Abstract
Background
Greece experienced an unprecedented increase in HIV cases among drug injectors in 2011 following economic crisis. Network level factors are increasingly understood to drive HIV transmission in emerging epidemics.
Methods
We examined the relationship between networks, risk behaviors and HIV serostatus among 1,404 people who inject drugs in Athens, Greece. We generated networks using the chain-referral structure within a large HIV screening program. Network proportions, the proportion of a respondent’s network with a given characteristic, were calculated. Multiple logistic regression were used to assess the relationship between network proportions and individual HIV seroprevalance, injection frequency and unprotected sex.
Results
1030 networks were generated. Respondent HIV seroprevalence was associated with greater proportions of network members who were HIV infected (i.e. those with ≥50% of network members HIV-positive vs. those with no network members HIV-positive) [AOR, 3.11; 95% CI, 2.10 to 4.62], divided drugs [AOR, 1.60; 95% CI, 1.10 to 2.35] or injected frequently [AOR, 1.50; 95% CI, 1.02 to 2.21]. Homelessness was the only sociodemographic characteristic associated with a risk outcome measure – high-frequency injecting [AOR, 1.41; 95% CI, 1.03 to 1.93]. These associations were weaker for more distal second and third degree networks and not present when examined within random networks.
Conclusion
Networks are an independently important contributor to the HIV outbreak in Athens Greece. Network associations were strongest for the immediate network, with residual associations for distal networks. Homelessness was associated with high frequency injecting. Prevention programs should consider including network-level interventions to prevent future emerging epidemics.
INTRODUCTION
There is growing consensus on the role that physical and social “risk configurations” 1 or “risk environments” 2 play in perpetuating HIV risk and transmission. The social risk environment consists of multiple levels of influence, from macro-level social institutions and structures, to meso-level social networks, to microlevel individual agency 3. Network analysis provides one method of examining the social environment in which risk factors interact to shape HIV transmission and behaviors.
Networks can function as pathways of disease transmission (risk networks) as well as channels for social influence (social networks) 4. Risk networks consist of people with whom an individual engages in HIV risk behaviors such as sex, injecting drugs or both. The recognition that risk networks influence transmission patterns of HIV has been established since early in the epidemic 5. Numerous studies have shown that risk connections between individuals rather than differences in individual risk behaviors alone, account for observed disease transmission in people who inject drugs (PWID)6–9.
Social networks consist of people who may affect an individual though social influence, social engagement and attachment or access to resources and material goods. Social networks have been shown to affect HIV risk behaviors among PWID 8. Injecting networks can produce strong social ties that promote mutual injecting and create norms for risky behaviors 9. For example, PWID networks in which equipment sharing is the norm have been found to sustain sharing among its members 10. Alternatively, networks that provide support and health information can promote risk reduction behaviors 11. For example, health advice and financial support networks are positively associated with norms promoting condom use 12.
PWID risk networks and social networks commonly overlap, and there have been analyses examining both types of networks within the same context and population 4. Understanding the different ways in which social and risk networks may affect HIV in a single population is of particular importance to understanding the dynamics in an emerging epidemic.
The 2011 outbreak of HIV in Greek PWID is one example of an emerging epidemic. Since early 2011 there has been a large increase in newly diagnosed cases of HIV among PWIDs in Greece. From 2000 to 2010, newly reported cases of HIV in people who inject drugs ranged between 9–19 cases, and represented 2–3% of all reported cases 13,14. In 2011, 266 HIV cases were attributed to PWID, representing 28% of all new reported HIV cases in Greece 15. In 2012, 547 newly diagnosed cases of HIV in PWID were reported, representing 46% of the newly reported infections for that year 16. Some studies have linked the outbreak to austerity measures, cuts in public spending, housing instability and unemployment resulting from the political and financial crises 15,17–19. The goal of this analysis is to determine the potential for network-based interventions in this context by exploring how networks, including more distal networks, are associated with individual HIV seroprevalence and risk behaviors. This analysis is conducted within the unique context of an emerging epidemic.
METHODS
Setting and Study Population
Network and respondent level data were collected from the first round of Aristotle, a large HIV testing and linkage to care program that took place in Athens, Greece from August to October 2012. Details of the Aristotle cohort have been described previously 19. In brief, the sample was generated using respondent driven sampling (RDS) 20. RDS has been widely applied to study difficult to reach populations including people who inject drugs, sex workers, and men who have sex with men (MSM) 21. With this approach, a small number of initial recruits or “seeds” are given coupons to recruit others from the target population, who in turn become recruiters. Individuals were eligible if they: a) presented a valid coupon, b) had injected drugs without a prescription in the past 12 months, c) lived in the Athens metropolitan area, d) had not previously participated in the study, e) were ≥ 18 years of age, f) were able to communicate in Greek or with the help of staff, and g) were willing and able to give informed consent.
Survey Instrument
The US National HIV Behavioral Surveillance System questionnaire22 was adapted for PWID in Greece. The survey included items for age, sex, nationality, relationship to the person who recruited the participant, homelessness (past 12 months and current), highest level of education completed, drug injected most often, frequency of using syringes, cookers, cottons or waters that someone else has used (past 12 months), frequency of using drugs that have been divided with a syringe that someone else has used (past 12 months), number of male and female sex partners (past 12 months), giving or receiving things like money or drugs in exchange for sex (during 12 months), condom usage (past 12 months), using alcohol or drugs before or during the last time having sex, ever participating in a drug treatment program, ever participating in an opiate substitution program, ever being tested for HIV, receiving free condoms from HIV prevention activities (past 12 months), and receiving syringes from prevention activities (past 12 months).
Laboratory testing
Microparticle EIA anti-HIV-1/2 (AxSYM HIV-1/2 gO, Abbott) was confirmed with Western Blot (MP Diagnostics).
Network Construction and Characteristics
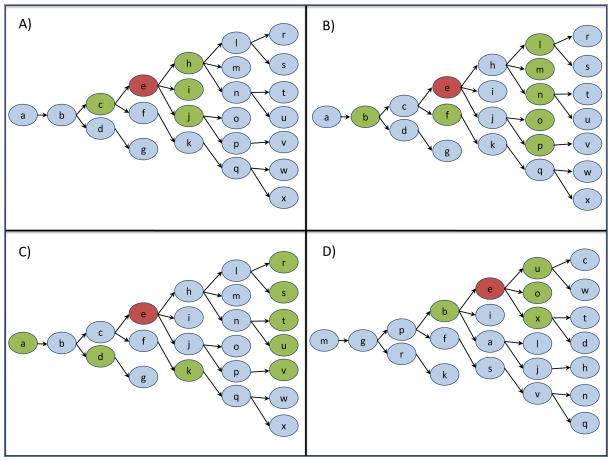
We used the RDS recruitment chain referral structure to generate personal networks. Figure 1 displays a hypothetical RDS recruitment structure used to illustrate how networks were generated from RDS linkages. In order to determine network effects over multiple degrees of social separation, second-degree and third-degree networks were also constructed (Figure 1, panels b and c). Studies have shown that network influences on individuals’ health behavior can extend beyond the first-degree network, though influence dissipates as social separation increases 23–25. In the present analyses, an individual’s second and third degree network consisted of all network members that were two and three distinct connections away from the index.
Figure 1. Personal network construction using Respondent Driven Sampling coupon referral scheme.
Reference individuals are highlighted in red, and panels A) first degree, B) second degree, C) third degree and D) random network members are highlighted in green. In panel A, individual e’s first degree network consists of network members directly connected to individual e: network member c (e’s recruiter) and network members h, i and j (the individuals recruited by e). In panel B, the second degree network consists of individuals two connections away from individual e. In panel C, the third degree network consists of individuals three connections away from individual e. In panel D, a random network was generated by randomly assigning respondents to different locations within the original RDS referral recruitment structure. Individual e’s position has shifted and new network members include individuals b, u, o and x.
Analytic Plan
The primary outcome of interest was HIV serostatus. Secondary outcomes included high frequency injecting (HFI) defined as injecting more than once per day 26, and unprotected sex (US) defined as wearing condoms only sometimes, rarely or never 27. Covariates of interest included network size and other characteristics of network members summarized as network proportions, i.e. the proportion of a respondent’s network with a given characteristic 28. For example, if an individual has 4 network members and 3 are HIV infected, that individual’s HIV infected network proportion would be 75%. HIV network proportions were coded for these analyses into 0%, 1%–49% or ≥50%. These categories were chosen based upon expected ease in interpretation of network proportion in the target population for future interventions (ie. Would you say that over half of your social circle are HIV infected, less than half or none?). Other covariates included various demographic, social and behavioral characteristics of the respondents. Analyses were restricted to participants with complete data on relevant variables. The first wave (seeds) and last wave were excluded from all analyses because their networks were limited by the sampling design to only the people they recruited or the person who recruited them, respectively.
We used a series of logistic regressions to examine the relationship between each outcome and the proportion of the network with that same characteristic, controlling for the various respondent-level covariates 29. Age and gender were included in all models, while other covariates were retained if their p-value was < 0.10. Network variables with a p-value < 0.05 were considered statistically significant. All analyses were performed using the Stata Release 13 statistical software package.
Sensitivity Analyses
One limitation of the logistic regression models is the assumption of independence between observations, which is unrealistic given the fact that respondents are directly linked via the network. Thus, we verified the statistical significance of the network proportion variables by conducting corresponding permutation tests. Each permutation or “random” network involved retaining the original network structure, but randomly relocating respondents (who retained all of their original, non-network characteristics) throughout the network (Figure 1d). This process represents the null hypothesis that one’s own HIV serostatus (or any other characteristic) is unrelated to that of the individuals in one’s egocentric network. Five-hundred permutations were performed to obtain an estimate of the permutation distribution for the odds ratio, and the observed odds ratio was evaluated relative to this distribution, yielding a two-sided permutation p-value.
In addition to the permutation tests, we also divided the network into 48 roughly equally sized clusters, each containing a contiguous subset of the network. The intention was to create clusters for which the within-cluster correlation exceeded the between-cluster correlation. We then used the clustered bootstrap to obtain estimates of the standard errors of the coefficients in the logistic models, and compared these to the naive standard errors 30. A second limitation of the logistic models is that the network proportion variable is subject to measurement error because it includes only a sample of the personal network. To address this limitation and characterize network proportions as continuous variables, we fit a generalized structural equation model (See Supplement).
Due to the significant proportion of individuals that were recruited by strangers (20.7%), we compared the full dataset to a dataset that excluded all connections with strangers. Logistic regression models using the no-stranger dataset were compared to results for the full network.
RESULTS
Respondent Driven Network Generation
Eleven seeds generated a study sample of 1404 participants over 10 waves of recruitment. The last wave of recruitment yielded 374 individuals; as described above, these together with the seeds have been excluded from analyses presented here. Individual-level variables for the 1030 respondents in waves two through nine can be found in Supplementary Table S1. The HIV seroprevalence rate for this subsample was 21%. Respondents were predominantly unemployed (73.9%), Greek (84.0%), and male (85.8%). Mean age was 35.2 years (SD=7.8). Homelessness in the past 12 months was reported by 36.0% of respondents.
A total of 1030 first-degree networks were generated from the referral contacts, one for each respondent in the sample. Network size ranged from 2–5. Supplementary Table S2 characterizes network HIV seroprevalence, high frequency injecting and unprotected sex by RDS wave. With subsequent waves of recruitment, study participants’ networks had less HIV, and reported less high frequency injecting.
Network-level Characteristics
Network-level variables for the 1030 networks can be found in Supplementary Table S3. On average, the prevalence of HIV among study participant networks was 22.1%. High frequency injecting and unprotected sex were reported on average by 44.8% and 48.2% of network members. Around 34% of the network members had never been tested for HIV before, and 63.4% had received syringes from prevention programs in the past 30 days.
We evaluated the relationships between network-level variables and respondent HIV seroprevalence (Table 1). Respondent HIV seroprevalence was associated with greater proportions of network members who were HIV infected (i.e. those with ≥50% of network members HIV-positive vs. those with no network members HIV-positive) [AOR, 3.11; 95% CI, 2.10 to 4.62], divided drugs [AOR, 1.60; 95% CI, 1.10 to 2.35] or injecting frequently [AOR, 1.50; 95% CI, 1.02 to 2.21]. Moderate network proportions that had been in jail as well as sharing cookers/filters/water were also associated with respondent HIV seroprevalence. Size of RDS-generated network was not found to be a significantly associated with respondent HIV seroprevalence. Clustered bootstrap standard errors were very similar to the naive standard errors, yielding similar p-values (Supplementary Table S4).
Table 1.
Network variablesa associated with individual HIV seroprevalence among people who inject drugs in Athens, Greece 2012.
| Univariate | Multivariableb | Clustered Bootstrapb, c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NETWORK LEVEL VARIABLESd | N | % HIV+ | Odds Ratio | 95% CI | P value | Adjusted Odds Ratio | 95% CI | P value | P value |
| Network HIV Seroprevalence | |||||||||
| HIV infected | |||||||||
| Low (0) | 671 | 13.0 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 109 | 33.0 | 3.31 | (2.09,5.24) | <0.001 | 2.98 | (1.76,5.05) | <0.001 | <0.001 |
| High (≥50) | 249 | 36.9 | 3.93 | (2.79,5.54) | <0.001 | 3.11 | (2.10,4.62) | <0.001 | <0.001 |
| Network Characteristics | |||||||||
| Homelesse | |||||||||
| Low (0) | 470 | 16.8 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 137 | 27 | 1.83 | (1.17,2.87) | 0.008 | 1.55 | (0.93,2.60) | 0.09 | 0.09 |
| High (≥50) | 422 | 23.5 | 1.52 | (1.09,2.11) | 0.01 | 1.13 | (0.77,1.66) | 0.52 | 0.43 |
| Primary school only | |||||||||
| Low (0) | 583 | 18.5 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 138 | 26.8 | 1.61 | (1.05,2.48) | 0.03 | 1.49 | (0.91,2.45) | 0.11 | 0.16 |
| High (≥50) | 308 | 22.7 | 1.29 | (0.92,1.81) | 0.14 | 1.02 | (0.69,1.51) | 0.92 | 0.93 |
| Ever in prison | |||||||||
| 0 | 308 | 15.6 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 87 | 29.9 | 2.31 | (1.33,4.01) | 0.003 | 2.82 | (1.48,5.37) | 0.002 | 0.004 |
| High (≥50) | 633 | 22.3 | 1.55 | (1.08,2.23) | 0.02 | 1.41 | (0.94,2.12) | 0.10 | 0.09 |
| Network Prevention Activities | |||||||||
| Receives condoms | |||||||||
| Low (0) | 263 | 16.7 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 61 | 9.8 | 0.54 | (0.22,1.34) | 0.18 | 0.41 | (0.13,1.26) | 0.12 | 0.16 |
| High (≥50) | 704 | 23.4 | 1.52 | (1.05,2.20) | 0.02 | 1.13 | (0.75,1.72) | 0.55 | 0.48 |
| Receives syringes | |||||||||
| Low (0) | 247 | 13.8 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 62 | 17.7 | 1.35 | (0.64,2.85) | 0.43 | 1.57 | (0.68,3.62) | 0.29 | 0.35 |
| High (≥50) | 720 | 23.6 | 1.94 | (1.30,2.89) | 0.001 | 1.46 | (0.93,2.28) | 0.10 | 0.14 |
| Network Risk Behaviorse | |||||||||
| Main drug non-heroin | |||||||||
| Low (0) | 717 | 18.7 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 115 | 24.3 | 1.40 | (0.88,2.23) | 0.16 | 1.40 | (0.82,2.39) | 0.21 | 0.33 |
| High (≥50) | 197 | 26.9 | 1.60 | (1.11,2.31) | 0.01 | 1.43 | (0.93,2.21) | 0.10 | 0.10 |
| Divides drugs | |||||||||
| Low (0) | 446 | 15.2 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 113 | 24.8 | 1.83 | (1.11,3.02) | 0.02 | 1.85 | (1.05,3.27) | 0.03 | 0.08 |
| High (≥50) | 465 | 25.2 | 1.87 | (1.34,2.61) | 0.0002 | 1.60 | (1.10,2.35) | 0.02 | 0.01 |
| High frequency injecting | |||||||||
| Low (0) | 405 | 14.6 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 97 | 17.5 | 1.25 | (0.69,2.25) | 0.46 | 1.11 | (0.58,2.12) | 0.75 | 0.80 |
| High (≥50) | 527 | 26.4 | 2.10 | (1.50,2.94) | <0.0001 | 1.50 | (1.02,2.21) | 0.04 | 0.11 |
| Shares cookers, filters or water | |||||||||
| Low (0) | 177 | 13.6 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 59 | 30.5 | 2.80 | (1.39,5.65) | 0.004 | 2.44 | (1.10,5.38) | 0.03 | 0.44 |
| High (≥50) | 793 | 21.8 | 1.78 | (1.12,2.82) | 0.01 | 1.54 | (0.92,2.57) | 0.10 | 0.29 |
| Shares syringes | |||||||||
| Low (0) | 406 | 18 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 129 | 22.5 | 1.32 | (0.81,2.15) | 0.07 | 1.30 | (0.75,2.27) | 0.35 | 0.44 |
| High (≥50) | 493 | 22.9 | 1.36 | (0.98,1.89) | 0.004 | 1.25 | (0.86,1.82) | 0.25 | 0.29 |
| Other Network Characteristics | |||||||||
| Acquaintance | |||||||||
| Low (0) | 488 | 19.1 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 172 | 18.6 | 0.97 | (0.62,1.52) | 0.90 | 0.91 | (0.54,1.52) | 0.71 | |
| High (≥50) | 370 | 24.6 | 1.39 | (1.00,1.92) | 0.05 | 1.28 | (0.87,1.86) | 0.21 | |
| Friend | |||||||||
| Low (0) | 434 | 17.7 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 194 | 23.7 | 1.44 | (0.95,2.18) | 0.08 | 1.26 | (0.78,2.03) | 0.34 | 0.35 |
| High (≥50) | 402 | 23.1 | 1.40 | (0.99,1.96) | 0.05 | 1.37 | (0.93,2.02) | 0.11 | 0.14 |
| Stranger | |||||||||
| Low (0) | 678 | 23.5 | 1.00 | 1.00 | |||||
| Moderate (1 – 49) | 158 | 16.5 | 0.64 | (0.41,1.01) | 0.06 | 0.63 | (0.38,1.04) | 0.07 | 0.14 |
| High (≥50) | 194 | 16 | 0.62 | (0.41,0.95) | 0.03 | 0.66 | (0.40,1.07) | 0.09 | 0.13 |
Each network variable represents a separate model. Multivariable models include the network variable indicated and individual-level covariates.
Individual-level covariates that had significant associations with HIV seroprevalence at the p<0.1 level in the univariable analyses were included for all multivariate models: age, sex, homelessness, highest level of education, history of incarceration, getting condoms from an HIV prevention activity, getting syringes from an HIV prevention activity, getting condoms from an HIV prevention activity, ever being in drug treatment, using divided drugs with a syringe that has already been used, frequency of injection, drug injected most often, sharing cookers/water or filters, sharing syringes, using alcohol or drugs with last sex partner and having unprotected sex.
Clustered Bootstrap model consisted of 48 roughly equal sized clusters sampled over approximately 1000 repetitions.
Network-level variables were measured as network proportions. For example, if an individual has 4 network members and 3 are HIV infected, that individual’s HIV infected network proportion is 0.75
Results reflect behavior in past 12 months
In the second set of analyses, bivariate and multivariate regressions were used to assess the relationship between network variables and two respondent risk behaviors: high frequency injecting (HFI) and unprotected sex (US) (Table 2). Having more network members experiencing homelessness was associated with high frequency injecting [AOR, 1.41; 95% CI, 1.03 to 1.93]. High frequency injecting was also associated with having more network members who were HIV infected [AOR, 1.61; 95% CI, 1.14 to 2.28], and injecting frequently [AOR, 1.66; 95% CI, 1.23 to 2.26]. Unprotected sex was associated with having more network members who were drug partners [AOR, 1.81; 95% CI, 1.03 to 3.17].
Table 2.
Network variablesa associated with individual high frequency injecting and unprotected sex among people who inject drugs in Athens, Greece 2012.
| High Frequency Injectingb | Unprotected Sexc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | Adjusted Odds Ratio | 95% CI | P value | N | % | Adjusted Odds Ratio | 95% CI | P value | |
| Network HIV Seroprevalence (%) | ||||||||||
| HIV infected | ||||||||||
| Low (0) | 669 | 38.7 | ref | 663 | 54.8 | - | - | - | ||
| Moderate (1 – 49) | 109 | 49.5 | 1.37 | (0.86,2.19) | 0.19 | 109 | 57.8 | - | - | - |
| High (≥50) | 248 | 57.3 | 1.61 | (1.14,2.28) | 0.007 | 243 | 50.2 | - | - | - |
| Network Risk Behaviors | ||||||||||
| Divides drugs | ||||||||||
| Low (0) | 444 | 40.8 | ref | 441 | 53.7 | - | - | - | ||
| Moderate (1 – 49) | 113 | 43.4 | 0.89 | (0.55,1.44) | 0.64 | 113 | 54 | - | - | - |
| High (≥50) | 464 | 48.1 | 1.11 | (0.82,1.51) | 0.51 | 456 | 54.2 | - | - | - |
| High frequency injecting | ||||||||||
| Low (0) | 403 | 33.5 | ref | 401 | 55.6 | - | - | - | ||
| Moderate (1 – 49) | 97 | 40.2 | 1.27 | (0.76,2.12) | 0.36 | 97 | 58.8 | - | - | - |
| High (≥50) | 526 | 53.4 | 1.66 | (1.23,2.26) | 0.001 | 517 | 51.8 | - | - | - |
| Shares syringes | ||||||||||
| Low (0) | 404 | 43.3 | - | - | - | 398 | 52 | ref | ||
| Moderate (1 – 49) | 129 | 41.1 | - | - | - | 129 | 61.2 | 1.32 | (0.81,2.17) | 0.27 |
| High (≥50) | 492 | 45.9 | - | - | - | 487 | 53.8 | 0.98 | (0.71,1.36) | 0.93 |
| Alcohol or drugs with last sex partner | ||||||||||
| Low (0) | 168 | 45.2 | - | - | - | 164 | 45.1 | ref | ||
| Moderate (1 – 49) | 147 | 42.9 | - | - | - | 146 | 56.2 | 1.42 | (0.83,2.43) | 0.20 |
| High (≥50) | 711 | 44.3 | - | - | - | 705 | 55.7 | 1.33 | (0.88,2.00) | 0.17 |
| Unprotected sex | ||||||||||
| Low (0) | 302 | 45 | - | - | - | 295 | 47.8 | ref | ||
| Moderate (1 – 49) | 154 | 39 | - | - | - | 155 | 61.9 | 1.66 | (1.03,2.67) | 0.04 |
| High (≥50) | 569 | 45.2 | - | - | - | 564 | 55.1 | 1.16 | (0.82,1.64) | 0.39 |
| Network Prevention Activities | ||||||||||
| Ever in OST | ||||||||||
| Low (0) | 586 | 45.9 | ref | 578 | 50.9 | ref | ||||
| Moderate (1 – 49) | 130 | 46.2 | 1.15 | (0.73,1.81) | 0.55 | 129 | 58.9 | 1.19 | (0.74,1.91) | 0.47 |
| High (≥50) | 296 | 39.5 | 0.89 | (0.64,1.24) | 0.50 | 294 | 58.5 | 1.38 | (0.98,1.95) | 0.07 |
| Receives condoms | ||||||||||
| Low (0) | 262 | 38.9 | ref | 261 | 53.3 | - | - | - | ||
| Moderate (1 – 49) | 61 | 29.5 | 0.63 | (0.31,1.29) | 0.21 | 61 | 60.7 | - | - | - |
| High (≥50) | 702 | 47.7 | 1.26 | (0.90,1.76) | 0.18 | 692 | 53.8 | - | - | - |
| Ever in drug treatment | ||||||||||
| Low (0) | 204 | 51 | ref | 204 | 49.5 | - | - | - | ||
| 1 – 49 | 56 | 57.1 | 1.07 | (0.52,2.17) | 0.86 | 55 | 60 | |||
| High (≥50) | 766 | 41.6 | 0.88 | (0.61,1.26) | 0.48 | 756 | 54.8 | - | - | - |
| Other Characteristics | ||||||||||
| Male | ||||||||||
| Low (0) | 71 | 33.8 | ref | 70 | 61.4 | - | - | - | ||
| Moderate (1 – 49) | 13 | 38.5 | 1.26 | (0.33,4.78) | 0.74 | 14 | 71.4 | - | - | - |
| High (≥50) | 942 | 45.2 | 1.47 | (0.82,2.63) | 0.20 | 931 | 53.2 | - | - | - |
| Homeless | ||||||||||
| Low (0) | 469 | 38.6 | ref | 462 | 53 | - | - | - | ||
| Moderate (1 – 49) | 136 | 43.4 | 1.22 | (0.79,1.89) | 0.38 | 137 | 51.1 | - | - | - |
| High (≥50) | 421 | 51.1 | 1.41 | (1.03,1.93) | 0.03 | 416 | 56 | - | - | - |
| Non-Greek | ||||||||||
| Low (0) | 781 | 41.2 | ref | 775 | 55.9 | ref | ||||
| Moderate (1 – 49) | 71 | 52.1 | 1.19 | (0.68,2.09) | 0.54 | 70 | 58.6 | 1.23 | (0.68,2.23) | 0.50 |
| High (≥50) | 174 | 55.2 | 1.23 | (0.79,1.91) | 0.35 | 170 | 43.5 | 0.95 | (0.63,1.46) | 0.83 |
| Primary school only | ||||||||||
| Low (0) | 583 | 39.6 | ref | 573 | 56 | ref | ||||
| Moderate (1 – 49) | 136 | 50 | 1.30 | (0.84,2.00) | 0.25 | 137 | 56.9 | 1.01 | (0.64,1.60) | 0.96 |
| High (≥50) | 307 | 50.8 | 1.33 | (0.97,1.83) | 305 | 48.9 | 0.90 | (0.64,1.26) | 0.54 | |
| Friend | ||||||||||
| Low (0) | 432 | 39.8 | ref | 427 | 54.6 | - | - | - | ||
| Moderate (1 – 49) | 193 | 46.1 | 1.25 | (0.84,1.86) | 192 | 58.3 | - | - | - | |
| High (≥50) | 402 | 48.3 | 1.30 | (0.94,1.78) | 397 | 51.4 | - | - | - | |
| Stranger | ||||||||||
| Low (0) | 675 | 47.3 | ref | 669 | 54.7 | - | - | - | ||
| Moderate (1 – 49) | 158 | 39.2 | 0.80 | (0.53,1.20) | 156 | 49.4 | - | - | - | |
| High (≥50) | 194 | 38.1 | 0.80 | (0.55,1.17) | 191 | 55.5 | - | - | - | |
| Drug Partner | ||||||||||
| Low (0) | 850 | 43.9 | - | - | - | 840 | 53.1 | ref | ||
| Moderate (1 – 49) | 91 | 50.5 | - | - | - | 90 | 52.2 | 0.87 | (0.51,1.50) | 0.62 |
| High (≥50) | 86 | 41.9 | 86 | 65.1 | 1.81 | (1.03,3.17) | 0.04 | |||
Each network variable represents an independent model. Multivariable models include the network variable indicated and individual-level covariates.
Covariates for multivariate HFI models included individual-level: HIV seroprevalence, age, sex, nationality, highest level of education, homelessness, history of incarceration, getting condoms from an HIV prevention activity, previously being tested for HIV, getting syringes from an HIV prevention activity, ever being in drug treatment, ever being in OST, using divided drugs with a syringe that has already been used, drug injected most often, sharing cookers/water or filters, sharing syringes, and multiple sex partners and having unprotected sex.
Covariates for multivariate US model included individual-level: HIV seroprevalence, age, sex, homelessness, highest level of education, ever being in drug treatment, using divided drugs with a syringe that has already been used, frequency of injection, drug injected most often, sharing cookers/water or filters, sharing syringes, exchange sex, using alcohol or drugs with last sex partner multiple sex partners
We also evaluated the association between more distal network member characteristics (second and third degree networks) and respondent HIV, high frequency injecting and unprotected sex (Figure 2). The number of significant associations diminished as degree of separation increased, indicating that network influence likely diminishes over social space.
Figure 2. Comparison of first degree network findings with second degree networks, third degree networks and 500 randomly generated networks.
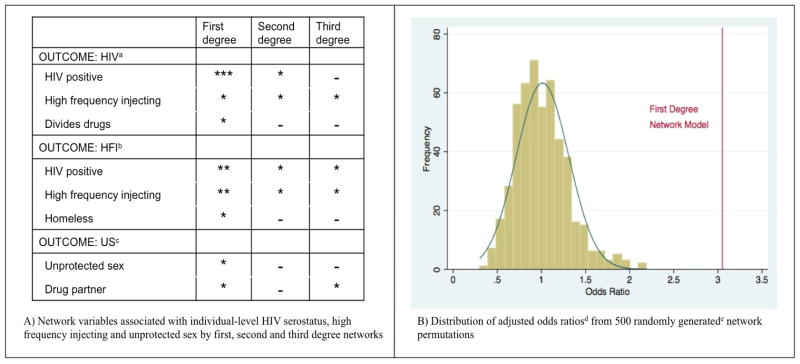
* p<0.05, ** p< 0.01, *** p< 0.001
aCovariates for multivariate HIV models included: age, sex, homelessness, highest level of education, history of incarceration, getting condoms from an HIV prevention activity, getting syringes from an HIV prevention activity, getting condoms from an HIV prevention activity, ever being in drug treatment, using divided drugs with a syringe that has already been used, frequency of injection, drug injected most often, sharing cookers/water or filters, sharing syringes, using alcohol or drugs with last sex partner and having unprotected sex.
bCovariates for multivariate HFI models included: age, sex, HIV seroprevalence, nationality, highest level of education, homelessness, history of incarceration, getting condoms from an HIV prevention activity, previously being tested for HIV, getting syringes from an HIV prevention activity, ever being in drug treatment, ever being in OST, using divided drugs with a syringe that has already been used, drug injected most often, sharing cookers/water or filters, sharing syringes, and multiple sex partners and having unprotected sex.
cCovariates for multivariate US model included: HIV seroprevalence, age, sex, homelessness, highest level of education, ever being in drug treatment, using divided drugs with a syringe that has already been used, frequency of injection, drug injected most often, sharing cookers/water or filters, sharing syringes, exchange sex, using alcohol or drugs with last sex partner multiple sex partners.
dOdds ratio of an individual having HIV if the proportion of their network having HIV is greater than half adjusted for age, sex, homelessness, highest level of education, history of incarceration, getting condoms from an HIV prevention activity, getting syringes from an HIV prevention activity, getting condoms from an HIV prevention activity, ever being in drug treatment, using divided drugs with a syringe that has already been used, frequency of injection, drug injected most often, sharing cookers/water or filters, sharing syringes, using alcohol or drugs with last sex partner and having unprotected sex.
eRandom networks were generated by randomly assigning respondents to different locations within the original RDS referral recruitment structure. The odds ratio from the first degree network model is notably outside the distribution of odds ratios from the randomly generated networks. This graph demonstrates that the significant association between the proportion of the immediate network that is HIV infected and the respondent being HIV infected is not likely due to random network effect.
Results of the permutation test for HIV serostatus are shown in Figure 2b. The observed odds ratio of 3.11 from the first-degree network model is located in the extreme tail of the permutation distribution, yielding an estimated p-value of < 0.002 (1/500). Similar results were obtained for the other network variables that were statistically significant in the primary analysis.
The logistic regression models predicting HIV serostatus were rerun on a subset of network data that had ties with strangers removed (Table 3). All network variables that were significantly associated with individual HIV in the full dataset were also significantly associated in the no-stranger dataset. When strangers were removed from the dataset, respondent HIV seroprevalence in the low and high network proportion groups remained the same as in the original model.
Table 3.
Network variablesa associated with individual HIV seroprevalence among people who inject drugs in Athens, Greece 2012 with connections to strangers removed.
| Multivariableb | |||||
|---|---|---|---|---|---|
| N | % | Odds Ratio | 95% CI | P value | |
| NETWORK LEVEL VARIABLESc | |||||
| Network HIV Seroprevalence (%) | |||||
| HIV infected | |||||
| Low (0) | 609 | 40.4 | 1.00 | ||
| Moderate (1 – 49) | 69 | 50.7 | 3.74 | (2.00,6.98) | <0.0001 |
| High (≥50) | 239 | 57.7 | 2.82 | (1.88,4.22) | <0.0001 |
| Network Risk Behaviorsd | |||||
| Divides drugs | |||||
| Low (0) | 429 | 42.2 | 1.00 | ||
| Moderate (1 – 49) | 66 | 50 | 3.12 | (1.61,6.04) | 0.001 |
| High (≥50) | 418 | 48.6 | 1.65 | (1.12,2.45) | 0.01 |
| High frequency injecting | |||||
| Low (0) | 389 | 35.2 | 1.00 | ||
| Moderate (1 – 49) | 51 | 33.3 | 2.17 | (0.99,4.76) | 0.05 |
| High (≥50) | 477 | 55.6 | 1.66 | (1.11,2.48) | 0.01 |
| Shares cookers, filters or water | |||||
| Low (0) | 189 | 39.7 | 1.00 | ||
| Moderate (1 – 49) | 36 | 55.6 | 3.50 | (1.42,8.60) | 0.006 |
| High (≥50) | 692 | 46.8 | 1.64 | (1.00,2.72) | 0.06 |
| Shares syringes | |||||
| Low (0) | 413 | 43.3 | 1.00 | ||
| Moderate (1 – 49) | 78 | 46.2 | 1.69 | (0.89,3.22) | 0.12 |
| High (≥50) | 425 | 47.8 | 1.48 | (1.00,2.19) | 0.05 |
| Other Characteristics | |||||
| Exchange Sexd | |||||
| Low (0) | 777 | 43.6 | 1.00 | ||
| Moderate (1 – 49) | 77 | 51.9 | 1.95 | (1.07,3.57) | 0.02 |
| High (≥50) | 109 | 48.6 | 0.56 | (0.30,1.04) | 0.07 |
| Homelessd | |||||
| Low (0) | 452 | 40.9 | 1.00 | ||
| Moderate (1 – 49) | 82 | 43.9 | 2.52 | (1.37,4.65) | 0.002 |
| High (≥50) | 383 | 51.7 | 1.31 | (0.88,1.95) | 0.18 |
| Primary school only | |||||
| Low (0) | 557 | 40.6 | 1.00 | ||
| Moderate (1 – 49) | 82 | 59.8 | 2.08 | (1.15,3.76) | 0.01 |
| High (≥50) | 278 | 51.8 | 1.37 | (0.92,2.04) | 0.12 |
| Ever in prison | |||||
| Low (0) | 289 | 41.9 | 1.00 | ||
| Moderate (1 – 49) | 58 | 41.4 | 3.55 | (1.67,7.55) | 0.001 |
| High (≥50) | 569 | 48 | 1.71 | (1.11,2.62) | 0.02 |
Each network variable represents an independent model. Multivariable models include the network variable indicated and individual-level covariates.
Covariates for multivariate HIV models included: Covariates for multivariate HIV models included individual-level: age, sex, homelessness, highest level of education, history of incarceration, getting condoms from an HIV prevention activity, getting syringes from an HIV prevention activity, getting condoms from an HIV prevention activity, ever being in drug treatment, using divided drugs with a syringe that has already been used, frequency of injection, drug injected most often, sharing cookers/water or filters, sharing syringes, using alcohol or drugs with last sex partner and having unprotected sex.
Network-level variables were measured as network proportions. For example, if an individual has 4 network members and 3 are HIV infected, that individual’s HIV infected network proportion is 0.75.
Results reflect behavior in past 12 months.
DISCUSSION
The results of this study support the importance of network characteristics in the emerging HIV outbreak among people who inject drugs in Athens, Greece. Greater network proportions of HIV infected PWID were positively associated with respondent HIV serostatus. Network proportions of risk behaviors such as dividing drugs and high frequency injecting were also associated with respondent HIV serostatus. These findings were attenuated with increasing degrees of separation. Comparison of results with randomly generated permutation networks further demonstrated that the observed network proportion associations were significant. In RDS, recruitment must proceed through a sufficiently large number of waves before the sample reaches equilibrium, thereby overcoming any bias from the nonrandom choice of seeds. The progression of network proportions over subsequent recruitment waves likely represents this process.
The sexual risk behaviors examined in these analyses were not associated with respondent HIV seroprevalence. This is consistent with other reports suggesting that the emerging HIV epidemic among PWID in Athens is primarily driven by high-risk injecting behaviors 13,15,19, rather than sex networks which, given our findings, appear to only play a limited role in HIV transmission at this stage of the epidemic. Other emerging epidemics have followed a similar pattern of early injection drug-driven transmission 31,32. In Athens, future interventions to prevent epidemics among other groups are warranted, however, given that 48% of men in Aristotle reported sex with non-PWID19. In addition, only 59% of men reported using condoms “always” or “usually”19. Considering the substantial proportion of respondents engaging in risky sex behaviors and reporting sex with non-PWID, the risk of increased future HIV spread via sexual networks will require additional intervention as the epidemic matures. Network interventions 33 that operate through sex networks may then become increasingly relevant.
Previous studies indicate that health behaviors and outcomes such as obesity and smoking may be influenced not only by immediate network members, but also by second and third degree network members 23–25. The observed network effects in Athens were particularly strong for the immediate (first degree) network, and dropped off with increasing social distance. There were still some residual effects of second and third degree networks members on respondent HIV serostatus, high frequency injecting and unprotected sex. These important primary network “effects” and more distal network effects support the diffusion of social behavior and HIV through networks and the potential impact that more distal network members might have on one’s health, behavior and circumstances.
The RDS networks used in this study likely include network members that exist in an individual’s social network, risk network or both. That is, the relationship with the recruit may be one of risk transmission (e.g. sharing syringes) without social influence, social influence without risk transmission or both social influence and risk transmission. Given the overlap that often occurs between social and risk PWID networks, studying their combined effect is likely important to fully understand network influences. To date, few analyses have explored the use of RDS recruitment for studying social or risk networks 34. In one study of drug injectors in Australia, researchers linked respondent information with recruiter information to form dyads and found associations between dyad characteristics and sharing of injection equipment 35. Another study used reported closeness to one’s recruiter as a predictor of HIV and Hepatitis C 36. We are unaware of other published studies that have used RDS recruitment ties as a proxy for an individual’s network beyond the dyad and that examined those networks for associations with HIV risk practices and HIV infection. This may be due to concerns about how well RDS-generated ties reflect the underlying social network 37. In our sample, a minority of an individual’s network on average was made up of “stranger” drug injectors. However, analyses with strangers removed yielded similar results to those utilizing the entire network. This similarity is not surprising given the potential effects that network members who are not close, but who are part of the social milieu may have on one’s risk behavior. As others have suggested 35, including other social network members not typically sampled in classic social network generation approaches 38 may be important as it includes strangers which may more closely resemble true social and risk networks and thus social norms. More research is needed to determine whether stranger status is assigned bi-directionally by two RDS linked individuals as well as the degree to which stranger networks play a role on influence and norms.
Other analyses have associated this HIV outbreak with recent economic, social and political instability in Greece, as well as the specific Greek austerity measures that were put in place to address the recession 18,39–41. Declines in various measures of population health have also been noted in other countries affected by the 2008 financial crisis.42 Though these other countries have not experienced significant increases in HIV incidence as seen in Greece, historical examples of HIV outbreaks following political and economic unrest are present in the literature 43. The recession has brought increasing levels of homelessness, which was the only sociodemographic characteristic associated with one of our risk outcome measures. This suggests an increase need for focus on homelessness at the network level.
Not enough is known about how risk and social networks function in response to socioeconomic crises 43. These crises that may result in structural changes such as greatly increased homelessness could impact risk of HIV acquisition 44. From 2009 to 2011, the estimated number of people experiencing homelessness rose 165% 45. A recent analysis found homelessness to be the most significant factor associated with HIV seroprevalence in the Athens PWID epidemic 19. In the current network analysis, we find that increased rates of homelessness in a respondent’s network, a key effect of economic crises on marginalized populations, was associated with increased frequency of injecting, which in turn may increase the likelihood that someone else in the network acquires HIV. These findings suggest that focus on the role of housing as an HIV protective intervention at the network level may be needed where emerging HIV epidemics are occurring within larger socio-economical disruptive contexts.
Unfortunately our data do not permit an examination of direct causal pathways between network characteristics and homelessness in this emerging epidemic. It also remains unclear the directionality of infection within these networks. However comparison of networks across localities and across time may elucidate how networks function in these types of outbreaks. This study may serve as a baseline for later studies in Greece as the economic situation changes or for future interventions that include provision of housing.
Large-scale structural factors such as financial crisis and increased homelessness are likely driving this HIV epidemic among PWID in Greece. As such, the response requires intervention at multiple levels 3. At the highest level, interventions may include anti-austerity programs or restructuring of Greek debt. These types of system-level approaches are currently under discussion within the Greek political arena. The data from this analysis indicate that future public policies and programs should also include meso-level network interventions. Network interventions focus at the level of the network with intervention effects measured both at the network as well as the individual levels. Many types of network interventions have been implemented and researched, such as identifying individuals within the network to disseminate information or interventions which stimulate peer to peer interaction and create cascades bio-behavioral diffusion 33. A network-level HIV prevention approach has recently started in Athens, Greece 46. Multi-level approaches including system-level and network-level interventions will be critical as we work to prevent emerging epidemics internationally.
Supplementary Material
Acknowledgments
Source of Funding: Aristotle is implemented under NSRF 2007-2013 (Operational Programme “Human Resources Development” 2007–2013, Priority Axis 14-Attika, Central Macedonia, Western Macedonia) and is co-funded by European Social Fund and national resources. Additional financial support was provided by the NIH (DP1 DA034989, 1R21MH098768 and R01DA033875) and the Hellenic Scientific Society for the study of AIDS and STDs. The International AIDS Society and the National Institute on Drug Abuse (NIDA) have supported the post-doc fellowship of GN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We are grateful for the support and energetic efforts of Aristotle, the Athens University Medical School, and the Organization Against Drugs (OKANA).
Footnotes
Conflicts of Interest: None to declare.
AUTHOR CONTRIBUTIONS
AH, JS, and SF obtained funding. VS, GN, DP and AH acquired data. JS and MT drafted the manuscript. PS, MT and JS performed statistical analysis and interpreted the data. All authors provided critical revision of the manuscript.
REFERENCE LIST
- 1.Rothenberg RB, Potterat JJ, Woodhouse DE. Personal risk taking and the spread of disease: beyond core groups. J Infect Dis. 1996;174 (Suppl 2):S144–149. doi: 10.1093/infdis/174.supplement_2.s144. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61(5):1026–1044. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Auerbach JD, Parkhurst JO, Cáceres CF. Addressing social drivers of HIV/AIDS for the long-term response: Conceptual and methodological considerations. Glob Public Health. 2011;6:S293–S309. doi: 10.1080/17441692.2011.594451. [DOI] [PubMed] [Google Scholar]
- 4.Neaigus A, Friedman SR, Curtis R, et al. The relevance of drug injectors’ social and risk networks for understanding and preventing HIV infection. Soc Sci Med. 1994;38(1):67–78. doi: 10.1016/0277-9536(94)90301-8. [DOI] [PubMed] [Google Scholar]
- 5.Klovdahl AS. Social networks and the spread of infectious diseases: The AIDS example. Soc Sci Med. 1985;21(11):1203–1216. doi: 10.1016/0277-9536(85)90269-2. [DOI] [PubMed] [Google Scholar]
- 6.Friedman SR, Kottiri BJ, Neaigus A, Curtis R, Vermund SH, Des Jarlais DC. Network-related mechanisms may help explain long-term HIV-1 seroprevalence levels that remain high but do not approach population-group saturation. Am J Epidemiol. 2000;152(10):913–922. doi: 10.1093/aje/152.10.913. [DOI] [PubMed] [Google Scholar]
- 7.Khan B, Dombrowski K, Saad M, McLean K, Friedman S. Network Firewall Dynamics and the Subsaturation Stabilization of HIV. Discrete Dyn Nat Soc. 2013 doi: 10.1155/2013/720818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latkin CA, Forman V, Knowlton A, Sherman S. Norms, social networks, and HIV-related risk behaviors among urban disadvantaged drug users. Soc Sci Med. 2003;56(3):465–476. doi: 10.1016/s0277-9536(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 9.De P, Cox J, Boivin J-F, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addict Abingdon Engl. 2007;102(11):1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 10.Stein MD, Charuvastra A, Anderson BJ. Social support and zero sharing risk among hazardously drinking injection drug users. J Subst Abuse Treat. 2002;23(3):225–230. doi: 10.1016/s0740-5472(02)00248-9. [DOI] [PubMed] [Google Scholar]
- 11.Friedman SR, Neaigus A, Jose B, et al. Networks, Norms and Solidaristic/Altruistic Action against Aids among the Demonized. Sociol Focus. 1999;32(2):127–142. doi: 10.1080/00380237.1999.10571131. [DOI] [Google Scholar]
- 12.Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: outcome of a network-oriented peer outreach intervention. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2003;22(4):332–339. doi: 10.1037/0278-6133.22.4.332. [DOI] [PubMed] [Google Scholar]
- 13.Paraskevis D, Nikolopoulos G, Tsiara C, et al. HIV-1 outbreak among injecting drug users in Greece, 2011: a preliminary report. Euro Surveill. 2011;16(36):19962. doi: 10.2807/ese.16.36.19962-en. [DOI] [PubMed] [Google Scholar]
- 14.Nikolopoulos G, Paraskevis D, Hatzakis A. HIV epidemiology in Greece. Future Microbiol. 2008;3(5):507–516. doi: 10.2217/17460913.3.5.507. [DOI] [PubMed] [Google Scholar]
- 15.Paraskevis D, Nikolopoulos G, Fotiou A, et al. Economic Recession and Emergence of an HIV-1 Outbreak among Drug Injectors in Athens Metropolitan Area: A Longitudinal Study. PloS One. 2013;8(11):e78941. doi: 10.1371/journal.pone.0078941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HIV/AIDS Surveillance Report in Greece, 31-12-2013. Athens: Hellenic Center for Disease Control and Prevention; 2013. http://www.keelpno.gr/Portals/0/Αρχεία/HIV/2014/Epidimiologiko_2013_final.pdf. [Google Scholar]
- 17.Bonovas S, Nikolopoulos G. High-burden epidemics in greece in the era of economic crisis. Early signs of a public health tragedy. J Prev Med Hyg. 2012;53(3):169–171. [PubMed] [Google Scholar]
- 18.Kondilis E, Giannakopoulos S, Gavana M, Ierodiakonou I, Waitzkin H, Benos A. Economic Crisis, Restrictive Policies, and the Population’s Health and Health Care: The Greek Case. Am J Public Health. 2013;103(6):973–979. doi: 10.2105/AJPH.2012.301126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sypsa V, Paraskevis D, Malliori M, et al. Homelessness and other risk factors for HIV infection in the current outbreak among injecting drug users in Athens, Greece. Am J Public Health. doi: 10.2105/AJPH.2013.301656. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heckathorn DD. Respondent-Driven Sampling: A New Approach to the Study of Hidden Populations. Soc Probl. 1997;44(2):174–199. doi: 10.2307/3096941. [DOI] [Google Scholar]
- 21.Abdul-Quader AS, Heckathorn DD, Sabin K, Saidel T. Implementation and Analysis of Respondent Driven Sampling: Lessons Learned from the Field. J Urban Health Bull N Y Acad Med. 2006;83(Suppl 1):1–5. doi: 10.1007/s11524-006-9108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lansky A, Abdul-Quader AS, Cribbin M, et al. Developing an HIV behavioral surveillance system for injecting drug users: the National HIV Behavioral Surveillance System. Public Health Rep Wash DC 1974. 2007;122 (Suppl 1):48–55. doi: 10.1177/00333549071220S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christakis NA, Fowler JH. The Spread of Obesity in a Large Social Network over 32 Years. N Engl J Med. 2007;357(4):370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 24.Christakis NA, Fowler JH. The Collective Dynamics of Smoking in a Large Social Network. N Engl J Med. 2008;358(21):2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centola D. The Spread of Behavior in an Online Social Network Experiment. Science. 2010;329(5996):1194–1197. doi: 10.1126/science.1185231. [DOI] [PubMed] [Google Scholar]
- 26.Neaigus A, Friedman SR, Goldstein M, Ildefonso G, Curtis R, Jose B. Using dyadic data for a network analysis of HIV infection and risk behaviors among injecting drug users. NIDA Res Monogr. 1995;151:20–37. [PubMed] [Google Scholar]
- 27.Shain RN, Piper JM, Newton ER, et al. A Randomized, Controlled Trial of a Behavioral Intervention to Prevent Sexually Transmitted Disease among Minority Women. N Engl J Med. 1999;340(2):93–100. doi: 10.1056/NEJM199901143400203. [DOI] [PubMed] [Google Scholar]
- 28.Schneider J, Michaels S, Bouris A. Family network proportion and HIV risk among black men who have sex with men. J Acquir Immune Defic Syndr 1999. 2012;61(5):627–635. doi: 10.1097/QAI.0b013e318270d3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox DR, Snell EJ. Analysis of Binary Data. London; New York: Chapman and Hall; 1989. [Google Scholar]
- 30.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall; 1994. [Google Scholar]
- 31.Lindan CP, Lieu TX, Giang LT, et al. Rising HIV infection rates in Ho Chi Minh City herald emerging AIDS epidemic in Vietnam. AIDS Lond Engl. 1997;11 (Suppl 1):S5–13. [PubMed] [Google Scholar]
- 32.Strathdee SA, Magis-Rodriguez C, Mays VM, Jimenez R, Patterson TL. The Emerging HIV Epidemic on the Mexico-US Border: An International Case Study Characterizing the Role of Epidemiology in Surveillance and Response. Ann Epidemiol. 2012;22(6):426–438. doi: 10.1016/j.annepidem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valente TW. Network Interventions. Science. 2012;337(6090):49–53. doi: 10.1126/science.1217330. [DOI] [PubMed] [Google Scholar]
- 34.Shah NS, Iveniuk J, Muth SQ, et al. Structural Bridging Network Position is Associated with HIV Status in a Younger Black Men Who Have Sex with Men Epidemic. AIDS Behav. 2014;18(2):335–345. doi: 10.1007/s10461-013-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paquette DM, Bryant J, De Wit J. Use of respondent-driven sampling to enhance understanding of injecting networks: A study of people who inject drugs in Sydney, Australia. Int J Drug Policy. 2011;22(4):267–273. doi: 10.1016/j.drugpo.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Eritsyan K, Heimer R, Barbour R, et al. Individual-level, network-level and city-level factors associated with HIV prevalence among people who inject drugs in eight Russian cities: a cross-sectional study. BMJ Open. 2013;3(6) doi: 10.1136/bmjopen-2013-002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heimer R. Critical Issues and Further Questions About Respondent-Driven Sampling: Comment on Ramirez-Valles, et al. (2005) AIDS Behav. 2005;9(4):403–408. doi: 10.1007/s10461-005-9030-1. [DOI] [PubMed] [Google Scholar]
- 38.Burt RS. Network items and the general social survey. Soc Netw. 1984;6(4):293–339. doi: 10.1016/0378-8733(84)90007-8. [DOI] [Google Scholar]
- 39.Paraskevis D, Nikolopoulos G, Fotiou A, et al. Economic recession and emergence of an HIV_1 outbreak in IDUs in Athens Metropolitan Area: a longitudinal study. Pap Submitt Publ (submitted) [Google Scholar]
- 40.Kentikelenis A, Karanikolos M, Papanicolas I, Basu S, McKee M, Stuckler D. Health effects of financial crisis: omens of a Greek tragedy. The Lancet. 2011;378(9801):1457–1458. doi: 10.1016/S0140-6736(11)61556-0. [DOI] [PubMed] [Google Scholar]
- 41.Kentikelenis A, Karanikolos M, Reeves A, McKee M, Stuckler D. Greece’s health crisis: from austerity to denialism. The Lancet. 2014;383(9918):748–753. doi: 10.1016/S0140-6736(13)62291-6. [DOI] [PubMed] [Google Scholar]
- 42.Karanikolos M, Mladovsky P, Cylus J, et al. Financial crisis, austerity, and health in Europe. [Accessed June 12, 2013];The Lancet. 2013 doi: 10.1016/S0140-6736(13)60102-6. http://www.sciencedirect.com/science/article/pii/S0140673613601026. [DOI] [PubMed]
- 43.Friedman SR, Rossi D, Braine N. Theorizing “Big Events” as a potential risk environment for drug use, drug-related harm and HIV epidemic outbreaks. Int J Drug Policy. 2009;20(3):283–291. doi: 10.1016/j.drugpo.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Galea S, Nandi A, Vlahov D. The Social Epidemiology of Substance Use. Epidemiol Rev. 2004;26(1):36–52. doi: 10.1093/epirev/mxh007. [DOI] [PubMed] [Google Scholar]
- 45.Giannetou K. The Social Problem of Homelessness. 2012;23:1–19. [Google Scholar]
- 46.Friedman SR, Downing MJ, Jr, Smyrnov P, et al. Socially-Integrated Transdisciplinary HIV Prevention. AIDS Behav. 2013 doi: 10.1007/s10461-013-0643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.