Abstract
Background
Ischaemic heart disease (IHD) is the most common cause of death worldwide.
Aim
To determine the long-term impact of organisational interventions for secondary prevention of IHD.
Design and setting
Systematic review and meta-analysis of studies from CENTRAL, MEDLINE®, Embase, and CINAHL published January 2007 to January 2013.
Method
Searches were conducted for randomised controlled trials of patients with established IHD, with long-term follow-up, of cardiac secondary prevention programmes targeting organisational change in primary care or community settings. A random-effects model was used and risk ratios were calculated.
Results
Five studies were included with 4005 participants. Meta-analysis of four studies with mortality data at 4.7–6 years showed that organisational interventions were associated with approximately 20% reduced mortality, with a risk ratio (RR) for all-cause mortality of 0.79 (95% confidence interval [CI] = 0.66 to 0.93), and a RR for cardiac-related mortality of 0.74 (95% CI = 0.58 to 0.94). Two studies reported mortality data at 10 years. Analysis of these data showed no significant differences between groups. There were insufficient data to conduct a meta-analysis on the effect of interventions on hospital admissions. Additional analyses showed no significant association between organisational interventions and risk factor management or appropriate prescribing at 4.7–6 years.
Conclusion
Cardiac secondary prevention programmes targeting organisational change are associated with a reduced risk of death for at least 4–6 years. There is insufficient evidence to conclude whether this beneficial effect is maintained indefinitely.
Keywords: cardiovascular disease, coronary artery disease, general practice, systematic review
INTRODUCTION
Ischaemic heart disease (IHD) is the most common cause of death worldwide, accounting for 12.7% of total global mortality in 2008.1 Interventions that halt or slow the progress of IHD once it has been detected (secondary prevention), to prevent further events among those with established heart disease, are widely advocated and promoted.2 Two systematic reviews of secondary cardiac prevention programmes conducted in 2001 and 2005 demonstrated improved processes of care3 and improved patient outcomes.4 However, both noted evidence of a ‘ceiling effect’, whereby interventions have a diminishing beneficial effect once certain levels of risk factor management are reached. Both reviews recommended rigorous evaluation of long-term clinical and economic outcomes.
In 2010, a Cochrane Review5 assessed the effectiveness of service organisation interventions that aimed to improve the delivery of secondary preventative care in primary care or community settings. The review concluded that there was weak evidence regarding the effectiveness of such interventions in improving compliance with target levels of cholesterol and blood pressure. The Cochrane Review considered data collected at the end of interventions, varying from 12 to 36 months. The present study aimed to systematically review, and where possible, conduct a meta-analysis of, the evidence from follow-up studies of randomised controlled trials (RCTs), evaluating the long-term effectiveness of organisational interventions for people with IHD in primary care or community settings. These organisational interventions aim to alter the way existing secondary prevention services are arranged; for example, by the introduction of a structured recall system and specific secondary prevention clinics, in which modifiable lifestyle risk factors and prescribed medications are reviewed. Primary care or a community setting is where most of the ongoing, long-term managing of IHD takes place and is defined as the first point of access to healthcare services, where clinicians are responsible for most personal healthcare needs and aim to develop and maintain a long-term relationship with patients and families.6
METHOD
The protocol for this systematic review is available on the PROSPERO register7 and the review was undertaken using Cochrane methodology.8
Inclusion criteria
Studies were included in which the original intervention lasted a minimum of 12 months and was evaluated in a RCT, randomised by individual or by cluster. Follow-up must have occurred a minimum of 12 months after the end of the intervention.
How this fits in
This systematic review shows that cardiac secondary prevention programmes for ischaemic heart disease targeting organisational change are associated with reduced mortality for at least 4–6 years, after the formal cessation of the original programmes. There is insufficient evidence to conclude whether this beneficial effect is maintained indefinitely. Further research is recommended on the long-term impact of organisational interventions in primary care on hospital admissions.
Interventions targeted at organisational change in primary or community care settings were included, where the intervention was aimed at improved clinician and patient adherence with recommendations on secondary prevention of IHD. It was noted that on study completion, interventions could have formally ended or could have continued and been delivered to control patients. The plan was to explore intervention activity and contamination of control patients during the observational follow-up period. The comparator was ‘usual care’, although it is likely that baseline levels of secondary prevention care varied significantly between different settings. Interventions that were delivered exclusively by research personnel or hospital-based staff, without a planned link to primary or community care clinicians were excluded.
Studies were included of patients with established IHD: documented myocardial infarction, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, coronary artery stent, or angina.
Search strategy and data extraction
The search was conducted on Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE®, Embase, and CINAHL in January 2013 (Appendix 1). The search was based on that used in the Cochrane Review in 2010,5 and limited to articles published from 2007 onwards, with no language restrictions. One author screened the titles for eligibility. Two authors independently screened the abstracts of the retained citations in duplicate and assessed full-text reports of all potentially relevant studies for eligibility. Two authors completed data extraction in duplicate, extracting details on patients, interventions, comparators, outcome measures, study design, follow-up data collection, risk of bias and informal intervention activity, and potential contamination during the follow-up period. Where insufficient data were reported, study authors were contacted for further information. Disagreements were resolved by consultation with another author when required.
Statistical analysis
The primary outcomes were mortality and hospital admissions (all-cause and cardiac). These outcomes were considered to be of greatest relevance to assessing long-term outcomes of interventions as they are direct measures of impact on people’s wellbeing and health. Secondary outcomes were risk factor management (blood pressure, cholesterol, and smoking status), appropriate prescribing of secondary prevention medications according to national guidelines, for example, prescribing of statins to reduce lipids to target levels, and health status, as measured by validated scales, for example, SF-12. Review Manager (RevMan, version 5.2) was used for all analyses.
Where possible and appropriate, data from included studies were combined for each outcome. A random-effects model was used to produce an overall summary of the combined treatment effect across studies. For dichotomous data, summary risk ratios (RR) were calculated with 95% confidence intervals [CIs], and for continuous data, mean differences with 95% CIs. If data from trials randomised by cluster were not already adjusted to take the cluster effect into account, the sample sizes were adjusted using the methods described in the Cochrane Handbook,8 using an estimate of the intracluster correlation coefficient (ICC) derived from the trial where available or from trials of similar populations.9
Risk of bias assessment of included studies was conducted separately for the original RCTs and then for the observational follow-up studies, using the appropriate risk of bias assessment methods recommended in the Cochrane Handbook.8 Statistical heterogeneity was assessed in each meta-analysis and heterogeneity regarded as substantial where τ2 >0 and either I2>50% or P<0.1 in the χ2 test for heterogeneity.
RESULTS
From the 10 294 citations identified in the search, and two additional citations identified from the bibliography of the Cochrane Review,5 the full text of 71 articles (56 studies) was reviewed (Figure 1). Sixty articles (51 studies) were excluded, most commonly because the intervention was not based in primary care.
Figure 1.
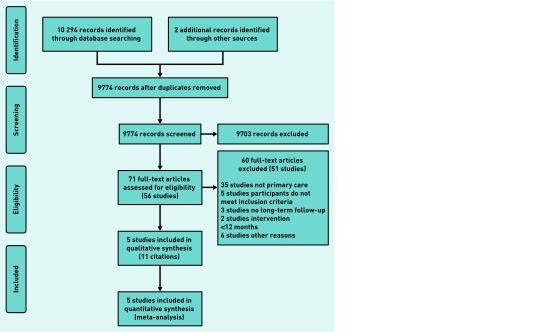
Selection process for eligible randomised controlled trials with long-term follow-up from all identified citations.
Included studies
Five randomised controlled trials were identified for inclusion, involving 4005 participants. Two trials were randomised by individual10,11 and three randomised by cluster, with randomisation by practice.12–14 All five trials were conducted in Western Europe: in Northern Ireland,10 Scotland,11 Spain,14 and Sweden,12 and the fifth in both the Republic of Ireland and Northern Ireland.13 Table 1 summarises the participant characteristics. Characteristics on the studies are available from the authors on request.
Table 1.
Summary of included studies and participant characteristics
| Study | Participants | Numbers randomised, age, sex | Length of intervention (length of follow-up) | What happened during follow-up? | Components of intervention | Outcomes at follow-up considered in this review |
|---|---|---|---|---|---|---|
| Cupples 199410a and 199915b Northern Ireland | Diagnosed angina Age <75 years | 688, 63 years, 59% male | 2 years (5 years) | Intervention ceased |
|
|
| Campbell 199811 Murchie 200316 Delaney 200819 Scotland |
Diagnosis of CHD or prescribed nitrates Age <80 years |
1343, 66 years, 58% male | 1 year (4.7 years) (10 years) | Intervention delivered to some patients in control and intervention |
|
|
| Kiesslingc 200212
201120 Sweden |
Diagnosed angina or diagnosed MI | 88, 62 years, 85% male | 2 years (10 years) | Intervention ceased |
|
|
| Munozc 200714 200817 Spain |
Diagnosed angina or MI within 6 years Age 30–79 years |
983, 64 years, 72% male | 3 years (5 years) | Intervention ceased |
|
|
| Murphyc 200913 201418 Republic of Ireland and Northern Ireland |
Previous MI, CABG, PTCA, diagnosed angina | 903, 67 years, 75% male | 18 months (6 years) | Intervention ceased |
|
|
First date refers to the date of publication of main study results.
Second (and subsequent) dates refer to date of publication of long-term follow-up results.
Randomisation by cluster. CABG = coronary artery bypass grafting. CHD = coronary heart disease. MI = myocardial infarction. PTCA = percutaneous transluminal coronary angioplasty.
Several sources of potential heterogeneity were evident, including differences between study populations, target levels for risk factors, outcomes measured, and in usual care. In addition, there was variation in the intervention components, the length of the original interventions (varied from 1–3 years), and the length of observational follow-up (four studies had follow-up data at 4.7–6 years,15–18 two studies had 10-year follow-up data19,20). Follow-up data were reported at two different times for the Scottish study: 4.7 years16 and 10 years.19 Five different intervention components were combined in different ways across the included studies (Table 1): patient education and pre-planned appointments (four studies) and clinician education, nurse-led interventions, and risk-factor management (three studies). The sample sizes were adjusted where required in all three cluster-randomised trials, using the methods recommended in the Cochrane Handbook.8
Care after the end of the formal intervention
The formal intervention ceased at the end of the original studies. In the three trials randomised by cluster, the risk of contamination of control patients during follow-up was slight, with no formal roll-out to control practices and cessation of support to intervention practices.17,18,20 In the trials randomised by individual,15,16 control, and intervention patients attended the same practices so some contamination may theoretically have occurred on study completion. This was formally assessed in the Scottish study,16 where many practices continued to offer the secondary prevention clinics to all patients. Fifty-five per cent of control patients had attended at least one secondary prevention clinic after 4 years.
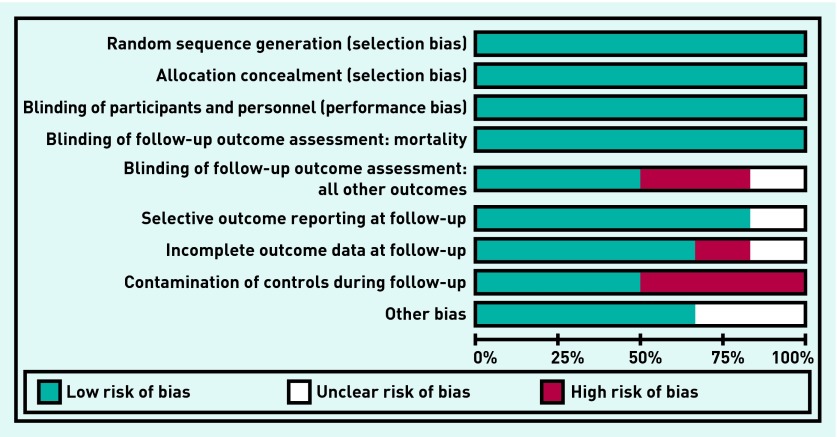
Risk of bias
Overall, the original intervention and the follow-up component of the five included studies were of reasonable quality (Figure 2). All studies conducted satisfactory random sequence generation and adequate allocation concealment. Apart from one study,12 participants and personnel were not blinded to allocation because of the nature of the intervention. Three studies15,18,20 had adequate blinding of outcome assessors at follow-up. The review authors concluded that lack of blinding of outcome assessors at follow-up did not affect mortality data but other outcome data collected from practice records were at high or unclear risk of bias.
Figure 2.
Risk of bias of included studies. Detection bias, attrition bias, or reporting bias at the end of the original trial are not included.
As outlined above, two of the five studies may have had some contamination of control patients in the follow-up period.15,16 Loss to long-term follow-up was very low for mortality data (<2%) but was >20% in all studies for other outcomes. Only one of the five included studies15 reported all the outcomes at follow-up that had been reported at the end of the original intervention study.
Effects of intervention
Mortality
Four studies15–18 reported data on 2803 participants for all-cause mortality at follow-up between 4.7 and 6 years. Murchie et al16 reported a significantly reduced risk ratio for the Scottish study, but the other three studies showed a non-significant reduced risk ratio favouring the intervention. Meta-analysis of all-cause mortality data produced a risk ratio of 0.79 (95% CI = 0.66 to 0.93) in favour of the intervention (Figure 3). This suggests that organisational interventions were associated with a reduction of overall risk of death of approximately 20%, a finding that is strengthened by the fact that no measurable statistical heterogeneity between studies was detected (τ 2 = 0.00; P = 0.90, I2 = 0%).
Figure 3.
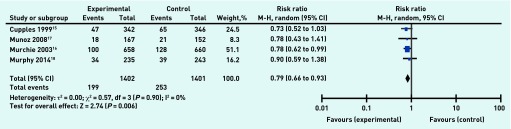
Organisational intervention versus usual care: all-cause mortality, 4.7–6 years.
Data on cause of death were reported or available from authors for the four studies. Cause of death was classified as cardiac, defined as death from any coronary or cardiovascular cause, or non-cardiac. One study15 showed a significantly reduced risk ratio favouring the intervention, but the other three studies showed a non-significant reduced risk ratio favouring the intervention. Meta-analysis of data on 2765 participants for cardiac-related mortality produced a risk ratio of 0.74 (95% CI = 0.58 to 0.94) in favour of the intervention (Figure 4). This suggests that organisational interventions were associated with a 26% reduction in risk of cardiac-related death, a finding that is strengthened by the measurable statistical heterogeneity detected between the studies (τ 2 = 0.01; P = 0.33, I2 = 12%).
Figure 4.
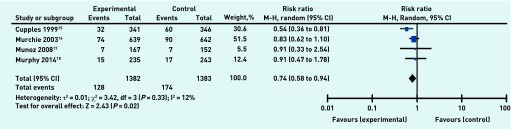
Organisational intervention versus usual care: death from cardiac causes, 4.7–6 years.
Meta-analysis of data from two studies19,20 including 1346 participants at 10-year follow-up indicated no statistically significant difference between the two groups for all- cause mortality (RR 0.91, 95% CI = 0.75 to 1.09) (Figure 5), with almost negligible statistical heterogeneity (τ 2 = 0.01; P = 0.31, I2 = 3%), or for cardiac-related mortality (RR 0.95, 95% CI = 0.79 to 1.16).
Figure 5.
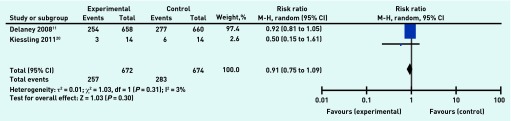
Organisational intervention versus usual care: all-cause mortality, 10 years.
Hospital admissions
Two studies provided data on admissions at long-term follow-up. Murphy et al13 originally reported a significant reduction in mean hospital admissions at the end of an 18-month organisational intervention, but this difference between groups was not sustained at 6-year follow-up.18 Delaney reported no significant difference in the total number of hospital admissions between groups after 10 years.19 It was not possible to pool data on hospital admissions.
Risk factor management and prescribing for secondary prevention
Meta-analysis of data from three studies15,16,18 showed that organisational interventions were not associated with a significant change in numbers with blood pressure (RR 0.95, 95% CI = 0.83 to 1.09) or cholesterol (RR 0.98, 95% CI = 0.89 to 1.07) within target levels (Table 2). Additional meta-analyses showed no significant changes in mean systolic blood pressure or mean total cholesterol associated with the interventions (Table 2). Only one study15 reported on smoking status at follow-up, self-reported current smoker (Yes/No) confirmed using a validated measurement, with no significant difference between the two groups.
Table 2.
Summary of meta-analysis results: categorical and continuous outcomes
| Outcome | Included studies, n | Intervention | Control | Risk ratio M-H, random (95% CI) | Heterogeneity, I2 | |||
|
|
|
|||||||
| Events | Total | Events | Total | |||||
| At 4.7–6 years: categorical outcomes | ||||||||
| Mortality | Mortality (all-cause) | 4 | 199 | 1402 | 253 | 1401 | 0.79 (0.66 to 0.93) | 0% |
|
| ||||||||
| Cardiac cause of death | 4 | 128 | 1384 | 174 | 1385 | 0.74 (0.58 to 0.94) | 12% | |
|
| ||||||||
| Risk factor management | BP within guidelines | 3 | 624 | 1016 | 623 | 1005 | 0.95 (0.83 to 1.09) | 61% |
|
| ||||||||
| Total cholesterol within guidelines | 3 | 417 | 950 | 397 | 879 | 0.98 (0.89 to 1.07) | 0% | |
|
| ||||||||
| Secondary prevention prescribing | Aspirin prescribed | 3 | 687 | 958 | 631 | 939 | 1.06 (1.00 to 1.12) | 39% |
|
| ||||||||
| Statins prescribed | 2 | 212 | 472 | 213 | 493 | 1.09 (0.77 to 1.53) | 75% | |
|
| ||||||||
| At 10 years: categorical outcomes | ||||||||
| Mortality | Mortality (all-cause) | 2 | 257 | 672 | 283 | 674 | 0.91 (0.75 to 1.09) | 3% |
|
| ||||||||
| Cardiac cause of death | 2 | 152 | 672 | 160 | 674 | 0.95 (0.79 to 1.16) | 0% | |
|
| ||||||||
| At 4.7–6 years: continuous outcomes | ||||||||
| Outcome | Included studies, n | Intervention, n | Control, n | Mean differenceb (95% CI) | Heterogeneity, I2 | |||
|
| ||||||||
| Risk factor management | Mean difference systolic BP (mmHg) | 2 | 469 | 466 | 1.94 (−3.02 to 6.91) | 70% | ||
|
| ||||||||
| Mean difference total cholesterol (mmol/l) | 2 | 362 | 358 | 0.03 (−0.12 to 0.18) | 0% | |||
BP = blood pressure.
Mantel-Haenszel, random effects.
Inverse variance, random effects.
Meta-analysis of data from three studies15,16,18 and two studies15,18 showed no significant change at follow-up in prescribing of aspirin (RR 1.06, 95% CI = 1.00 to 1.12) or statins (RR 1.09, 95% CI = 0.77 to 1.53), respectively, associated with the interventions. As illustrated in Table 2, there was substantial clinical and statistical heterogeneity in some of the analyses around risk factor management and prescribing, so these results need to be interpreted with caution.
Patient-reported health status was measured in three studies, with none showing a significant difference between groups at follow-up.15,18,21 Meta-analysis was not possible because of differences between measurement scales.
DISCUSSION
Summary
This review considered whether cardiac secondary prevention programmes that targeted organisational change in primary care or community settings were associated with sustained improvements in patient outcomes. There were statistically significant differences in all-cause and cardiac-related mortality between intervention and control patients at follow-up over a 4.7–6 year time period, with approximately 20% reduction in all-cause mortality and 26% reduction in risk of cardiac-related death in intervention patients.
Only two studies provided follow-up data at 10 years and no significant reduction in mortality was observed. However, given this small number of studies it is not possible to say whether this relates to dissipation of the intervention effect on mortality over time or that this analysis was underpowered to detect a difference.
There were no significant differences in risk factor management or prescribing for secondary prevention over the 4.7–6 year follow-up period, and the one study that had originally reported significant reductions in hospital admissions found no significant differences at follow-up. After a minimum follow-up period of 4.7 years in the studies included in this review, substantial numbers of intervention patients with known IHD have blood pressure and total cholesterol above target levels (blood pressure 38% above target; total cholesterol 55% above target). However, differences between included studies in the study populations, the intervention components, and risk factor target levels over time make interpretation of the risk factor and prescribing data difficult; this also suggests that other factors contribute to the apparent improved survival but there is insufficient evidence available to reach any conclusion regarding other factors, such as diet or exercise.
Strengths and limitations
This systematic review was conducted using Cochrane methodology and reported using the PRISMA guidelines.8,22 The protocol was published on the PROSPERO register. Potential limitations relate to generalisability of findings, as the populations included in the trials were predominantly males with a mean age range of 62–67 years and located in Western Europe, and to the clinical heterogeneity of interventions. In addition, the small number of included studies did not allow for sensitivity analysis based on risk of contamination of controls.
In general, there is insufficient evidence to determine how long a service organisation intervention can continue to have an impact after the intervention has ended, the impact of contamination because of cross-over during the follow-up periods, and the changes in usual care practices over the follow-up period.
Implications for policy and research
This review raises important questions about components and sustainability of effects of organisational interventions for secondary prevention of IHD. There was considerable heterogeneity in interventions in the included studies and many studies did not describe exact details of intervention components as has been recommended recently.23 Most studies included pre-planned appointments and patient education, although the intensity of both varied between studies. Therefore, there is limited information about how these interventions brought about changes in patient outcomes and the process of care. Although the interventions did vary, this reflects what would happen in practices if a health system introduced secondary cardiovascular prevention programmes with an organisational focus. This is consistent with the pragmatic nature of the studies included in this review.
This review suggests that organisational interventions are associated with clinically important outcomes in relation to mortality. The data included suggest that further improvements in risk factor management for those with levels above guideline targets is possible and could improve outcomes further. They indicate that mortality benefits occur for at least 4–6 years after formal cessation of interventions, despite potential contamination of control patients, suggesting that resources supporting targeted interventions over shorter time periods can lead to sustained benefits that persist beyond the duration of the interventions.
Further research to measure the long-term impact of service organisation interventions on hospital admissions for IHD is recommended, especially in the current climate of increased costs of inpatient care and constrained budgets.
Therefore, there is limited information about causal pathways between interventions aimed at behavioural change and outcomes. Future research should test specific theories about how interventions work24 in terms of effecting patient and practitioner behaviour change. It is also necessary to identify for how long these behavioural changes persist without ongoing or intermittent exposure to interventions.
Cardiac secondary prevention programmes appear to have an impact on mortality in the longer-term, even after the formal cessation of such programmes and in spite of potential contamination of original control groups because of cross-over. This provides reassurance to practitioners that current efforts in secondary prevention are worthwhile. Consideration should be given to targeting secondary prevention programmes at populations with persistently high levels of risk and where the current level of care is suboptimal.
Acknowledgments
Thanks to Dr Neil Campbell and Dr Miguel Angel Muñoz Pérez who provided extra data for their studies, and to all other authors who provided additional information.
Appendix 1. Search strategy
The authors searched CENTRAL (The Cochrane Library, Issue 12, December 2012), MEDLINE® on OVID (including MEDLINE® Daily Update 22 January 2013 and Ovid MEDLINE® In-Process & Other Non-Indexed Citations 22 January 2013), EMBASE on Ovid (January 2007 to January 2013) and CINAHL on EBSCO (January 2007 to January 2013). The search was based on that used in the Cochrane Review in 2010, ENREF 5 modified to reflect the focus in this review on long-term outcomes. The search was limited to articles published from 2007 onwards to cover the period following that review. No language or other limitations were imposed. Appendix 2 contains the MEDLINE search strategy. Contact was made with authors of studies included in the Cochrane Review ENREF 5 and authors of studies published since 2007 that met the inclusion criteria apart from the requirement for follow-up, to establish whether any long-term follow-up data had been collected but had not yet been published.
| 1 | exp Myocardial Ischemia/ | 335 677 | 46 | (ambulatory adj care).tw. | 6246 |
| 2 | Heart Diseases/ | 52 711 | 47 | (discharg$ adj3 program$).tw. | 617 |
| 3 | angina.tw. | 42 059 | 48 | (practice adj guideline).tw. | 2428 |
| 4 | (heart adj3 disease$).tw. | 120 550 | 49 | (discharg$ adj3 planning).tw. | 2134 |
| 5 | (coronary adj3 disease$).tw. | 101 291 | 50 | (comprehensive adj3 care).tw. | 5669 |
| 6 | myocardial infarct$.tw. | 133 877 | 51 | (treatment adj3 plan$).tw. | 38 159 |
| 7 | exp Myocardial revascularization/ | 75 836 | 52 | (nurse$ adj3 led).tw. | 1862 |
| 8 | (coronary adj3 bypass$).tw. | 35 114 | 53 | (disease adj management).tw. | 6939 |
| 9 | cabg.tw. | 11 519 | 54 | multi-disciplin$.tw. | 2916 |
| 10 | (coronary adj3 angioplast$).tw. | 13 338 | 55 | multidisciplin$.tw. | 38 309 |
| 11 | ptca.tw. | 6062 | 56 | exp Communication/ | 333 646 |
| 12 | (heart adj3 infarct$).tw. | 5476 | 57 | (service$ adj3 organi$).tw. | 5122 |
| 13 | postmyocardial infarct$.tw. | 795 | 58 | Nurse’s Role/ | 30 889 |
| 14 | or/1–13 | 517 186 | 59 | Nurse Practitioners/ | 14 627 |
| 15 | delivery of health care/ | 59 683 | 60 | Community Health Services/ | 25 576 |
| 16 | patient care management/ | 2080 | 61 | Medical Audit/ | 13 932 |
| 17 | comprehensive health care/ | 5925 | 62 | Nursing Audit/ | 2908 |
| 18 | nursing process/ | 6198 | 63 | audit. tw. | 20 307 |
| 19 | exp nursing assessment/ | 29 817 | 64 | secondary prevention clinic$.tw. | 41 |
| 20 | exp patient care planning/ | 49 053 | 65 | general practi$.tw. | 57 322 |
| 21 | Patient-Centered Care/ | 8972 | 66 | primary care.tw. | 62 204 |
| 22 | “delivery of health care, integrated”/ | 7454 | 67 | reminder$.tw. | 6120 |
| 23 | exp managed care programs/ | 38 300 | 68 | family practic$.tw. | 7133 |
| 24 | disease management/ | 9340 | 69 | recall$.tw. | 41 338 |
| 25 | exp patient care team/ | 51 103 | 70 | (Nurse adj3 clinic$).tw. | 4408 |
| 26 | exp Primary Health Care/ | 71 178 | 71 | (secondary prevention adj3 intervention$).tw. | 231 |
| 27 | Physicians, Family/ | 14 346 | 72 | (secondary prevention adj3 program$).tw. | 396 |
| 28 | Family Practice/ | 58 725 | 73 | “Appointments and Schedules”/ | 6492 |
| 29 | Physicians, General Pract$/ | 1165 | 74 | appointment$.tw. | 11 880 |
| 30 | General Practice/ | 2144 | 75 | (follow$up or long$term or aftercare).ti,ab. | 22 777 |
| 31 | Reminder Systems/ | 1943 | 76 | or/15–75 | 1 048 082 |
| 32 | interdisciplinary communication/ | 8495 | 77 | randomized controlled trials/ | 82 540 |
| 33 | Guideline Adherence/ | 17 546 | 78 | random allocation/ | 75 988 |
| 34 | “Continuity of Patient Care”/ | 13 241 | 79 | double-blind method/ | 117 201 |
| 35 | home care services/ | 26 352 | 80 | single-blind method/ | 16 890 |
| 36 | home nursing/ | 7784 | 81 | (clin$ adj25 trial$).ti,ab. | 230 106 |
| 37 | ambulatory care/ | 33 910 | 82 | ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. |
119 106 |
| 38 | patient discharge/ | 17 015 | |||
| 39 | (manag$ adj3 care).tw. | 31 702 | 83 | research design/ | 72114 |
| 40 | (management adj3 program$).tw. | 10 433 | 84 | random$.ti,ab. | 623 725 |
| 41 | (case adj3 manag$).tw. | 12 226 | 85 | or/77–84 | 954 576 |
| 42 | (patient adj3 management).tw. | 20 622 | 86 | 14 and 76 and 85 | 2868 |
| 43 | (home adj3 intervention$).tw. | 1346 | 87 | exp animal/ not humans/ | 3 750 242 |
| 44 | (home adj3 care).tw. | 19 309 | 88 | 86 not 87 | 2866 |
| 45 | (home adj visit).tw. | 1247 | 89 | limit 88 to yr=”2007 – 2013” | 1086 |
| 90 | remove duplicates from 89 | 1078 |
Funding
This study was funded by the Health Research Board, Ireland. The funder had no part in the design of the study, the collection, analysis, and interpretation of the data, the writing of the report, and the decision to submit the article for publication (reference HRA_HSR/2011/12).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
All authors, with the exception of Brian Buckley, were investigators on the SPHERE follow-up study, and Margaret E Cupples was an investigator in the study conducted in Northern Ireland; both studies are included in this review. The authors declare no other competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol. 2013;168(2):934–945. doi: 10.1016/j.ijcard.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.British Cardiac Society. British Hypertension Society. Diabetes UK. HEART UK. Primary Care Cardiovascular Society. Stroke Association JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(Suppl 5):v1–52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAlister FA, Lawson FM, Teo KK, Armstrong PW. Randomised trials of secondary prevention programmes in coronary heart disease: systematic review. BMJ. 2001;323(7319):957–962. doi: 10.1136/bmj.323.7319.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005;143(9):659–672. doi: 10.7326/0003-4819-143-9-200511010-00010. [DOI] [PubMed] [Google Scholar]
- 5.Buckley BS, Byrne MC, Smith SM. Service organisation for the secondary prevention of ischaemic heart disease in primary care. Cochrane Database Syst Rev. 2010;(3):CD006772. doi: 10.1002/14651858.CD006772.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson MS, Yordy KD, Lohr K, Vanselow NA. Primary care: America’s health in a new era. Washington DC: National Academic Press; 1996. [PubMed] [Google Scholar]
- 7.Murphy E, Vellinga A, Murphy A, et al. Long-term outcomes of organizational interventions for the secondary prevention of ischaemic heart disease in primary care and community settings. 2012. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42012003136 (accessed 20 May 2015).
- 8.Higgins JPT, Green S, editors; Cochrane Collaboration, editor. Cochrane handbook for systematic reviews of interventions. The Cochrane Library. Version 5.1. [updated March 2011]. www.cochrane-handbook.org (accessed 20 May 2015).
- 9.Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. J Health Serv Res Policy. 2000;5(1):12–16. doi: 10.1177/135581960000500105. [DOI] [PubMed] [Google Scholar]
- 10.Cupples ME, McKnight A. Randomised controlled trial of health promotion in general practice for patients at high cardiovascular risk. BMJ. 1994;309(6960):993–996. doi: 10.1136/bmj.309.6960.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell NC, Ritchie LD, Thain J, et al. Secondary prevention in coronary heart disease: a randomised trial of nurse led clinics in primary care. Heart. 1998;80(5):447–452. doi: 10.1136/hrt.80.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiessling A, Henriksson P. Efficacy of case method learning in general practice for secondary prevention in patients with coronary artery disease: randomised controlled study. BMJ. 2002;325(7369):877–880. doi: 10.1136/bmj.325.7369.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy AW, Cupples ME, Smith SM, et al. Effect of tailored practice and patient care plans on secondary prevention of heart disease in general practice: cluster randomised controlled trial. BMJ. 2009;339:b4220. doi: 10.1136/bmj.b4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz MA, Vila J, Cabanero M, et al. Efficacy of an intensive prevention program in coronary patients in primary care, a randomised clinical trial. Int J Cardiol. 2007;118(3):312–320. doi: 10.1016/j.ijcard.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Cupples ME, McKnight A. Five year follow up of patients at high cardiovascular risk who took part in randomised controlled trial of health promotion. BMJ. 1999;319(7211):687–688. doi: 10.1136/bmj.319.7211.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murchie P, Campbell NC, Ritchie LD, et al. Secondary prevention clinics for coronary heart disease: four year follow up of a randomised controlled trial in primary care. BMJ. 2003;326(7380):84. doi: 10.1136/bmj.326.7380.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz MA, Subirana I, Ramos R, et al. Eficacia de un programa intensivo de prevencion secundaria de cardiopatia isquemica tras 5 anos de intervencion [Efficacy of an intensive prevention programme of coronary heart disease: 5 year follow-up outcomes] Med Clin (Barc) 2008;130(14):521–525. doi: 10.1157/13119713. [DOI] [PubMed] [Google Scholar]
- 18.Murphy AW, Cupples ME, Vellinga A, et al. Six year follow-up of the SPHERE cluster randomised controlled trial: effect of tailored practice and patient care plans on secondary prevention of heart disease in general practice; NAPCRG; 11 November; Ottawa, Canada: 2014. [Google Scholar]
- 19.Delaney EK, Murchie P, Lee AJ, et al. Secondary prevention clinics for coronary heart disease: a 10-year follow-up of a randomised controlled trial in primary care. Heart. 2008;94(11):1419–1423. doi: 10.1136/hrt.2007.126144. [DOI] [PubMed] [Google Scholar]
- 20.Kiessling A, Lewitt M, Henriksson P. Case-based training of evidence-based clinical practice in primary care and decreased mortality in patients with coronary heart disease. Ann Fam Med. 2011;9(3):211–218. doi: 10.1370/afm.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murchie P, Campbell NC, Ritchie LD, et al. Effects of secondary prevention clinics on health status in patients with coronary heart disease: 4 year follow-up of a randomized trial in primary care. Fam Pract. 2004;21(5):567–574. doi: 10.1093/fampra/cmh514. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michie S, Webb TL, Sniehotta FF. The importance of making explicit links between theoretical constructs and behaviour change techniques. Addiction. 2010;105(11):1897–1898. doi: 10.1111/j.1360-0443.2010.03161.x. [DOI] [PubMed] [Google Scholar]