Abstract
Aryloxysulfonyl azides can be effectively activated by commercially available cobalt(II) complex of meso-tetraphenylporphyrin ([Co(TPP)]) at room temperature under neutral and nonoxidative conditions for selective radical aziridination of alkenes via metalloradical catalysis. The [Co(TPP)]-catalyzed radical aziridination system is suitable for different combinations of olefin substrates and aryloxysulfonyl azides, producing various N-aryloxysulfonyl aziridine derivatives in good to excellent yields. In addition to generating the environmentally benign N2 as the only byproduct, this Co(II)-based metalloradical aziridination process features mild reaction conditions and operational simplicity.
Keywords: Metalloradical, Radical, Cobalt, Aziridination, Azide, Porphyrin
1. Introduction
As stable metalloradicals with well-defined open-shell doublet d7 electronic structure, cobalt(II) complexes of porphyrins, [Co(Por)], have shown to be effective catalysts for aziridination of a broad spectrum of alkenes with different classes of nitrogen sources via a unique stepwise radical process (Scheme 1).1–2 Of various Co(II)-based metalloradical catalysts, Co(II) complexes supported by porphyrin ligands bearing amide functionalities [Co(AmidoPor)] have shown to be particularly effective in activating various organic azides, including phosphoryl azides,3 sulfonyl azides,4 and aryl azides,5 for radical olefin aziridination, producing the corresponding aziridines in high yields under mild conditions. The high catalytic efficiency of [Co(AmidoPor)] metalloradical catalysts is attributed to the stabilization of the key α-Co(III)-aminyl radical (also known as Co(III)-nitrene radical) intermediate through hydrogen-bonding interaction with the amide group of the porphyrin ligand (Scheme 1: A1).1–5 While the effectiveness of [Co(AmidoPor)] have been clearly demonstrated (especially in the case of enantioselective aziridination), it would be practically more desirable if the commercially available Co(II) complex of meso-tetraphenylporphyrin ([Co((TPP)]) could be applied effectively as metalloradical catalyst for radical olefin aziridination with azides when the concern of enantioselectivity is unnecessary. This practical desire is translated to the fundamental question of whether [Co((TPP)], which lacks of the hydrogen-bonding capability, can effectively activate azides to generate the corresponding α-Co(III)-aminyl radical (Scheme 1: A2) to serve as active intermediates for the radical aziridination process.
Scheme 1.
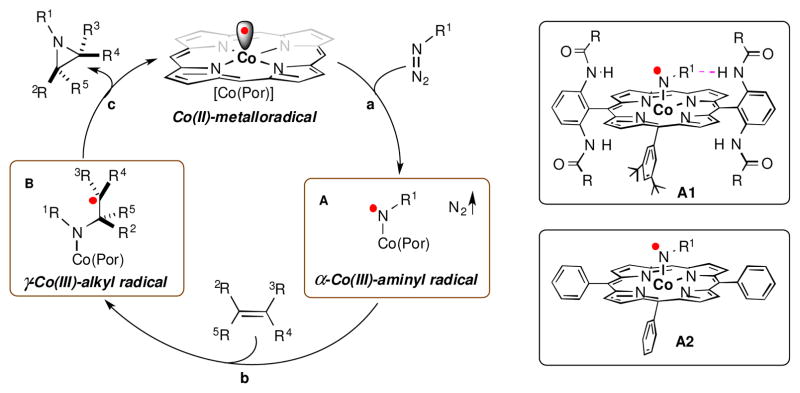
(Left) Pathways of proposed mechanism of radical aziridination via Co(II)-based metalloradical catalysis: a) generation of α-Co(III)-aminyl radical via metalloradical activation of azide by [Co(Por)]; b) formation of γ-Co(III)-alkyl radical through radical addition of α-Co(III)-aminyl radical onto olefin; and c) production of aziridine via 3-exo-tet radical cyclization of γ-Co(III)-alkyl radical. (Right) Structures of postulated α-Co(III)-aminyl radical intermediates supported by amidoporphyrins (A1) and TPP (A2). For clarity, one 3,5-DitBu-Phenyl group on the meso-position of the amidoporphyrin (A1) and one phenyl group on the meso-position of TPP (A2) were omitted.
Several different types of organic azides have been previously investigated as nitrogen sources for catalytic olefin aziridination by metalloradical [Co(TPP)], including diphenylphosphoryl azide3a and 4-nitrophenyl azide.6–7 In addition to limited substrate scope and low product yields, these existing [Co(TPP)]-based systems typically required the use of high catalyst loading (8–10 mol %) and elevated reaction temperature (75–100 °C).3a, 6 Driven by the desire to utilize the commercially available [Co(TPP)] as a practical catalyst for olefin aziridination, we embarked on a project to search for more reactive organic azides that can be activated by [Co(TPP)] to generate the corresponding key α-Co(III)-aminyl radicals under mild conditions. As the outcome of this effort, we herein report our findings that aryloxysulfonyl azides (ArOSO2N3) are a new type of organic azides that can be effectively activated by [Co(TPP)] even at room temperature for selective radical aziridination (eq 1). The newly developed Co(II)-catalyzed aziridination can utilize ArOSO2N3 with different aryl substituents and is suitable for a wide range of aromatic olefins with diverse electronic and steric properties, affording a series of N-aryloxysulfonyl aziridines in good to excellent yields. In addition to generating the environmentally benign N2 as the only byproduct, the new metalloradical aziridination system enjoys several practical attributes associated with neutral and nonoxidative condition as well as the commercial availability of the catalyst [Co(TPP)].
 |
(1) |
2. Results and discussion
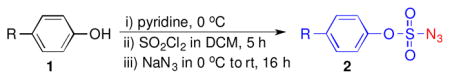
After examining different organic azides, we turned our attention to aryloxysulfonyl azides (ArOSO2N3), which have not been previously explored as precursors of α-Co(III)-aminyl radicals for Co(II)-based metalloradical catalysis.8 In comparison with arylsulfonyl azides (ArSO2N3), which has been previously shown to be ineffective for catalytic aziridination by [Co(TPP)],4a,9 ArOSO2N3 should be more electron-deficient due to the electron-withdrawing effect of the aryloxy groups and might be easier to be activated by [Co(TPP)]. With this assumption in mind, we synthesized a series of aryloxysulfonyl azides from the corresponding phenols in a one-pot procedure by following the literature methods.4b,10 For example, azides 2a–e (Table 1), which contain H, Cl, Br, F, and Ph substituents, respectively, at the para-position of the aryl ring, were readily prepared from the reactions of the commercially available phenols with SO2Cl2 in the presence of pyridine, followed by treatment of the resulting aryloxysulfonyl chlorides with NaN3.10
Table 1.
Preparation of aryloxysulfonyl azides [a]
| ||
|---|---|---|
| entry | product | yield (%)[b] |
| 1 |
 2a |
40 |
| 2 |
2b |
39 |
| 3 |
2c |
40 |
| 4 |
2d |
41 |
| 5 |
2e |
45 |
Reaction conditions: phenols 1 (10 mmol), sulfuryl chloride (22 mmol), NaN3 (30 mmol).
Isolated yields.
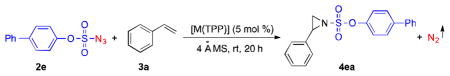
With 4-phenylphenoxysulfonyl azide (2e) as a model azide reagent, we studied the catalytic aziridination reaction of styrene and found that [Co(TPP)] was a highly effective metalloradical catalyst to activate the azide even at room temperature for the production of the corresponding aziridine 4ea (Table 2). Further experiments demonstrated that the effectiveness of the [Co(TPP)]-catalyzed aziridination reaction could be dramatically impacted by the molar ratio of the azide reagent to styrene substrate. When 10 equivalents of styrene were used, aziridine 4ea was obtained in 95% yield (Table 2, entry 1). The yield slightly decreased to 82% in the presence of 5 equivalents of styrene (Table 2, entry 2). The aziridination by [Co(TPP)] became much less effective when a stoichiometric amount of styrene was employed (Table 2, entry 3).11 It was found that the solvent could also influence the outcome of the metalloradical aziridination process by [Co(TPP)]. While chlorobenzene was identified to be an optimal solvent, it was shown that coordinative and more polar solvents such as acetonitrile and THF completely suppressed the catalytic process, leading to no formation of the aziridine product (Table 2, entries 4–5). In stark contrast to the catalytic capability of its Co(II) metalloradical complex, other transition metal complexes of TPP, such as Fe(III), Mn(III), Zn(II), V(IV), Cu(II), Ni(II) and Cr(III) complexes (Figure 1), were found to be unable to activate aryloxysulfonyl azide 2e effectively for aziridination of styrene (Table 2, entries 6–12). It is evident that the metalloradical character of [Co(TPP)] is key to the success of the catalytic aziridination process with the azide (Scheme 1).
Table 2.
Catalytic aziridination of styrene with 4-phenylphenoxysulfonyl azide by metalloporphyrin complexes [a]
| ||||
|---|---|---|---|---|
| entry | Azide:Styrene | [M(TPP)] | solvent | yield (%)[b] |
| 1 | 1:10 | [Co(TPP)] | PhCl | 95 |
| 2 | 1:5 | [Co(TPP)] | PhCl | 82 |
| 3 | 1:1.2 | [Co(TPP)] | PhCl | 12 |
| 4 | 1:5 | [Co(TPP)] | CH3CN | NR |
| 5 | 1:5 | [Co(TPP)] | THF | NR |
| 6 | 1:10 | [Fe(TPP)Cl] | PhCl | 8 |
| 7 | 1:10 | [Mn(TPP)Cl] | PhCl | 8 |
| 8 | 1:10 | [Zn(TPP)] | PhCl | 3 |
| 9 | 1:10 | [VO(TPP)] | PhCl | 6 |
| 10 | 1:10 | [Cu(TPP)] | PhCl | 8 |
| 11 | 1:10 | [Ni(TPP)] | PhCl | 4 |
| 12 | 1:10 | [Cr(TPP)Cl] | PhCl | 4 |
Reaction conditions: azide 2e (0.1 mmol), styrene 3a (1 mmol) unless otherwise stated, 4Å MS (100 mg), catalyst (5 mol %) unless otherwise stated, solvent (1 mL), 20 h, rt.
1H-NMR yields.
Figure 1.
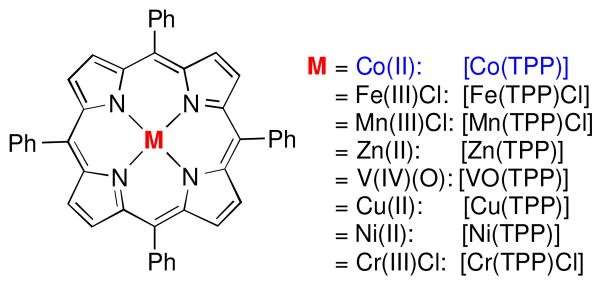
Structures of porphyrin complexes with metal ions.
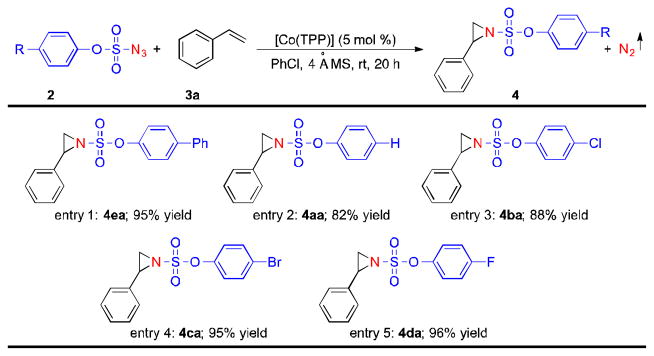
The commercially available metalloradical catalyst [Co(TPP)] was demonstrated to be effective in activating different aryloxysulfonyl azides at room temperature for radical aziridination. As summarized in Table 3, in addition to 4-phenylphenoxysulfonyl azide 2e, phenoxysulfonyl azide 2a could be activated by [Co(TPP)] to aziridinate styrene to give the corresponding aziridine 4aa in a high yield as well (Table 3, entry 2). Various halogenated phenoxysulfonyl azides, including chloro-, bromo- and fluoro-substituted azides (2b–d), could also be applied for [Co(TPP)]-catalyzed aziridination of styrene, affording the corresponding aziridine products 4ba, 4ca and 4da, respectively, in excellent yields (Table 3, entries 3–5).
Table 3.
|
Reaction conditions: azide 2 (0.1 mmol), styrene 3a (0.5 mmol), 4Å MS (100 mg), [Co(TPP)] (5 mol %), PhCl (1 mL), 20 h, rt.
Isolated yields.
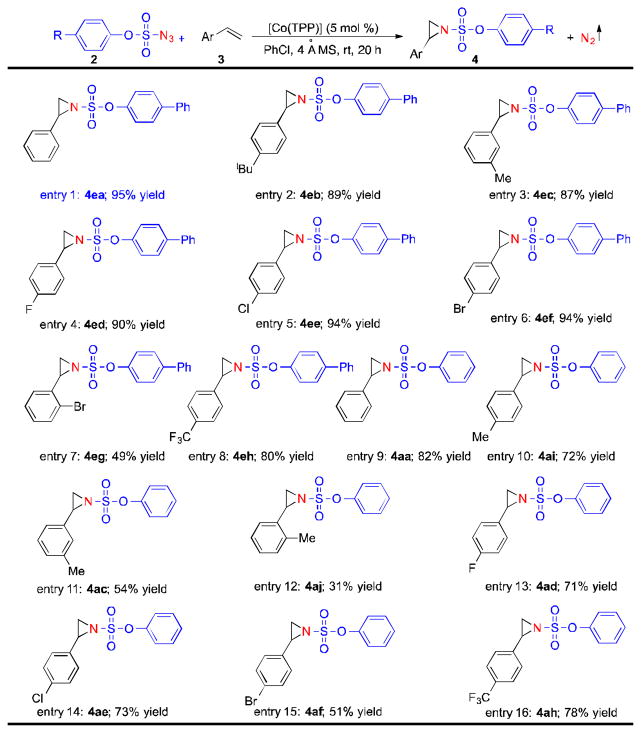
To investigate the substrate scope of the Co(II)-based catalytic system, 4-phenylphenoxysulfonyl azide (2e) and phenoxysulfonyl azide (2a) were selected as representative nitrogen sources for aziridination reactions of different olefins. As shown in Table 4, the [Co(TPP)]-catalyzed room-temperature aziridination was demonstrated to be suitable for a wide range of aromatic olefins with various steric and electronic properties. For example, styrene derivatives with electron-donating substituents, including methyl and tert-butyl groups, could serve as effective substrates for the metalloradical aziridination, yielding the desired aziridines in high yields (Table 4, entries 1–3 and entries 9–12). As well, fluoro-, chloro- and bromo-substituted styrenes underwent the aziridination reactions smoothly by [Co(TPP)], affording the corresponding aziridine products effectively (Table 4, entries 4–7 and entries 13–15). Moreover, the electron-deficient 4-trifluoromethylstyrene could also be aziridinated by both azides 2e and 2a to produce the aziridines 4eh and 4ah, respectively, in good yields (Table 4, entries 8 and 16). It was noted that the reactivity of the [Co(TPP)]-catalyzed aziridination could be significantly influenced by the steric hindrance of the aromatic olefins. While the aziridination reactions of para- and meta-substituted styrenes typically gave good to excellent yields of the corresponding aziridines, more sterically hindered ortho-substituted styrenes, such as 2-bromo- and 2-methylstyrenes, could be only aziridinated by [Co(TPP)] in moderate yields (Table 4).
Table 4.
|
Reaction conditions: azide 2 (0.1 mmol), olefin 3 (0.5 mmol), 4Å MS (100 mg), [Co(TPP)] (5 mol %), PhCl (1 mL), 20 h, rt.
Isolated yields.
3. Conclusions
In summary, we have identified aryloxysulfonyl azides (ArOSO2N3) as a new type of nitrogen sources for radical aziridination by Co(II)-based metalloradical catalysis. Due to their high reactivity, we have shown that aryloxysulfonyl azides can be even activated by simple metalloradical catalyst [Co(TPP)] even at room temperature to effect productive aziridination reactions of different aromatic olefins. The [Co(TPP)]/ArOSO2N3-based aziridination system has several practical attributes associated with metalloradical catalysis. In addition to the neutral and mild reaction conditions, environmentally benign dinitrogen is the only byproduct from the catalytic process. The commercial availability of [Co(TPP)] should further enhance the practicality of this room-temperature aziridination method. The resulting N-aryloxysulfonyl aziridines, which can be readily converted to unprotected N–H aziridine derivatives through facile removal of the aryloxysulfonyl groups,12 may find applications for the synthesis of nitrogen-containing compounds of pharmaceutical importance.12b–c,13 More broadly, the demonstrated high reactivity of aryloxysulfonyl azides may stimulate the exploration of their applications for the development of other Co(II)-based metalloradical processes where the α-Co(III)-aminyl radicals serve as the key intermediates (Scheme 1).
Supplementary Material
Acknowledgments
We are grateful for financial support by NSF (CHE-1152767) and NIH (R01-GM098777).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/XXXXXX.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.For related reviews, see Cenini S, Gallo E, Caselli A, Ragaini F, Fantauzzi S. Coord Chem Rev. 2006;250:1234–1253.Lu H, Zhang XP. Chem Soc Rev. 2011;40:1899–1909. doi: 10.1039/c0cs00070a.Pellissier H, Clavier H. Chem Rev. 2014;114:2775–2823. doi: 10.1021/cr4004055.Jiang H-L, Zhang XP. In: Oxidation: C–N Bond Formation by Oxidation (Aziridines) In Comprehensive Chirality. Carreira EM, Yamamoto H, editors. Vol. 5. Elsevier; Amsterdam: 2012. pp. 168–182.Cui X, Zhang XP. Cobalt-Mediated Carbene Transfer Reactions. In: Moss RA, Doyle MP, editors. Contemporary Carbene Chemistry. Chapter 15. John Wiley & Sons; 2013. pp. 491–525.
- 2.For studies on the radical mechanism of [Co(Por)]-catalyzed olefin aziridination, see: Olivos Suarez AI, Jiang HJ, Zhang XP, de Bruin B. Dalton Trans. 2011;40:5697–5705. doi: 10.1039/c1dt10027k.Hopmann KH, Ghosh A. ACS Catalysis. 2011;1:597–600.For related studies on the radical mechanism of [Co(Por)]-catalyzed C–H amination, including EPR observation of α-Co(III)-aminyl radical (also known as Co(III)-nitrene radical) intermediates, see: Lyaskovskyy V, Suarez AIO, Lu HJ, Jiang HL, Zhang XP, de Bruin B. J Am Chem Soc. 2011;133:12264–12273. doi: 10.1021/ja204800a.For detailed studies on the similar radical mechanism involving α-Co(III)-alkyl radical (also known as Co(III)-carbene radical) intermediates, see: Dzik WI, Xu X, Zhang XP, Reek JNH, de Bruin B. J Am Chem Soc. 2010;132:10891–10902. doi: 10.1021/ja103768r.Belof JL, Cioce CR, Xu X, Zhang XP, Space B, Woodcock HL. Organometallics. 2011;30:2739–2746. doi: 10.1021/om2001348.Lu HJ, Dzik WI, Xu X, Wojtas L, de Bruin B, Zhang XP. J Am Chem Soc. 2011;133:8518–8521. doi: 10.1021/ja203434c.
- 3.(a) Gao GY, Jones JE, Vyas R, Harden JD, Zhang XP. J Org Chem. 2006;71:6655–6658. doi: 10.1021/jo0609226. [DOI] [PubMed] [Google Scholar]; (b) Jones JE, Ruppel JV, Gao GY, Moore TM, Zhang XP. J Org Chem. 2008;73:7260–7265. doi: 10.1021/jo801151x. [DOI] [PubMed] [Google Scholar]; (c) Tao J, Jin LM, Zhang XP. Beilstein J Org Chem. 2014;10:1282–1289. doi: 10.3762/bjoc.10.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Ruppel JV, Jones JE, Huff CA, Kamble RM, Chen Y, Zhang XP. Org Lett. 2008;10:1995–1998. doi: 10.1021/ol800588p. [DOI] [PubMed] [Google Scholar]; (b) Subbarayan V, Ruppel JV, Zhu S, Perman JA, Zhang XP. Chem Commun. 2009:4266–4268. doi: 10.1039/b905727g. [DOI] [PubMed] [Google Scholar]
- 5.Jin LM, Xu X, Lu HJ, Cui X, Wojtas L, Zhang XP. Angew Chem-Int Ed. 2013;52:5309–5313. doi: 10.1002/anie.201209599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Caselli A, Gallo E, Ragaini F, Ricatto F, Abbiati G, Cenini S. Inorg Chim Acta. 2006;359:2924–2932. [Google Scholar]; (b) Caselli A, Gallo E, Fantauzzi S, Morlacchi S, Ragaini F, Cenini S. Eur J Inorg Chem. 2008:3009–3019. [Google Scholar]
- 7.For select recent examples of other catalytic aziridination systems, see: Pellissier H. Adv Synth Catal. 2014;356:1899–1935.Jat JL, Paudyal MP, Gao H, Xu QL, Yousufuddin M, Devarajan D, Ess DH, Kuerti L, Falck JR. Science. 2014;343:61–65. doi: 10.1126/science.1245727.Chan KH, Guan X, Lo VK, Che CM. Angew Chem Int Ed. 2014;53:2982–2987. doi: 10.1002/anie.201309888.Lebel H, Parmentier M, Leogane O, Ross K, Spitz C. Tetrahedron. 2012;68:3396–3409.Cramer SA, Jenkins DM. J Am Chem Soc. 2011;133:19342–19345. doi: 10.1021/ja2090965.Han J, Li Y, Zhi S, Pan Y, Timmons C, Li G. Tetrahedron Lett. 2006;47:7225–7228.
- 8.For an example of the use of trichloroethyloxysulfonyl azide for metalloradical aziridination, see: ref 4b.
- 9.For an example of the use of arylsulfonyl azide for intramolecular C–H amination, see: Ruppel JV, Kamble RM, Zhang XP. Org Lett. 2007;9:4889–4892. doi: 10.1021/ol702265h.
- 10.(a) Yang T, Cui H, Zhang C, Zhang L, Su CY. Chem Cat Chem. 2013;5:3131–3138. [Google Scholar]; (b) Maryanoff BE, Nortey SO, Gardocki JF, Shank RP, Dodgson SP. J Med Chem. 1987;30:880–887. doi: 10.1021/jm00388a023. [DOI] [PubMed] [Google Scholar]; (c) Hedayatullah M, Hugueny JC. Synth Commun. 1981;11:643–646. [Google Scholar]
- 11.No significant improvements were observed when the reaction was conducted in higher catalyst loadings, at elevated temperatures or using excess azides, due to the limited solubility of [Co(TPP)] and decomposition of the azide.
- 12.(a) Cohen SB, Halcomb RL. J Am Chem Soc. 2002;124:2534–2543. doi: 10.1021/ja011932l. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Huang X, Snyder SA, Bheema Rao P, Bella M, Reddy MV. Angew Chem Int Ed. 2002;41:834–838. doi: 10.1002/1521-3773(20020301)41:5<834::aid-anie834>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]; (c) Atfani M, Wei L, Lubell WD. Org Lett. 2001;3:2965–2968. doi: 10.1021/ol016225b. [DOI] [PubMed] [Google Scholar]
- 13.(a) Kiefer L, Gorojankina T, Dauban P, Faure H, Ruat M, Dodd RH. Bioorg Med Chem Lett. 2010;20:7483–7487. doi: 10.1016/j.bmcl.2010.10.006. [DOI] [PubMed] [Google Scholar]; (b) Williams AJ, Chakthong S, Gray D, Lawrence RM, Gallagher T. Org Lett. 2003;5:811–814. doi: 10.1021/ol027418h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


