Abstract
OBJECTIVE
Constraint-induced movement therapy (CIMT) leads to improvement in upper extremity movement and cortical reorganization after stroke. Direct comparison of the differential degree of cortical reorganization according to chronicity in stroke subjects receiving CIMT has not been performed and was the purpose of this study. We hypothesized that a higher degree of cortical reorganization would occur in the early (less than 9 months post-stroke) compared to the late group (more than 12 months post-stroke).
METHODS
17 early and 9 late subjects were enrolled. Each subject was evaluated using transcranial magnetic stimulation (TMS) and the Wolf Motor Function Test (WMFT) and received CIMT for 2 weeks.
RESULTS
The early group showed greater improvement in WMFT compared with the late group. TMS motor maps showed persistent enlargement in both groups. The map shifted posteriorly in the late stroke group.
CONCLUSION
CIMT appears to lead to greater improvement in motor function in early phase after stroke. Greater cortical reorganization associated with shift in map position occurred in late group.
SIGNIFICANCE
The contrast between larger functional gains in the early group vs larger map expansion in the late group may indicate that cortical reorganization depends upon different neural substrates in the late stroke group.
Keywords: plasticity, recovery, transcranial magnetic stimulation, upper extremity, motor
Introduction
Stroke continues to be a major public health concern in the United States (Rosamond et al., 2008). In the past decade, knowledge has dramatically proliferated regarding the potential of the mature brain to reorganize in continual response to novel external and/or internal demands, including those present after stroke (Hallett, 2001, Kleim and Jones, 2008). This potential of the brain to reorganize may play a crucial role in functional recovery after stroke (Nudo, 2003). The term “neuronal plasticity” describes enduring morphological or functional changes in neuronal properties (Kleim and Jones, 2008, Nudo, 2003). Plastic changes can occur via modification of synaptic strength, axonal sprouting, and altered synaptic activation (Hallett et al., 1993, Kaas, 1991, Nudo, 2003, Pascual and Torres, 1993). Related research has focused on translating this knowledge to novel rehabilitative approaches that optimize functional recovery after stroke. For example, a series of studies has provided evidence that constraint-induced movement therapy (CIMT) improves motor recovery after stroke. CIMT originated from studies of forearm deafferentation in non-human primates (Taub and Wolf, 1997). CIMT for recovery after stroke consists of restraining the less-affected arm with a mitt for 90% of waking hours for 2–3 weeks, during which time participants engage in daily repetitive and mass practice of sensorimotor tasks. Several small-scale studies applying CIMT in early (Alberts et al., 2004, Blanton and Wolf, 1999, Dromerick et al., 2000, Page et al., 2005, Ro et al., 2006) or late (Bonifer et al., 2005, Liepert et al., 1998, Miltner et al., 1999, Wittenberg et al., 2003) phase after stroke have reported superior results compared with standard rehabilitative methods. A large multi-center trial enrolling 222 subjects who had predominantly ischemic strokes within the previous 3 to 9 months (i.e., the Extremity Constraint Induced Therapy Evaluation [EXCITE] trial) has shown statistically significant and clinically relevant improvements in the motor ability and use of the paretic arm compared with participants receiving usual and customary care (Wolf et al., 2006, Wolf et al., 2008). A separate study reported that stroke subjects receiving CIMT within 3 to 9 months post-stroke had greater improvement in motor function compared to subjects receiving identical intervention after 12 months post-stroke(Wolf et al., 2010). However, there was no statistical difference in motor function between the 2 groups after 24 months (Wolf, Thompson, 2010).
Current research associates motor cortical activation and motor recovery after stroke as a dynamic process depending on the time elapsed from the stroke, motor functional level, site, and size of the lesion (Carey et al., 2006, Foltys et al., 2003, Marshall et al., 2000, Rossini et al., 2003). Previous studies applying CIMT in the late phase of stroke recovery have demonstrated expansion of transcranial magnetic stimulation (TMS) motor maps (Liepert et al., 2001, Wittenberg, Chen, 2003). Our study in the early phase also demonstrated an increase in motor map size compared to the group receiving usual and customary care (Sawaki et al., 2008a). TMS has been extensively used in humans to evaluate brain reorganization associated with simple motor training (Classen et al., 1998, Kaelin-Lang et al., 2005, Sawaki et al., 2003b), motor skill acquisition (Pascual-Leone et al., 1993, Pascual-Leone et al., 1995), and peripheral (Roricht et al., 1999, Ziemann et al., 1998)or central lesions (Bastings et al., 2002, Liepert, Miltner, 1998, Wittenberg, Chen, 2003). Because the effect of time after stroke (chronicity) on this type of plastic change has not been thoroughly investigated, we tested the hypothesis that subjects early after stroke (3 to 9 months post-stroke) receiving 2 weeks of CIMT would show an increased TMS motor map volume in the ipsilesional primary motor cortex compared with subjects receiving the identical intervention late after stroke (more than 12 months post-stroke). We further hypothesized that this increase would persist at the 4-month follow-up. We expected that the degree of map expansion would be positively correlated with improvement in upper extremity motor function.
Methods
Subjects
Inclusion criteria were identical to those of the EXCITE trial (Wolf, Winstein, 2006, Wolf, Winstein, 2008); and a portion of the subjects were also enrolled in EXCITE. Briefly, active movement in the paretic arm had to include at least 20 degrees of wrist extension and 10 degrees of extension at the thumb and 2 other digits (Wolf, Winstein, 2006, Wolf, Winstein, 2008). To ensure the safe use of TMS and to minimize potential confounding variables, exclusion criteria included: a) a history of seizures, alcohol or drug abuse, psychiatric illness, and/or head injury; b) cognitive deficits severe enough to preclude informed consent; c) a positive pregnancy test or being of childbearing age and not using appropriate contraception; d) ferromagnetic material in the cranium; and e) cardiac or neural pacemakers. After a careful screening process, 17 early subjects (3 to 9 months post stroke; age ± SEM: 54.4 ± 3.8) and 9 late subjects (more than 12 months post-stroke; age ± SEM: 57.6±3.8) were found eligible for this study. Each individual participant gave informed consent. The protocol was approved by the Institutional Review Boards at each participating site (Wake Forest University, Emory University, and The Ohio State University).
Study design
Each subject participated in 10 consecutive weekdays of CIMT-based, intensive upper extremity therapy, during which time he/she donned a padded mitt covering the non-paretic hand. The mitt was also worn for at least 90% of waking hours over the 2-week period, including 2 weekends (Wolf, Winstein, 2006, Wolf, Winstein, 2008). Treatment focused on unimanual skill acquisition and functional retraining and was based on the principles of intensive task-oriented training (Panyan M. Lawrence, 1980, Taub et al., 1994) that can also be described in terms of motor learning (Kleim and Jones, 2008, Schmidt RA, 1999, Winstein, 1991). Tasks emphasized grasp as well as manipulation and release of objects. Subjects also performed general activities related to daily living and fine motor coordination. Task difficulty was progressively increased by using a training strategy in which targets for motor ability goals were kept just beyond the level of performance already achieved(Wolf, Winstein, 2006).
Outcome measures
Wolf Motor Function Test (WMFT)
The WMFT was chosen as the primary clinical outcome measure (Wolf et al., 2001, Wolf, Winstein, 2006) and was performed by blinded evaluators at all sites. This test encompasses a battery of 15 time-based tasks and 2 force-based tasks (item 7: lift weight and item 14: grip strength). The WMFT has established reliability and validity and has been used extensively to evaluate upper extremity motor function in CIMT trials (Wolf, Winstein, 2006).
Neurophysiological assessment (TMS)
Comparability of TMS data collection techniques across all 3 sites was ensured prior to enrollment of subjects. After each institutional research team completed intensive training for TMS data acquisition, data from 2 healthy volunteers and 2 subjects with late stroke were acquired at each site. Additionally, 1 healthy volunteer (GW) was tested at the 3 sites to ensure reproducibility.
Testing was conducted on 3 occasions (at baseline, at 2 weeks upon completion of intervention, and at 4-month follow-up). Bipolar adhesive monitoring electrodes (H59P, Kendall soft-E™, Chicopee, MA) were placed over the belly of the extensor digitorum communis (EDC) muscle bilaterally, with the reference electrode placed proximally and inter-electrode distance of approximately 1.5 cm (Wolf et al., 2004). To ensure reproducibility across sites, similar equipment and techniques were used at all three sites. The EDC muscle was selected as the target muscle because it is the primary effector of finger extension, which is one of the minimal motor criteria for inclusion in the study. A template was created for each subject at baseline using a sheet of polyester film to guarantee reproducibility of electrode placements at different time points. Any volume conduction of motor-evoked potentials (MEPs) from neighboring muscles would most likely capture functionally-related wrist extensor activity (Wolf, Butler, 2004). The electromyographic (EMG) signals were amplified and filtered (band-pass 30 Hz to 1 kHz) using an isolated bioelectric amplifier (James Long Co., Caroga Lake, NY) and fed into a laboratory computer for off-line analysis. Auditory feedback of EMG was used to ensure quiescence of target muscle activity prior to stimulation. TMS was delivered using a Magstim 200 stimulator with a figure-eight coil (Magstim, Whitland, Dyfed, UK). The coil handle was pointed in a posterior direction, yielding approximately posterior-to-anterior current flow across the central sulcus (Brasil-Neto et al., 1992, Kobayashi and Pascual-Leone, 2003) and allowing consistent positioning during mapping. The coil was placed on the frontoparietal region contralateral to the target muscle and moved until the optimal position for stimulation of the muscle (i.e., the hot-spot) was found. Resting motor thresholds: The resting motor threshold (rMT) was defined as the minimum TMS intensity required to elicit at least 5 out of 10 MEPs ≥ 25 μV on consecutive trials, a modification of the standard (Rossini et al., 1994) because of the smaller potentials recorded with bipolar vs belly-tendon montages. Active motor thresholds: The active motor threshold (aMT) was defined as the lowest TMS intensity resulting in MEPs of about 200 μV on 50% of trials during isometric contraction of the EDC muscle (Rossini, Barker, 1994). Mapping of motor cortex: Stimulus sites were located using a latitude/longitude-based coordinate system(Wassermann et al., 1992). Subjects wore a tight-fitting cap (Electro Cap Intl., Eaton, Ohio) pre-marked with a 1 cm grid referenced to the vertex (Cz). Latitude (x) was defined as the medio-lateral distance from Cz; and longitude (y), as the distance from Cz along a line of constant latitude from the inter-aural line. Stimulation intensity was set at 110% of rMT, and 10 stimuli were delivered to each scalp site at a rate of 1 stimulus every 5 seconds. Stimulation was continued on each site until a border position without a response of at least 25 μV peak-to-peak in 5 successive, or less than 50%, of 10 stimulations was encountered. The average response of every series of 10 stimuli was calculated off-line. Map volume: The normalized map volume (nMV) is a simple measure of the spread of the motor representation over multiple scalp sites. It is calculated as the sum of the normalized MEP (nMEP – the mean MEP at each location, divided by the largest mean MEP) over all locations. The nMV ranges from 1 (for a map with only one active location) to a value that is equal to the number of active locations, if all locations gave equal responses. Center of gravity mapping: The center of gravity (COG) is an estimate of the center of the motor map and is an average of all active location vectors, each weighted by the MEP amplitude at that location (Wassermann, McShane, 1992). If there are n locations, the center of gravity is calculated by for the x coordinate (COG x) and similarly for the y coordinate (COG y) (Liepert, Miltner, 1998). Recruitment curves: To study changes in cortical excitability in a range relevant to map acquisition, limited recruitment (stimulus-response) curve (RC) measurements were performed (Devanne et al., 1997). The coil was kept at the hot-spot of the EDC muscle. The stimulus intensity was increased in 10% steps between 90 and 150% of rMT, and 10 MEPs were recorded at each stimulus intensity. Silent period: The silent period (SP) is a measure of cortical inhibition that can be measured using a single stimulator (Rossini, Barker, 1994, Rothwell, 1991). Stimulation was delivered at 150% of aMT during active contraction of the EDC muscle. Five SPs were recorded, and the post-stimulus analysis time was 500 ms (Inghilleri et al., 1993). Duration of SP were visually measured and averaged; trials without consistent background EMG activity were discarded (Chen et al., 1999).
Data analyses
All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC). Statistical analyses were conducted on performance assessment (log WMFT) outcomes (for both paretic and non-paretic sides) and TMS measures (rMT, aMT, nMV, COG x, COG y, RC, and SP for both hemispheres). We compared baseline measures for the two groups (early vs late). The normality of each measure was assessed using the Kolmogorov-Smirnov test. Because of the skewed distribution of the WMFT, a logarithmic transformation was performed on the 15 time-based WMFT measures (Wolf et al., 2005). Student t-tests were used to compare normally distributed measures (either before or after transformations). Transformations did not correct non-normality of aMT, SP, and COG y of the less-affected side; therefore, the non-parametric Wilcoxon rank-sums test was used for these three measures to make comparisons between groups. An analysis of variance (ANOVA) model with repeated measures was fitted to each dependent variable, in order to evaluate group (early vs late) and visit (2-weeks and 4-month) main effects, adjusting for baseline values by including the baseline as a covariate in the model. The interaction effect between group and visit was also included in the initial model. If the interaction was not significant at the level of 0.05, then it was removed from the final model. For all tests, significance level was set at 0.05.
Results
Wolf Motor Function Test (WMFT)
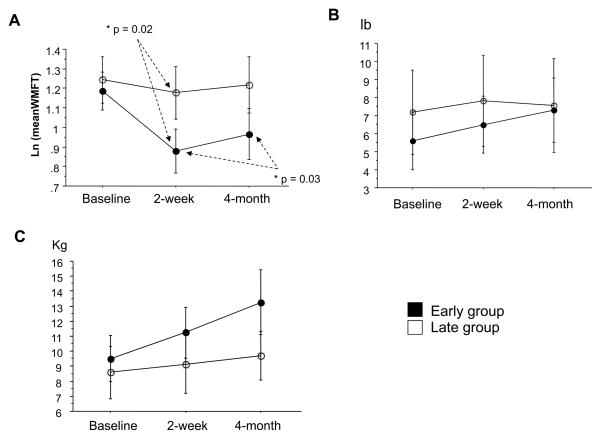
At baseline motor function testing with WMFT, there was no significant difference for time- and force-based measures between groups. Both groups improved on time- and force-based measures on the paretic side immediately after CIMT after adjustment for baseline measure; however, only the early group showed significantly better performance on time-based measures (P = .02, Figure 1A). At 4-month follow-up, time-based measures showed slightly but significantly slower performances compared with measurements taken immediately after CIMT in early subjects (P = .03, Figure 1A). The force-based measures (i.e, grip strength and liftable weight) tended to reflect greater improvement in the early than in the late group, but these between-group differences were not statistically significant (Figure 1B and 1C). There was no significant difference for time- and force-based measures between groups at 4-month follow-up. Time- and force-based measures remained stable on the less-affected side following the 2-week intervention (Table 1).
Figure 1.
Effect of CIMT on Wolf Motor Function Test (WMFT) collected at baseline, at 2 weeks (ie, completion of intervention), and at 4-month follow-up. A: Log mean of time-based evaluations (scores indicate time to complete tasks; smaller scores indicate greater improvement). B: Mean of force-based measure (lift weight lb; higher scores indicate more weight lifted). C: Mean of force-based measure (grip strength kg; higher scores indicate more grip strength). Note that both early (solid dots) and late (open dots) stroke groups showed improvement in all performance measures at 2 weeks and at 4-month follow-up. Time-based measures in early group showed significant improvement compared with the late group immediately after CIMT (P = .024). Data are expressed as mean ± SE.
Table 1.
Functional Measures of the Less Affected Hand
| Early | Late | |
|---|---|---|
| Ln meanWFMT (baseline) | 0.36±0.04 | 0.42±0.09 |
| Ln meanWFMT (2-week) | 0.32±0.43 | 0.39±0.09 |
| Ln meanWFMT (4-month) | 0.42±0.07 | 0.31±0.05 |
|
| ||
| Lift lb (baseline) | 17.21±1.01 | 16.27±1.85 |
| Lift (2-week) | 17.67±0.74 | 17.00±1.41 |
| Lift (4-month) | 17.53±0.90 | 18.67±1.21 |
|
| ||
| Grip Kg (baseline) | 30.41±2.81 | 31.05±3.05 |
| Grip (2-week) | 32.70±2.56 | 29.60±3.16 |
| Grip (4-month) | 31.99±3.95 | 28.16±2.17 |
Natural Log (Ln) Mean of 15-time based measures, force-based subtests 7 (Lift) and 14 (Grip) collected at baseline, after 2 weeks and at a 4-month follow-up. Data are expressed as mean ± SE.
Neurophysiological findings – Ipsilesional hemisphere
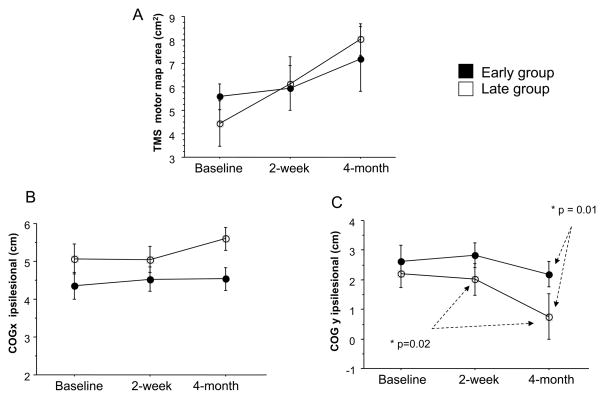
No significant between-group differences for TMS measures were found at baseline. Both groups had a non-significant trend towards an increased TMS motor map volume of the EDC at 4-month follow-up, but no significant difference in EDC map volume was found between the groups at any time (Figure 2A). After adjusting for differences in baseline measures, no significant shift was observed in the ipsilesional COG x direction for either group (Figure 2B). On the other hand, ipsilesional COG y at the 4-month follow-up showed significant differences: 1) in the late group, there was a posterior shift compared with measurements taken immediately after CIMT (P=.02, Figure 2C); and 2) there was a between groups difference, with the late group more posterior than the early group (P = .01, Figure 2C). An illustration of these longitudinal changes in TMS motor map volume and location in two representative subjects is shown in Figure 3. No significant differences were found over time in rMT, aMT and SP in the ipsilesional hemisphere (Table 2). Also, no significant changes were found in RC (Figure 4, upper graphs). However, it appears that the late group shows decreased excitability in response to TMS in the ipsilesional hemisphere compared to the early group (Figure 4, upper graphs). This pattern reverses at the 4-month follow-up.
Figure 2.
Longitudinal changes in TMS motor map volume (A), COG x (B) and COG y (C) on the ipsilesional hemisphere. Note that both early (solid dots) and late (open dots) stroke groups exhibit increased map volume after 2 weeks and at 4-month follow-up. There is, however, a significantly further posterior shift of COG y from its position at 2 weeks to its position at 4-month follow-up for the late group as compared with that of the early group (Figure 2C, P = .01). Additionally, there is a significant difference of COG y between the early and late groups at 4-month follow-up (Figure 2C, P = .02), suggesting a differential pattern of reorganization between the groups. Data are expressed as mean ± SE.
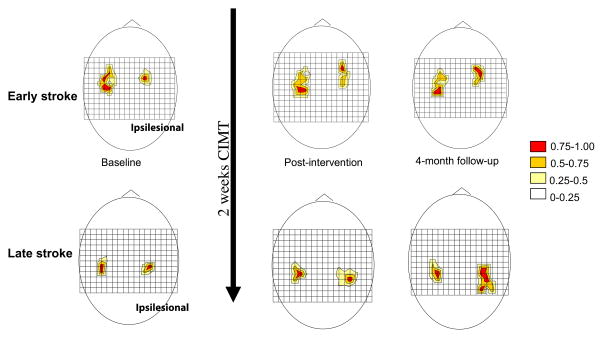
Figure 3.
Longitudinal changes in TMS motor map volume of 2 representative subjects. The grid size is 1 cm, and (0,0) is Cz in the 10–20 EEG system. Motor responses at each scalp position are color-coded by MEP size (relative to the maximal response). Increased TMS motor map volume of ipsilesional hemisphere was observed in both an early subject (top diagrams) and a late subject (bottom diagrams) over a 4-month period. The 4-month follow-up for the late subject showed a significantly posterior shift of TMS motor map.
Table 2.
TMS measures of the ipsilesional side in the two treatment groups
| Early | Late | |
|---|---|---|
| RMT (baseline) | 64.54±5.62 | 55.33±5.68 |
| RMT (2-week) | 62.54±4.45 | 63.40±7.86 |
| RMT (4-month) | 62.00±4.82 | 52.70±6.95 |
|
| ||
| AMT (baseline) | 58.08±5.94 | 47.80±4.81 |
| AMT (2-week) | 52.73±5.01 | 52.50±6.24 |
| AMT (4-month) | 52.27±4.40 | 48.50±7.18 |
|
| ||
| SP (baseline) | 139.69±10.52 | 166.49±14.25 |
| SP (2-week) | 154.95±9.46 | 165.72±19.99 |
| SP (4-month) | 168.19±24.70 | 177.41±24.52 |
Resting motor threshold (rMT), active motor threshold (aMT), and silent period (SP) collected in the extensor digitorum communis (EDC) muscle of the more-affected forearm at baseline (1), after 2 weeks (2), and at a 4-month follow-up (3). Data are expressed as mean ± SE.
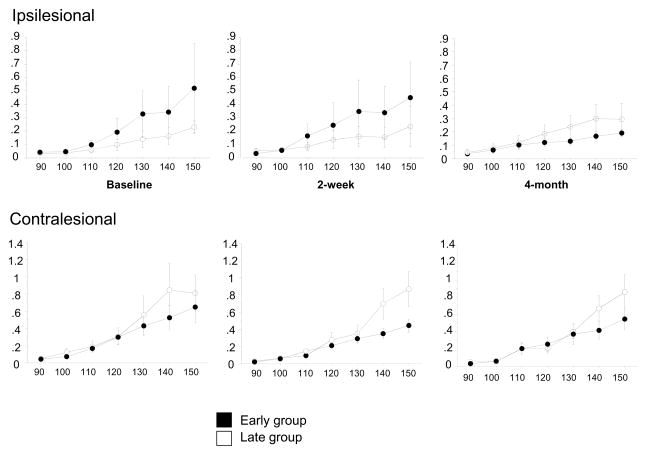
Figure 4.
Longitudinal changes in recruitment curves for ipsilesional (upper graphs) and contralesional (bottom graphs). Note that while there is no significant difference between the early (solid dots) and late (open dots) groups at any time, there is a reversed pattern in the ipsilesional and contralesional hemispheres particularly in the late group. That is to say that, the ipsilesional hemisphere in the late group appears to be less excitable in response to TMS at baseline and the contralesional hemisphere exhibits higher corticomotor excitability. This pattern is, however, lost at 4-month follow up. Data are expressed as mean ± SE.
Contralesional hemisphere
Baseline TMS measures of the contralesional hemisphere differed significantly between groups only with regard to map position: COG y for the early group was more anterior (mean ± SE, 2.76 ± 2.28) compared with COG y for the late group (mean ± SE, 0.68 ± 0.33) (p = .01, Table 3). Other TMS measures including rMT, aMT and SP were stable over time and did not differ significantly between groups (Table 3). There was no significant difference between the 2 groups in RC over time (Figure 4, bottom graphs). However, the late group appears to exhibit higher degree of excitability in response to TMS compared to the early group (Figure 4, bottom graphs).
Table 3.
TMS measures of the contralesional side in the two treatment groups
| Early | Late | |
|---|---|---|
| RMT (baseline) | 52.06±3.16 | 44.22±3.39 |
| RMT (2-week) | 51.31±2.69 | 44.88±3.05 |
| RMT (4-month) | 52.44±2.52 | 45.50±2.80 |
|
| ||
| AMT (baseline) | 45.28±3.72 | 36.63±2.99 |
| AMT (2-week) | 42.00±2.90 | 38.83±4.37 |
| AMT (4-month) | 45.69±3.02 | 39.83±3.28 |
|
| ||
| Motor map area (baseline) | 4.87±0.42 | 4.89±0.86 |
| Motor map area (2-week) | 5.42±0.54 | 5.49±0.89 |
| Motor map area (4-month) | 4.62±0.63 | 5.90±0.46 |
|
| ||
| COG x (baseline) | 4.70±0.27 | 4.70±0.31 |
| COG x (2-week) | 4.58±0.24 | 4.72±0.38 |
| COG x (4-month) | 4.65±0.28 | 4.86±0.42 |
|
| ||
| COG y (baseline) | 2.76±0.57 * | 0.68±0.33* |
| COG y (2-week) | 2.95±0.57 | 0.26±0.54 |
| COG y (4-month) | 2.62±0.54 | 0.53±0.93 |
|
| ||
| SP (baseline) | 143.88±14.68 | 148.08±19.99 |
| SP (2-week) | 151.14±20.65 | 167.89±8.78 |
| SP (4-month) | 145.64±10.72 | 162.65±18.67 |
Resting motor threshold (rMT), active motor threshold (aMT), TMS motor map area, center of gravity for the x (COG x) and y (COG y) coordinates, and silent period (SP) collected in the extensor digitorum communis (EDC) muscle of the non-paretic forearm at baseline, after 2 weeks, and at a 4-month follow-up. Motor map area expressed in cm2. Data are expressed as mean ± SE.
COG y of the contralesional hemisphere of the chronic group is significantly more posterior than that of the subacute group at baseline (p=0.01).
Correlation of changes with chronicity
We found significant correlation between changes in log WMFT and chronicity (F = 10.446, P = .01). No significant correlation was found between measures of changes in motor performance (WMFT) and TMS motor map volume (F = 0.181, P = 0.67). No significant correlation was found between changes in TMS map (F = .372, P = .55), changes in COG y (F = 0.355, P = .56), or changes in COG x (F = 2.644, P = .12) and chronicity.
Discussion
The goal of this study was to investigate differential patterns of cortical reorganization underlying improved motor function following CIMT during early and late periods after stroke. As noted previously, extensive precedent exists for the use of TMS to measure cortical reorganization underlying improved motor function in humans. For example, Bastings et al. evaluated 12 subjects with late stroke and demonstrated that those with good recovery had larger TMS motor maps recorded from the first dorsal interosseous muscle in the ipsilesional hemisphere when compared with age-matched healthy volunteers (Bastings, Greenberg, 2002). Liepert et al demonstrated expansion of TMS motor maps generated for thenar muscles in people with late stroke receiving CIMT(Liepert, Miltner, 1998). This motor map expansion may be the human correlate of similar expansion in motor maps after intensive motor training of non-human primates with cortical lesions. In both instances, areas near the infarcted area may reorganize functionally to take over the activities chiefly executed by primary motor cortex. In another study, Wittenberg et al reported expansion of ipsilesional and decrease of contralesional TMS motor maps after CIMT in late stroke (Wittenberg, Chen, 2003). More recently, we demonstrated in the first multicenter trial of CIMT that such therapy can produce statistically significant enlargement of ipsilesional TMS motor maps that persists for at least 4 months in early stroke subjects compared with subjects receiving standard care (Sawaki et al., 2008b).
In the present study, there was a marked improvement in motor function immediately after CIMT in early stroke subjects, with a lesser degree of improvement for late stroke subjects. Furthermore, contrary to our initial hypothesis, enlargement of the ipsilesional TMS-evoked motor map volume showed no statistically significant differences between early and late groups. However, map volume still nearly doubled for the late group between baseline and 4-month follow-up, with a less notable expansion apparent for the early group. The contrast between relatively larger functional gains in the early group vs larger map expansion in the late group implies that the role of map expansion as an index of motor recovery may differ according to time elapsed since stroke and is not tightly linked to function. Map size may continue to shrink for several months after stroke, so that earlier intervention affects a map that has not yet fully regressed. Additionally, posterior shift of the COG was significant in the late stroke subjects. These findings may suggest that motor cortical reorganization in response to a structured motor training protocol such as CIMT may differ quantitatively in early and late periods after stroke. TMS motor map volume expansion occurred in the absence of significant changes in rMT, aMT, RC, and SP. Hence, corticomotor excitability changes are not likely to confound the interpretation of map volume changes.
The ipsilesional posterior shift of the COG after CIMT in our late group is expected, given prior evidence for such shift (Calautti et al., 2003, Carey, Abbott, 2006, Pineiro et al., 2001, Rossini et al., 1998). For instance, data from Dancause and colleagues, who used microelectrodes to record neuronal activity in adult squirrel monkeys, demonstrated major neuroanatomical reorganization of somatosensory cortical area in response to ischemic infarct to the M1 hand area(Dancause et al., 2005). It is conceivable that the ipsilesional posterior shift in motor map may well reflect adaptive neuroplastic change expressed as increased activation of the somatosensory cortex in addition to the motor cortex as a form of compensation after prolonged deprivation of activity. Interestingly, Barbay et al demonstrated that early vs late motor training can yield comparable improvement in motor skills in squirrel monkeys (Barbay et al., 2006); but mechanisms underlying motor recovery associated with motor training were distinct in the acute and late stages. Using intracortical microstimulation applied to primary motor cortex (M1) hand area, the investigators demonstrated that delaying the training results in a significant decrease in the spared-hand representation compared with the early training. They concluded that timing of rehabilitative training can have a differential effect upon reorganization of movement representations in M1 after stroke (Barbay, Plautz, 2006). Posterior shift and expansion of upper extremity motor representation appears to be a consistent finding after stroke in animal models; however, the association between this shift and motor recovery in humans remains unclear. While, the posterior shift was substantial in the late group in our study, there exists the possibility that subtle changes in the corticomotor excitability could have accounted for the shift. Our TMS recruitment curve data shows a reverse pattern in the ipsilesional and contralesional hemisphere in the late group (cf. Figure 4). Such features are possibly attributable to a longer period of maladaptive plasticity prior to reactivation of the motor cortex affected by stroke particularly in the late group.
Other findings from the present study showed that the contralesional COG y in the early group is anterior to that of the late group at baseline. The anterior localization of contralesional COG y at baseline in our early subjects may indicate an early recruitment of certain cortical areas, such as the premotor area, that goes on to decrease or become less prominent in later stages of recovery. Such a differential change may reflect an aspect of early spontaneous recovery, at which time contralesional premotor cortex could be more active.
The main limitation of this study is the small sample size. Also, while we attempted to enroll equal number of early and late stroke subjects in this study, sample size was unequal in the 2 groups and could be considered as a confounding factor. These are possibly the main underlying reasons for the small improvement in motor function in our late group. In fact, Wolf et al. compared the effects of CIMT in 98 subjects receiving early to 78 subjects receiving late intervention. On the other hand, changes of motor function after CIMT in our study was similar to the data reported by Wolf and colleagues in that the early group showed greater improvement compared to the late group. Additionally, they also found no statistical difference between the 2 groups in the long-term follow-up. Another limitation is the fact that we did not monitor for CNS-active medications during the study. It is known that several drugs could exert beneficial or detrimental effects on motor recovery(Boroojerdi et al., 2001, Butefisch et al., 2002, Goldstein, 1990, Goldstein and Davis, 1990, Sawaki et al., 2002, Sawaki et al., 2003a, Ziemann et al., 1999). Finally, co-registration of the TMS data with a neuronavigation system could have been beneficial to better understand the shifts of center of gravity in our study. However, if posterior shift does in fact occur as a slow change protracted over time (cf. Figure 2C), then approaches to rehabilitation, including task training, may need to differ according to time elapsed since stroke.
In conclusion, this study provides evidence that map expansion of the cortical representation of EDC is a consistent phenomenon in both early and late groups insofar as other measurable changes of excitability and inhibition remain stable. However, the potential for cortical reorganization of late stroke subjects appears to be greater, as evidenced by a larger posterior shift in motor maps and a more dramatic increase in map size. Future research should include longitudinal TMS studies early after stroke and with protracted follow-ups (i.e., over a year follow-up) to better understand the dynamics of cortical reorganization associated with CIMT and degree of motor function.
Supplementary Material
Acknowledgments
This study was sponsored by NICHD RO1 HD-40984 and partially sponsored by the Cardinal Hill Endowment in Stroke and Spinal Cord Rehabilitation. We thank the therapists, nurses, and research assistants from all sites for invaluable work during data collection. We also extend thanks for editing by Cheryl Carrico, MS, OT/L.
References
- Alberts JL, Butler AJ, Wolf SL. The effects of constraint-induced therapy on precision grip: a preliminary study. Neurorehabil Neural Repair. 2004;18:250–8. doi: 10.1177/1545968304271370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbay S, Plautz EJ, Friel KM, Frost SB, Dancause N, Stowe AM, et al. Behavioral and neurophysiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Exp Brain Res. 2006;169:106–16. doi: 10.1007/s00221-005-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastings EP, Greenberg JP, Good DC. Hand motor recovery after stroke: a transcranial magnetic stimulation mapping study of motor output areas and their relation to functional status. Neurorehabil Neural Repair. 2002;16:275–82. doi: 10.1177/154596802401105207. [DOI] [PubMed] [Google Scholar]
- Blanton S, Wolf SL. An application of upper-extremity constraint-induced movement therapy in a patient with subacute stroke. Phys Ther. 1999;79:847–53. [PubMed] [Google Scholar]
- Bonifer NM, Anderson KM, Arciniegas DB. Constraint-induced therapy for moderate chronic upper extremity impairment after stroke. Brain Inj. 2005;19:323–30. doi: 10.1080/02699050400004302. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Ziemann U, Chen R, Butefisch CM, Cohen LG. Mechanisms underlying human motor system plasticity. Muscle Nerve. 2001;24:602–13. doi: 10.1002/mus.1045. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG. Topographic mapping of the human motor cortex with magnetic stimulation: factors affecting accuracy and reproducibility. Electroencephalogr Clin Neurophysiol. 1992;85:9–16. doi: 10.1016/0168-5597(92)90095-s. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Sawaki L, Waldvogel D, Classen J, Kopylev L, et al. Modulation of use-dependent plasticity by d-amphetamine. Ann Neurol. 2002;51:59–68. doi: 10.1002/ana.10056. [DOI] [PubMed] [Google Scholar]
- Calautti C, Leroy F, Guincestre JY, Baron JC. Displacement of primary sensorimotor cortex activation after subcortical stroke: a longitudinal PET study with clinical correlation. Neuroimage. 2003;19:1650–4. doi: 10.1016/s1053-8119(03)00205-2. [DOI] [PubMed] [Google Scholar]
- Carey LM, Abbott DF, Egan GF, O’Keefe GJ, Jackson GD, Bernhardt J, et al. Evolution of brain activation with good and poor motor recovery after stroke. Neurorehabil Neural Repair. 2006;20:24–41. doi: 10.1177/1545968305283053. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539–42. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert A, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–23. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–79. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–38. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Dromerick AW, Edwards DF, Hahn M. Does the application of constraint-induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke? Stroke. 2000;31:2984–8. doi: 10.1161/01.str.31.12.2984. [DOI] [PubMed] [Google Scholar]
- Foltys H, Krings T, Meister IG, Sparing R, Boroojerdi B, Thron A, et al. Motor representation in patients rapidly recovering after stroke: a functional magnetic resonance imaging and transcranial magnetic stimulation study. Clin Neurophysiol. 2003;114:2404–15. doi: 10.1016/s1388-2457(03)00263-3. [DOI] [PubMed] [Google Scholar]
- Goldstein LB. Pharmacology of recovery after stroke. Stroke. 1990;21:III139–42. [PubMed] [Google Scholar]
- Goldstein LB, Davis JN. Restorative neurology. Drugs and recovery following stroke. Stroke. 1990;21:1636–40. doi: 10.1161/01.str.21.11.1636. [DOI] [PubMed] [Google Scholar]
- Hallett M. Plasticity of the human motor cortex and recovery from stroke. Brain Res Brain Res Rev. 2001;36:169–74. doi: 10.1016/s0165-0173(01)00092-3. [DOI] [PubMed] [Google Scholar]
- Hallett M, Cohen LG, Pascual-Leone A, Brasil-Neto JP, Wassermann EM, Cammarota AN. Plasticity of the human motor cortex. In: Thilmann AF, Rymer WZ, Burke DJ, editors. Spasticity Mechanisms and management. Berlin: Springer; 1993. pp. 67–81. [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol (Lond) 1993;466:521–34. [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci. 1991;14:137–67. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Sawaki L, Cohen LG. Role of voluntary drive in encoding an elementary motor memory. J Neurophysiol. 2005;93:1099–103. doi: 10.1152/jn.00143.2004. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–39. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Liepert J, Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- Liepert J, Uhde I, Graf S, Leidner O, Weiller C. Motor cortex plasticity during forced-use therapy in stroke patients: a preliminary study. J Neurol. 2001;248:315–21. doi: 10.1007/s004150170207. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–61. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E. Effects of constraint-induced movement therapy on patients with chronic motor deficits after stroke: a replication. Stroke. 1999;30:586–92. doi: 10.1161/01.str.30.3.586. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys Med Rehabil Clin N Am. 2003;14:S57–76. doi: 10.1016/s1047-9651(02)00054-2. [DOI] [PubMed] [Google Scholar]
- Page SJ, Levine P, Leonard AC. Modified constraint-induced therapy in acute stroke: a randomized controlled pilot study. Neurorehabil Neural Repair. 2005;19:27–32. doi: 10.1177/1545968304272701. [DOI] [PubMed] [Google Scholar]
- Panyan M, Lawrence K. How to Use Shaping. H & H Enterprises; 1980. [Google Scholar]
- Pascual LA, Torres F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Cammarota A, Wassermann EM, Brasil NJ, Cohen LG, Hallett M. Modulation of motor cortical outputs to the reading hand of braille readers. Ann Neurol. 1993;34:33–7. doi: 10.1002/ana.410340108. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–45. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Functional MRI detects posterior shifts in primary sensorimotor cortex activation after stroke: evidence of local adaptive reorganization? Stroke. 2001;32:1134–9. doi: 10.1161/01.str.32.5.1134. [DOI] [PubMed] [Google Scholar]
- Ro T, Noser E, Boake C, Johnson R, Gaber M, Speroni A, et al. Functional reorganization and recovery after constraint-induced movement therapy in subacute stroke: case reports. Neurocase. 2006;12:50–60. doi: 10.1080/13554790500493415. [DOI] [PubMed] [Google Scholar]
- Roricht S, Meyer BU, Niehaus L, Brandt SA. Long-term reorganization of motor cortex outputs after arm amputation. Neurology. 1999;53:106–11. doi: 10.1212/wnl.53.1.106. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Caltagirone C, Castriota-Scanderbeg A, Cicinelli P, Del Gratta C, Demartin M, et al. Hand motor cortical area reorganization in stroke: a study with fMRI, MEG and TCS maps. Neuroreport. 1998;9:2141–6. doi: 10.1097/00001756-199806220-00043. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Physiological studies of electric and magnetic stimulation of the human brain. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:29–35. [PubMed] [Google Scholar]
- Sawaki L, Boroojerdi B, Kaelin-Lang A, Burstein AH, Butefisch CM, Kopylev L, et al. Cholinergic influences on use-dependent plasticity. J Neurophysiol. 2002;87:166–71. doi: 10.1152/jn.00279.2001. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008a;22:505–13. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki L, Butler AJ, Xiaoyan L, Wassenaar PA, Mohammad YM, Blanton S, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008b;22:505–13. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki L, Werhahn KJ, Barco R, Kopylev L, Cohen LG. Effect of an alpha(1)-adrenergic blocker on plasticity elicited by motor training. Exp Brain Res. 2003a;148:504–8. doi: 10.1007/s00221-002-1328-x. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Yaseen Z, Kopylev L, Cohen LG. Age-dependent changes in the ability to encode a novel elementary motor memory. Ann Neurol. 2003b;53:521–4. doi: 10.1002/ana.10529. [DOI] [PubMed] [Google Scholar]
- Schmidt RALT. Human Kinetics. 1999. Motor Control and Learning: A Behavioral Emphasis. [Google Scholar]
- Taub E, Crago JE, Burgio LD, Groomes TE, Cook EW, 3rd, DeLuca SC, et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994;61:281–93. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Wolf SL. Constraint induced movement techniques to facilitate upper extremity use in stroke patients. Top Stroke Rehabil. 1997;3:38–61. doi: 10.1080/10749357.1997.11754128. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- Winstein C. Designing practice for motor learning: clinical impressions. In: Lister M, editor. Contemporary Management of Motor Control Problems: Proceedings of the II Step Conference. Alexandria, Va: Foundation for Physical Therapy; 1991. [Google Scholar]
- Wittenberg GF, Chen R, Ishii K, Bushara KO, Eckloff S, Croarkin E, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Butler AJ, Campana GI, Parris TA, Struys DM, Weinstein SR, et al. Intra-subject reliability of parameters contributing to maps generated by transcranial magnetic stimulation in able-bodied adults. Clin Neurophysiol. 2004;115:1740–7. doi: 10.1016/j.clinph.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–9. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Thompson PA, Morris DM, Rose DK, Winstein CJ, Taub E, et al. The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Thompson PA, Winstein CJ, Miller JP, Blanton SR, Nichols-Larsen DS, et al. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. 2010;41:2309–15. doi: 10.1161/STROKEAHA.110.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Jama. 2006;296:2095–104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Hallett M, Cohen LG. Mechanisms involved in up-regulation of deafferentation-induced plasticity in human motor cortex. Neurology. 1998;50 (Suppl 4):A332. [Google Scholar]
- Ziemann U, Tam A, Butefisch C, Cohen LG. Effects of dextroamphetamine on deafferentation- and stimulation-induced plasticity in human motor cortex. Neurology. 1999;52(Suppl 2):A468. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.