIGHTS.
Novel psychophysical test used dynamically changing levels of noxious stimulation.
This pain test assessed sensitization and desensitization within the same paradigm.
A conditioning stimulus enhanced sensitization and desensitization in older adults.
A conditioning stimulus decreased pain sensitivity of older and younger adults.
Acknowledgments
This research was supported by NIH-NIA Grant R01AG039659 (J.L.R.) and NIH Grant T32 T32NS045551-06 (K.M.N.).
Footnotes
Conflict of interest
Andre Mauderli is an officer of Neuroanalytics Corportation. There are no other conflicts of interest, or any financial interests, to report with regard to this work for any of the other authors.
Author and contributions
JLR, APM, and CJV made substantial contributions to the conception or design of the work and interpretation of the data for the work.
KMN and YCA made substantial contributions to the acquisition, analysis, and interpretation of data for the work.
All authors listed aided in drafting the work or revising it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Uncited reference
[32].
References
- 1.Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2008;136:142–149. doi: 10.1016/j.pain.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain. 2009;144:16–19. doi: 10.1016/j.pain.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 3.van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain. 2010;11:408–419. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Riley JL, III, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain. 2010;150:153–160. doi: 10.1016/j.pain.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC) I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 6.Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2007;27:729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vierck CJ, Riley JL, III, Wong F, King CD, Mauderli AP. Psychophysical demonstration of bidirectional pain modulation (sensitization and desensitization) by ascending or descending progressions of thermal stimulus intensity. Brain Res. 2010;1347:58–64. doi: 10.1016/j.brainres.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Vierck CJ, Wong F, King CD, Mauderli AP, Schmidt S, Riley JL., III Characteristics of sensitization associated with chronic pain conditions. Clin J Pain. 2014;30:119–128. doi: 10.1097/AJP.0b013e318287aac7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong F, Vierck CJ, Riley JL, III, King C, Mauderli AP. A new thermal stimulation method for human psychophysical studies: pain intensity clamping. J Neurosci Methods. 2010;188:83–88. doi: 10.1016/j.jneumeth.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doll RJ, Buitenweg JR, Meijer HG, Veltink PH. Tracking of nociceptive thresholds using adaptive psychophysical methods. Behav Res Methods. 2014;46:55–66. doi: 10.3758/s13428-013-0368-4. [DOI] [PubMed] [Google Scholar]
- 12.Lariviere M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23:506–510. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- 13.Washington LL, Gibson SJ, Helme RD. Age-related differences in the endogenous analgesic response to repeated cold water immersion in human volunteers. Pain. 2000;89:89–96. doi: 10.1016/S0304-3959(00)00352-3. [DOI] [PubMed] [Google Scholar]
- 14.Grashorn W, Sprenger C, Forkmann K, Wrobel N, Bingel U. Age-dependent decline of endogenous pain control: exploring the effect of expectation and depression. PLoS One. 2013;8(9):e75629. doi: 10.1371/journal.pone.0075629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley JL, Cruz-Almeida Y, Glover TL, et al. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain. 2014;15(3):272–282. doi: 10.1016/j.jpain.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 17.Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variables. Pain. 2003;106:427–437. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;9:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain. 2001;2:307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 21.Harkins SW, Davis MD, Bush FM, Kasberger J. Suppression of first pain and slow temporal summation of second pain in relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:M260–M265. doi: 10.1093/gerona/51a.5.m260. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Kosinski M, Dewey JE. How to score version two of the SF-36 health survey. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 23.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 24.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. East Norwalk, CT: Appleton & Lange; 1993. [Google Scholar]
- 26.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 27.Chakour MC, Gibson SJ, Bradbeer M, Helme RD. The effect of age on A delta- and C-fibre thermal pain perception. Pain. 1996;64:143–152. doi: 10.1016/0304-3959(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 28.Ochoa J, Mair WG. The normal sural nerve in man II. Changes in the axons and Schwann cells due to ageing. Acta Neuropathol. 1969;13:217–239. doi: 10.1007/BF00690643. [DOI] [PubMed] [Google Scholar]
- 29.Walsh NE, Schoenfeld L, Ramamurthy S, Hoffman J. Normative model for cold pressor test. Am J Phys Med Rehabil. 1989;68:6–11. doi: 10.1097/00002060-198902000-00003. [DOI] [PubMed] [Google Scholar]
- 30.McCaul KD, Malott JM. Distraction and coping with pain. Psychol Bull. 1984;95:516–533. [PubMed] [Google Scholar]
- 31.Moont R, Pud D, Sprecher E, Sharvit G, Yarnitsky D. ‘Pain inhibits pain’ mechanisms: is pain modulation simply due to distraction? Pain. 2010;150:113–120. doi: 10.1016/j.pain.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Harkins SW, Price DD, Martelli M. Effects of age on pain perception: thermonociception. J Gerontol. 1986;41:58–63. doi: 10.1093/geronj/41.1.58. [DOI] [PubMed] [Google Scholar]
- 33.Farrell M, Gibson S. Age interacts with stimulus frequency in the temporal summation of pain. Pain Med. 2007;8:514–520. doi: 10.1111/j.1526-4637.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 34.Price DD, Hayes RL, Ruda M, Dubner R. Neural representation of cutaneous after sensations by spinothalamic tract neurons. Fed Proc. 1978;37:2237–2239. [PubMed] [Google Scholar]
- 35.Hashmi JA, Davis KD. Effect of static and dynamic heat pain stimulus profiles on the temporal dynamics and interdependence of pain qualities, intensity, and affect. J Neurophysiol. 2008;100:1706–1715. doi: 10.1152/jn.90500.2008. [DOI] [PubMed] [Google Scholar]
- 36.Tousignant-Laflamme Y, Page S, Goffaux P, Marchand S. An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Res. 2008;1230:73–79. doi: 10.1016/j.brainres.2008.06.120. [DOI] [PubMed] [Google Scholar]
- 37.Goodin BR, Kronfli T, King CD, Glover TL, Sibille K, Fillingim RB. Testing the relation between dispositional optimism and conditioned pain modulation: does ethnicity matter? J Behav Med. 2012;36(2):165–174. doi: 10.1007/s10865-012-9411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 39.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia – imaging a shared neuronal network. Science. 2006;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]