Abstract
Two new HIV-inhibitory depsipeptides, stellettapeptins A (1) and B (2), were isolated from an extract of the marine sponge Stelletta sp., collected from northwestern Australia. Structures of these cyclic nonribosomal peptides were elucidated on the basis of extensive NMR data analysis, and chemical degradation and derivatization studies. Stellettapeptins contain numerous nonproteinogenic amino acid residues and they are the first peptides reported to contain a 3-hydroxy-6,8-dimethylnon-4-(Z)-enoic acid moiety. Compounds 1 and 2 potently inhibit infection of human T-lymphoblastoid cells by HIV-1RF with EC50 values of 23 and 27 nM, respectively.
Keywords: Depsipeptide, Sponge peptide, Stelletta, Anti-HIV
Marine sponges in the genus Stelletta have proven to be an extremely rich source of structurally diverse and biologically active natural products.1–3 Marine sponges frequently contain nonribosomal peptides that have unusual amino acid residues and aliphatic moieties reminiscent of the mixed nonribosomal peptide synthetase (NRPS)–polyketide synthase (PKS) pathways of microorganisms, which suggests the involvement of symbiotic microbes in their production.4 In the course of our search for bioactive metabolites from marine organisms, we obtained two new cyclic depsipeptides from a sponge of the genus Stelletta. We report herein the isolation, structure determination, and HIV-inhibitory properties of stellettapeptins A (1) and B (2).
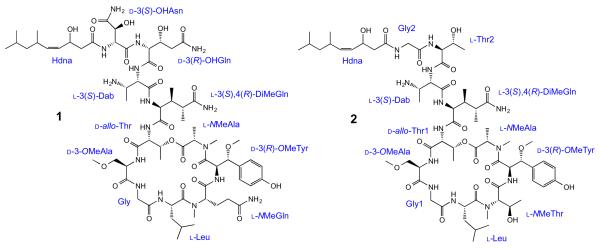
Stellettapeptin A (1) was isolated as an amorphous solid, which had a molecular formula of C67H108N16O23 from analysis of HRESIMS data coupled with 1H and 13C NMR spectral data (Table 1). The presence of a large number of exchangeable amide NH protons (δH 6.80–9.98 ppm) and carbonyl resonances (δC 170.0–182.4 ppm) in the 1H and 13C NMR spectra of 1 was characteristic of a peptide derivative. Detailed analysis of 2D NMR data enabled us to assign 11 amino acid residues: N-methylalanine (NMeAla), β-methoxytyrosine (β-OMeTyr), N-methylglutamine (NMeGln), leucine (Leu), glycine (Gly), 3-methoxyalanine (3-OMeAla), threonine (Thr), 3,4-dimethylglutamine (3,4-DiMeGln), 2,3-diaminobutanoic acid (Dab), 3-hydroxyglutamine (3-OHGln), and 3-hydroxyasparagine (3-OHAsn) (Table 1). Additionally, a 3-hydroxy-6,8-dimethylnon-4-enoic acid (Hdna) moiety was identified (Fig. 1).
Table 1.
NMR spectral data for 1 (600 MHz, CD3OH)
| Position | δ C | δH (J in Hz) | HMBC |
|---|---|---|---|
| NMeAla | |||
| 1 | 171.4 | ||
| 2 | 51.9 | 5.42 q (7.0) | 1, 3, N-Me, 13-OMeTyr |
| 3 | 13.3 | 1.28 d (7.0) | 1, 2 |
| N-Me | 29.5 | 2.68 s | 2, 13-OMeTyr |
| 3-OMeTyr | |||
| 1 | 170.0 | ||
| 2 | 53.8 | 4.92b dd (10.5, 9.8) | 1, 3 |
| 3 | 84.4 | 4.53a d (9.8) | 1, 2, 4, 5, 9 |
| 3-OMe | 56.9 | 3.10 s | 3 |
| 4 | 129.5 | ||
| 5, 9 | 131.5 | 7.17 d (8.3) | 3, 6, 7, 8 |
| 6, 8 | 116.2 | 6.80 d (8.3) | 4, 7 |
| 7 | 158.8 | ||
| NH | 8.23 d (10.5) | 2, 1NMeGln | |
| NMeGln | |||
| 1 | 170.8 | ||
| 2 | 55.8 | 4.76a m | 1, 4, N-Me, 1Leu |
| 3 | 25.1 | 1.29 m | 5 |
| 1.54 m | 2, 4, 5 | ||
| 4 | 32.1 | 1.61 m | 2, 3, 5 |
| 1.69 m | 2, 3, 5 | ||
| 5 | 177.9 | ||
| 5-NH2 | 6.80 br s | 4, 5 | |
| 7.05 br s | 5 | ||
| N-Me | 30.4 | 2.93 s | 2, 1Leu |
| Leu | |||
| 1 | 174.0 | ||
| 2 | 49.4 | 4.72 m | 3, 1Gly |
| 3 | 40.7 | 1.23 m | |
| 1.61 m | 5 | ||
| 4 | 26.2 | 1.66 m | 3 |
| 5 | 21.5 | 0.90a d (6.5) | 3, 4, 6 |
| 6 | 23.6 | 0.95 d (6.5) | 3, 4, 5 |
| NH | 7.20 d (9.2) | 1Gly | |
| Gly | |||
| 1 | 172.3 | ||
| 2 | 44.2 | 3.51 dd (17.0, 5.2) | 1, 13-OMeAla |
| 3.95 dd (17.0, 6.1) | 1, 13-OMeAla | ||
| NH | 9.08 dd (6.1, 5.2) | 2, 13-OMeAla | |
| 3-OMeAla | |||
| 1 | 172.8a | ||
| 2 | 55.4 | 4.48 q (7.2) | 1, 3, 1Thr |
| 3 | 71.6 | 3.74 m | 1, 2, 3-OMe |
| 3.79 m | 1, 2, 3-OMe | ||
| 3-OMe | 59.5 | 3.39 s | 3 |
| NH | 8.47 d (7.2) | 2, 3, 1Thr | |
| Thr | |||
| 1 | 172.9a | ||
| 2 | 57.4 | 5.20 dd (10.2, 2.8) | 1, 3, 4 |
| 3 | 71.6 | 5.60 dq (6.6, 2.6) | 4, 1NMeAla |
| 4 | 14.8 | 1.18 d (6.3) | 2, 3 |
| NH | 8.93 d (10.2) | 2, 13,4-DiMeGln | |
| 3,4-DiMeGln | |||
| 1 | 174.1 | ||
| 2 | 59.3 | 4.09 dd (9.3, 2.9) | 1, 3, 3-Me, 4 |
| 3 | 36.8 | 2.47 m | 2, 3-Me, 4-Me, 5 |
| 3-Me | 17.3 | 1.28 d (6.9) | 2, 3, 4 |
| 4 | 44.9 | 2.72 m | 2, 3, 3-Me, 4-Me, 5 |
| 4-Me | 14.0 | 1.32 d (7.1) | 3, 4, 5 |
| 5 | 182.4 | ||
| 5-NH2 | 7.14 br s | 4, 5 | |
| 7.87a br s | 5 | ||
| NH | 9.98 br s | 1Dab | |
| Dab | |||
| 1 | 171.6 | ||
| 2 | 56.4 | 4.56 t (6.1) | 1, 3, 4, 13-OHGln |
| 3 | 49.5 | 3.95 m | |
| 3-NH2 | 7.73 2H, br s | ||
| 4 | 16.8 | 1.43 d (6.8) | 2, 3 |
| NH | 8.75 d (6.9) | 2, 3, 13-OHGln | |
| 3-OHGln | |||
| 1 | 172.9a | ||
| 2 | 58.7 | 4.52a dd (8.5, 1.3) | 1, 3 |
| 3 | 68.7 | 4.75a m | |
| 4 | 40.6 | 2.37 m | 5 |
| 2.45 m | 2, 3, 5 | ||
| 5 | 176.0 | ||
| 5-NH2 | 6.88 br s | 4, 5 | |
| 7.54 br s | 5 | ||
| NH 7.88a d (8.5) | 2, 3, 13-OHAsn | ||
| 3-OHAsn | |||
| 1 | 171.8 | ||
| 2 | 59.5 | 4.73 dd (6.9, 3.9) | 1, 3, 4, 1Hdna |
| 3 | 72.7 | 4.61 br d (3.9) | 4 |
| 4 | 176.6 | ||
| 4-NH2 | 7.36 br s | 3, 4 | |
| 7.80 br s | 4 | ||
| NH | 8.25 d (6.9) | 1Hdna | |
| Hdna | |||
| 1 | 175.2 | ||
| 2 | 45.4 | 2.36 m | 1, 3, 4 |
| 2.61 m | 1, 3, 4 | ||
| 3 | 66.6 | 4.90b m | |
| 4 | 131.0 | 5.37 dd (10.5, 9.1) | 2, 6 |
| 5 | 139.3 | 5.21 dd (10.5, 5.0) | 3, 4, 6, 6-Me, 7 |
| 6 | 31.3 | 2.63 m | 4, 5, 6-Me, 7 |
| 6-Me | 22.1 | 0.92a d (6.7) | 5, 6, 7 |
| 7 | 48.2 | 1.13 2H, m | 5, 6, 6-Me, 8 |
| 8 | 26.9 | 1.57 m | 6, 7, 8-Me |
| 8-Me | 23.7 | 0.87a d (6.7) | 7, 8 |
| 9 | 23.0 | 0.88a d (6.7) | 7, 8 |
Signals overlapped.
Buried under CD3OH signal.
Figure 1.
Structures and composition of stellettapeptins A (1) and B (2).
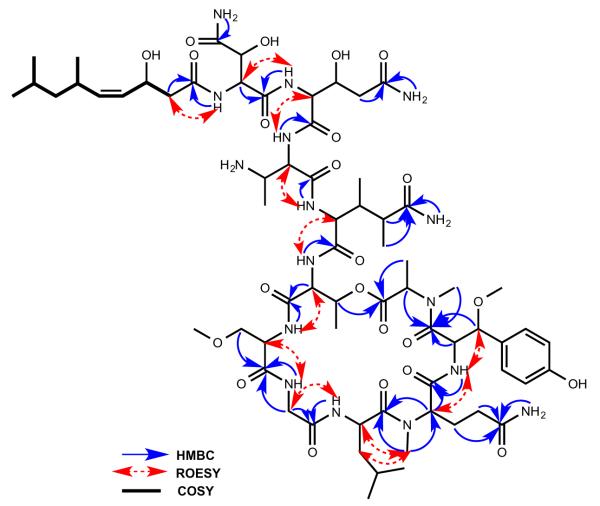
The sequence of amino acid residues in stellettapeptin A (1) was deduced from inter-residue NH/CHα ROE interactions, acquired in CD3OH, and HMBC correlations as shown in Figure 2. The Hdna unit was defined by 1H–1H COSY and heteronuclear correlation NMR data, and its double bond assigned Z geometry based on the 10.5 Hz vicinal coupling between the H-4 (δH 5.37) and H-5 (δH 5.21) olefin protons. The Hdna moiety was linked to the N-terminus of the 3-OHAsn residue by an HMBC correlation from the 3-OHAsn NH (δH 8.25) to the carbonyl (δC 175.2) of Hdna. This linkage was also supported by ROESY correlations between the 3-OHAsn amide proton and the Hdna H2-2 (δH 2.36, 2.61) methylene protons. The secondary amide NH signal (δH 7.88) of 3-OHGln correlated with the C-1 carbonyl (δC 171.8) of 3-OHAsn in the HMBC spectrum, which connected these residues, while the 3-OHGln α-methine (δH 4.52) showed a ROESY correlation with the amide NH (δH 8.75) of Dab. The amino acid sequence was further extended by a ROESY between the Dab α-methine (δH 4.56) and the secondary amide NH (δH 9.98) of 3,4-DiMeGln. An HMBC correlation from the Thr NH (δH 8.93) to C-1 (δC 174.1) of 3,4-DiMeGln linked these two residues, while an HMBC from 3-OMeAla NH (δH 8.47) to the Thr carbonyl (δC 172.9) joined these subunits. ROESY interactions observed between the α-methine (δH 4.48) of 3-OMeAla and the Gly NH (δH 9.08), and between the Gly methylene resonances (δH 3.51, 3.95) and the Leu NH (δH 7.20) helped define neighboring residues. HMBC correlations between the methyl group (δH 2.93) of NMeGln and the Leu carbonyl (δC 174.0), and between the 3-OMeTyr NH (δH 8.23) and C-1 (δC 170.8) of NMeGln linked these residues. An HMBC correlation from the methyl group (δH 2.68) of NMeAla to the 3-OMeTyr carbonyl (δC 170.0) completed the amino acid sequence. An ester bond linking NMeAla and the Thr residue was evident from the deshielded chemical shift of the Thr oxymethine (δH 5.60) and an HMBC correlation from this proton to the NMeAla carbonyl (δC 171.4). Thus the planar structure of 1 was elucidated.
Figure 2.
Key HMBC, ROESY, and COSY correlations for 1.
The absolute configurations of l-NMeAla, l-NMeGln, l-Leu, d-allo-Thr, d-3-OMeAla, (2R,3R)-3-OHGln, and (2R,3S)-3-OHAsn were determined by LC–MS analysis of the acid hydrolysate derivatized with Marfey’s reagent and comparison with appropriate amino acid standards. Both the l- and d-FDLA derivatives were prepared and analyzed for amino acids with multiple chiral centers for which a complete set of standards was not available.5 Due to decomposition of β-OMeTyr during acid hydrolysis of 1, this residue was converted to β-OMeAsp via ozonolysis prior to acid hydrolysis and derivatization, as described by Zampella et al.6 Samples of 1, callipeltin A,7 and papuamide B8 were ozonized and hydrolyzed, and then subjected to Marfey’s analysis. Both the l- and d-FDLA derivatives were prepared and ion selective monitoring for β-OMeAsp [m/z 458, (M+H)+] of the hydrolysates from all three peptides showed the same retention times (Supplementary data) corresponding to (2R,3S)-3-OMeAsp. Thus the configuration of the β-OMeTyr residue in 1 was assigned as 2R,3R.6 The coupling constant (9.8 Hz) between H-2 (δH 4.92) and H-3 (δH 4.53) of this residue was in good agreement with those of callipeltin A and papuamide B, which also have a (2R,3R)-3-OMeTyr, while neamphamide9 contains (2S,3R)-β-OMeTyr and its oxymethine proton (H-3, dH 5.03) appears as a broadened singlet. These data supported the (2R,3R)-configuration of β-OMeTyr in 1. Comparison by LC–MS of both the l- and d-FDLA derivatives of 3,4-DiMeGln and Dab of 1 with similar derivatives from the hydrolysates of authentic samples of callipeltin A and/or papuamide B indicated these residues have the same configuration in all three peptides. Thus, their configurations were assigned as (2S,3S,4R)-DiMeGln and (2S,3S)-Dab. As (2S,3S)-Dab is a common fragment of papuamides and callipeltin A, considerable effort has been made toward its synthesis.10–13 In these synthetic studies, the diastereomers of Dab showed distinct differences in the coupling constants, with the H-2/H-3 coupling constant in the (2R,3S)-isomer being ~3 Hz, while in the (2S,3S)-diastereoisomer it is 6.1–7 Hz, supporting the presence of (2S,3S)-Dab in 1. Thus the absolute configurations of all 17 stereogenic carbons in the amino acid portion of stellettapeptin A (1) were successfully established. The polyketide moiety was unstable and it decomposed during repeated cleavage attempts, so the configuration of this portion of 1 could not be assigned.
Stellettapeptin B (2) analyzed for a molecular formula of C63H103N13O20 by HRESIMS coupled with 1H and 13C NMR spectroscopic data (Supplementary data). Its 1H and 13C NMR spectra were also characteristic of a peptide and suggested that 2 was similar in structure to 1. Detailed analysis of 2D NMR data of 2 led to the identification of 12 residues: NMeAla, β-OMeTyr, NMeThr, Leu, Gly (2), 3-OMeAla, Thr (2), 3,4-DiMeGln, Dab, and Hdna (Fig. 1). The sequence of these residues was deduced from analysis of ROESY and HMBC correlations, as in 1. The structure of 2 differed from 1 by the presence of NMeThr, Thr, and Gly in place of NMeGln, 3-OHGln, and 3-OHAsn residues, respectively. Similar to compound 1, macrocyclic ring closure in 2 was established by an HMBC correlation from the Thr1 oxymethine (δH 5.65) and the carbonyl (δC 171.7) of the C-terminal NMeAla residue. The absolute configurations of the amino acid residues of 2 were also determined by Marfey’s analysis of the acid hydrolysate using a similar strategy employed with 1. Compound 2 was shown to have the same absolute configuration for each amino acid residue it has in common with 1. It was also analyzed for l-NMeThr and both a d-allo-Thr and a l-Thr residue. Based on the close structural similarity between compounds 1 and 2, and the fact that all cyclic depsipeptides in this structural class that form a macrocycle via esterification of the C-terminus with the hydroxyl group of a threonine utilize a d-allo-Thr residue, the l-Thr was assigned to be adjacent to the N-terminal Gly residue in 2.
The anti-HIV activity of 1 and 2 was evaluated in an XTT based cell viability assay using the human T-cell line CEM-SS infected with HIV-1RF.14 After a six day incubation period, compounds 1 and 2 effectively inhibited the cytopathic effect of HIV-1 infection with EC50 values (concentration at which 50% of the target cells are protected from death by the virus) of 23 and 27 nM, respectively (Fig. 3 and Supplementary data). Direct cytotoxicity of 1 and 2 against the host cells was observed with IC50 values (concentration at which 50% of cells are killed by the test sample) of 367 and 373 nM, respectively.
Figure 3.
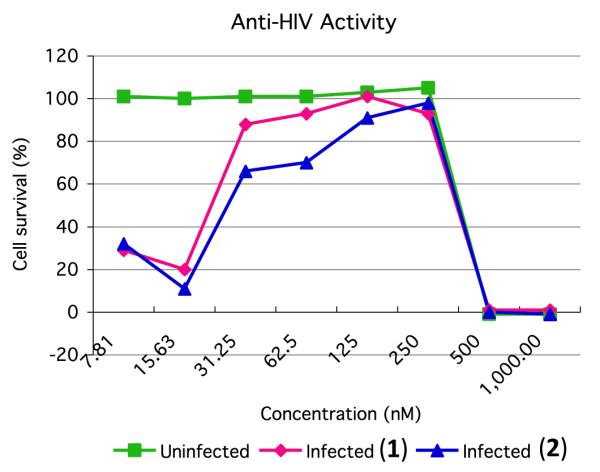
Anti-HIV activity of compounds 1 and 2. Uninfected cell cultures reveal direct cytotoxicity of the test samples. HIV infected cultures show cytoprotective effects of 1 and 2 against viral infection at lower concentrations, and cytotoxic effects at higher concentrations.
In conclusion, stellettapeptins A (1) and B (2) are new depsipeptides with structural features characteristic of the family of anti-HIV peptides which includes the callipeltins,6,7,15–17 papuamides,8 mirabamides,1,18 and neamphamide.9 Compounds 1 and 2 contain a previously undescribed polyketide subunit, 3-hydroxy-6,8-dimethylnon-4-enoic acid, and the 3-OHGln and 3-OHAsn residues in 1 are rarely found in peptides. Compounds 1 and 2 potently inhibited the cytopathic effect of HIV-1 infection, providing additional evidence that this class of peptides may hold promise as anti-HIV agents. Isolation of the stellettapeptins from Stelletta sp., which is phylogenetically distinct from sponges the callipeltins and papuamides were isolated from, and the fact that 1 and 2 have characteristic structural features of nonribosomal peptides, suggest a possible microbial origin for the stellettapeptins.
Supplementary Material
Acknowledgments
We thank professors Apurba Dutta, University of Kansas, and Anders Broberg, Swedish University of Agricultural Sciences, for synthetic samples of (2S,3R)- and (2R,3S)-3-OH-aspartic acid, and threo- and erythro-3-OH-glutamic acid, respectively. We thank the late Prof. Luigi Minale for a sample of callipeltin A, Catherine Hixson (SAIC) for the hydrolysis of 1 and 2, and S. Tarasov and M. Dyba, Biophysics Resource, Structural Biophysics Laboratory for mass spectral analysis. This project was funded in part with federal funds from the Frederick National Laboratory for Cancer Research, National Institutes of Health (contract HHSN261200800001E). This research was supported in part by the Intramural Research Program of NIH, National Laboratory for Cancer Research, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported in part by the Korea Institute of Ocean Science & Technology (Grant PE98816 to H.J.S.).
Footnotes
Supplementary data (1D and 2D NMR spectral data, ozonolysis, amino acid analysis, anti-HIV data, and isolation of 1 and 2) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet.2015.05.058. These data include MOL files and InChiKeys of the most important compounds described in this article.
References and notes
- 1.Lu Z, Van Wagoner RM, Harper MK, Baker HL, Hooper JNA, Bewley CA, Ireland CM. J. Nat. Prod. 2011;74:185. doi: 10.1021/np100613p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Naggar M, Piggott AM, Capon RJ. Org. Lett. 2008;10:4247. doi: 10.1021/ol8016512. [DOI] [PubMed] [Google Scholar]
- 3.Wegerski CJ, France D, Cornell-Kennon S, Crews P. Bioorg. Med. Chem. 2004;12:5631. doi: 10.1016/j.bmc.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 4.Piel J. Nat. Prod. Rep. 2004;21:519. doi: 10.1039/b310175b. [DOI] [PubMed] [Google Scholar]
- 5.Fujii K, Ikai Y, Oka H, Suzuki M, Harada K-I. Anal. Chem. 1997;69:5146. [Google Scholar]
- 6.Zampella A, D’Orsi R, Sepe V, Casapullo A, Monti MC, D’Auria MV. Org. Lett. 2005;7:3585. doi: 10.1021/ol0513600. [DOI] [PubMed] [Google Scholar]
- 7.Zampella A, D’Auria MV, Gomez-Paloma L, Casapullo A, Minale L, Debitus C, Henin Y. J. Am. Chem. Soc. 1996;118:6202. [Google Scholar]
- 8.Ford PW, Gustafson KR, McKee TC, Shigematsu N, Maurizi LK, Pannel LK, Williams DE, de Silva ED, Lassota P, Allen TM, Soest RV, Andersen RJ, Boyd MR. J. Am. Chem. Soc. 1999;121:5899. [Google Scholar]
- 9.Oku N, Gustafson KR, Cartner LK, Wilson JA, Shigematsu N, Hess S, Pannell LK, Boyd MR, McMahon JB. J. Nat. Prod. 2004;67:1407. doi: 10.1021/np040003f. [DOI] [PubMed] [Google Scholar]
- 10.Bunnage ME, Burke AJ, Davies SG, Millican NL, Bicholson RL, Proberts PM, Smith AD. Org. Biomol. Chem. 2003;1:3708. doi: 10.1039/b306936m. [DOI] [PubMed] [Google Scholar]
- 11.Han H, Yoon J, Janda KD. J. Org. Chem. 1998;63:2045. [Google Scholar]
- 12.Robinson AJ, Stanislawski P, Dulholland D, He L, Li H-Y. J. Org. Chem. 2001;66:4148. doi: 10.1021/jo001152f. [DOI] [PubMed] [Google Scholar]
- 13.Burke AJ, Davies SG, Hedgecock CJR. Synlett. 1996:621. [Google Scholar]
- 14.Gulakowski RJ, McMahon MB, Staley PG, Moran RA, Boyd MR. J. Virol. Methods. 1991;33:87. doi: 10.1016/0166-0934(91)90010-w. [DOI] [PubMed] [Google Scholar]
- 15.D’Auria MV, Zampella A, Gomez-Paloma L, Minale L, Debitus C, Roussakis C, Le Bert V. Tetrahedron. 1996;52:9589. [Google Scholar]
- 16.Zampella A, Randazzo A, Borbone N, Luciani S, Trevisi L, Debitus C, D’Auria MV. Tetrahedron Lett. 2002;43:6163. [Google Scholar]
- 17.Sepe V, D’Orsi R, Borbone N, D’Auria MV, Bifulco G, Monti MC, Catania A, Zampella A. Tetrahedron. 2006;62:833. [Google Scholar]
- 18.Plaza A, Gustchina E, Baker HL, Kelly M, Bewley CA. J. Nat. Prod. 2007;70:1753. doi: 10.1021/np070306k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.