Abstract
The criteria to evaluate response to treatment in acute myeloid leukemia (AML) have changed little in the past sixty years. It is now possible to use higher sensitivity tools to measure residual disease burden in AML. Such minimal or measurable residual disease (MRD) measurements provide a deeper understanding of current patient status and allow stratification for risk of subsequent clinical relapse. Despite these obvious advantages, and after over a decade of laboratory investigation and pre-clinical validation, MRD measurements are not currently routinely used for clinical decision-making or drug development in non-APL AML. We review here some potential constraints that may have delayed adoption including a natural hesitancy of end users, economic impact concerns, misperceptions regarding the meaning of and need for assay sensitivity, the lack of one single MRD solution for all AML patients and finally the need to involve patients in decision making based on such correlates. It is our opinion that none of these issues represent insurmountable barriers and our hope is that by providing potential solutions we can help map a path forward to a future where our patients will be offered personalized treatment plans based on the amount of AML they have left remaining to treat.
After nearly 15 years of methodological validation and fine-tuning, the concept of minimal or measurable residual disease (MRD) is reaching the point where the collective amount of data accumulated is now almost sufficient to support integration into overall clinical strategy and for individualized patient decision-making. This is perhaps best evidenced by the series of seminars held under the auspices of the FDA in early 2013, where this concept was debated in different diseases, including AML [1]. The purpose was to evaluate whether this approach to the extent of remaining disease in our patients should supersede the traditional – and arguably by and large obsolete – morphological evaluation.
Given that the literature is replete with reviews on the methodological features and potential prognostic implications of MRD in AML [2–10] we instead here attempt to distill the data from the current clinically oriented literature to assemble a picture of how this concept might be most efficiently integrated into the routine care of our AML patients. Rather than argue the strong points favoring the use of MRD we instead briefly summarize the problems facing its clinical integration and suggest ways to speed up this process.
There is no single MRD assay in AML
AML is the prototype of a heterogeneous cancer, the complexity of which is amply covered in the current literature [11–16]. Consequently, the potential MRD targets in AML are nearly as diverse ranging from fusion transcripts [17, 18], somatic mutations [19] to individual [20, 21] or, more recently, combinations [22, 23] of overexpressed genes. With the emerging NGS option and the increasing use of flow cytometry you should theoretically be spoiled for choice. This however masks a situation in which the tradition of diagnostic procedures in a given center often dictates the choice of methodology, be it molecular biology or flow cytometry. What should be imperative, however, is that whatever method is applied, it is validated not only per se, but also in terms of continuous proficiency testing in a multicenter setting.
Next generation sequencing (NGS) is rapidly emerging as a third way of performing MRD in the hematological malignancies [24–36]. While ideal for identification of clone specific rearranged immune receptors as found in the lymphoid malignancies NGS faces several limitations to immediate application in myeloid malignancies. Firstly, identification of single nucleotide variant mutations present at variant allele frequencies less than 2% is currently challenging due to the intrinsic error rate associated with this technology although innovative approaches to overcome this limitation are being developed [37–39]. Secondly, there is increasing appreciation that somatic mutations recurrently observed in patients with MDS/AML are not in themselves disease defining and may also be found in such patients enjoying very long term remissions consistent with cure [40] and even in apparently clinically healthy older adults [41–44]. The clinical significance of detection of such mutations as a consequence of clonal hematopoiesis from a potentially pre-leukemic hematopoietic stem cell pool [16, 45, 46] in otherwise healthy individuals remains to be fully established. Thirdly, NGS is a rapidly evolving scientific field and the stability and regulation of “locked down” reagent, technical and bioinformatics pipelines required for clinical biomarker development is not yet complete [47–49]. This problem may be exacerbated by the lack of dominant commercial or academic suppliers of NGS testing for AML MRD. Despite these limitations, and the fact it currently lacks the validation which has been afforded qPCR and flow cytometry, we do foresee that NGS will be a major tool in the management of AML diagnosis and monitoring in the future, given high throughput capacity and the ability to delineate inter- and intrapatient heterogeneities.
Present status of clinical evaluation of MRD
The value of MRD determination in the clinical setting in AML is at the present time based predominantly on retrospective data. The clinical value of such high sensitivity measurement of remaining disease burden appears to be uniformly positive with the caveat that the typical approach was not to intervene with additional malignant cytoreduction, but rather correlate the ultimate course of the disease to an MRD value at a given time, usually at first CR evaluation or prior to transplantation. Irrespective of the time point chosen MRD has invariably been shown able to stratify patients in complete remission into distinct cohorts with different risk of hematological relapse. It is important to realize that this has been true not only for the 1st CR evaluation setting, but to an equal extent for later ones e.g. end of consolidation. Importantly this information on the amount of leukemia left after treatment (MRD) can, in some cases, supersede pre-treatment prognostic risk stratification based on surrogates of disease biology (ie: the biology of the leukemia that was present before treatment) [50].
What are the constraints that impact AML MRD and its adoption into the clinical setting?
We realize that incorporation of MRD into routine clinical practice faces several challenges. Shortly after introduction, the major challenge was the mere fact that it involved new technologies, mainly the quantitative real-time PCR (qPCR) hardware. As with all other technological advances, this has been not only made less expensive, but also simplified. The same concept only partially applies to flow cytometry, where the identification of leukemia-associated phenotypes (LAIPs) is still a basic scientific challenge despite advances in hardware similar to those seen for QPCR. Nevertheless, other variables are to our minds of major concern.
1) Overcoming the natural hesitancy of the end-users
Undoubtedly, the most important hindrance in adopting MRD as a gold standard for following leukemia patients is a psychological one. Thus, it has been a continuous struggle throughout the 2000s to convince hematologist colleagues that a conversion to MRD positivity invariably signals an impending clinical relapse [51–59]. We strongly feel that the fear of false positivity and overtreatment of potentially already cured patients is over-emphasized given the above abundant literature with the single caveat that a confirmatory second positive MRD test taken within 2–4 weeks of the first is necessary to make a diagnosis of molecular relapse [4].
Support for MRD as “actionable” evidence of persistent disease should come from the eagerly anticipated results of ongoing clinical trials where MRD is incorporated prospectively either in a monitor/not monitor setting (e.g. the MRC AML17 trial, ISRCTN55675535) or in post treatment, risk based, selection of transplant type [60]. In the meantime data presentation of MRD results remains a challenge, but we have found that the software package we recently published and which is being used in UK AML trials, has had a significant impact in explaining MRD data to both clinicians and patients [61].
2) Economics
A second qualm raised about the application of MRD assays concerns the expenditure entailed. There is a clear incremental cost to performing molecular or flow cytometric assessments of post-treatment disease burden in addition to the historical standard of morphological evaluation. These expenses must be framed however in reference to the cost of treatment itself; initial induction-related hospitalization costs have been estimated at around 60,000–80,000 USD [62–64] and the costs of allogeneic hematopoietic stem cell transplantation have been estimated to be on average approximately 90,000 USD for the procedure alone (range 26,580 to $200,000 USD) [65–67]. These costs are likely to increase, for example a course of a single newly developed immune checkpoint inhibitor drug (not approved for AML but currently in clinical trials) typically exceeds $100,000, with optimal therapy likely to involve combinations of these agents [68].
In contrast the cost of MRD assessment by molecular and NGS methods is likely to fall with increasing automation [69] together with increased capacity and decreased hardware costs [70]. Accurately determining treatment response and triaging to appropriate therapy or continued surveillance, a clear clinical and ethical imperative, is likely to cost at least one and perhaps two orders of magnitude less than any therapy given. The “number needed to test” for cost-neutrality would likely be highly favorable given the considerable economic consequences of preventing an inappropriate transplant for example. Furthermore, given the rising costs of drug development and efficiency in the conduct of clinical trials required for drug approval [71] and increased focus on targeted relapse prevention with maintenance therapy [72] having quantifiable evidence of efficacy for interventions performed in the remission setting is likely to have benefit.
3) Assay sensitivity
We are therefore left with more technical issues relating to e.g. sensitivity and data presentation as constraints for integrating MRD into clinical practice as a routine measure and not only a tool in treatment protocols. As we have argued elsewhere, the sensitivity issue is context dependent [3]. Thus, in the vast majority of clinical scenarios the sensitivity afforded by all assays, be they QPCR or flow cytometry based, will exceed the current standard of care. Importantly in the low residual disease setting the accuracy of MRD testing will be limited not by assay sensitivity but rather sampling constraints and threshold setting [73]. A strictly technical focus on finding “the best MRD assay” most “ready for primetime” defined by artificial estimates of sensitivity may obscure the clinical reality that most responses to therapy in AML patients, both in clinical practice and in investigational trials, are currently measured using criteria not substantially modified since their initial introduction in 1956[74]. The initial objective should therefore not be to find the unquestionably one perfect AML MRD assay, but instead adopt standardized easily comparable response criteria better than that most currently used based only on blast counting by morphology. Even a one or two log increased sensitivity of disease detection beyond the current 5% threshold (Figure 1) would likely offer real clinical benefit.
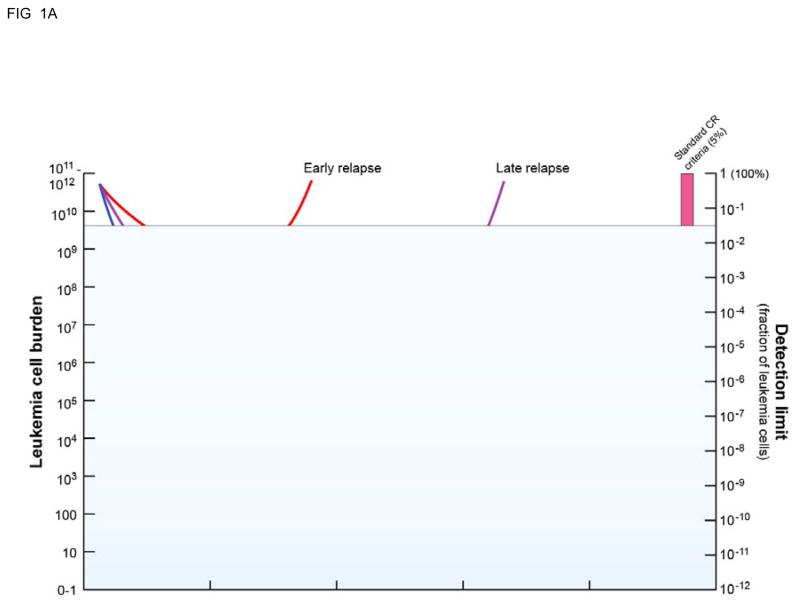
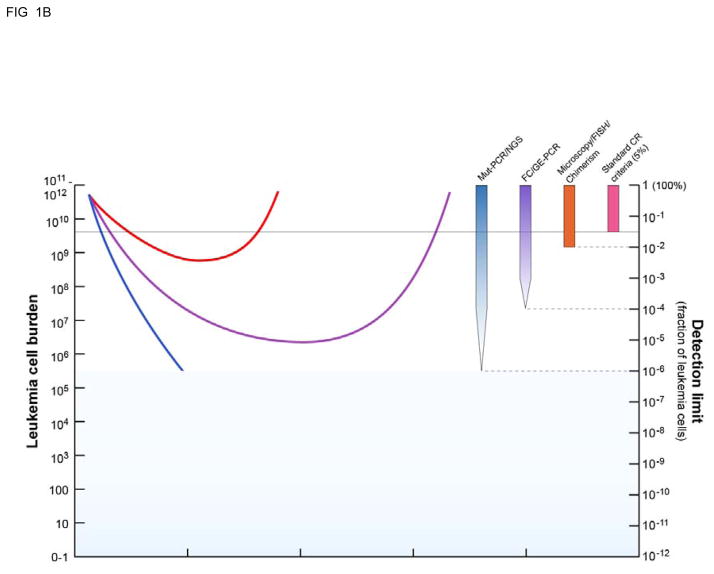
Figure 1. High sensitivity measurements of residual disease burden allows for a more complete understanding of AML treatment response and clinical “relapse”.
A: Standard clinical response criteria mean that most (50–75%) of patients treated with chemotherapy achieve an initial “complete remission” but many will subsequently relapse. B: Use of measurable residual disease (MRD) techniques with higher sensitivity than morphological examination (>5% blasts) for example FISH/chimerism (orange bar, ~10−2 sensitivity), flow cytometry or gene expression RQ-PCR (purple bar, ~10−4 sensitivity) or PCR for re-arranged or mutated sequence/next generation sequencing (blue bar, ~10−6 sensitivity) allows for better understanding of response to treatment and greater ability to predict subsequent clinical relapse. Adapted from [6].
The various ways of describing assay sensitivity for MRD has resulted in some confusion that is unhelpful to the goals of adoption and standardization. Sensitivity for fusion transcripts were initially calculated as the ability of a given assay to distinguish a leukemic cell diluted in either water or a non-leukemia background, for example T-cells. Sensitivity however should only be defined in the context of thresholds for positivity set based on the background target expression levels in real world healthy or regenerating hematopoiesis. These normal thresholds will vary according to sample source [75] and by MRD target selected [41–44]. A final additional weakness in claims of sensitivity particularly prominent in flow cytometry is the practice of not applying any correction to account for the fraction of total leukemia burden represented by the marker or immunophenotype used to track MRD. LAIP’s often encompass no more than 5–10% of the original diagnostic sample, with multiple LAIPs typically reported per patient at presentation [76]. The reporting of the ability to detect for example 0.1% LAIP positive cells in a remission sample will therefore lead to a leukemia detection sensitivity of not 1 in 1000, but rather as low as 1 in 50, if the original LAIP encompassed only 5% percent of the malignant population.
4) Patient heterogeneity and antigenic drift
Acute myeloid leukemia is a broad diagnosis that encompasses a genetically and phenotypically diverse set of myeloid malignancies [11]. In addition to this inter-patient variation there is also significant intra-patient variability with the potential multiple malignant clones to be present within any individual patient at initial diagnosis [16, 75] with the observation that the predominant clone at presentation is not necessarily the one ultimately responsible for post treatment relapse and death [15]. While both of these factors increase the difficulty associated with the adoption of MRD for AML in clinic, neither is insurmountable.
AML is not a single entity. It is therefore clear that a search for one single universal marker for MRD assessment in AML is likely to be a suboptimal strategy. An extensive program by a consortium of 26 European university laboratories was required to standardize QPCR detection of fusion gene transcripts [17] and by 11 laboratories within the European Leukemia Network to validate WT1, the lead overexpressed gene candidate for residual AML detection [76]. More recently MRD assays involving detection of somatic mutations [27, 54, 59, 77, 78] and QPCR array panels incorporating multiple targets have been reported in both pediatric [22] and adult [23] AML. While there is no one molecular MRD assay suitable for all cases of AML, every AML patient has at least one assay that can be used. Similarly, in the right hands flow cytometry can identify an informative immunophenotype at diagnosis in the vast majority (95%) of AML patients [79, 80].
There is a justifiable fear that any MRD monitoring target or immunophenotype selected at diagnosis may be lost at relapse. For molecular targets those mutations occurring later in the evolutionary development of an AML [16, 81], for example FLT3-ITD [82–84], may be either gained or lost at relapse. Initiating driver mutations such as the balanced chromosomal translocations seen in core binding factor leukemia [85, 86] or acute promyelocytic leukemia [87, 88] and those seen in the epigenetic regulators DNMT3A, TET2 and ASXL1 are more stable but, as previously stated, may also be found at some level in patients in long-term remissions [40] and in pre-leukemic cells in otherwise healthy leukemia-free individuals [16, 41–46]. NPM1-mutation appears to represent a useful and stable marker of AML MRD [27, 55, 59, 77, 89–91] frequently seen in those with normal cytogenetics [92]. Discrepancies in those leukemia associated aberrant immunophenotypes detectable by flow cytometry in a patient between presentation and ultimate relapse are very well documented [75, 93–96]. While analysis strategies have been developed to mitigate the impact of this “antigenic shift” they require investment of considerable expertise and effort. There is no way of denying that flow cytometry of malignant myelopoiesis is far more complicated than that of e.g. early B-cell development in pre-B ALL or that of detecting the remaining mature B-cell compartment in patients with multiple myeloma. In our view any universally acceptable and widely used test of MRD in AML is ultimately likely to be molecular (QPCR or NGS) rather than flow cytometry based.
5) Involving the patient
Last, but not least, an important component is the patient experience and understanding of the results of any MRD testing. Remission is defined as the absence of detectable disease, which is dependent on the sensitivity of the modality used for disease detection (Figure 1). Traditional response criteria thresholds are based not on any underlying biological reality but rather on the technical sensitivity of tests used for disease detection, creating a comfortable but artificial dichotomy between clinical remission and clinical relapse, with the consequence that two AML patients achieving a “complete remission” may have markedly different risks of subsequent clinical relapse. Communicating, in a balanced way, the advantages and limitations of the tools used to track low levels of residual disease requires deep understanding on the part of the physician and a significant investment of time. Here the graphic depiction as mentioned above [61] can be of great help (“we have a handle on your disease”) and it is our experience that once the patient has grasped the concept, much time can be spared in later out-patient consultations.
It may be possible for a patient in CR to have peripheral blood samples for surveillance monitoring taken at her/his primary care physician or the referring department. This would require extra care in ensuring correct tubes are at hand and that the delivery to the lab is speedy, and inclusion of the internal quality controls for QPCR (inclusion of household genes as controls) and flow cytometry (inclusion of same tube viability markers) to identify samples of inferior quality. While sensitivity from bone marrow (BM) samples is superior for most, BM aspiration is only necessary for a fraction of the MRD assays presently being used in clinical trials [3, 4]. The time schedule for the interval between each such monitoring test could be set based on the knowledge of AML subtype relapse kinetics [3, 4, 54, 56, 58]. When an MRD sample turns positive, the procedure adopted by many centers is to test another PB sample taken in at least two, but no more than four, weeks time. If this shows unaltered or increasing positivity, the patient should be called in for a BM aspiration, which will invariably confirm the relapse detected in the PB. The consistent inclusion of MRD in AML in a setting like the one described will ultimately result in a smoother and more evidence-based methodology for following these patients. The impact of such an individualized monitoring strategy on patient anxiety and post-treatment health-related quality of life (HRQOL) should be studied [97].
How to move forward?
The transitional phase MRD is going through at the present time should be as brief as possible. In order to accomplish that, it is, however, necessary to realize some issues and focus on other ones.
Firstly, the hematology community needs to abandon the antiquated reliance on “blast counting.” While perfectly appropriate based on the technology available at the time when first proposed as a measure of residual disease burden in 1956 [74], this is not a biologically relevant approach on which to make important treatment decisions in the 21st century [6, 98]. Acknowledging that we can no longer reasonably base recommendations to our patients regarding potentially fatal intervention e.g. allogeneic transplantation (where molecular biology has advanced our knowledge considerably) solely upon the counting of a few hundred cells in a stained histology smear is an important first step on the path to change.
Taking the next steps towards incorporating higher sensitivity measurements of residual disease burden in our routine clinical assessment will, on the other hand, require a considerable amount of stringency and international cooperation. In this respect, the initial efforts from the U.S Food and Drug Administration (FDA) to raise in 2013 the possibility of using minimal residual disease as a surrogate endpoint in acute myeloid leukemia clinical trials [1, 99] were highly commendable and we await with interest the upcoming written guidance from this agency on this matter. In Table 1 we outline a few key points, which we feel are of importance for the hematological community to deal with, preferably in a coordinated manner internationally. We believe that these goals – while not belonging to the low-hanging fruit category – are reachable within a few years and that this part of the process is clearly manageable. What is called for is, however, that steering committees are established to act as advisors and proponents for those designing new treatment protocols with MRD endpoints integral to the trial design. Hopefully, such groups will be able to secure a degree of standardization in applying MRD assays. We propose holding an international working group meeting on “high sensitivity measurement of acute myeloid leukemia as clinical endpoints” in 2017 on the fourth anniversary of the FDA workshop to review progress and reach consensus on what assays can be considered sufficiently robust and validated to be adopted clinically and which should remain in a developmental research stage.
Table 1.
Outstanding issues for implementing MRD in clinical decision-making in AML
| Issue | Present Problems | Moving forward |
|---|---|---|
| Acceptance by clinicians | Understandable reluctance | Awaiting more clinical trials, e.g. AML17 |
| Different platforms | Confusion, particularly for flow | Formalized proficiency testing |
| Expressing sensitivity | Different approaches | Agreement between working groups of common standard |
| MRD based decision-making | Scantily applied | Upfront incorporation, e.g. whether to transplant |
| What method to employ? | Lack of consistency | Initiate prospective comparisons between flow and RQ-PCR |
| Data reporting | Difficulties in interpretation | Common reporting packages incorporating graphics |
Finally, having accomplished that, we will look forward to the day when the MRD abbreviations can be abandoned. AML disease burden after treatment has been artificially dichotomized into either “complete” remission or not on the basis of the historical artifact of the low sensitivity tools used to quantify AML sixty years ago [74]. In reality AML patients in complete remission have a high diverse clinical status ranging from those definitively cured to those patients with a huge residual leukemia burden only just less than clinically obvious relapse (Figure 1) [6]. Therefore while the recent suggestion that the MRD abbreviation should be retained but renamed not minimal but “measurable” residual disease [100] is an improvement it would be most honest to also classify any residual leukemia detected by traditional low sensitivity methods in this same category, acknowledging that any distinctions between high levels of “MRD” and low levels of clinically evident relapse are arbitrary and without biological meaning. We would suggest therefore that rather than try to preserve the acronym it may be more helpful if we simply instead adopted the term RD (for residual disease) for measurements consistent with the presence of residual leukemia when found by any methodology including traditional morphology, cytogenetics, chimerism, qPCR, MFC or NGS. This term may be modified by descriptors of the method(s) used and estimates of the quantity remaining, but would more honestly reflect our biological understanding of tumor burden, better reflect our appreciation that our ability to detect residual disease is based on the sensitivity of the tools we use to do so, and better signal our confidence to determine the extent to which our therapeutic ventures have succeeded or not.
Acknowledgments
This work was supported by the Intramural Research Program of the National Heart, Lung, Blood Institute of the National Institutes of Health.
The authors thank Alan Hoofring and Ethan Tyler of the NIH Medical Arts Service for assistance.
Footnotes
CONFLICT-OF-INTEREST DISCLOSURE
Authors report no relevant conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hokland P, Cotter F. Readying the minimal residual disease concept in acute myeloid leukaemia for prime time - the American way. Br J Haematol. 2013;162(4):429–30. doi: 10.1111/bjh.12419. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”? Blood. 2014;124(23):3345–55. doi: 10.1182/blood-2014-05-577593. [DOI] [PubMed] [Google Scholar]
- 3.Hokland P, et al. Sensitivity of minimal residual disease in acute myeloid leukaemia in first remission--methodologies in relation to their clinical situation. Br J Haematol. 2012;158(5):569–80. doi: 10.1111/j.1365-2141.2012.09203.x. [DOI] [PubMed] [Google Scholar]
- 4.Hokland P, Ommen HB. Towards individualized follow-up in adult acute myeloid leukemia in remission. Blood. 2011;117(9):2577–84. doi: 10.1182/blood-2010-09-303685. [DOI] [PubMed] [Google Scholar]
- 5.Roug AS, et al. Diagnosing and following adult patients with acute myeloid leukaemia in the genomic age. Br J Haematol. 2014;167(2):162–76. doi: 10.1111/bjh.13048. [DOI] [PubMed] [Google Scholar]
- 6.Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nat Rev Clin Oncol. 2013;10(8):460–71. doi: 10.1038/nrclinonc.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paietta E. Minimal residual disease in acute myeloid leukemia: coming of age. Hematology Am Soc Hematol Educ Program. 2012;2012:35–42. doi: 10.1182/asheducation-2012.1.35. [DOI] [PubMed] [Google Scholar]
- 8.Coustan-Smith E, Campana D. Should evaluation for minimal residual disease be routine in acute myeloid leukemia? Curr Opin Hematol. 2013;20(2):86–92. doi: 10.1097/MOH.0b013e32835dd90a. [DOI] [PubMed] [Google Scholar]
- 9.Kayser S, et al. Evidence-based focused review of minimal residual disease-directed therapy in acute myeloid leukemia. Blood. 2015 doi: 10.1182/blood-2014-11-578815. [DOI] [PubMed] [Google Scholar]
- 10.Appelbaum FR. Measurement of minimal residual disease before and after myeloablative hematopoietic cell transplantation for acute leukemia. Best Pract Res Clin Haematol. 2013;26(3):279–84. doi: 10.1016/j.beha.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–74. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes AE, et al. Clonal architecture of secondary acute myeloid leukemia defined by single-cell sequencing. PLoS Genet. 2014;10(7):e1004462. doi: 10.1371/journal.pgen.1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klco JM, et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer Cell. 2014;25(3):379–92. doi: 10.1016/j.ccr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goswami M, et al. Expression of putative targets of immunotherapy in acute myeloid leukemia and healthy tissues. Leukemia. 2014;28(5):1167–70. doi: 10.1038/leu.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding L, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506–10. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welch JS, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–78. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabert J, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003;17(12):2318–57. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 18.Tobal K, Frost L, Liu Yin JA. Quantification of DEK-CAN fusion transcript by real-time reverse transcription polymerase reaction in patients with t(6;9) acute myeloid leukemia. Haematologica. 2004;89(10):1267–9. [PubMed] [Google Scholar]
- 19.Gorello P, et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia. 2006;20(6):1103–8. doi: 10.1038/sj.leu.2404149. [DOI] [PubMed] [Google Scholar]
- 20.Cilloni D, et al. Quantitative assessment of WT1 expression by real time quantitative PCR may be a useful tool for monitoring minimal residual disease in acute leukemia patients. Leukemia. 2002;16(10):2115–21. doi: 10.1038/sj.leu.2402675. [DOI] [PubMed] [Google Scholar]
- 21.Inoue K, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994;84(9):3071–9. [PubMed] [Google Scholar]
- 22.Steinbach D, et al. Prospective validation of a new method of monitoring minimal residual disease in childhood acute myeloid leukemia. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-1999. [DOI] [PubMed] [Google Scholar]
- 23.Goswami M, et al. A multigene array for measurable residual disease detection in AML patients undergoing SCT. Bone Marrow Transplant. 2015 doi: 10.1038/bmt.2014.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hourigan CS. Next Generation MRD. Biol Blood Marrow Transplant. 2014;20(9):1259–60. doi: 10.1016/j.bbmt.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logan AC, et al. Immunoglobulin and T Cell Receptor Gene High-Throughput Sequencing Quantifies Minimal Residual Disease in Acute Lymphoblastic Leukemia and Predicts Post-Transplantation Relapse and Survival. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan AC, et al. Minimal residual disease quantification using consensus primers and high-throughput IGH sequencing predicts post-transplant relapse in chronic lymphocytic leukemia. Leukemia. 2013;27(8):1659–65. doi: 10.1038/leu.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thol F, et al. Next-generation sequencing for minimal residual disease monitoring in acute myeloid leukemia patients with FLT3-ITD or NPM1 mutations. Genes Chromosomes Cancer. 2012;51(7):689–95. doi: 10.1002/gcc.21955. [DOI] [PubMed] [Google Scholar]
- 28.Ladetto M, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia. 2013 doi: 10.1038/leu.2013.375. [DOI] [PubMed] [Google Scholar]
- 29.Duployez N, et al. Minimal residual disease monitoring in t(8;21) acute myeloid leukemia based on RUNX1-RUNX1T1 fusion quantification on genomic DNA. Am J Hematol. 2014;89(6):610–5. doi: 10.1002/ajh.23696. [DOI] [PubMed] [Google Scholar]
- 30.Faham M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120(26):5173–80. doi: 10.1182/blood-2012-07-444042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mailankody S, et al. Minimal residual disease in multiple myeloma: bringing the bench to the bedside. Nat Rev Clin Oncol. 2015 doi: 10.1038/nrclinonc.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Lopez J, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123(20):3073–9. doi: 10.1182/blood-2014-01-550020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrappe M. Detection and management of minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2014;2014(1):244–9. doi: 10.1182/asheducation-2014.1.244. [DOI] [PubMed] [Google Scholar]
- 34.Weng WK, et al. Minimal residual disease monitoring with high-throughput sequencing of T cell receptors in cutaneous T cell lymphoma. Sci Transl Med. 2013;5(214):214ra171. doi: 10.1126/scitranslmed.3007420. [DOI] [PubMed] [Google Scholar]
- 35.Wu D, et al. Detection of minimal residual disease in B lymphoblastic leukemia by high-throughput sequencing of IGH. Clin Cancer Res. 2014;20(17):4540–8. doi: 10.1158/1078-0432.CCR-13-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu D, et al. High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med. 2012;4(134):134ra63. doi: 10.1126/scitranslmed.3003656. [DOI] [PubMed] [Google Scholar]
- 37.Young AL, et al. Quantifying ultra-rare pre-leukemic clones via targeted error-corrected sequencing. Leukemia. 2015 doi: 10.1038/leu.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robasky K, Lewis NE, Church GM. The role of replicates for error mitigation in next-generation sequencing. Nat Rev Genet. 2014;15(1):56–62. doi: 10.1038/nrg3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lou DI, et al. High-throughput DNA sequencing errors are reduced by orders of magnitude using circle sequencing. Proc Natl Acad Sci U S A. 2013;110(49):19872–7. doi: 10.1073/pnas.1319590110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ploen GG, et al. Persistence of DNMT3A mutations at long-term remission in adult patients with AML. Br J Haematol. 2014;167(4):478–86. doi: 10.1111/bjh.13062. [DOI] [PubMed] [Google Scholar]
- 41.Jaiswal S, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genovese G, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–87. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie M, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472–8. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busque L, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44(11):1179–81. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corces-Zimmerman MR, et al. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci U S A. 2014;111(7):2548–53. doi: 10.1073/pnas.1324297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jan M, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med. 2012;4(149):149ra118. doi: 10.1126/scitranslmed.3004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pant S, Weiner R, Marton MJ. Navigating the rapids: the development of regulated next-generation sequencing-based clinical trial assays and companion diagnostics. Front Oncol. 2014;4:78. doi: 10.3389/fonc.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chin EL, da Silva C, Hegde M. Assessment of clinical analytical sensitivity and specificity of next-generation sequencing for detection of simple and complex mutations. BMC Genet. 2013;14:6. doi: 10.1186/1471-2156-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson CA, Simonyan V. FDA’s Activities Supporting Regulatory Application of “Next Gen” Sequencing Technologies. PDA J Pharm Sci Technol. 2014;68(6):626–30. doi: 10.5731/pdajpst.2014.01024. [DOI] [PubMed] [Google Scholar]
- 50.Jourdan E, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013;121(12):2213–23. doi: 10.1182/blood-2012-10-462879. [DOI] [PubMed] [Google Scholar]
- 51.Stentoft J, et al. Minimal residual core binding factor AMLs by real time quantitative PCR--initial response to chemotherapy predicts event free survival and close monitoring of peripheral blood unravels the kinetics of relapse. Leuk Res. 2006;30(4):389–95. doi: 10.1016/j.leukres.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 52.Ommen HB, et al. Relapse prediction in acute myeloid leukaemia patients in complete remission using WT1 as a molecular marker: development of a mathematical model to predict time from molecular to clinical relapse and define optimal sampling intervals. Br J Haematol. 2008;141(6):782–91. doi: 10.1111/j.1365-2141.2008.07132.x. [DOI] [PubMed] [Google Scholar]
- 53.Bornhauser M, et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica. 2009;94(11):1613–7. doi: 10.3324/haematol.2009.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ommen HB, et al. Strikingly different molecular relapse kinetics in NPM1c, PML-RARA, RUNX1-RUNX1T1, and CBFB-MYH11 acute myeloid leukemias. Blood. 2010;115(2):198–205. doi: 10.1182/blood-2009-04-212530. [DOI] [PubMed] [Google Scholar]
- 55.Sockel K, et al. Minimal residual disease-directed preemptive treatment with azacitidine in patients with NPM1-mutant acute myeloid leukemia and molecular relapse. Haematologica. 2011;96(10):1568–70. doi: 10.3324/haematol.2011.044388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ommen HB, et al. Relapse kinetics in acute myeloid leukaemias with MLL translocations or partial tandem duplications within the MLL gene. Br J Haematol. 2014;165(5):618–28. doi: 10.1111/bjh.12792. [DOI] [PubMed] [Google Scholar]
- 57.Platzbecker U, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26(3):381–9. doi: 10.1038/leu.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ommen HB, et al. The kinetics of relapse in DEK-NUP214 positive acute myeloid leukemia patients. Eur J Haematol. 2015 doi: 10.1111/ejh.12511. [DOI] [PubMed] [Google Scholar]
- 59.Schnittger S, et al. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood. 2009;114(11):2220–31. doi: 10.1182/blood-2009-03-213389. [DOI] [PubMed] [Google Scholar]
- 60.Venditti A, et al. Risk-Adapted, MRD-Refined Therapeutic Approach for the Treatment of Acute Myeloid Leukemia: From a Single Center Experience to the Cooperative Gimema Protocol AML1310. ASH Annual Meeting Abstracts. 2012;120(21):1422. [Google Scholar]
- 61.Ostergaard M, et al. Development of standardized approaches to reporting of minimal residual disease data using a reporting software package designed within the European LeukemiaNet. Leukemia. 2011;25(7):1168–73. doi: 10.1038/leu.2011.69. [DOI] [PubMed] [Google Scholar]
- 62.Nerich V, et al. Induction-related cost of patients with acute myeloid leukaemia in France. Int J Clin Pharm. 2011;33(2):191–9. doi: 10.1007/s11096-010-9462-1. [DOI] [PubMed] [Google Scholar]
- 63.Leunis A, et al. The costs of initial treatment for patients with acute myeloid leukemia in the Netherlands. Leuk Res. 2013;37(3):245–50. doi: 10.1016/j.leukres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 64.Bradley CJ, et al. Acute myeloid leukemia. Cancer. 2011;117(20):4772–4778. doi: 10.1002/cncr.26095. [DOI] [PubMed] [Google Scholar]
- 65.Thao V, et al. Variation in inpatient costs of hematopoietic cell transplantation among transplant centers in the United States. JAMA Intern Med. 2014;174(8):1409–12. doi: 10.1001/jamainternmed.2014.2302. [DOI] [PubMed] [Google Scholar]
- 66.Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012;120(8):1545–51. doi: 10.1182/blood-2012-05-426783. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez-Ortega I, et al. Cost-effectiveness of primary antifungal prophylaxis with posaconazole versus itraconazole in allogeneic hematopoietic stem cell transplantation. J Med Econ. 2013;16(6):736–43. doi: 10.3111/13696998.2013.791301. [DOI] [PubMed] [Google Scholar]
- 68.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masmas TN, et al. Evaluation and automation of hematopoietic chimerism analysis based on real-time quantitative polymerase chain reaction. Biol Blood Marrow Transplant. 2005;11(7):558–66. doi: 10.1016/j.bbmt.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Sheridan C. Illumina claims $1,000 genome win. Nat Biotechnol. 2014;32(2):115. doi: 10.1038/nbt0214-115a. [DOI] [PubMed] [Google Scholar]
- 71.Walter RB, et al. Shortcomings in the clinical evaluation of new drugs: acute myeloid leukemia as paradigm. 2010;116:2420–2428. doi: 10.1182/blood-2010-05-285387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hourigan CS, McCarthy P, de Lima M. Back to the future! The evolving role of maintenance therapy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(2):154–63. doi: 10.1016/j.bbmt.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butturini A, Klein J, Gale RP. Modeling minimal residual disease (MRD)-testing. Leuk Res. 2003;27(4):293–300. doi: 10.1016/s0145-2126(02)00166-2. [DOI] [PubMed] [Google Scholar]
- 74.Bisel HF. Criteria for the evaluation of response to treatment in acute leukemia. Blood. 1956;11:676–677. [Google Scholar]
- 75.Zeleznikova T, Babusikova O. The impact of cell heterogeneity and immunophenotypic changes on monitoring minimal residual disease in acute myeloid leukemia. Neoplasma. 2006;53(6):500–6. [PubMed] [Google Scholar]
- 76.Cilloni D, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27(31):5195–201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 77.Kronke J, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29(19):2709–16. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- 78.Shayegi N, et al. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood. 2013;122(1):83–92. doi: 10.1182/blood-2012-10-461749. [DOI] [PubMed] [Google Scholar]
- 79.Campana D, Leung W. Clinical significance of minimal residual disease in patients with acute leukaemia undergoing haematopoietic stem cell transplantation. Br J Haematol. 2013;162(2):147–61. doi: 10.1111/bjh.12358. [DOI] [PubMed] [Google Scholar]
- 80.Al-Mawali A, et al. Incidence, sensitivity, and specificity of leukemia-associated phenotypes in acute myeloid leukemia using specific five-color multiparameter flow cytometry. Am J Clin Pathol. 2008;129(6):934–45. doi: 10.1309/FY0UMAMM91VPMR2W. [DOI] [PubMed] [Google Scholar]
- 81.Welch JS. Mutation position within evolutionary subclonal architecture in AML. Semin Hematol. 2014;51(4):273–81. doi: 10.1053/j.seminhematol.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Shih LY, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100(7):2387–92. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- 83.Kottaridis PD, et al. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood. 2002;100(7):2393–8. doi: 10.1182/blood-2002-02-0420. [DOI] [PubMed] [Google Scholar]
- 84.Nazha A, et al. Activating internal tandem duplication mutations of the fms-like tyrosine kinase-3 (FLT3-ITD) at complete response and relapse in patients with acute myeloid leukemia. Haematologica. 2012;97(8):1242–5. doi: 10.3324/haematol.2012.062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miyamoto T, Weissman IL, Akashi K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci U S A. 2000;97(13):7521–6. doi: 10.1073/pnas.97.13.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyamoto T, et al. Persistence of multipotent progenitors expressing AML1/ETO transcripts in long-term remission patients with t(8;21) acute myelogenous leukemia. Blood. 1996;87(11):4789–96. [PubMed] [Google Scholar]
- 87.Tobal K, Liu Yin JA. RT-PCR method with increased sensitivity shows persistence of PML-RARA fusion transcripts in patients in long-term remission of APL. Leukemia. 1998;12(9):1349–54. doi: 10.1038/sj.leu.2401133. [DOI] [PubMed] [Google Scholar]
- 88.Tobal K, et al. Persistence of RAR alpha-PML fusion mRNA detected by reverse transcriptase polymerase chain reaction in patients in long-term remission of acute promyelocytic leukaemia. Br J Haematol. 1995;90(3):615–8. doi: 10.1111/j.1365-2141.1995.tb05592.x. [DOI] [PubMed] [Google Scholar]
- 89.Jain P, et al. Mutated NPM1 in patients with acute myeloid leukemia in remission and relapse. Leuk Lymphoma. 2014;55(6):1337–44. doi: 10.3109/10428194.2013.840776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kristensen T, et al. NPM1 mutation is a stable marker for minimal residual disease monitoring in acute myeloid leukaemia patients with increased sensitivity compared to WT1 expression. Eur J Haematol. 2011;87(5):400–8. doi: 10.1111/j.1600-0609.2011.01673.x. [DOI] [PubMed] [Google Scholar]
- 91.Shayegi N, et al. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood. 2013 doi: 10.1182/blood-2012-10-461749. [DOI] [PubMed] [Google Scholar]
- 92.Falini B, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254–66. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 93.Bene MC, Kaeda JS. How and why minimal residual disease studies are necessary in leukemia: a review from WP10 and WP12 of the European LeukaemiaNet. Haematologica. 2009;94(8):1135–50. doi: 10.3324/haematol.2008.004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cui W, et al. Leukemia-associated aberrant immunophenotype in patients with acute myeloid leukemia: changes at refractory disease or first relapse and clinicopathological findings. Int J Lab Hematol. 2014;36(6):636–49. doi: 10.1111/ijlh.12193. [DOI] [PubMed] [Google Scholar]
- 95.Langebrake C, et al. Immunophenotypic differences between diagnosis and relapse in childhood AML: Implications for MRD monitoring. Cytometry B Clin Cytom. 2005;63(1):1–9. doi: 10.1002/cyto.b.20037. [DOI] [PubMed] [Google Scholar]
- 96.Voskova D, et al. Stability of leukemia-associated aberrant immunophenotypes in patients with acute myeloid leukemia between diagnosis and relapse: comparison with cytomorphologic, cytogenetic, and molecular genetic findings. Cytometry B Clin Cytom. 2004;62(1):25–38. doi: 10.1002/cyto.b.20025. [DOI] [PubMed] [Google Scholar]
- 97.Cheng MJ, Hourigan CS, Smith TJ. Adult Acute Myeloid Leukemia Long-term Survivors. J Leuk (Los Angel) 2014;2(2) doi: 10.4172/2329-6917.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ouyang J, et al. The clinical significance of negative flow cytometry immunophenotypic results in a morphologically scored positive bone marrow in patients following treatment for acute myeloid leukemia. Am J Hematol. 2015 doi: 10.1002/ajh.23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Public Workshop on Minimal Residual Disease (MRD) as a Surrogate Endpoint in Acute Myeloid Leukemia (AML) [Accessed February 17, 2015];2013 Mar 4; http://www.fda.gov/Drugs/NewsEvents/ucm341421.htm. 05/22/2013.
- 100.Goldman JM, Gale RP. What does MRD in leukemia really mean? Leukemia. 2014;28(5):1131. doi: 10.1038/leu.2013.318. [DOI] [PubMed] [Google Scholar]