Hepatitis D virus (HDV) can only infect those with hepatitis B virus (HBV). HBV vaccination efforts have controlled HDV to some extent, but HDV remains an issue for injecting drug users and those in endemic HDV areas.
Abstract
Hepatitis D is caused by the hepatitis D virus (HDV), a unique RNA pathogen that requires the hepatitis B surface antigen (HBsAg) to infect. Hepatitis D is transmitted by the parenteral route. The main susceptible group is patients with chronic HBsAg infection who become superinfected with the virus. Hepatitis D occurs throughout the globe, but control of hepatitis B virus (HBV) in the last two decades has consistently diminished the circulation of HDV in industrialized countries. However, hepatitis D remains a medical issue for injecting drug users (IDUs), as well as immigrants from endemic HDV areas, who are reintroducing the infection in Europe.
The 1970s were a time of great excitement in hepatology. The discovery of the hepatitis B surface antigen (HBsAg) opened the gate to the mysteries of viral hepatitis. The hepatitis B virus (HBV) virion and its nucleocapsid were characterized at the beginning of the 1970s. The discovery of the hepatitis A virus followed suit, providing an explanation for the two forms of viral hepatitis predicted by early epidemiological analysis. In contrast, the discovery of hepatitis D virus (HDV) was unexpected. The identification of this virus followed the description of the delta antigen–antibody system in carriers of the HBsAg in Torino, Italy in the mid-1970s (Rizzetto et al. 1977). The studies in Italy showed that delta antigen was distinct from the other known antigenic specificities of HBV, yet was associated with HBV infection because the HBsAg was also present in the blood of all patients with the new antigen.
Delta would have most likely been confused with the many subtypes of HBV that were described at the time, but fortunately the National Institutes of Health (NIH) in Bethesda, MD took interest and research moved in the late 1970s to the United States of America. With the availability of chimpanzees for transmission experiments and the expertise at the NIH, it was rapidly recognized that delta was not an antigen of HBV, but a new and unique human RNA virus, HDV. With a circular RNA genome of ∼1700 bases, HDV is the smallest infectious agent known in man (Rizzetto 1983). Eight genotypes have, so far, been identified. The most common in Europe, the Middle East, North America, and North Africa is genotype 1; genotype 2 is present in the Far East and genotype 3 has been found in the Amazon Basin (Le Gal et al. 2006; see Taylor 2015 for a more detailed discussion).
OVERVIEW
HDV is spread like HBV, that is, by parenteral exposure (Rizzetto et al. 1991). Direct parenteral inoculation is the most efficient way of transmission, in particular, in HBsAg-positive hosts. Infectivity titration studies in chimpanzees showed that the virus could be passaged to HBsAg-positive animals with HDV-positive serum diluted as much as 10−11-fold (Ponzetto et al. 1988). In contrast to HBV, vertical transmission from mother to offspring, homosexual promiscuity, or nosocomial exposure seem to be inconspicuous risk factors for the transmission of the HDV.
HDV is pathogenic (Smedile et al. 1994). Clinical studies from all continents have shown that HDV infection aggravates the natural history of the underlying HBV infection. Hepatitis D is considered the most severe form of viral hepatitis in humans, accelerating progression to cirrhosis and leading to early decompensation of liver function compared with HBV monoinfection (Rizzetto and Alavian 2013). In Greece, Samoa, and the Far East, HDV was also associated with benign clinical conditions or normal liver function, suggesting that disease expression may vary, possibly related to different HDV genotypes (Rizzetto and Ciancio 2012).
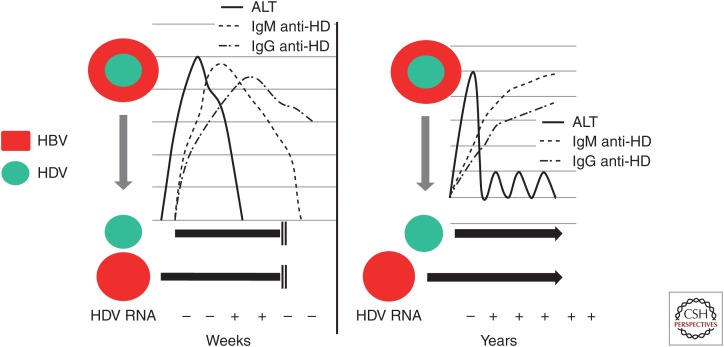
Diagnostic assays for the antibody to the hepatitis delta (HD) antigen (anti-HD) were rapidly developed after the discovery of HDV, their use expediting the understanding of the global epidemiology of hepatitis D. The concept of coinfection and superinfection, extrapolated from infectivity studies in chimpanzees, provided the rationale for interpreting the natural history of hepatitis D (Rizzetto et al. 1990). The performance of anti-HD as an epidemiologic marker varies depending on the clinical setting. In self-limited coinfections, serological responses are delayed and transitory (Fig. 1, left). In this setting, recognition of past infection is often impossible. Instead, superinfection of HDV in HBsAg carriers is followed by strong serological responses that persist over time, and can be detected in random blood samples of the superinfected patient (Fig. 1, right). Therefore, HBsAg carriers superinfected by HDV are the only reliable source of information on the epidemiology of HDV, and prevalence data should be limited to this category. Surveys should be oriented to HBsAg carriers with chronic liver disease. Healthy inactive carriers of the HBsAg are unlikely to have experienced an HDV superinfection, as this event would have changed their asymptomatic status into chronic liver disease.
Figure 1.
Virologic and serologic markers in coinfection (hepatitis D virus [HDV]/hepatitis B virus [HBV]) and superinfection (hepatitis B surface antigen [HBsAg]/HDV).
Many studies have reported prevalence rates of HDV among healthy HBsAg carriers as representative of local prevalence; the data are misleading as these individual are unlikely to harbor HDV. To be comparable, prevalence rates of HDV should refer to a denominator of HBsAg-positive liver disease. The failure to realize that rates of HDV determined in the general HBsAg population, or blood banks are not representative of the true prevalence of HDV infection may explain discrepancies reported in the epidemiology of hepatitis D throughout the world.
Surveys in the 1980s showed that HDV was present worldwide and its local prevalence was a function of the prevalence of the HBV (Rizzetto et al. 1991). However, although overlapping with HBV, the prevalence of HDV did not always coincide with that of HBV; in the Far East, rates of HDV were often low compared with a distinct prevalence of HBV. Variations in the penetration of HDV among HBsAg populations may be determined by differences in local social behaviors, the virulence of HDV, or the genetic susceptibility of the carrier.
At the end of the 1980s, it was estimated that ∼5% of HBsAg carriers were infected with HDV worldwide, corresponding to ∼15 million individuals, most of which had severe and progressive liver disease. Since then, the epidemiologic scenario of HDV has changed in industrialized countries. In the past 20 years, HBV vaccination, the improvement of sanitation and living conditions, and sexual restraints prompted by the risk of AIDS have led to the containment of HBV with a significant reduction of domestic networks of HBsAg carriers. Deprived of HBsAg, the circulation of HDV has markedly declined in the Western world. This decline has been age dependent, with rates of infection diminishing in younger HBsAg carriers, but not in older ones (Wedemeyer and Manns 2010; Rizzetto and Ciancio 2012).
At the end of the last century, the decline of HDV in Southern Europe led to the assumption that hepatitis D was no longer a relevant medical problem (Gaeta et al. 2000). This perception diminished awareness of the threat posed by HDV, so that testing for the virus has often been neglected in recent years (Cross et al. 2008; Holmberg and Ward 2010). However, hepatitis D has not vanished from Europe, but is on the rise because of immigration (Rizzetto 2009). Aging domestic populations of HDV patients are being replaced by immigrants from areas in which HDV remains endemic. Domestic patients are old, have a long-standing infection, and advanced liver disease. Immigrants with HDV infections are younger, have more recent HDV infections, and often show an aggressive hepatitis, such as described in Southern Europe in the 1980s (Farci 2003). The clinical features of hepatitis D have also changed. In Italy in the 1980s, most patients had a florid chronic hepatitis; by the end of 1990s, 70% had, instead, cirrhosis residual to burned-out inflammation (Rosina et al. 1999). In Barcelona, patients recruited from 1983 to 1995 were younger, had acquired HDV mainly by coinfection, were often injecting drug users (IDUs), and frequently coinfected by hepatitis C virus (HCV) and human immunodeficiency virus (HIV); patients recruited from 1996 to 2008 were older, with a higher proportion of immigrants, most with chronic hepatitis D acquired by superinfection (Buti et al. 2011).
The epidemic of HDV diminished also in IDUs, but the problem of hepatitis D in these patients remains widespread in Europe (Wedemeyer and Manns 2010; Rizzetto and Alavian 2013). The situation is puzzling in the United States. Although surveys in the 1980s–1990s showed a conspicuous presence of HDV in IDUs, no further survey was performed for the next 15 years, leading to the perception that HDV had disappeared. Recent studies have revived interest and awareness, leading to reconsideration of the impact of hepatitis D also in the United States.
In contrast to the control of HDV in the developed world, its epidemiology has not changed in regions of the developing world in which HBV remains unchecked. In recent years, awareness of hepatitis D has increased in many places in Asia and Africa, where facilities for testing have been implemented (Rizzetto and Ciancio 2012).
EPIDEMIOLOGY OF HDV AT THE FINGERTIPS IN THE 1980s–1990s
In the 1980s, initial surveys showed that HDV infections were endemic worldwide, but there were great variations in prevalence and some intriguing contrasts. In poor areas of South America, Africa, and India, HDV was transmitted primarily by superinfection as a secondary event in the context of HBV endemicity. In the Amazon basin, HDV occurred frequently in children and adolescents chronically infected early in life with HBV (Hadler et al. 1991). By adulthood, most of the HBsAg carriers had contracted HDV and many died in periodic outbreaks of fulminant hepatitis D. Unique to this area were infections sustained by HDV genotype 3 and liver histology, displaying a cytopathic noninflammatory process of liver microsteatosis. HDV continues to be an important health issue in the Brazilian Amazon despite the implementation of HBV vaccination in rural areas; in blood collected from HBsAg carriers along the banks of the Purus River in 2005–2006, the prevalence of anti-HD was 41.9% (Braga et al. 2012). Outbreaks of severe and fulminant hepatitis developing on a background of high-HBV endemicity have been reported in the last 20 years, also from Russia (Flodgren et al. 2000), Greenland (Børresen et al. 2010), and Mongolia (Tsatsralt-Od et al. 2006).
As expected, in the 1980s, the endemicity of HDV was low in regions of the world in which the prevalence of HBV was low, but it was low also in Japan, Korea, and Indonesia, where rates of HBV were, instead, conspicuous. Recent surveys have confirmed that the infection did not penetrate in Korea despite contemporary migratory exchanges and the facilitating role of a consistent population of HBsAg carriers at risk. The prevalence of anti-HD was only 0.32% in a mixed-hospital HBsAg population collected in the country from 2008 to 2010 (Kim et al. 2011). There has been no report of significant HDV also, in Japan, except for a high prevalence in the Mikayo Islands, Okinawa (Abbas et al. 2010), where up to 23% of the HBsAg carriers were found to be infected with HDV in the 1990s; the prevalence of HDV in the area had decreased to 8.5% in 2000 (Arakawa et al. 2000).
In the 1980s, epidemiological analyses focused primarily on the Mediterranean Basin and Taiwan, where facilities to investigate the extent and pattern of HDV infection became soon available, with Italy providing the prototype scenario. In these countries, the spreading of HDV resulted from an epidemic pattern in subjects at high risk, mainly the IDUs, and an endemic pattern in the general population. In IDUs sharing contaminated paraphernalia, HDV was acquired predominantly by coinfection, the virus transmitting from acute case to acute case through the recruitment of new drug users. In the general population, transmission occurred mainly by close and intimate contacts through superinfection from a reservoir of chronic HDV cases; cohabitation with a HDV carrier within an overcrowded family unit represented a major risk factor in Southern Italy (Sagnelli et al. 1992). In Italy, infection peaked in the fourth decade of life; males were more affected than females. In the early 1990s, in Taiwan, on a significant background of anti-HD in the general population (∼55% of anti-HD among HBsAg prostitutes), the infection reached an exceedingly high prevalence of 91% in HBsAg-positive IDUs (Rizzetto and Ciancio 2012).
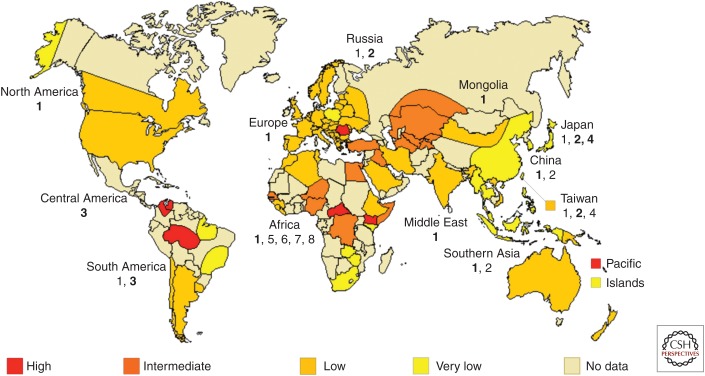
Throughout the 1980s, many other limited surveys were performed to establish local prevalences of HDV. Most studies considered only few cases, with different medical features. Information, therefore, was scattered. Often, prevalence rates were disparate and uneven also at regional levels; in Brazil, Saudi Arabia, Kenya, and China, pockets of hyperendemic infection were identified close to areas in which the prevalence of HDV was negligible (Rizzetto et al. 1991). Data were more consistent when analyses were performed by medical categories; clinical studies of chronic HBsAg hepatitis, and acute liver illnesses confirmed that hepatitis D was a major cause of cirrhosis and fulminant hepatitis worldwide. The main areas of HDV distribution globally are shown in Figure 2, in which the predominant HDV genotype for each geographical area is also shown.
Figure 2.
Schematic representation of the main areas of HDV globally onto which the predominant hepatitis D virus (HDV) genotype for each geographical area has been superimposed. (From Negro 2014; reproduced from © 2013 John Wiley and Sons.)
EPIDEMIOLOGICAL CHANGES IN THE LAST TWO DECADES IN EUROPE
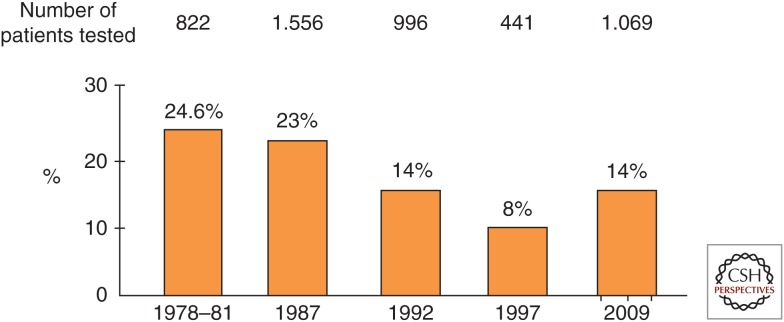
In 1983, the prevalence of anti-HD was 24.6% in carriers with liver disease in Italy. In a survey in 1987, the endemicity of HDV remained stable, with 23% of the carriers showing anti-HD and a 40% peak prevalence among cirrhotic patients. However, in two subsequent Italian surveys, the rate of anti-HD had declined to 14% in 1992 and 8.3% in 1997 (Rizzetto and Ciancio 2012). In Spain, rates of anti-HD declined from 15% in 1975–1985 to 7.9% in 1986–1992; in Taiwan, the rate of HDV superinfections diminished from 23.7% in 1983 to 4.2% in 1996. In Turkey, the prevalence of anti-HD in chronic HBsAg liver disease diminished from 31% to 11% in 1980–2005.
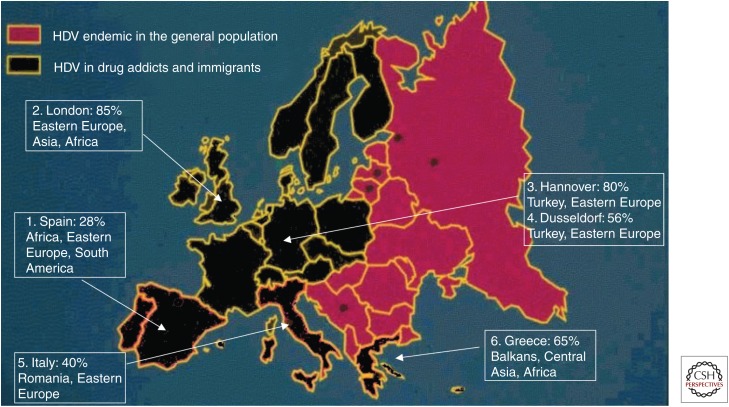
The prevalence of HDV has not diminished further in Europe. In blood samples collected from HBsAg patients in 2006–2007 in Italy, the overall prevalence of anti-HD remained 8.1% with no further reduction compared with 1997. However, among incident cases, the prevalence was 14.3%, suggesting the arrival of a new wave of HDV-infected individuals (Fig. 3) (Stroffolini et al. 2009). In a recent study in Italy, the prevalence of anti-HD was 8.4%; the antibody was found in 7.4% of Italians, but in 11.5% of immigrants (Brancaccio et al. 2014). In the last decade, rates of anti-HD also remained stable in Germany, England, and France. In Germany, 8%–10% of the HBsAg-positive patients had anti-HD; >75% were coming from Turkey, Eastern Europe, and the former Soviet Union (Wedemeyer and Manns 2010). In the years 2000–2006 in South London, the prevalence of anti-HD in ∼1000 carriers of the HBsAg with chronic liver disease was 8.5% (Cross et al. 2008); most of the patients were from Africa and Eastern Europe. The prevalence of HDV has increased over the last 15 years in blood donors in France (Servant-Delmas et al. 2014). At present, African immigrants account for the majority of the HDV population in this country (Le Gal et al. 2007). Immigrants also account for the larger proportion of chronic hepatitis D in Greece (Fig. 4) (Rizzetto and Ciancio 2012).
Figure 3.
Changes in the prevalence of anti-HD in HBsAg-carriers with liver disease in Italy; 2009 are incident cases.
Figure 4.
Epidemiology of hepatitis D virus (HDV) in Europe in 2012. Prevalence of immigrants among HDV+. (1) Data from Buti et al. (2011), (2) data from Cross et al. 2008, (3) data from Wedemeyer and Manns 2010, (4) data from Rizzetto and Ciancio 2012, and (5) data from Brancaccio et al. 2014.
HDV is present, also, in other countries of Northern Europe, but confined to risk groups, with a prevalence ∼5% in Austria, Ireland, Poland, Belgium, the Czech Republic, and Switzerland (Genné and Rossi 2011). Most HDV cases are found in IDUs, but the proportion of immigrants is increasing; in the United Kingdom, Germany, and Spain, >70% of the HDV-infected patients born in these countries were IDUs. Among 1319 HBsAg-positive patients in the EuroSIDA study (Soriano et al. 2011), the prevalence of anti-HD was 14.5% and a similar figure has been reported in HIV/HBV subjects in Romania (Ionescu and Mihăescu 2011); in this population, HDV increased the risk of liver-related deaths and the overall mortality.
The endemicity of HDV remains constant in countries of Eastern Europe and Turkey. Anti-HD was detected in 20.4% of 1094 patients with chronic hepatitis B (CHB) recruited in 2006 in Bucharest (Popescu et al. 2013). However, among institutionalized children with CHB in southeast Romania, the prevalence of HDV declined from 33% to 21% from 2000 to 2009 (Rizzetto and Ciancio 2012). Prevalence rates of anti-HD have varied from 14% to 39% in Moldova and Serbia, and from 7% to 10.29% in Albanian patients with chronic liver disease. Antibody rates were low in Croatia and Bulgaria (Rizzetto and Ciancio 2012). Only one individual was positive in 1287 health care workers in Kosovo (Quaglio et al. 2008). Between 2002 and 2004, 27.5% of 120 patients with CHB had anti-HD in Dicle, southeast Turkey (Bayan et al. 2007). In 282 HBsAg patients collected between 2006 and 2009 in Elazig, eastern Turkey, anti-HD was found in 45.5% and HDV RNA in 23% (Bahcecioglu et al. 2011).
The issue of HDV infection has been reinvestigated in the United States. In a recent study, 50% of the chronically HBV-infected IDUs in Baltimore, MD, had anti-HD (Kucirka et al. 2010), and an 8% prevalence of anti-HD was found in 499 HBsAg carriers in northern California (Gish et al. 2013). In this study, HDV-positive patients had higher rates of cirrhosis than those with HBV monoinfection; 69% were Caucasian non-Hispanic, 10% came from Asia and the Pacific Islands.
HDV remains endemic in and around the Middle East (Amini et al. 2013). In patients with chronic HBsAg hepatitis, the pooled prevalence of HDV was 47.36% in Somalia, 24.37% in Egypt, and 8.15% in Saudi Arabia. Among cirrhotics and in patients with hepatocellular carcinoma, it was 33.2% in Somalia and 29.6% in Egypt (Rizzetto and Alavian 2013). The prevalence of anti-HD was 4.7% in HBsAg-positive blood donors in Ismailia, Egypt in 2012 (Gomaa et al. 2013).
A number of recent surveys are providing a map of the burden of hepatitis D in Asia and Africa (Fig. 2). HDV is highly endemic in Pakistan. In Larkana, Pakistan (Shaikh et al. 2011) and Pubjab, India (Zaidi et al. 2010), 23.6% and 88.8%, respectively, of the HBsAg carriers with liver disease were found to have anti-HD. In HBsAg patients in Karachi, Pakistan, the antibody prevalence has varied from 35% to 59% (Moatter et al. 2007; Baig et al. 2009). Throughout Iran, anti-HD prevalence of 5.7%–12.7% were found from 2002 through 2009 in patients with chronic HBsAg liver disease (Rizzetto and Ciancio 2012). In a series of blood samples collected from 2008 to 2011 in Zahedan, the prevalence of anti-HD was 16.3% in chronic active hepatitis and 65.9% in cirrhotic patients (Bakhshipour et al. 2013). The antibody rate was, instead, low (3.1%) in 2012 in Biriand city, east of Iran (Ziaee and Azarkar 2013). A 15% rate of anti-HD was reported in Tajikistan in HBsAg cirrhosis and hepatocellular carcinoma (Khan et al. 2008). In India, a 10.6% and 5.9% prevalence of anti-HD was reported in HBsAg carriers, respectively, in 2005 in New Delhi (Chakraborty et al. 2005) and Chennai, India in 2008 (Saravanan et al. 2008). Overall, 5%–10% of the patients with HBV-related liver disease appear to have been exposed to the HDV in the country (Rizzetto and Ciancio 2012). The prevalence of anti-HD was detected also in resident tribes of the Nicobar and Andaman Islands (Murhekar et al. 2005). HDV seems to be of low prevalence in the general HBsAg population of Malaysia, Thailand, and the Philippines. However, rates of anti-HD of 20%–34% and 21.8%, were reported in drug addicts, respectively, from Malaysia (Duraisamy et al. 1994) and Thailand (Theamboonlers et al. 2002). HDV appears to be endemic in the North of Vietnam; HDV-RNA was found in 15.4% of HBsAg carriers and in 43.3% of those with acute hepatitis (Sy et al. 2013). Variable figures were reported from China. The prevalence of anti-HD was 13% in Shijiazkiang, China but only 0.15% in Hong Kong; in Shandong Province, China, 13.15% of hepatitis B patients were anti-HD positive (Ciancio and Rizzetto 2002). In a 2006 study in Wuhan, China, only 2.22% of the intravenous drug users were found to be infected (Li et al. 2006).
Consistent rates of anti-HD were recently reported from countries in Africa. In 2009, an antibody rate of 66.7% was reported in Gabon (Makuwa et al. 2009). In Cameroon (Foupouapouognigni et al. 2011), anti-HD was found in 17.6% of 233 patients with CHB at two medical centers. In Nigeria, anti-HD was found in 12.5% of 96 patients with HBsAg liver disease (Nwokediuko and Ijeoma 2009), and in Mauritania, in 14.7% of HBsAg-positive pregnant women (Mansour et al. 2012).
CONCLUDING REMARKS
The HDV remains a medical scourge in poor countries of the globe in which HBV remains endemic. Knowledge of HDV infection throughout the world is increasing, but no information is yet available from many areas of Asia, Africa, Central, and South America. The infection is coming under control in the developed world, in which HBV vaccination has been implemented. In industrialized countries, hepatitis D remains, nevertheless, a major medical issue in drug addicts and immigrants coming from areas in which HDV infection remains endemic.
REFERENCES
*Reference is also in this collection.
- Abbas Z, Jafri W, Raza S. 2010. Hepatitis D: Scenario in the Asia-Pacific region. World J Gastroenterol 16: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini N, Alavian SM, Kabir A, Aalaei-Andabili SH, Saiedi Hosseini SY, Rizzetto M. 2013. Prevalence of hepatitis D in the eastern Mediterranean region: Systematic review and meta analysis. Hepat Mon 13: e8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa Y, Moriyama M, Taira M, Hayashi N, Tanaka N, Okubo H, Sugitani M. 2000. Molecular analysis of hepatitis D virus infection in Miyako Island, a small Japanese island. J Viral Hepat 7: 375–381. [DOI] [PubMed] [Google Scholar]
- Bahcecioglu IH, Aygun C, Gozel N, Poyrazoglu OK, Bulut Y, Yalniz M. 2011. Prevalence of hepatitis delta virus (HDV) infection in chronic hepatitis B patients in eastern Turkey: Still a serious problem to consider. J Viral Hepat 18: 518–524. [DOI] [PubMed] [Google Scholar]
- Baig S, Siddiqui AA, Ahmed WU, Qureshi H, Arif A. 2009. Frequency of hepatitis C and D super infection in patients with hepatitis B related complex liver disorders. J Coll Physicians Surg Pak 19: 699–703. [PubMed] [Google Scholar]
- Bakhshipour A, Mashhadi M, Mohammadi M, Nezam SK. 2013. Seroprevalence and risk factors of hepatitis delta virus in chronic hepatitis B virus infection in Zahedan. Acta Med Iran 51: 260–264. [PubMed] [Google Scholar]
- Bayan K, Yilmaz S, Tuzun Y, Yildirim Y. 2007. Epidemiological and clinical aspects of liver cirrhosis in adult patients living in Southeastern Anatolia: Leading role of HBV in 505 cases. Hepatogastroenterology 54: 2198–2202. [PubMed] [Google Scholar]
- Børresen ML, Olsen OR, Ladefoged K, McMahon BJ, Hjuler T, Panum I, Simonetti J, Jones C, Krarup H, Koch A. 2010. Hepatitis D outbreak among children in a hepatitis B hyper-endemic settlement in Greenland. J Viral Hepat 17: 162–170. [DOI] [PubMed] [Google Scholar]
- Braga WS, Castilho Mda C, Borges FG, Leão JR, Martinho AC, Rodrigues IS, Azevedo EP, Barros Júnior GM, Paraná R. 2012. Hepatitis D virus infection in the Western Brazilian Amazon—Far from a vanishing disease. Rev Soc Bras Med Trop 45: 691–695. [DOI] [PubMed] [Google Scholar]
- Brancaccio G, Giuberti T, Verucchi G, Levantesi M, Sacchini D, Fattovich G, Madonia S, Fasano M, Gavrila C, Nardi A, et al. 2014. Epidemiological evolution of chronic hepatitis delta in Italy. An analysis of the master-B cohort. Dig Liv Dis 46: e12–e13. [Google Scholar]
- Buti M, Homs M, Rodriguez-Frias F, Funalleras G, Jardí R, Sauleda S, Tabernero D, Schaper M, Esteban R. 2011. Clinical outcome of acute and chronic hepatitis delta over time: A long-term follow-up study. J Viral Hepat 18: 434–442. [DOI] [PubMed] [Google Scholar]
- Chakraborty P, Kailash U, Jain A, Goyal R, Gupta RK, Das BC, Kar P. 2005. Seroprevalence of hepatitis D virus in patients with hepatitis B virus-related liver diseases. Indian J Med Res 122: 254–257. [PubMed] [Google Scholar]
- Ciancio A, Rizzetto M. 2002. Clinical patterns, epidemiology and disease burden of hepatitis D virus chronic liver disease (ed. Margolis H, Alter M, Liang T, Dienstag J), pp. 271–275. International Medical Press, London. [Google Scholar]
- Cross TJ, Rizzi P, Horner M, Jolly A, Hussain MJ, Smith HM, Vergani D, Harrison PM. 2008. The increasing prevalence of hepatitis delta virus (HDV) infection in south London. J Med Virol 80: 277–282. [DOI] [PubMed] [Google Scholar]
- Duraisamy G, Zuridah H, Ariffin Y, Kek CS. 1994. Hepatitis delta virus in intravenous drug users in Kuala Lumpur. Med J Malaysia 49: 212–216. [PubMed] [Google Scholar]
- Farci P. 2003. Delta hepatitis: An update. J Hepatol 39: S212–S219. [DOI] [PubMed] [Google Scholar]
- Flodgren E, Bengtsson S, Knutsson M, Strebkova EA, Kidd AH, Alexeyev OA, Kidd-Ljunggren K. 2000. Recent high incidence of fulminant hepatitis in Samara, Russia: Molecular analysis of prevailing hepatitis B and D virus strains. J Clin Microbiol 38: 3311–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foupouapouognigni Y, Noah DN, Sartre MT, Njouom R. 2011. High prevalence and predominance of hepatitis delta virus genotype 1 infection in Cameroon. J Clin Microbiol 49: 1162–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaeta GB, Stroffolini T, Chiaramonte M, Ascione T, Stornaiuolo G, Lobello S, Sagnelli E, Brunetto MR, Rizzetto M. 2000. Chronic hepatitis D: A vanishing disease? An Italian multicenter study. Hepatology 32: 824–827. [DOI] [PubMed] [Google Scholar]
- Genné D, Rossi I. 2011. Hepatitis delta in Switzerland: A silent epidemic. Swiss Med Wkly 141: w13176. [DOI] [PubMed] [Google Scholar]
- Gish RG, Yi DH, Kane S, Clark M, Mangahas M, Baqai S, Winters MA, Proudfoot J, Glenn JS. 2013. Coinfection with hepatitis B and D: Epidemiology, prevalence and disease in patients in Northern California. J Gastroenterol Hepatol 28: 1521–1525. [DOI] [PubMed] [Google Scholar]
- Gomaa NI, Metwally LA, Nemr N, Younis S. 2013. Seroprevalence of HDV infection in HBsAg positive population in Ismailia, Egypt. Egypt J Immunol 20: 23–28. [PubMed] [Google Scholar]
- Hadler SC, Alcala de Monzon M, Bensabath G, Martinez Duran M, Schatz G, Fields HA. 1991. Epidemiology of hepatitis delta virus infection in less developed countries. Prog Clin Biol Res 364: 21–31. [PubMed] [Google Scholar]
- Holmberg SD, Ward JW. 2010. Hepatitis delta: Seek and ye shall find. J Infect Dis 202: 822–824. [DOI] [PubMed] [Google Scholar]
- Ionescu B, Mihăescu G. 2011. Hepatitis B, C and D coinfection in HIV-infected patients: Prevalence and progress. Roum Arch Microbiol Immunol 70: 129–133. [PubMed] [Google Scholar]
- Khan A, Kurbanov F, Tanaka Y, Elkady A, Sugiyama M, Dustov A, Mizokami M. 2008. Epidemiological and clinical evaluation of hepatitis B, hepatitis C, and delta hepatitis viruses in Tajikistan. J Med Virol 80: 268–276. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim SJ, Park HW, Shin WG, Kim KH, Lee JH, Kim HY, Jang MK. 2011. Prevalence and clinical significance of hepatitis D virus co-infection in patients with chronic hepatitis B in Korea. J Med Virol 83: 1172–1177. [DOI] [PubMed] [Google Scholar]
- Kucirka LM, Farzadegan H, Feld JJ, Mehta SH, Winters M, Glenn JS, Kirk GD, Segev DL, Nelson KE, Marks M, et al. 2010. Prevalence, correlates, and viral dynamics of hepatitis delta among injection drug users. J Infect Dis 202: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal F, Gault E, Ripault MP, Serpaggi J, Trinchet JC, Gordien E, Dény P. 2006. Eighth major clade for hepatitis delta virus. Emerg Infect Dis 12: 1447–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal F, Castelneau C, Gault E, et al. 2007. Hepatitis D virus infection. Not a vanishing disease in Europe! Hepatology 45: 1332–1333. [DOI] [PubMed] [Google Scholar]
- Li J, Wang J, Tian K, Wang Y, Zhang L, Huang H. 2006. Epidemiology of hepatitis B, C, D and G viruses and cytokine levels among intravenous drug users. J Huazhong Univ Sci Technolog Med Sci 26: 221–224. [DOI] [PubMed] [Google Scholar]
- Makuwa M, Mintsa-Ndong A, Souquière S, Nkoghé D, Leroy EM, Kazanji M. 2009. Prevalence and molecular diversity of hepatitis B virus and hepatitis delta virus in urban and rural populations in northern Gabon in central Africa. J Clin Microbiol 47: 2265–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour W, Malick FZ, Sidiya A, Ishagh E, Chekaraou MA, Veillon P, Ducancelle A, Brichler S, Le Gal F, Lo B, et al. 2012. Prevalence, risk factors, and molecular epidemiology of hepatitis B and hepatitis delta virus in pregnant women and in patients in Mauritania. J Med Virol 84: 1186–1198. [DOI] [PubMed] [Google Scholar]
- Moatter T, Abbas Z, Shabir S, Jafri W. 2007. Clinical presentation and genotype of hepatitis delta in Karachi. World J Gastroenterol 13: 2604–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar MV, Murhekar KM, Arankalle VA, Sehgal SC. 2005. Hepatitis delta virus infection among the tribes of the Andaman and Nicobar Islands, India. Trans R Soc Trop Med Hyg 99: 483–484. [DOI] [PubMed] [Google Scholar]
- Negro F. 2014. Structure and molecular virology. In Viral hepatitis, 4th ed. (ed. Thomas HC, Lok ASF, Locarnini SA, Zuckerman AJ), pp. 395–402. Wiley, Blackwell. [Google Scholar]
- Nwokediuko SC, Ijeoma U. 2009. Seroprevalence of antibody to HDV in Nigerians with hepatitis B virus-related liver diseases. Niger J Clin Pract 12: 439–442. [PubMed] [Google Scholar]
- Ponzetto A, Negro F, Popper H, Bonino F, Engle R, Rizzetto M, Purcell RH, Gerin JL. 1988. Serial passage of hepatitis delta virus in chronic hepatitis B virus carrier chimpanzees. Hepatology 8: 1655–1661. [DOI] [PubMed] [Google Scholar]
- Popescu GA, Otelea D, Gavriliu LC, Neaga E, Popescu C, Paraschiv S, Fratila M. 2013. Epidemiology of hepatitis D in patients infected with hepatitis B virus in Bucharest: A cross-sectional study. J Med Virol 85: 769–774. [DOI] [PubMed] [Google Scholar]
- Quaglio G, Ramadani N, Pattaro C, Cami A, Dentico P, Volpe A, Pellizzer G, Berisha A, Smacchia C, Figliomeni M, et al. 2008. Prevalence and risk factors for viral hepatitis in the Kosovarian population: Implications for health policy. J Med Virol 80: 833–840. [DOI] [PubMed] [Google Scholar]
- Rizzetto M. 1983. The delta agent. Hepatology 3: 729–737. [DOI] [PubMed] [Google Scholar]
- Rizzetto M. 2009. Hepatitis D: Thirty years after. J Hepatol 50: 1043–1050. [DOI] [PubMed] [Google Scholar]
- Rizzetto M, Alavian SM. 2013. Hepatitis delta: The rediscovery. Clin Liver Dis 17: 475–487. [DOI] [PubMed] [Google Scholar]
- Rizzetto M, Ciancio A. 2012. Epidemiology of hepatitis D. Semin Liver Dis 32: 211–219. [DOI] [PubMed] [Google Scholar]
- Rizzetto M, Canese MG, Aricò S, Crivelli O, Trepo C, Bonino F, Verme G. 1977. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 18: 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M, Ponzetto A, Forzani I. 1990. Hepatitis delta virus as a global health problem. Vaccine 8: S10–S14; discussion S21–S23. [DOI] [PubMed] [Google Scholar]
- Rizzetto M, Ponzetto A, Forzani I. 1991. Epidemiology of hepatitis delta virus: Overview. Prog Clin Biol Res 364: 1–20. [PubMed] [Google Scholar]
- Rosina F, Conoscitore P, Cuppone R, Rocca G, Giuliani A, Cozzolongo R, Niro G, Smedile A, Saracco G, Andriulli A, et al. 1999. Changing pattern of chronic hepatitis D in Southern Europe. Gastroenterology 117: 161–166. [DOI] [PubMed] [Google Scholar]
- Sagnelli E, Stroffolini T, Ascione A, Bonino F, Chiaramonte M, Colombo M, Craxi A, Giusti G, Manghisi OG, Pastore G, et al. 1992. The epidemiology of hepatitis delta infection in Italy. J Hepatol 15: 211–215. [DOI] [PubMed] [Google Scholar]
- Saravanan S, Velu V, Kumarasamy N, Shankar EM, Nandakumar S, Murugavel KG, Balakrishnan P, Solomon S, Thyagarajan SP. 2008. Seroprevalence of hepatitis delta virus infection among subjects with underlying hepatic diseases in Chennai, southern India. Trans R Soc Trop Med Hyg 102: 793–796. [DOI] [PubMed] [Google Scholar]
- Servant-Delmas A, Le Gal F, Gallian P, Gordien E, Laperche S. 2014. Increasing prevalence of HDV/HBV infection over 15 years in France. J Clin Virol 59: 126–128. [DOI] [PubMed] [Google Scholar]
- Shaikh MA, Shaikh WM, Solangi GA, Shaikh BA, Soomro MA. 2011. Frequency of hepatitis D virus infection in hepatitis B surface antigen-positive liver diseases. J Coll Physicians Surg Pak 21: 23–25. [PubMed] [Google Scholar]
- Smedile A, Rizzetto M, Gerin JL. 1994. Advances in hepatitis D virus biology and disease. Prog Liver Dis 12: 157–175. [PubMed] [Google Scholar]
- Soriano V, Grint D, d’Arminio Monforte A, Horban A, Leen C, Poveda E, Antunes F, de Wit S, Lundgren J, Rockstroh J, et al. 2011. Hepatitis delta in HIV-infected individuals in Europe. AIDS 25: 1987–1992. [DOI] [PubMed] [Google Scholar]
- Stroffolini T, Almasio PL, Sagnelli E, Mele A, Gaeta GB; Italian Hospitals’ Collaborating Group. 2009. Evolving clinical landscape of chronic hepatitis B: A multicenter Italian study. J Med Virol 81: 1999–2006. [DOI] [PubMed] [Google Scholar]
- Sy BT, Ratsch BA, Toan NL, Song le H, Wollboldt C, Bryniok A, Nguyen HM, Luong HV, Velavan TP, Wedemeyer H, et al. 2013. High prevalence and significance of hepatitis D virus infection among treatment-naïve HBsAg-positive patients in Northern Vietnam. PLoS ONE 8: e78094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Taylor JM. 2015. Hepatitis D virus replication. Cold Spring Harb Perspect Med 10.1101/cshperspect.a021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theamboonlers A, Hansurabhanon T, Verachai V, Chongsrisawat V, Poovorawan Y. 2002. Hepatitis D virus infection in Thailand: HDV genotyping by RT-PCR, RFLP and direct sequencing. Infection 30: 140–144. [DOI] [PubMed] [Google Scholar]
- Tsatsralt-Od B, Takahashi M, Endo K, Buyankhuu O, Baatarkhuu O, Nishizawa T, Okamoto H. 2006. Infection with hepatitis A, B, C, and delta viruses among patients with acute hepatitis in Mongolia. J Med Virol 78: 542–550. [DOI] [PubMed] [Google Scholar]
- Wedemeyer H, Manns MP. 2010. Epidemiology, pathogenesis and management of hepatitis D: Update and challenges ahead. Nat Rev Gastroenterol Hepatol 7: 31–40. [DOI] [PubMed] [Google Scholar]
- Zaidi G, Idrees M, Malik FA, Amin I, Shahid M, Younas S, Hussain R, Awan Z, Tariq A, Parveen K. 2010. Prevalence of hepatitis delta virus infection among hepatitis B virus surface antigen positive patients circulating in the largest province of Pakistan. Virol J 26: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaee M, Azarkar G. 2013. Prevalence of hepatitis d virus infection among patients with chronic hepatitis B attending birjand hepatitis clinic (East of Iran) in 2012. Hepat Mon 13: e11168. [DOI] [PMC free article] [PubMed] [Google Scholar]