Abstract
Classic galactosemia is a rare genetic disease of the galactose metabolism, resulting from deficient activity of galactose-1-phosphate uridylyltransferase (GALT). The current standard of care is lifelong dietary restriction of galactose, which however fails to prevent the development of long-term complications. Structural-functional studies demonstrated that the most prevalent GALT mutations give rise to proteins with increased propensity to aggregate in solution. Arginine is a known stabilizer of aggregation-prone proteins, having already shown a beneficial effect in other inherited metabolic disorders.
Herein we developed a prokaryotic model of galactose sensitivity that allows evaluating in a cellular context the mutations’ impact on GALT function, as well as the potential effect of arginine in functionally rescuing clinically relevant variants.
This study revealed that some hGALT variants, previously described to exhibit no detectable activity in vitro, actually present residual activity when determined in vivo. Furthermore, it revealed that arginine presents a mutation-specific beneficial effect, particularly on the prevalent p.Q188R and p.K285N variants, which led us to hypothesize that it might constitute a promising therapeutic agent in classic galactosemia.
Keywords: Arginine, Classic galactosemia, Galactose sensitivity, Missense mutations, Protein aggregation
Introduction
Classic galactosemia (OMIM #230400) is a rare metabolic disease resulting from deficient activity of galactose-1-phosphate uridylyltransferase (GALT, EC 2.7.7.12), the second enzyme of the Leloir pathway (Fridovich-Keil and Walter 2008). This inherited metabolic disorder is a potentially lethal disease that develops in the neonatal period, upon exposure to galactose in milk (Berry and Walter 2012; Fridovich-Keil and Walter 2008). The present standard of care is a lifelong galactose-restricted diet, which, notwithstanding its irrefutable life-saving role in the neonatal period, fails to prevent long-term cognitive, motor, and fertility impairments (Bosch 2006; Fridovich-Keil and Walter 2008; Waggoner et al. 1990), and thus intense research has been dedicated to the pursuit of a more effective therapy.
Classic galactosemia is caused by mutations in the GALT gene, and more than 260 variations have already been described, the majority being missense mutations (>60%) (Calderon et al. 2007; Leslie et al. 1992). Structural and functional studies demonstrated that clinically relevant GALT mutations result in misfolded protein variants (Coelho et al. 2014; McCorvie et al. 2013). A decrease in thermal and conformational stability has been observed for several GALT variants (McCorvie et al. 2013), although the most frequent GALT mutations actually affect the variants’ aggregation propensity, particularly the p.Q188R variant, responsible for >60% of galactosemic phenotypes (Coelho et al. 2014; Suzuki et al. 2001).
Arginine has been previously described as therapeutically beneficial for a pyruvate dehydrogenase complex-deficient patient whose biochemical and clinical symptoms significantly improved upon arginine aspartate intake (Silva et al. 2009). A positive role of arginine has also been observed in the peroxisome function of cultured cells from peroxisome biogenesis disorder patients with mutations in PEX1, PEX6, and PEX12 (Berendse et al. 2013). Furthermore, arginine is a long-recognized protein stabilizer that has been proposed to exert an anti-aggregation effect by increasing the activation energy of protein aggregation (Baynes et al. 2005). In line with this, we sought to evaluate whether arginine exerts a protective stabilizing effect towards clinically relevant GALT variants. Accordingly, this study aimed to evaluate the effect of arginine on rescuing variant GALT function and alleviating galactose-induced toxicity in a prokaryotic model of galactose sensitivity.
Materials and Methods
Cloning and Mutagenesis
The Escherichia (E.) coli K-12ΔgalT strain (JW0741-1;ΔgalT730::kan), with a deletion of the endogenous galT gene, was purchased from the Coli Genetic Stock Center (Baba et al. 2006). The human GALT (hGALT) cDNA sequence, including an N-terminal hexa-histidyl tag-encoding sequence, was cut from the pET24b-based construct reported in (Coelho et al. 2014) with the BamHI and HindIII restriction enzymes and cloned into the pTrcHisA expression vector (Invitrogen). The mutations originating the studied variants (p.S135L, p.G175D, p.P185S, p.Q188R, p.R231C, pR231H, p.K285N, and p.N314D) were introduced by site-directed mutagenesis, as previously described (Coelho et al. 2014), and confirmed by direct sequencing. As a negative control, we employed a pTrcHisA-based vector containing the cDNA encoding the human enzyme phenylalanine hydroxylase (hPAH) (Leandro et al. 2000).
Cell Cultures and Growth Media
Non-transformed and wild-type hGALT-transformed E. coli ΔgalT were first grown in M9 minimal medium (Maniatis et al. 1982) containing either 1% glucose (with or without 1% galactose), 1% glycerol, or 1% galactose as carbon sources, to evaluate the ability of wild-type hGALT to alleviate galactose toxicity.
Cultures expressing all hGALT variants and hPAH were grown at 37°C in M9 minimal medium (Maniatis et al. 1982) containing glycerol (1%) as sole carbon source, from a starting optical density at 600 nm (OD600nm) of 0.05. At OD600nm = 0.3, 250 μM isopropyl-d-thiogalactoside (IPTG) was added to all cultures to induce protein expression, as well as 25 mM arginine to cultures III and IV (Table 1). After 1 h of protein expression induction, 1% galactose was added to cultures II and IV (Table 1). Cultures growth was followed by hourly measurements of OD600nm, starting at induction time (t = 0 h) and up to 9 h.
Table 1.
Cell culture conditions to evaluate the ability of GALT variants to alleviate galactose toxicity and the effect of arginine
| Supplementationa | ||
|---|---|---|
| Galactose | Arginine | |
| I | − | − |
| II | + | − |
| III | − | + |
| IV | + | + |
aAll cultures were grown in M9 minimal medium containing 1% glycerol as carbon source, supplemented with 100 μM ferrous sulfate and 100 μM zinc sulfate
Growth curves were obtained from plotting OD600nm from cultures I and II (Table 1) as a function of time (Figs. 1, 2, and 3 show representative graphics from multiple independent experiments). To directly evaluate the arginine effect on the rescue from galactose toxicity, ratios r and rarg were calculated according to Eqs. (1) and (2), respectively, and were plotted as a function of time (Figs. 2 and 3 show representative graphics from multiple independent experiments). Recombinant production of hGALT and hPAH was confirmed by immunoblotting analysis, using, respectively, the anti-GALT and anti-His primary antibodies (sc-365577, Santa Cruz Biotechnology; 27-4710-01, GE Healthcare Biosciences).
| 1 |
| 2 |
Fig. 1.
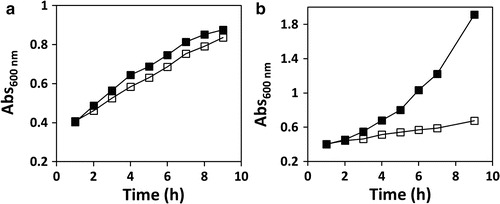
Galactose toxicity is alleviated by expression of human GALT. Growth profiles of Escherichia coli ΔgalT transformed with vectors encoding wild-type hGALT (Panel a, positive control) or wild-type hPAH (Panel b, negative control), in the absence or presence of galactose. Bacteria were grown at 37°C in M9 minimal medium with 1% glycerol as carbon source. At OD600nm =0.3, protein expression was induced with 250 μM IPTG. After 1 h, vehicle (water, full squares) or 1% galactose (hollow squares) was added to the cultures, and the growth was followed by hourly measurements of OD600nm up to 9 h. Growth curves are representative graphics from several independent experiments (n = 12 for wild-type hGALT and n = 12 for PAH)
Fig. 2.
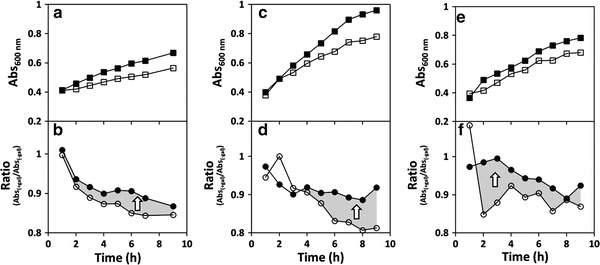
Arginine improves the function of p.Q188R, p.K285N, and p.G175D hGALT. Growth profiles of Escherichia coli ΔgalT expressing the p.Q188R, p.K285N, and p.G175D hGALT variants (Panels a, c, and e, respectively) in the absence or presence of galactose. Bacteria were grown at 37°C in M9 minimal medium with 1% glycerol as carbon source. At OD600nm =0.3, protein expression was induced with 250 μM IPTG. After 1 h, vehicle (water, full squares) or 1% galactose (hollow squares) was added to the cultures, and the growth was followed by hourly measurements of OD600nm up to 9 h. Simultaneously with water/galactose, 25 mM arginine or vehicle (water) was added to the cultures. Panels b, d, and f depict the ratio curves for bacteria expressing, respectively, p.Q188R, p.K285N, and p.G175D hGALT, obtained by dividing, at each time point, the OD600nm in the presence or absence of galactose (black circles, in the presence of 25 mM arginine; hollow circles, absence of arginine). The gray-shaded areas and the white arrows depict the effect of arginine in improving the ability of these variants to alleviate galactose toxicity, highlighted by white arrows. Growth curves are representative graphics from several independent experiments (n = 4 for p.Q188R and n = 3 for p.G175D and p.K285N)
Fig. 3.
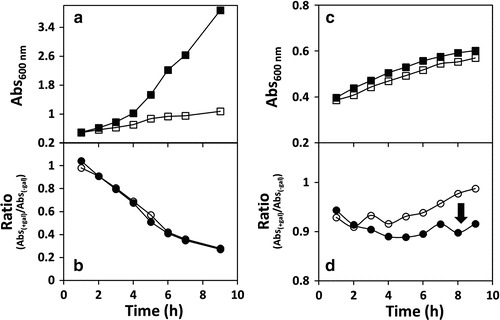
Absent or negative effect of arginine on p.P185S and p.R231C hGALT function. Growth profiles of Escherichia coli ΔgalT expressing the p.P185S and p.R231C hGALT variants (Panels a and c, respectively), in the absence or presence of galactose. Bacteria were grown at 37°C in M9 minimal medium with 1% glycerol as carbon source. At OD600nm =0.3, protein expression was induced with 250 μM IPTG. After 1 h, vehicle (water, full squares) or 1% galactose (hollow squares) was added to the cultures, and the growth was followed by hourly measurements of OD600nm up to 9 h. Simultaneously with water/galactose, 25 mM arginine or vehicle (water) was added to the cultures. Panels b and d depict the ratio curves for bacteria expressing, respectively, p.P185S and p.R231C hGALT, obtained by dividing, at each time point, the OD600nm in the presence or absence of galactose (black circles, in the presence of 25 mM arginine; hollow circles, absence of arginine). Growth curves of E. coli ΔgalT cultures expressing the p.P185S variant (Panel a) suggest a galactose-sensitivity profile similar to that of hPAH (negative control), and the ratio curves (Panel b) show complete functional unresponsiveness to arginine supplementation. Growth curves of ΔgalT E. coli expressing p.R231C (Panel c) suggest a very mild galactose-sensitivity profile, and the ratio curves (Panel d) show a puzzlingly negative effect of arginine. Growth curves are representative graphics from several independent experiments (n = 2 for p.P185S and n = 3 for p.R231C)
Results and Discussion
We have developed a prokaryotic model of galactose sensitivity using the E. coli ΔgalT strain, with a deletion of the endogenous galT gene (Baba et al. 2006). Expressing hGALT variants in this E. coli strain, and assaying the cultures sensitivity to galactose in the culture medium, allows evaluating the mutations severity in a cellular context, as well as testing stabilizing compounds. Previous studies reported a yeast model of galactose sensitivity (Riehman et al. 2001; Ross et al. 2004), which is however technically more demanding and time-consuming comparatively to E. coli.
Effect of Carbon Sources upon the Growth of Non-transformed and Wild-Type hGALT-Transformed E. coli ΔgalT
Non-transformed and wild-type hGALT-transformed E. coli ΔgalT were initially grown in the presence of different carbon sources. For non-transformed E. coli ΔgalT cultured under glucose or glucose plus galactose, the growth curves were indistinguishable, since glucose is the preferred carbon source and galactose represents no toxicity in the presence of this hexose. This absent toxicity of galactose likely results from carbon catabolite repression exerted by glucose, which represses the galactose uptake systems GalP and Mgl (Görke and Stülke 2008; Misko et al. 1987; Shimizu 2013; Steinsiek and Bettenbrock 2012). In the presence of glycerol, transported into E. coli through facilitated diffusion (Chu et al. 2002), the growth rate was approximately half of that observed in the presence of glucose, confirming that this polyol is not as efficient as glucose as a carbon source, as previously described (Chu et al. 2002). In the presence of galactose, the growth was practically arrested, indicating the inability of this strain to use galactose as carbon source and/or its high toxicity. In turn, E. coli ΔgalT expressing wild-type hGALT presented growth curves identical to the non-transformed strain in all conditions, except when galactose was the single carbon source, in which the growth was significantly higher than that of the non-transformed bacteria. This strongly suggests that the expression of hGALT enables the utilization of galactose as a carbon source and/or abolishes the galactose sensitivity of the E. coli ΔgalT strain (Fig. 1a).
Galactose Sensitivity of Bacteria Expressing Different hGALT Variants
To evaluate the ability of the studied hGALT variants to sustain growth in the presence of galactose when expressed in E. coli ΔgalT, the bacteria expressing wild-type hGALT were used as the positive control, whose growth was barely affected by the presence of 1% galactose (Fig. 1a). Bacteria expressing wild-type hPAH were used as the negative control, with galactose severely arresting the culture growth (Fig. 1b). Taken together, these observations confirm the toxicity of galactose in our model, since the PAH-expressing cells are still able to use glycerol as carbon source, while being severely affected by galactose. By expressing an unrelated protein, we aimed to subject the control bacterial cultures to the same metabolic demands associated with protein production as the cultures expressing hGALT. Interestingly, using glycerol as sole carbon source (condition I, Table 1), the hPAH-producing culture exhibited a much higher growth rate than hGALT (wild-type and variants) cultures in the same conditions (Fig. 1b, upper panel). This may arise from the fact that producing different recombinant proteins has different energy costs to the bacterial cells.
Bacterial cultures expressing the variants p.N314D, p.S135L, and p.R231H showed essentially no growth arrest upon galactose addition, presenting a galactose growth curve essentially superimposable to that of glycerol alone (not shown). Cells expressing the p.Q188R, p.K285N, p.G175D, and p.R231C variants exhibited variable levels of galactose toxicity (Figs. 2a, c, e and 3c). On the other hand, cultures expressing p.P185S showed a highly galactose-sensitive profile, similarly to the negative control (Fig. 3a). No clear correlation could be established between the variants’ toxicity and the in vitro specific activity (Coelho et al. 2014), suggesting that some hGALT variants present some residual activity in vivo that, however, is not detectable in vitro, particularly p.K285N, p.R231C, and p.R231H (Coelho et al. 2014).
Arginine Rescue of the hGALT Variants
Wild-type hGALT ratio curves (r and rarg) revealed a slight degree of response to the medium supplementation with arginine (not shown), which is not surprising since it has been described that arginine is also able to stabilize the proteins’ native state (Arakawa and Tsumoto 2003). In contrast, wild-type hPAH ratio curves (r and rarg) are superimposable, which rules out any indirect effect of arginine in improving the growth profiles of the cultures expressing the hGALT variants.
Interestingly, p.N314D, which is believed to be the ancestral allele (Carney et al. 2009), appears to be insensitive to arginine, as well as p.S135L and p.R231H which show essentially overlapping ratio curves. Notably, the p.Q188R, p.K285N, and p.G175D variants were partially rescued by arginine, with rarg > r (Fig. 2b, d and f). The rescue of p.Q188R is remarkable, due to its high prevalence and to its classification as a severe mutation particularly prone to aggregation (Coelho et al. 2014; Shield 2000; Suzuki et al. 2001). Immunoblotting analysis of the soluble lysate revealed that this variant is actually highly expressed (higher than the wild type or any other variant), which may account for the unexpected high tolerance of the cultures even in the absence of arginine (not shown). The p.R231C variant displays ratio curves with the puzzling feature of a slightly increased toxicity in the presence of arginine (Fig. 3d). In turn, p.P185S ratio curves are very similar to the hPAH negative control, revealing its severe functional impairment and unresponsiveness to the potential stabilizing effect of arginine (Fig. 3b and d).
The reproducibility of the ratio curves clearly indicates that arginine’s mode of action is mutation specific, showing it is indeed functionally improving variants p.Q188R, p.K285N, and p.G175D in alleviating the galactose toxicity.
Conclusion
We have developed a prokaryotic model of galactose sensitivity that allows evaluating the impact of human GALT mutations on the function of GALT variants, with the additional advantage of being assessed in a cellular context. In fact, this study has revealed that some hGALT variants present residual activity in cell cultures that, however, is not detectable when determined in vitro with the recombinant protein (Coelho et al. 2014). Additionally, this bacterial model of galactose sensitivity has also proven useful in evaluating potential therapeutic agents for new therapeutic approaches against classic galactosemia. Previous studies on the molecular basis of classic galactosemia associated with missense mutations reported that the main pathogenic mechanism relates to protein misfolding, resulting in increased protein aggregation (Coelho et al. 2014) or decreased thermal and conformational stability (McCorvie et al. 2013). Accordingly, we have studied the potential beneficial effect of arginine, a well-established suppressant of protein aggregation (Baynes et al. 2005). Arginine has shown a mutation-specific effect, with p.Q188R, p.K285N, and p.G175D variants being rescued, suggesting that this amino acid could be of some therapeutic benefit in patients carrying these mutations. Notably, p.Q188R results from the most prevalent mutation in Western countries, accounting for over 60% of GALT mutant alleles (Suzuki et al. 2001), which entails a great therapeutic impact of arginine in classic galactosemia.
These results lay a foundation for future studies using the prokaryotic model of galactose sensitivity and put forward the hypothesis that arginine might be of some benefit in classic galactosemia, setting the stage for further studies.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia (SFRH/BD/48259/2008 to AIC and PEst-OE/SAU/UI4013/2011) and by Sociedade Portuguesa de Doenças Metabólicas Grant to IR.
Synopsis
Arginine supplementation functionally improves, in a mutation-specific manner, clinically relevant human GALT variants expressed in a bacterial model of galactose sensitivity, opening new perspectives towards the therapeutic potential of arginine for classic galactosemia.
Compliance with Ethics Guidelines
Conflict of Interest
All the authors, Ana I. Coelho, Matilde Trabuco, Maria João Silva, Isabel Tavares de Almeida, Paula Leandro, Isabel Rivera, and João B. Vicente, declare that they have no conflict of interest.
Informed Consent
No patients were included in this study.
Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributions
Experimental design – AI Coelho, MJ Silva, I Tavares de Almeida, P Leandro, I Rivera, JB Vicente
Experimental work – AI Coelho, M Trabuco, JB Vicente
Data analysis and interpretation – AI Coelho, P Leandro, I Rivera, JB Vicente
Writing of the manuscript – AI Coelho, P Leandro, I Rivera, JB Vicente
Footnotes
Competing interests: None declared
Contributor Information
João B. Vicente, Email: joaovicente@ff.ulisboa.pt
Collaborators: Johannes Zschocke
References
- Arakawa T, Tsumoto K. The effects of arginine on refolding of aggregated proteins: not facilitate refolding, but suppress aggregation. Biochem Biophys Res Commun. 2003;304:148–152. doi: 10.1016/S0006-291X(03)00578-3. [DOI] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M et al (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:1–11 [DOI] [PMC free article] [PubMed]
- Baynes BM, Wang DIC, Trout BL. Role of arginine in the stabilization of proteins against aggregation. Biochemistry. 2005;44:4919–4925. doi: 10.1021/bi047528r. [DOI] [PubMed] [Google Scholar]
- Berendse K, Ebberink MS, IJlst L, Wanders RJ, Waterham HR. Arginine improves peroxisome functioning in cells from patients with a mild peroxisome biogenesis disorder. Orphanet J Rare Dis. 2013;8:138–145. doi: 10.1186/1750-1172-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry GT, Walter JH (2012). Disorders of galactose metabolism. In: Saudubray JM, van den Berghe G, Walter JH (eds). Inborn metabolic diseases: diagnosis and treatment. Springer, Germany
- Bosch AM. Classical galactosaemia revisited. J Inherit Metab Dis. 2006;29:516–525. doi: 10.1007/s10545-006-0382-0. [DOI] [PubMed] [Google Scholar]
- Calderon FR, Phansalkar AR, Crockett DK, Miller M, Mao R. Mutation database for the galactose-1-phosphate uridyltransferase (GALT) gene. Hum Mutat. 2007;28:939–943. doi: 10.1002/humu.20544. [DOI] [PubMed] [Google Scholar]
- Carney AE, Sanders RD, Garza KR, et al. Origins, distribution and expression of the Duarte-2 (D2) allele of galactose-1-phosphate uridylyltransferase. Hum Mol Genet. 2009;18:1624–1632. doi: 10.1093/hmg/ddp080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Han C, Shimizu H, Wong B. The effect of fructose, galactose, and glucose on the induction of β-galactosidase in Escherichia coli. J Exp Microbiol Immunol. 2002;2:1–5. [Google Scholar]
- Coelho AI, Trabuco M, Ramos R et al (2014) Functional and structural impact of the most prevalent missense mutations in classic galactosemia. Mol Genet Genomic Med 2:484–496 [DOI] [PMC free article] [PubMed]
- Fridovich-Keil JL, Walter JH. Galactosemia. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, editors. The online metabolic and molecular bases of inherited disease. New York: Mc-Graw Hill; 2008. pp. 1–92. [Google Scholar]
- Görke B, Stülke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- Leslie ND, Immerrman EB, Flach JE, Florez M, Fridovich-Keil JL, Elsas LJ., II The human galactose-1-phosphate uridyltransferase gene. Genomics. 1992;14:474–480. doi: 10.1016/S0888-7543(05)80244-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular cloning. A laboratory manual. New York: Cold Spring Harbor; 1982. [Google Scholar]
- McCorvie TJ, Gleason TJ, Fridovich-Keil JL, Timson DJ. Misfolding of galactose 1-phosphate uridylyltransferase can result in type I galactosemia. Biochim Biophys Acta. 2013;1832:1279–1293. doi: 10.1016/j.bbadis.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko TP, Mitchell W, Meadow N, Roseman S. Sugar transport by the bacterial phosphotransferase system. Reconstitution of inducer exclusion in Salmonella typhimurium membrane vesicles. J Biol Chem. 1987;262:16261–16266. [PubMed] [Google Scholar]
- Riehman K, Crews C, Fridovich-Keil JL. Relationship between genotype, activity, and galactose sensitivity in yeast expressing patient alleles of human galactose-1-phosphate uridylyltransferase. J Biol Chem. 2001;276:10634–10640. doi: 10.1074/jbc.M009583200. [DOI] [PubMed] [Google Scholar]
- Ross KL, Davis CN, Fridovich-Keil JL. Differential roles of the Leloir pathway enzymes and metabolites in defining galactose sensitivity in yeast. Mol Genet Metab. 2004;83:103–116. doi: 10.1016/j.ymgme.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Shield JPH. The relationship of genotype to cognitive outcome in galactosaemia. Arch Dis Child. 2000;83:248–250. doi: 10.1136/adc.83.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K (2013) Metabolic regulation of a bacterial cell system with emphasis on Escherichia coli metabolism. ISRN Biochem 2013:645983:1–645983:47 [DOI] [PMC free article] [PubMed]
- Silva MJ, Pinheiro A, Eusebio F, Gaspar A, Tavares de Almeida I, Rivera I. Pyruvate dehydrogenase deficiency: identification of a novel mutation in the PDHA1 gene which responds to amino acid supplementation. Eur J Pediatr. 2009;168:17–22. doi: 10.1007/s00431-008-0700-7. [DOI] [PubMed] [Google Scholar]
- Steinsiek S, Bettenbrock K. Glucose transport in Escherichia coli mutant strains with defects in sugar transport systems. J Bacteriol. 2012;194:5897–5908. doi: 10.1128/JB.01502-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, West C, Beutler E. Large-scale molecular screening for galactosemia alleles in a pan-ethnic population. Hum Genet. 2001;109:210–215. doi: 10.1007/s004390100552. [DOI] [PubMed] [Google Scholar]
- Waggoner DD, Buist NRM, Donnel GN. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis. 1990;13:802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]


