Abstract
This meta-analysis was aimed to assess the diagnostic performance of acoustic radiation force impulse (ARFI) elastography for the differentiation of malignant and benign breast lesions. The databases of PubMed, Web of ScienceTM, WanFang, Vip, SinoMed and China National Knowledge Infrastructure were searched for all studies that evaluated the diagnostic performance of ARFI including virtual touch tissue quantification (VTQ) and virtual touch tissue imaging (VTI). All the studies were published prior to Mar. 21, 2014. The studies published in English or Chinese were collected. A total of 11 studies, including 1,408 breast lesions from 1,245 women, were analyzed. The values of summary sensitivity and summary specificity were 0.843 (95% confidence interval [CI]: 0.811-0.872) and 0.932 (95% CI: 0.913-0.948) for VTQ of ARFI, and 0.864 (95% CI: 0.799-0.914) and 0.882 (95% CI: 0.832-0.922) for VTI of ARFI, respectively. Subgroup analysis excluding mucinous carcinoma and carcinoma in situ showed higher summary sensitivity (0.877 95% CI: 0.835-0.911), higher summary specificity (0.943 95% CI: 0.921-0.960) and lower heterogeneity (I2=23.5%). The cut-off values for shear wave velocity of VTQ ranged widely from 2.89 to 6.71 m/s, while the VTI ranged narrowly from 1.37 to 1.66. In general, ARFI elastography seems to be a good method for differentiation between benign and malignant breast lesions. However, its usefulness for identifying breast mucinous carcinoma and breast carcinoma in situ is limited. VTI seems to be more reliable and repeatable than VTQ.
Keywords: Breast lesion, acoustic radiation force impulse, ultrasound, elastography, meta-analysis
Introduction
Breast cancer is the leading cause of cancer-related death among females in economically developing countries [1]. Many studies have shown that early diagnosis of breast cancer can improve the prognosis of patients. The Breast Imaging Reporting and Data System (BI-RADS) [2,3] has been widely used to stratify the malignancy risk of breast lesions. Compared with mammography, magnetic resonance imaging (MRI) and biopsy, ultrasound (US) remains an important modality due to its advantages such as free of radiation, low cost, easy-performance and non-invasiveness. Over the past 10 years, US elastography has been introduced into clinical practice for the diagnosis of breast cancer. US elastography can provide qualitative and semi-quantitative information about the stiffness of breast lesions, which is useful for identifying malignant and benign breast lesions [4-7]. The principle of US elastography is that the stiffness of malignant lesions is greater than that of benign ones. However, it did not suitable for all breast lesions especially some “soft cancers”, since it would lead to false negative results. Recently, a quantitative elastography has been developed on the basis of acoustic radiation force impulse (ARFI) technology [8-18]. It could generate and trace wave propagation and tissue displacement to calculate shear wave velocity (SWV). ARFI elastography has two modes: the tissue displacement at longitudinal direction provides a qualitative response for virtual tissue imaging (VTI), which measures qualitatively by the area ratio (AR); and a quantitative response for virtual touch tissue quantification (VTQ), which measures transverse SWV values in m/sec. Compared with previous conventional strain elastography, ARFI could evaluate the breast tissue stiffness without external compression and provide qualitative and quantitative information to help differentiation between malignant and benign breast lesions [19-22].
As increasing literatures regarding the value of ultrasound elastography for the differentiation of breast lesions have emerged, thus it is necessary to make a summary of the current US elastography techniques. Sadigh et al. 2012 [7] evaluated the diagnostic performance of two different strain measurements in ultrasound elastography, which contains strain ration and length ration [7]. Li et al. 2013 [6] have performed a meta-analysis on shear wave elastography containing supersonic shear imaging (SSI) and acoustic radiation force impulse (ARFI), aiming to evaluate its ability for differentiation between benign and malignant solid breast lesions [6]. However, to the best of our knowledge, no meta-analysis only focusing on ARFI in the diagnosis of breast lesions has been performed. Therefore, we conducted this meta-analysis to evaluate the performance of ARFI in the diagnosis of breast cancer.
Materials and methods
Eligibility criteria
We collected the studies that evaluated the breast lesions using ARFI elastography and all the studies were published before Mar. 21, 2014. These studies used postoperative pathology or US guided core needle biopsy or vacuum-assisted biopsy as reference standard for diagnosis. No matter published in English or Chinese, all included studies provided necessary data to calculate the true positive (TP), false positive (FP), true negative (TN) and false negative (FN) diagnostic results of ARFI elastography for the differentiation of benign and malignant breast lesions based on the cut-off values. The studies that reported necessary data but failed to perform the statistical analysis were excluded. There was no limit on the age and sex. To obtain good reliability, the studies included less than 30 patients and those studies with overlapping patient samples were also excluded.
Data sources and searches
We searched the databases of PubMed, the Cochrane Library, the Web of ScienceTM, WanFang, Vip, SinoMed and China National Knowledge Infrastructure for studies that evaluated the diagnostic performance of ARFI for breast lesions (Figure 1). The following keywords were used: “acoustic radiation force impulse”, “ARFI” “virtual touch tissue quantification”, “virtual touch tissue imaging”, “breast”, “breast neoplasms”, “breast lesions”, “breast cancer”, “breast carcinoma”, mammary, “mammary neoplasms”, “mammary lesions”, “mammary cancer”, “mammary carcinoma”.
Figure 1.
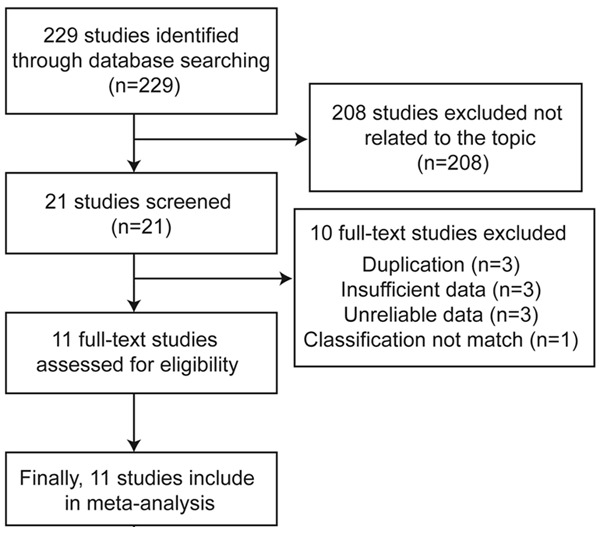
Flow chart of study selection.
Study selection and data extraction
Two authors independently assessed the eligible studies twice at different times. All eligible studies for the synthesis were retrospective studies. The following data were extracted: country of origin, year of publication, number of patients, number of breast lesions available for analysis, rate of malignant breast lesions, histological type of the lesions, reference standard for the diagnosis, SWV cut-offs, and BI-RADS categories of breast lesions. TP, FP, TN and FN were extracted allowing the calculation of sensitivity and specificity for each reported test threshold (Table 1).
Table 1.
Main characteristics of the studies evaluating the performance of ARFI for the differentiation of breast lesions
| Author year | Studies | Country | No. | Age (years) | size (mm) | Malignant rate (%) | Lesions types | ARFI cut off | BIRADS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| patient | lesions | SWV (m/s) | AR | ||||||||
| Bai 2012 | [8] | China | 108 | 143 | 44 (19-87) | 8-41 | 28.70% | FA, IDP, AS, IDC, DCIS, NC, basal-like carcinoma | 3.065 | - | ALL |
| Gong 2013 | [18] | China | 72 | 90 | 47 (19-76) | _ | 42.2 | FA, ILC, DCIS, MC | 6.2 | - | ALL |
| Jin 2012 | [9] | China | 95 | 122 | 43.5 (18-69) | 6.4-38.8 | 45.9 | BPT, PA, AS, HN,FA, MC, MPT, MEC, ILC, IDC, DCIS | 3.65 | 1.37 | 4 |
| Li 2013 | [15] | China | 68 | 75 | 40.8 (27-61) | 8.2-46.3 | 45.3 | IDC, ILC, MEC, FA, HN, IDP | 6.45 | 1.53 | 4 |
| Meng 2011 | [10] | China | 86 | 76 | 45.6 (17-78) | 7-46 | 35.5 | FA, FM, PA; IDC, PC, ILC, Paget’s disease | 6.37 | 1.54 | ALL |
| Ou 2013 | [17] | China | 103 | 126 | 36.9 (19-73) | 4.5-38 | 18.3 | FA, FM, IDP, BPT, INF, IDC, DCIS, NC, MC, MPT, PC | 6.64 | - | ALL |
| Tamaki 2013 | [11] | Japan | 180 | 182 | 55 (23-91) | 0-60 | 85.7 | IDC, DCIS, MC, ILC, FA, FCC, IDP, BPT | 2.89 | - | ALL |
| Tozaki 2012 | [12] | Japan | 158 | 161 | 52 (26-80) | 4.5-33 (M) 4.3-31 (B) | 56.5 | HN, HN, complicated cyst, IDC, MC, FA, IDP, BPT, DCIS, ILC | 3.59 | - | ALL |
| Xiong 2012 | [16] | China | 90 | 102 | 42 (18-65) | 8-61 | 36.3 | FA, IDP, HN, INF | 6.71 | 1.66 | ALL |
| Zhang H 2013 | [14] | China | 175 | 227 | 38 (16-75) | 8-40 (M) 6-48 (B) | 21.1 | FA, AS, IDP, INF, IDC, ILC, spindle cell tumor, IDC | 3.29 | - | ALL |
| Zhang L 2013 | [13] | China | 110 | 104 | 40 (18-71) | 7-52 | 28.8 | HN, FA, IDP, INF, BPT, AS, cysts, MC, DCIS | 3.655 | - | ALL |
IDC: invasive ductal carcinomas; DCIS: ductal carcinomas in situ; ILC: invasive lobular carcinoma; MEC: medullary carcinoma; MC: mucinous carcinoma; MPT: malignant phyllodes tumor; NC: neuroendocrine carcinoma; PC: papillocarcinoma; FA: fibroadenoma; AS: adenosis; IDP: intraductal papilloma; FM: fibrocystic mastopathy; PA: papilloma; BPT: benign phyllodes tumor; HN: hyperplastic nodule; INF: inflammation; FCC: fibrocystic change.
Quality assessment
The quality of the studies was assessed using the Quality Assessment of Studies on Diagnostic Accuracy included in Systematic Review (QUADAS-2 tool) (Figure 2), which was designed to assess the quality of primary diagnostic accuracy of studies. To judge the bias and applicability, the first part of each domain was answered as “yes”, “no”, or “unclear”. Applicability sections were structured in a similar way to the bias sections [22-24].
Figure 2.
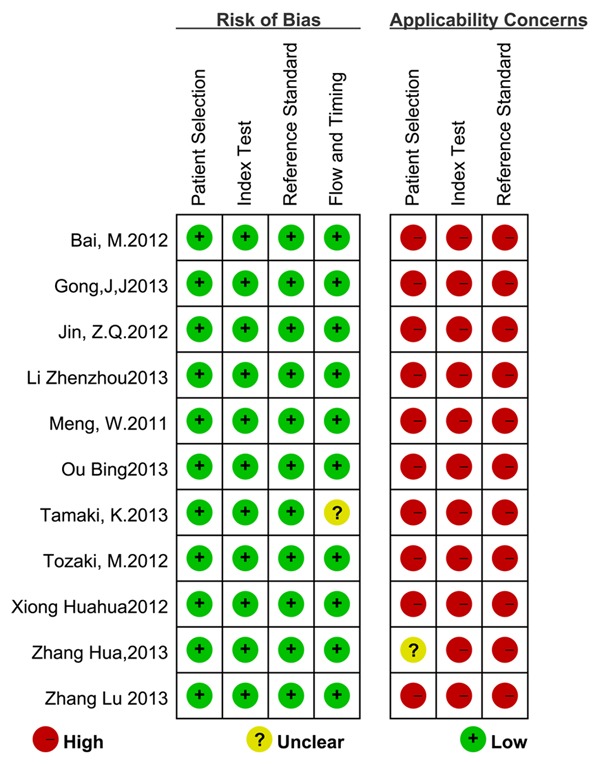
Quality assessment of included studies using QUADAS-2.
Data synthesis and analysis
If not specified, data were reported with the mean value and 95% confidence intervals (CIs). Due to the different cutoff values of SWV and AR in individual study, firstly, we examined the threshold effects of all data. If the threshold effect was not statistically significant, then we can carry out synthesis. We used sensitivity (the proportion of test positives among reference standard positives) and specificity (the proportion of test negatives among reference standard positives) as standard measures and diagnostic odds ratio as a global measure of diagnostic test accuracy. A summary receiver operating characteristic (SROC) curve was also plotted to graphically present the results. Random effects models were explored to account for variability among studies and the study heterogeneity was evaluated by I2 computing and Q statistics. When the value of I2 exceeds 70%, the data of studies cannot be directly merged and subgroup analysis is necessary. All I2 values of subgroup analysis were presented in line graph (Figure 3).
Figure 3.
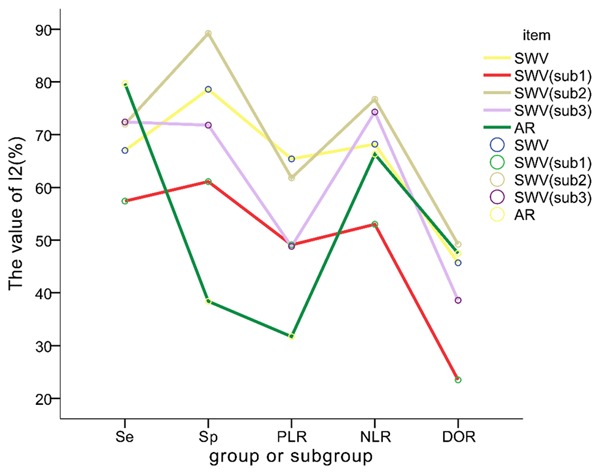
The value of I2 of each group or subgroup. Yellow line stands for the I2 value of the group SWV, which contains all 11 studies. Red line stands for the I2 value of the group SWV (sub1), which contains all 7 studies that exclude studies containing both carcinoma in situ and mucinous carcinoma. Brown line stands for the I2 value of the group SWV (sub2), which includes 4 studies containing both carcinoma in situ and mucinous carcinoma. Violet line stands for the I2 value of the group SWV (sub3), which contains all 9 studies that exclude studies obtaining breast lesions from the BI-RADS category 4. Green line stands for the I2 value of the group AR, which contains all 4 studies. It is shown that the red line representing the subgroup named SWV (sub1) decreases significantly and its position is lower than other lines, which indicates the type of breast lesions has a significant effect on diagnosis performance of VTQ method. We can predict that the diagnosis performance of VTQ for the breast lesions without either carcinoma in situ or mucinous carcinoma has better repeatability.
All the pooled indexes (pooled sensitivity, pooled specificity and pooled ROC) in our study were analyzed by the random effect model, because the values of I2 were lower than 70% and higher than 25%. Two-tailed P < 0.05 indicated a statistically significant difference. All statistical analysis was performed by Review Manager (RevMan) (Version 5.2 .7), Meta-disc (Version 1.4, Javier Zamora) and SPSS (Version 20.0) softwares for Windows.
Results
On the basis of the described searching strategies, a total of 21 studies were retrieved. After evaluation, 3 were excluded for the duplication of results, 6 were excluded either because the authors did not provide all the necessary data or the reference standard was not the gold standard. Another 1 was excluded because the classification of VTQ did not match our study. Finally, 11 studies (all full-length) were included, including 1,245 patients [8-12] (Figure 1).
The main characteristics of the studies included in the meta-analysis are summarized in Table 1. We presented the risk of bias and the concerns regarding the applicability of the studies included in this meta-analysis (Figure 2). A total of 1,408 breast lesions in 1,245 patients, consisting of 581 malignant and 827 benign lesions, were evaluated.
All the results of the studies
The diagnostic accuracy of VTQ for the differentiation between benign and malignant breast lesions was evaluated. All included studies had tested the usefulness of SWV (measured in m/s). The summary sensitivity was 0.843 (95% CI: 0.811-0.872) and the summary specificity was 0.932 (95% CI: 0.913-0.948) (Figure 4). The summary positive likelihood ratio (LR+) analyzed using the random effects model was 10.868 (95% CI: 6.971-16.945), while the summary negative likelihood ratio (LR-) analyzed by the random effects model was 0.150 (95% CI: 0.100-0.223). The summary diagnostic odds ratio was 87.237 (95% CI: 48.445-157.0). The area under the curve (AUC) of SROC was 0.955 (Figure 5).
Figure 4.
Summary sensitivity and Summary specificity of SWV. A and B. Displayed the sensitivity and specificity of the group SWV that including all 11 studies. C and D. Displayed the sensitivity and specificity of the the group SWV(sub1), which contained all 7 studies that apart from studies containing both carcinoma in situ and mucinous carcinoma.
Figure 5.
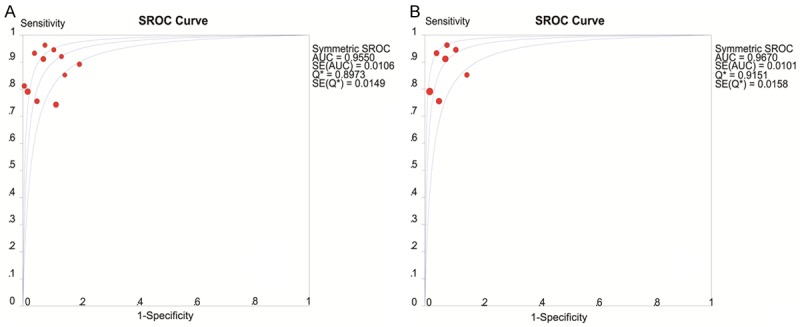
SROC curve of SWV for diagnosis performance of breast lesions. A. Was the SROC curve of the group SWV and it was shown that the area under the curve (AUC) was 0.956. B. Stands for the SROC curve of the group SWV (sub 1) and the AUC was 0.97. Both of the AUCs were in the high level.
The diagnostic accuracy of VTI for the differentiation between benign and malignant breast lesions was also evaluated. Only four of the included studies had reported the AR (non-dimensional). The summary sensitivity was 0.864 (95% CI: 0.799-0.914) and the summary specificity was 0.882 (95% CI: 0.832-0.922). The summary positive likelihood ratio (LR+) analyzed by random effects model was 6.970 (95% CI: 4.483-10.835), while the summary negative likelihood ratio (LR-) analyzed by the random effects model was 0.147 (95% CI: 0.062-0.344). The summary diagnostic odds ratio was 56.117 (95% CI: 19.514-161.38).
The results of the subgroups
Since some of I2 values were greater than 70%, all studies were divided into two subgroups after careful analysis of the disease spectrum. The studies containing both carcinoma in situ and mucinous carcinoma were classified as one subgroup named SWV (sub2); and the rest of the studies were classified as the other subgroup, which was called SWV (sub1). And then summary sensitivity (Figure 4), summary specificity (Figure 4), LR-, LR+ and summary diagnostic odds ratio of these studies were calculated respectively. All results are shown in Table 2. The SROC of SWV (sub2) is shown in Figure 5.
Table 2.
Subgroup analysis of the studies evaluating the performance of ARFI for the diagnosis of breast lesions
| Item | No. of study | Threshold effect | SSe (95% CI)11 | SSp (95% CI)11 | +LR (95% CI)11 | -LR (95% CI)11 | DOR (95% CI)11 | SROC’s AUC | Q (SDOR) | P (SDOR) | I2 (%) (SDOR) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SWV | 11 | no | 0.843 (0.811-0.872) | 0.932 (0.913 0.948) | 10.868 (6.971-16.945) | 0.150 (0.100-0.223) | 87.237 (48.445-157.09) | 0.955 | 18.4 | 0.049 | 45.7 |
| SWV (sub1) | 7 | no | 0.877 (0.835-0.911) | 0.943 (0.921-0.960) | 13.192 (8.303-20.962) | 0.138 (0.086-0.220) | 115.03 (61.976-213.49) | 0.967 | 7.84 | 0.25 | 23.5 |
| SWV (sub2) | 4 | no | 0.806 (0.754-0.851) | 0.907 (0.864-0.940) | 7.019 (3.363-14.651) | 0.173 (0.084-0.356) | 50.848 (18.223-141.89) | 0.935 | 5.19 | 0.116 | 49.2 |
| SWV (sub3) | 9 | no | 0.837 (0.801-0.869) | 0.948 (0.930 0.963) | 13.305 (8.528 20.758) | 0.145 ( 0.089-0.237) | 114.49 (59.992-218.51) | 0.965 | 13.03 | 0.111 | 38.6 |
| VTI | 4 | no | 0.864 (0.799-0.914) | 0.882 (0.832-0.922) | 6.970 (4.483-10.835) | 0.147 (0.062-0.344) | 56.117 (19.514-161.38) | 0.9585 | 5.72 | 0.126 | 47.5% |
SWV: shear wave velocity; VTI: virtual tissue imaging; SSe: summary sensitivity; SSp: summary specificity; +LR: positive likelihood ratio; -LR: negative likelihood ratio; DOR: diagnostic odds ratio; SROC: summary receiver operating characteristic; AUC: area under the curve; I2 inconsistency index; 95% CI: 95% confidence interval.
For the reason that the data of Jin et al. 2012 and Li et al. 2013 were obtained from the BI-RADS category 4 breast lesions, it was necessary to classify the other 9 studies as another subgroup, which was called SWV (sub3). The diagnostic accuracy of VTQ was analyzed one more time. The results were as follows: summary sensitivity, 0.837 (95% CI: 0.801-0.869); summary specificity, 0.948 (95% CI: 0.930-0.963); summary positive likelihood ratio (LR+) analyzed using the random effects model, 13.305 (95% CI: 8.528-20.758); summary negative likelihood ratio (LR-) analyzed by the random effects model, 0.145 (95% CI: 0.089-0.237); and summary diagnostic odds ratio (SDOR), 56.117 (95% CI: 19.514-161.38). The area under the curve (AUC) of summary receiver operating characteristic (SROC) curve was 0.965. All these results are also shown in Table 2.
Discussion
Breast cancer is one of the most frequently diagnosed cancers globally and also the main cause of cancer-related death among women [1]. AFRI, as a new US-based elastography, can provide quantitative and semi-quantitative measurements without invasiveness or radiation. In this meta-analysis, we evaluated the performance of ARFI in the differentiation of breast lesions. The results indicated that ARFI elastography has a high sensitivity and specificity for the diagnosis of malignant and benign breast lesions.
Analysis on all studies of VTQ and VTI
Due to the small sample size and high variation coefficients, the 11 included studies were all analyzed by random effects model, setting the value of α equal to 0.1 so as to increase the test performance. Except for the summary sensitivity of VTI or summary specificity of SWV, the Q value of summary sensitivity and summary specificity for other groups in this study were lower than 70%. Therefore, heterogeneity of the included studies was acceptable and the values of summary results were reliable. In this meta-analysis, the summary sensitivity and summary specificity were inspiriting. The values of summary sensitivity and summary specificity were 0.843 (95% CI: 0.811-0.872) and 0.932 (95% CI: 0.913-0.948) for VTQ, and 0.864 (95% CI: 0.799-0.914) and 0.822 (95% CI: 0.882-0.922) for VTI, respectively. However, test for heterogeneity still presented moderate heterogeneity (Table 3), which indicated that a subgroup analysis was necessary.
Table 3.
The value of I2 of all group or subgroup (%)
| Item | SSe | SSp | LR+ | LR- | SDOR |
|---|---|---|---|---|---|
| SWV | 67 | 78.6 | 65.4 | 68.2 | 45.7 |
| SWV (sub1) | 57.4 | 61.1 | 49.1 | 53 | 23.5 |
| SWV (sub2) | 72 | 89.2 | 61.8 | 76.7 | 49.2 |
| SWV (sub3) | 72.4 | 71.8 | 48.8 | 74.3 | 38.6 |
| VTI | 79.7 | 38.4 | 31.7 | 66.3 | 47.5 |
I2 inconsistency index; SWV: shear wave velocity; VTI: virtual tissue imaging; SSe: summary sensitivity; SSp: summary specificity; +LR: positive likelihood ratio; -LR: negative likelihood ratio; SDOR: summary diagnostic odds ratio.
Analysis on subgroups of SWV (sub1) and SWV (sub2)
It was difficult to detect breast carcinomas with small size by US. Therefore, the measurements of SWV or AR were also infeasible in these cases. The other fact is that breast mucinous carcinoma contains a lot of jelly-like substance and it is softer than other breast cancers though it is malignant. When four studies containing mucinous carcinoma and carcinoma in situ were excluded [9,11,17,18], the summary sensitivity, summary specificity, summary LR+, summary DOR of the SWV (sub1) increased. And the value of I2 decreased to 23.5%, which indicated that the heterogeneity of subgroup studies was low. It also indicated that the type of breast lesions had a significant influence on the diagnostic performance of VTQ method of ARFI elastography. The I2 value of all groups and the subgroups are shown in Figure 3. It was shown that the red line of SWV (sub1) decreased significantly and was lower than other lines. Therefore, we can predict that except for carcinoma in situ and mucinous carcinoma, the diagnostic performance of VTQ for other breast lesions is more valuable and has better repeatability.
Analysis on subgroups of SWV (sub3)
Considering the selected lesions from the studies of Jin et al. 2013 [9] and Li et al. 2013 [15] were in BI-RADS category 4, we did the statistical analysis on the remaining nine studies excluding these two studies. It showed low value of Q (13.03), high summary sensitivity and summary specificity and significant increase of summary positive likelihood ratio (LR+) and the summary diagnostic odds ratio (SDOR).
We also found that the cut-off values of SWV on VTQ from the included studies were in a wide range of 2.89 to 6.71 m/s, while the range of VTI AR cut-off values (from 1.37 to 1.66) was narrow. The difference of lesions in BI-RADS category and pathological spectrum, as well as the stiffness of breast parenchyma and adipose tissue content, has an unpredictable effect on the measurements of VTQ and VTI. However, the effect on VTQ was more obvious than on VTI. So we predict that VTI is more reliable than VTQ, despite the VTI-area ratio (AR) measurement is a semi-quantitative method while VTQ is a quantitative method.
Recently, two measurement methods of ARFI have been improved. One is Virtual Touch IQ (VTIQ), which is an improvement on the basis of VTQ. Compared with VTQ that just provides single point velocity data, VTIQ is a new two-dimensional shear wave imaging technique, which is capable of measuring multi-point shear wave speed [25]. VTIQ software synthesizes information from up to 256 sequential acquisition beam lines inside a two-dimensional user-defined region of interest and then displays a qualitative and quantitative map of shear wave velocities ranging from 0.5 to 10 m/s, as well as qualitative maps for shear wave quality, travel time and tissue displacement [26]. Both VTIQ and VTQ calculate the SWV by tracking the displacement of region of interest. Ianculescu et al. 2014 showed that the sensitivity and specificity of VTIQ were 92% and 64.6% respectively for assessing breast lesions, while Tozaki et al. 2013 reported that the sensitivity was 86% and the specificity was 90%. However, both of their studies showed that diagnostic performance of VTIQ did not have a significant advantage over VTQ for differentiation between benign and malignant breast lesions. The other measurement method is gray level quantification (GLQ) in virtual touch tissue imaging, which analyzes the gray levels ranging from 0 (pure black) to 255 (pure white) with matrix laboratory software named MATLAB [27]. Similar to VTI, GLQ also calculates the ratio of the elasticity image, but uses different calculation methods. In the study of Li et al. 2014, the sensitivity and specificity of GLQ for differentiation between benign and malignant breast lesions were 86.5% and 93.1% respectively at a cutoff value of 52.31, implying that VTI of ARFI technology has a great development potential.
Some limitations of this study should be taken into account. Unpublished studies and articles in other languages were not included in this study. Although just 11 eligible articles were included, the high value of Q suggested the possibility of heterogeneity biases. The body of evidence included a large number of studies conducted in Asia (China, Japan). It is known that different settings including clinical practice, device settings, and baseline risks are likely to lead to different results, [28] and affect synthesis of this meta-analysis. Therefore, the findings in this study must be interpreted cautiously. In our study, the publication biases cannot be eliminated and further study with more clinical cases is needed.
In general, ARFI elastography seems to be a good method for differentiation between benign and malignant breast lesions. However, it may not be applicable for identifying breast mucinous carcinoma and breast carcinoma in situ. Moreover, VTI seems to be more reliable and repeatable than VTQ.
Acknowledgements
This work was supported in part by Grant 14441900900 from Science and Technology Commission of Shanghai Municipality, and Grant 2012045 of Shanghai Talent Development Project from Shanghai Human Resource and Social Security Bureau.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS lexicon for US and mammography: interobserver variability and positive predictive value. Radiology. 2006;239:385–391. doi: 10.1148/radiol.2392042127. [DOI] [PubMed] [Google Scholar]
- 3.Vanel D. The American College of Radiology (ACR) Breast Imaging and Reporting Data System (BI-RADS): a step towards a universal radiological language? Eur J Radiol. 2007;61:183. doi: 10.1016/j.ejrad.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Hiltawsky KM, Kruger M, Starke C, Heuser L, Ermert H, Jensen A. Freehand ultrasound elastography of breast lesions: clinical results. Ultrasound Med Biol. 2001;27:1461–1469. doi: 10.1016/s0301-5629(01)00434-3. [DOI] [PubMed] [Google Scholar]
- 5.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, Yamakawa M, Matsumura T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Li DW, Fang YX, Song YJ, Deng ZJ, Gao J, Xie Y, Yin TS, Ying L, Tang KF. Performance of shear wave elastography for differentiation of benign and malignant solid breast masses. PLoS One. 2013;8:e76322. doi: 10.1371/journal.pone.0076322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadigh G, Carlos RC, Neal CH, Dwamena BA. Accuracy of quantitative ultrasound elastography for differentiation of malignant and benign breast abnormalities: a meta-analysis. Breast Cancer Res Treat. 2012;134:923–931. doi: 10.1007/s10549-012-2020-x. [DOI] [PubMed] [Google Scholar]
- 8.Bai M, Du L, Gu J, Li F, Jia X. Virtual touch tissue quantification using acoustic radiation force impulse technology: initial clinical experience with solid breast masses. J Ultrasound Med. 2012;31:289–294. doi: 10.7863/jum.2012.31.2.289. [DOI] [PubMed] [Google Scholar]
- 9.Jin ZQ, Li XR, Zhou HL, Chen JX, Huang X, Dai HX, Li JW, Chen XD, Xu XH. Acoustic radiation force impulse elastography of breast imaging reporting and data system category 4 breast lesions. Clin Breast Cancer. 2012;12:420–427. doi: 10.1016/j.clbc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Meng W, Zhang G, Wu C, Wu G, Song Y, Lu Z. Preliminary results of acoustic radiation force impulse (ARFI) ultrasound imaging of breast lesions. Ultrasound Med Biol. 2011;37:1436–1443. doi: 10.1016/j.ultrasmedbio.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Tamaki K, Tamaki N, Kamada Y, Uehara K, Miyashita M, Ishida T, Sasano H. A non-invasive modality: the us virtual touch tissue quantification (VTTQ) for evaluation of breast cancer. Jpn J Clin Oncol. 2013;43:889–895. doi: 10.1093/jjco/hyt098. [DOI] [PubMed] [Google Scholar]
- 12.Tozaki M, Isobe S, Sakamoto M. Combination of elastography and tissue quantification using the acoustic radiation force impulse (ARFI) technology for differential diagnosis of breast masses. Jpn J Radiol. 2012;30:659–670. doi: 10.1007/s11604-012-0106-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Zhou P, Deng J, Tian SM, Qian Y, Wu XM, Ma SH. Comparative Study of Convential Ultrasound and Acoustic Radiation Force Impulse (ARFI) in the Differential Dignosis of Benign and Malignant Lesions. Medical Innovation of China. 2013;10:100–103. [Google Scholar]
- 14.Zhang H, Ran H, Zhang P, Wang Z, Song W, You X. Acoustic radiation force impulse technology in quantitative differential disgnosis of benign and malignant breast lesions. Chin J Med Imaging Technol. 2013;29:407–410. [Google Scholar]
- 15.Li Z, Luo C, Liu Q, Chen S, Li QS, Zou X, Li Q. Acoustice radiation force impulse in differential diagnosis of benign and malignant BI-RADS 4 breast lesions. Chin J Med Imaging Technol. 2013;29:727–730. [Google Scholar]
- 16.Xiong H, LI Q, Chen S, Guo G, Luo C, Sun Y. Preliminary study of elasticity detection of benign and malignant breast lesions be acoustic radiation force impulse. Journal of Ultrasound in Clinical Medicine. 2012;14:266–269. [Google Scholar]
- 17.Ou B, Luo BM, Yang HY. Combination of BI-RADS and Virtual Tissue Quantification in Differentiating Benign or Malignant of Breat Lesions. Chinese J Ultrasound Med. 2013;29:321–323. [Google Scholar]
- 18.Gong JJ, Sun DS, Zhong JY, Zhang Y, Shi Y, Lu SK, Yu ZQ. Study of acoustic radiation force impulse technology in differential diagnosis of benign and malignant breast tumors. J Clin Ultrasound in Med. 2013;15:29–31. [Google Scholar]
- 19.Bouchard RR, Hsu SJ, Wolf PD, Trahey GE. In vivo cardiac, acoustic-radiation-force-driven, shear wave velocimetry. Ultrason Imaging. 2009;31:201–213. doi: 10.1177/016173460903100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003;29:1715–1723. doi: 10.1016/j.ultrasmedbio.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 22.Tanter M, Bercoff J, Athanasiou A, Deffieux T, Gennisson JL, Montaldo G, Muller M, Tardivon A, Fink M. Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound Med Biol. 2008;34:1373–1386. doi: 10.1016/j.ultrasmedbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Leeflang MM, Moons KG, Reitsma JB, Zwinderman AH. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clin Chem. 2008;54:729–737. doi: 10.1373/clinchem.2007.096032. [DOI] [PubMed] [Google Scholar]
- 24.Whiting PF, Rutjes AW, Westwood ME, Mallett S. A systematic review classifies sources of bias and variation in diagnostic test accuracy studies. J Clin Epidemiol. 2013;66:1093–1104. doi: 10.1016/j.jclinepi.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Tozaki M, Saito M, Benson J, Fan L, Isobe S. Shear wave velocity measurements for differential diagnosis of solid breast masses: a comparison between virtual touch quantification and virtual touch IQ. Ultrasound Med Biol. 2013;39:2233–2245. doi: 10.1016/j.ultrasmedbio.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Ianculescu V, Ciolovan LM, Dunant A, Vielh P, Mazouni C, Delaloge S, Dromain C, Blidaru A, Balleyguier C. Added value of Virtual Touch IQ shear wave elastography in the ultrasound assessment of breast lesions. Eur J Radiol. 2014;83:773–777. doi: 10.1016/j.ejrad.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Sun J, Zhang J, Hu D, Wang Q, Peng K. Quantification of acoustic radiation force impulse in differentiating between malignant and benign breast lesions. Ultrasound Med Biol. 2014;40:287–292. doi: 10.1016/j.ultrasmedbio.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Panagiotou OA, Contopoulos-Ioannidis DG, Ioannidis JP. Comparative effect sizes in randomised trials from less developed and more developed countries: meta-epidemiological assessment. BMJ. 2013;346:f707. doi: 10.1136/bmj.f707. [DOI] [PMC free article] [PubMed] [Google Scholar]



