Endometrial gene expression profiles have been obtained for the entire menstrual cycle. These studies have led to the development of the Endometrial Receptivity Array, which can assist patients in timing embryo transfer.
Abstract
Human endometrium has been extensively investigated in the search for markers capable of predicting its receptive status. The completion of the Human Genome Project has triggered a rapid development of new fields in molecular biology, the “transcriptomics” being a major turning point in the knowledge acquisition of endometrial receptivity. Based on this, a customized Endometrial Receptivity Array (ERA) has been developed, which is capable of identifying the genomic signature of receptivity. This diagnostic tool showed that the window of implantation (WOI) is displaced in one out of four patients with implantation failure, allowing the identification of their personalized WOI. This strategy allows performing a personalized embryo transfer (pET) on the day in which the endometrium is receptive. The combination of a systems biology approach and next-generation sequencing will overcome the limitations of microarrays, and will, in the future, allow elucidation of the mechanisms involved in embryo implantation.
The endometrium, which lines the inside of the uterine cavity, undergoes cyclic changes that are regulated by ovarian steroids. It can be subdivided into the basal layer that is responsible for its regenerative capacity, and the functional layer that undergoes proliferation, secretion, and tissue degeneration every month from menarche to menopause. Its aim is to prepare the optimal moment for embryonic implantation known as the window of implantation (WOI).
The endometrial cycle comprises the menstrual, proliferative, and secretory phases. The proliferative phase, which corresponds to the ovarian follicular phase and increased production of estrogens, lasts until ovulation occurs. During this stage, the increasing estrogen levels cause the proliferation of the stromal cells as well as the elongation of the spiral arteries. After ovulation, with the appearance of progesterone secreted by the granulosa-luteal cells, the secretory phase begins. If implantation does not occur, the secretory phase ends, and the corpus luteum degenerates. Menstruation occurs owing to the drop of estrogen and progesterone, which resets the endometrium until pregnancy occurs. The secretory phase can be divided into early-, mid-, and late-secretory. The most important phase is the mid-secretory, because at this point the endometrium acquires a receptive phenotype. A peak of progesterone characterizes this period, known as the WOI. The WOI lasts between 12 h to 2 d and may vary in length from patient to patient (Fig. 1). During this phase, implantation will occur if a viable blastocyst is present and finds a receptive endometrium, and a synchronized dialogue is established between them.
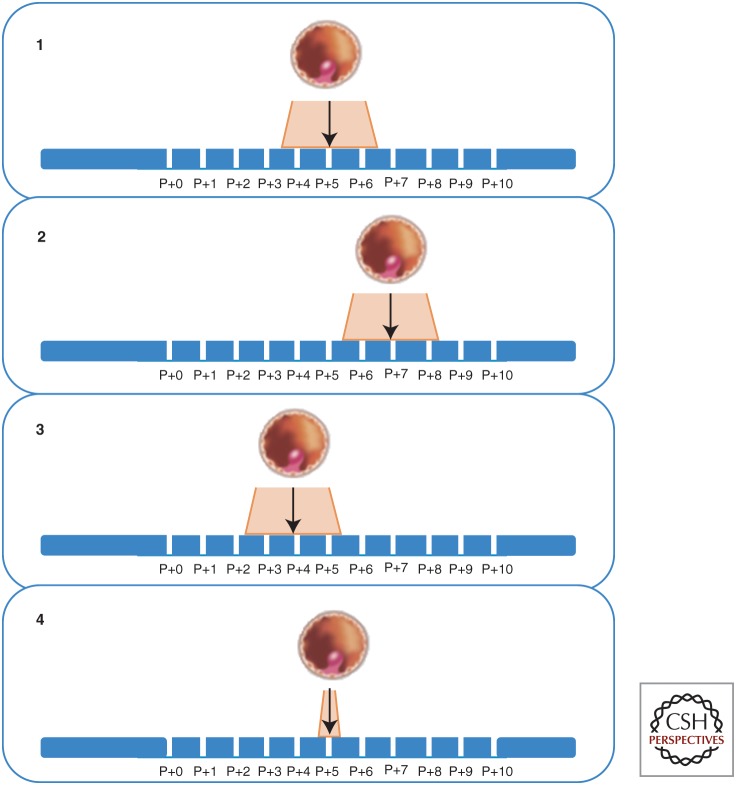
Figure 1.
Displacement of the window of implantation (WOI). It has been assumed that the WOI is constant in time in all women (1). However, the genomic signature of the endometrium shows the existence of a displacement of the WOI in up to 25% of patients that can be delayed (2), advanced (3), or shorter than expected (4). P + x refers to the days after progesterone administration. (Based on Galliano et al. 2014.)
Endometrial receptivity is a widely studied process; its understanding is providing better and more comprehensive knowledge of the reproductive process. Histological criteria have been used since the 1950s to date the endometrium (Noyes et al. 1950, 1975). However, morphological criteria have major limitations for predicting endometrial receptivity, as shown in randomized studies (Coutifaris et al. 2004; Murray et al. 2004). The diagnosis obtained may vary depending on the subjective interpretation from each observer and histological variations at the moment the endometrial biopsy was obtained. In addition, these morphological markers do not properly recognize the phenotype of the receptive endometrium.
Endometrial receptivity has also been unsuccessfully investigated at the single molecular and biochemical levels. However, the identification of a search for the transcriptomic signature was a major turning point in the understanding of this function.
The completion of the Human Genome Project (Venter et al. 2001) triggered a rapid development of new fields in molecular biology that are described as the “omics revolution.” Omics refers to the application of high-throughput techniques and massive data analysis, allowing molecular profiling and changes between groups or individuals to be investigated. Among the “omics sciences”, there is the transcriptomics, in which analyses of patterns of gene expression in a specific tissue, under specific conditions, helps to diagnose physiological functions and pathological conditions.
Microarray-based gene expression technology, which allows simultaneous monitoring of the expression of thousands of genes, has been the most widely used platform for transcriptomic analysis (Altmäe et al. 2013). Data obtained are further curated using statistical analysis and exploratory methods. The most common exploratory method is clustering or principal component analysis (PCA). Other visual methods, such as heatmap representations, display the differential expression patterns among different conditions (Díaz-Gimeno et al. 2014).
Over the last decade, the transcriptomics of the human endometrium has been widely investigated (Ruíz-Alonso et al. 2012). Several areas have been covered, from the transcriptomic expression throughout the menstrual cycle to the changes identified under different treatments or gynecological conditions. However, the main interest has been the identification of the specific transcriptomic signature that can diagnose the receptive function and improve the effectiveness of reproductive treatments.
TRANSCRIPTOMICS OF THE ENDOMETRIUM
Endometrial transcriptomic studies have covered the identification of the physiological endometrial profile throughout the menstrual cycle with special attention to the WOI (Carson et al. 2002; Kao et al. 2002; Borthwick et al. 2003; Riesewijk et al. 2003; Horcajadas et al. 2004a,b; Ponnampalam et al. 2004; Mirkin et al. 2005; Puynadeera et al. 2005; Yanaihara et al. 2005; Critchley et al. 2006; Talbi et al. 2006; Haouzi et al. 2009a; Kuokkanen et al. 2010; Tseng et al. 2010; Van Verenbergh et al. 2010; Díaz-Gimeno et al. 2011; Revel et al. 2011). Endometrial transcriptomic profiles have also been investigated under different controlled ovarian stimulation protocols (Simon et al. 2005; Horcajadas et al. 2008), or in the context of unexplained pathologies such as recurrent implantation failure (Tapia et al. 2008; Koler et al. 2009; Altmäe et al. 2010), or other endometrial conditions (Table 1) (Habermann et al. 2011; Matsuzaki 2011).
Table 1.
Original studies on endometrial transcriptomics in assisted reproductive medicine
| References | Endometrial biopsy timing (in days) | Study |
|---|---|---|
| Carson et al. 2002 | LH + (2–4) versus LH + (7–9) | ES versus MS |
| Kao et al. 2002 | CD 8–10 versus LH + (8–10) | LP versus MS |
| Borthwick et al. 2003 | CD 9–11 versus LH + (6–8) | LP versus MS |
| Riesewijck et al. 2003 | LH + 2 versus LH + 7 | ES versus MS |
| Mirkin et al. 2004 | LH + 8 versus hCG + 9 | Ag versus Atg versus NC |
| Ponnampalam et al. 2004 | Complete cycle, dating by Noyes | EP versus MP versus LP versus ES versus MS versus LS versus M |
| Horcajadas et al. 2005 | LH(+ 2; + 7) versus hCG + 7 | NC versus COH |
| Mirkin et al. 2005 | LH + 3 versus LH + 8 | ES versus MS |
| Punyadeera et al. 2005 | CD 2–5 versus CD 11–14 | M versus LP |
| Simon et al. 2005 | LH (+ 2; + 7) versus hCG (+ 2; + 7) | Ag versus Atg versus NC |
| Yanahaira et al. 2005 | CD 9–11 | Epithelial versus stromal cells in proliferative phase |
| Critchley et al. 2006 | Dating by Noyes | MS versus LS |
| Talbi et al. 2006 | Complete cycle, dating by Noyes | EP versus MP versus LP versus ES versus MS versus LS |
| Horcajadas et al. 2008 | LH + (1–9) versus hCG+ (1–9) | NC versus COS |
| Liu et al. 2008 | LH + 7 versus hCG + 7 | NC versus COS |
| Macklon et al. 2008 | LH + 5 versus hCG + 2 | NC versus COS |
| Haouzi et al. 2009b | LH (+ 2; + 7) versus hCG + (+ 2; + 5) | NC versus COS |
| Haouzi et al. 2009a | LH + 2 versus LH + 7 | ES versus MS |
| Koler et al. 2009 | CD 21 | Fertility versus infertility |
| Altmäe et al. 2010 | LH + 7 | Fertility versus infertility |
| Haouzi et al. 2011 | LH (+ 2; + 7) versus hCG (+ 2; + 5) | Ag versus Atg versus NC |
| Tseng et al. 2010 | Dating by Noyes | ES versus MS versus LS |
| Van Vaerenbergh et al. 2010 | LH + (5–7) | MS versus pregnant |
| Blockeel et al. 2011 | Oocyte retrieval | rFSH versus low-dose hCG |
| Diaz-Gimeno et al. 2011 | LH + 1, + 3, + 5 versus LH + 7 LH + (1–5) versus LH + 7 versus CD 8–12 | LP versus ES versus MS |
| Labarta et al. 2011 | hCG + 7 | Different serum progesterone level |
| Van Vaerenbergh et al. 2011 | Oocyte retrieval | Different serum progesterone level |
| Evans et al. 2012 | LH + 2 versus LH + 7 | Epithelial versus stromal cells in proliferative phase |
| Petracco et al. 2012 | CD 1–3 versus CD 5–8 versus CD 11–13 | EP versus MP versus LP |
| Diaz-Gimeno et al. 2013 | Dating by Noyes versus ERA prediction | MP versus ES versus MS versus LS |
| Ruíz-Alonso et al. 2013 | P + 5/LH + 7 RIF versus controls | pWOI/pWOI delayed/pWOI advanced |
| Bermejo et al. 2014 | Oocyte retrieval COS | Comparing 4 GnRH-a protocols |
| Haouzi et al. 2014 | hCG + 2 versus hCG + 7 | Different serum progesterone level |
| Ruíz-Alonso et al. 2014 | P + 5 versus P + 7 | ET versus pET |
Note that endometrial disorders such as cancer, endometriosis, and myomas are not considered in this table.
Based on the data published in Díaz-Gimeno et al. 2014.
Abbreviations: Ag, agonist; Atg, antagonist; CD, cycle day; COH, controlled ovarian hyperstimulation; COS, controlled ovarian stimulation; EP, early-proliferative; ERA, Endometrial Receptivity Array; ES, early-secretory; GnRH-a, Gonadotropin releasing hormone-agonist; hCG + , hCG administration + days; LH + , LH surge + days; LP, late-proliferative; LS, late-secretory; M, menstrual; MP, mid-proliferative; MS, mid-secretory; NC, natural cycle; P + , progesterone + days; pWOI, personalized window of implantation; rCG + , rCG administration + days; RIF, recurrent implantation failure.
Most of these studies investigate the transcriptomic signature in the whole endometrial tissue without separating the different compartments. However, in some studies, laser capture micro-dissection has facilitated specific compartment gene expression profiles (Yanaihara et al. 2005; Evans et al. 2012; Ulbrich et al. 2013). Even the specific profiles for stromal cells and glands at different depths in the endometrium have been reported (Gaide Chevronnay et al. 2009).
Several groups have used transcriptomics to search for the molecular diagnosis of the different phases of the human endometrium (Carson et al. 2002; Kao et al. 2002; Borthwick et al. 2003; Riesewijk et al. 2003). Ponnampalam et al. (2004) were the first to propose the transcriptomic characterization of the human endometrium throughout the menstrual cycle. Based on data extracted from samples taken at different cycle phases, they identified seven main groups of genes with a similar expression pattern throughout the cycle. Each of these groups had an expression peak in one of the seven subphases (menstrual, early-proliferative, mid-proliferative, late-proliferative, early-secretory, mid-secretory, and late-secretory). This finding was later reinforced by Talbi et al. (2006), who found groups of genes with a peak of expression in all the analyzed stages (proliferative, early-secretory, mid-secretory, and late-secretory).
TRANSCRIPTOMICS OF ENDOMETRIAL RECEPTIVITY
The most commonly used strategy to search for the human endometrial receptivity signature was to compare expression profiles during the WOI versus other stages of the menstrual cycle (Carson et al. 2002; Kao et al. 2002; Borthwick et al. 2003; Riesewijk et al. 2003; Horcajadas et al. 2004a,b; Mirkin et al. 2005; Critchley et al. 2006; Franchi et al. 2008; Kuokkanen et al. 2010; Tseng et al. 2010; Díaz-Gimeno et al. 2011).
The most frequent comparisons that have been performed are the receptive phase (mid-secretory) versus prereceptive (early-secretory) (Carson et al. 2002; Riesewijk et al. 2003; Mirkin et al. 2005; Franchi et al. 2008; Haouzi et al. 2009b; Tseng et al. 2010; Díaz-Gimeno et al. 2011); receptive versus proliferative (Kao et al. 2002; Borthwick et al. 2003; Kuokkanen et al. 2010); and receptive versus postreceptive (late-secretory) (Critchley et al. 2006; Tseng et al. 2010) (reviewed in Horcajadas et al. 2007). There was a lack of consensus on the results obtained, which was likely caused by the variability in some of the parameters such as samples taken from the same or different patients, the decision to use a pool of samples or not, the day of the cycle on which samples were taken, or the type of data analysis undertaken (Horcajadas et al. 2004a,b; Giudice 2006; Simmen and Simmen 2006; Haouzi et al. 2011).
Despite the differences among all the studies performed, they all agreed on the existence of a specific transcriptomic profile during the WOI. This characteristic profile suggested that a unique transcriptional process occurs to achieve a receptive phenotype (Borthwick et al. 2003; Riesewijk et al. 2003; Horcajadas et al. 2008; Haouzi et al. 2009a,b; Díaz-Gimeno et al. 2011), being the gatekeeper is the progesterone receptor activation.
Overexpressed genes include those involved in the processes of metabolism, glandular secretion, cell differentiation, cell communication, innate immune response, response to stress, response to wounding, cell adhesion, and proteolysis regulation (reviewed at Ruíz-Alonso et al. 2012).
The implantation of the blastocyst in the endometrium activates the production of cytokines that modulate receptivity by regulating the expression of adhesion molecules in mammals (Simon et al. 1997). Leukemia inhibitory factor (LIF) is a cytokine that has been the focus of many studies (Aghajanova et al. 2008; Allegra et al. 2009; Rashid et al. 2011) because of its clear functional effect in the mouse model. Osteopontin (SPP1) has been the gene with the greatest consensus among most endometrial transcriptomic studies. The glycoprotein encoded by this gene, regulated by progesterone, is a ligand for αvβ3 integrin and mediates cellular adhesion and migration during implantation (Apparao et al. 2001). There are several studies indicating that regulation of the immune response occurs during embryonic implantation (Hannan and Salamonsen 2007; Salamonsen et al. 2007). It is consistent with the transcriptomics studies showing that during mid-secretory stage, an activation of responses to stress, defense, humoral immunity, innate immunity and injuries occurs (Díaz-Gimeno et al. 2011; Ruíz-Alonso et al. 2012). Among the genes up-regulated involved in these processes are: glycodelin, which decreases maternal immunological responses to the implanting embryo (Aghajanova et al. 2008); CXCL14, a chemokine that is thought to be the major recruitment stimulus for immune cells during the WOI (Talbi et al. 2006) as well as chemotaxis of natural killer cells to cluster around epithelial glands (Mokhtar et al. 2010); and IL15, which plays important roles in uNK cell proliferation and differentiation (Okada et al. 2000) involved in the recruitment of peripheral blood CD16(–) NK cells (Kitaya et al. 2005). Moreover, the protection of the embryo against several agents as free radicals and heavy metals is very important. Metallothioneins and GPXs (antioxidants) are also overexpressed at this stage (Talbi et al. 2006; Ruíz-Alonso et al. 2012).
It is worth highlighting the relevance of the l-selectin ligands for the conversion to the receptive phenotype. Whereas l-selectin is expressed in the blastocyst (Genbacev et al. 2003), the endometrial epithelial cells express its ligand (Wang et al. 2008).
CLINICAL TRANSLATION OF THE TRANSCRIPTOMIC SIGNATURE OF ENDOMETRIAL RECEPTIVITY
To prove the clinical applicability of endometrial transcriptomics as a diagnostic tool, it was necessary to find that samples clearly grouped according to the stage to which they belonged. During the past decade, our group has worked in this topic from basic research (Riesewijk et al. 2003) to the publication of the transcriptomic signature that identifies the expression level of 238 genes related to endometrial receptivity (Díaz-Gimeno et al. 2011). This molecular tool, named ERA, has been designed to identify the endometrial receptivity status in natural cycles at LH + 7, or in hormonal replacement cycles (HRT) 5 d after progesterone administration (P + 5) previously primed with estradiol. This customized array is coupled to a computational predictor that can identify the endometrium of a given patient regardless of its histological appearance (Díaz-Gimeno et al. 2011, 2013).
To train the computational predictor, gene expression profiles obtained from samples at different stages of the menstrual cycle (proliferative, prereceptive, receptive, and postreceptive) were used. This classification has a specificity and sensitivity of 0.8857 and 0.99758, respectively (Díaz-Gimeno et al. 2011). ERA is more accurate than histological dating and is a highly reproducible method, in the same patient using the same type of treatment even up to 40 mo apart (Díaz-Gimeno et al. 2011, 2013). For the first time, a molecular tool based on the expression of a cluster of genes has been clinically applied in reproductive medicine to assess the endometrial factor in patients with recurrent implantation failure (Ruíz-Alonso et al. 2013) with proven accuracy and consistency.
Although the endometrial WOI has been considered standard and constant in all women, transcriptomics have helped to figure out that personalization also applies to the endometrium. Once the personalized WOI is identified, a personalized embryo transfer (pET) plan is developed to transfer the embryo according to the day in which the endometrium is receptive (Fig. 1).
The diagnostic and clinical value of the ERA test in patients with recurrent implantation failure (RIF) has been analyzed in a prospective interventional, multicenter, clinical trial (Ruíz-Alonso et al. 2013). An RIF group of 85 patients with at least three previous failed ovum donation cycles, or IVF patients younger than 40 yr old with at least three failed IVF cycles was compared with a control group (with one or no previous failed cycle). In this trial, RIF and control patients underwent ERA-based endometrial receptivity diagnosis using an endometrial biopsy obtained either on day LH + 7 in a natural cycle or on day P + 5 in an HRT cycle. Whereas the ERA test identified 12% of the patients with displaced WOI in the control group, in the RIF group 26% of patients have a displaced WOI. This means that in one out of four patients with RIF, the endometrial factor is responsible. In these patients, a second ERA test confirmed the suspected WOI displacement, and personalized embryo transfer was performed accordingly. The clinical outcome of pET shows a 50% pregnancy rate (PR) and a 38.5% implantation rate (IR), which is similar to the control group (Ruíz-Alonso et al. 2013). Now, an international prospective randomized clinical trial on the effectiveness of the ERA test in the infertility work-up in the first appointment is ongoing (NCT:954758, see http://clinicaltrials.gov/).
CLINICAL APPLICABILITY OF ENDOMETRIAL TRANSCRIPTOMICS
Clinicians used to believe that the WOI length was ∼2 d, and it was the same in all women. Published data cited in the above section shows that the WOI as diagnosed by its transcriptomic signature is displaced in one out of four RIF patients (Ruíz-Alonso et al. 2013), and in 20% of the general population. Furthermore, no ongoing pregnancy results after embryo transfer in a patient with no receptive endometrium diagnosed by ERA, whereas a personalized embryo transfer in the same group of patients leads to 60% PR and 40% IR (Ruíz-Alonso et al. 2014).
This finding implies relevant clinical implications and points out that the endometrial factor should be properly diagnosed and considered, defining the personalized WOI of each woman to improve clinical results.
NEW PERSPECTIVES: NEXT-GENERATION SEQUENCING
Microarray-based expression profiling has technical limitations because it is limited by the nature of the probes included in the given platform as well as their sensitivity and specificity (Reis-Filho 2009; Díaz-Gimeno et al. 2014). Nowadays, next generation sequencing (NGS) is an emerging technology that allows measurement of gene expression by RNA sequencing (RNA-seq). This new technology is capable of sequencing all the mRNAs present in a given sample, even the 25% of genes with low expression that remain undetected with standard microarray technologies (Mane et al. 2009; Wang et al. 2009; Díaz-Gimeno et al. 2014).
Therefore, although transcriptomics based on microarray technology has sufficiently standardized procedures to allow its clinical applicability, it is likely to be challenged by newer global gene-expression analysis technologies, as reported by the MAQC consortium (Mane et al. 2009). Massively parallel sequencing requires an availability of high-performance computing and bioinformatics support that goes way beyond many research laboratories. Furthermore, quality control and standardization of the massively parallel sequencing experiments and data reporting are important issues to consider (Reis-Filho 2009). Nevertheless, progress in this direction is rapid, and the way is paved for the implementation of NGS for routine clinical diagnosis.
CONCLUSIONS
For decades, the study of endometrial receptivity has been restricted to the use of morphological criteria under the concept of anatomical medicine. Technological advances in recent years have enabled us the transition from anatomical to molecular medicine for the diagnosis of the human endometrium discovering the endometrial transcriptome during the menstrual cycle. This strategy has enabled the development of a molecular diagnostic test (ERA) that inform us about the timing when the WOI is open in a given patient. The clinical translation of this test allows a personalized embryo transfer that, as has already been shown, increases the success of embryo implantation in patients with recurrent implantation failure of endometrial origin. Although the transcriptome has rapidly advanced, next-generation sequencing technologies is now an emerging reality that will allow analysis of not only mRNAs, but also small RNAs and noncoding RNA, providing a more comprehensive view of the transcriptomic of the endometrium.
REFERENCES
- Aghajanova L, Simon C, Horcajadas JA. 2008. Are favourite molecules of endometrial receptivity still in favour? Expert Rev Obstet Gynecol 3: 487–501. [Google Scholar]
- Allegra A, Marino A, Coffaro F, Lama A, Rizza G, Scaglione P, Sammartano F, Santoro A, Volpes A. 2009. Is there a uniform basal endometrial gene expression profile during the implantation window in women who became pregnant in a subsequent ICSI cycle? Hum Reprod 24: 2549–2557. [DOI] [PubMed] [Google Scholar]
- Altmäe S, Martinez-Conejero JA, Salumets A, Simon C, Horcajadas JA, Stavreus-Evers A. 2010. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod 16: 178–187. [DOI] [PubMed] [Google Scholar]
- Altmäe S, Esteban FJ, Stavreus-Evers A, Simón C, Giudice L, Lessey BA, Horcajadas JA, Macklon NS, D’Hooghe T, Campoy C, et al. 2013. Guidelines for the design, analysis and interpretation of ‘omics’ data: Focus on human endometrium. Hum Reprod Update 20: 12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apparao KB, Murray MJ, Fritz MA, Meyer WR, Chambers AF, Truong PR, Lessey BA. 2001. Osteopontin and its receptor αvβ3 integrin are coexpressed in the human endometrium during the menstrual cycle but regulated differentially. J Clin Endocrinol Metab 86: 4991–5000. [DOI] [PubMed] [Google Scholar]
- Bermejo A, Cerrillo M, Ruíz-Alonso M, Blesa D, Simón C, Pellicer A, Garcia-Velasco JA. 2014. Impact of final oocyte maturation using gonadotropin-releasing hormone agonist triggering and different luteal support protocols on endometrial gene expression. Fertil Steril 101: 138–146.e3. [DOI] [PubMed] [Google Scholar]
- Blockeel C, Van Vaerenbergh I, Fatemi HM, Van Lommel L, Devroey P, Bourgain C. 2011. Gene expression profile in the endometrium on the day of oocyte retrieval after ovarian stimulation with low-dose hCG in the follicular phase. Mol Hum Reprod 17: 33–41. [DOI] [PubMed] [Google Scholar]
- Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, Smith SK. 2003. Determination of the transcript profile of human endometrium. Mol Hum Reprod 9: 19–33. [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. 2002. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod 8: 871–879. [DOI] [PubMed] [Google Scholar]
- Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern PG, Schlaff WD, Carr BR, Steinkampf MP, et al. 2004. NICHD National Cooperative Reproductive Medicine Network. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril 82: 1264–1272. [DOI] [PubMed] [Google Scholar]
- Critchley HO, Robertson KA, Forster T, Henderson TA, Williams AR, Ghazal P. 2006. Gene expression profiling of mid to late secretory phase endometrial biopsies from women with menstrual complaint. Am J Obstet Gynecol 195: 406.e1–406.e16. [DOI] [PubMed] [Google Scholar]
- Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alama P, Pellicer A, Simón C. 2011. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril 95: 50–60. [DOI] [PubMed] [Google Scholar]
- Díaz-Gimeno P, Ruíz-Alonso M, Blesa D, Bosch N, Martínez-Conejero JA, Alama P, Garrido N, Pellicer A, Simón C. 2013. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril 99: 508–517. [DOI] [PubMed] [Google Scholar]
- Díaz-Gimeno P, Ruíz-Alonso M, Blesa D, Simón C. 2014. Transcriptomics of the human endometrium. Int J Dev Biol 58: 127–137. [DOI] [PubMed] [Google Scholar]
- Evans GE, Martinez-Conejero JA, Phillipson GT, Simon C, McNoe LA, Sykes PH, Horcajadas JA, Lam EY, Print CG, Sin IL, et al. 2012. Gene and protein expression signature of endometrial glandular and stromal compartments during the wiondow of implantation. Fertil Steril 97: 1365–1373.e2. [DOI] [PubMed] [Google Scholar]
- Franchi A, Zaret J, Zhang X, Bocca S, Oehninger S. 2008. Expression of immunomodulatory genes, their protein products and specific ligands/receptors during the window of implantation in the human endometrium. Mol Hum Reprod 14: 413–421. [DOI] [PubMed] [Google Scholar]
- Gaide Chevronnay HP, Galant C, Lemoine P, Courtoy PJ, Marbaix E, Henriet P. 2009. Spatiotemporal coupling of focal extracellular matrix degradation and reconstruction in the menstrual human endometrium. Endocrinology 150: 5094–5105. [DOI] [PubMed] [Google Scholar]
- Galliano D, Bellver J, Díaz-Garcia C, Simon C, Pellicer A. 2014. ART and uterine pathology: How relevant is the maternal side for implantation? Hum Reprod Update 21: 13–38. [DOI] [PubMed] [Google Scholar]
- Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. 2003. Trophoblast l-selectin-mediated adhesion at the maternal–fetal interface. Science 299: 405–408. [DOI] [PubMed] [Google Scholar]
- Giudice LC. 2006. Application of functional genomics to primate endometrium: Insights into biological processes. Reprod Biol Endocrinol 4: S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann JK, Bundgen NK, Gemoll T, Hautaniemi S, Lundgren C, Wangsa D, Doering J, Bruch HP, Nordstroem B, Roblick UJ, et al. 2011. Genomic instability influences the transcriptome and proteome in endometrial cancer subtypes. Mol Cancer 10: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan NJ, Salamonsen LA. 2007. Role of chemokines in the endometrium and in embryo implantation. Curr Opin Obstet Gynecol 19: 266–272. [DOI] [PubMed] [Google Scholar]
- Haouzi D, Mahmoud K, Fourar M, Bendhaou K, Dechaud H, De Vos J, Reme T, Dewailly D, Hamamah S. 2009a. Identification of new biomarkers of human endometrial receptivity in the natural cycle. Hum Reprod 24: 198–205. [DOI] [PubMed] [Google Scholar]
- Haouzi D, Assou S, Mahmoud K, Tondeur S, Reme T, Hedon B, De Vos J, Hamamah S. 2009b. Gene expression profile of human endometrial receptivity: Comparison between natural and stimulated cycles for the same patients. Hum Reprod 24: 1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouzi D, Dechaud H, Assou S, Monzo C, De Vos J, Hamamah S. 2011. Transcriptome analysis reveals dialogues between human trophectoderm and endometrial cells during the implantation period. Hum Reprod 26: 1440–1449. [DOI] [PubMed] [Google Scholar]
- Haouzi D, Bissonnette L, Gala A, Assou S, Entezami F, Perrochia H, Dechaud H, Hugues JN, Hamamah S. 2014. Endometrial receptivity profile in patients with premature progesterone elevation on the day of HCG administration. Biomed Res Int 2014: 951937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horcajadas JA, Riesewijk A, Dominguez F, Cervero A, Pellicer A, Simon C. 2004a. Determinants of endometrial receptivity. Ann NY Acad Sci 1034: 166–175. [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Riesewijk A, Martin J, Cervero A, Mosselman S, Pellicer A, Simon C. 2004b. Global gene expression profiling of human endometrial receptivity. J Reprod Immunol 63: 41–49. [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Riesewijk A, Polman J, van Os R, Pellicer A, Mosselman S, Simón C. 2005. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod 11: 195–205. [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Pellicer A, Simon C. 2007. Wide genomic analysis of human endometrial receptivity: New times, new opportunities. Hum Reprod Update 13: 77–86. [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Minguez P, Dopazo J, Esteban FJ, Dominguez F, Giudice LC, Pellicer A, Simon C. 2008. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab 93: 4500–4510. [DOI] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. 2002. Global gene profiling in human endometrium during the window of implantation. Endocrinology 143: 2119–2138. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Yamaguchi T, Honjo H. 2005. Central role of interleukin-15 in postovulatory recruitment of peripheral blood CD16(−) natural killer cells into human endometrium. J Clin Endocrinol Metab 90: 2932–2940. [DOI] [PubMed] [Google Scholar]
- Koler M, Achache H, Tsafrir A, Smith Y, Revel A, Reich R. 2009. Disrupted gene pattern in patients with repeated in vitro fertilization (IVF) failure. Hum Reprod 24: 2541–2548. [DOI] [PubMed] [Google Scholar]
- Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. 2010. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod 82: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarta E, Martínez-Conejero JA, Alamá P, Horcajadas JA, Pellicer A, Simón C, Bosch E. 2011. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: A functional genomics analysis. Hum Reprod 26: 1813–1825. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lee KF, Ng EH, Yeung WS, Ho PC. 2008. Gene expression profiling of human peri-implantation endometria between natural and stimulated cycles. Fertil Steril 90: 2152–2164. [DOI] [PubMed] [Google Scholar]
- Macklon NS, van der Gaast MH, Hamilton A, Fauser BC, Giudice LC. 2008. The impact of ovarian stimulation with recombinant FSH in combination with GnRH antagonist on the endometrial transcriptome in the window of implantation. Reprod Sci 15: 357–365. [DOI] [PubMed] [Google Scholar]
- Mane SP, Evans C, Cooper KL, Crasta OR, Folkerts O, Hutchison SK, Harkins TT, Thierry-Mieg D, Thierry-Mieg J, et al. 2009. Transcriptome sequencing of the Microarray Quality Control (MAQC) RNA reference samples using next generation sequencing. BMC Genomics 12: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki S. 2011. DNA microarray analysis in endometriosis for development of more effective targeted therapies. Front Biosci (Elite Ed) 3: 1139–1153. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Nikas G, Hsiu JG, Díaz J, Oehninger S. 2004. Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab 89: 5742–5752. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, Williams S, Bocca S, Oehninger S. 2005. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod 20: 2014–2117. [DOI] [PubMed] [Google Scholar]
- Mokhtar NM, Cheng CW, Cook E, Bielby H, Smith SK, Charnock-Jones DS. 2010. Progestin regulates chemokine (C–X–C motif) ligand 14 transcript level in human endometrium. Mol Hum Reprod 16: 170–177. [DOI] [PubMed] [Google Scholar]
- Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, Zeng D, Fritz MA. 2004. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril 81: 1333–1343. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Herting AT, Rock J. 1950. Dating the endometrial biopsy. Fertil Steril 1: 3–25. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Herting AT, Rock J. 1975. Dating the endometrial biopsy. Am J Obstet Gynecol 122: 262–263. [DOI] [PubMed] [Google Scholar]
- Okada H, Nakajima T, Sanezumi M, Ikuta A, Yasuda K, Kanzaki H. 2000. Progesterone enhances interleukin-15 production in human endometrial stromal cells in vitro. J Clin Endocrinol Metab 85: 4765–4770. [DOI] [PubMed] [Google Scholar]
- Petracco RG, Kong A, Grechukhina O, Krikun G, Taylor HS. 2012. Global gene expression profiling of proliferative phase endometrium reveals distinct functional subdivisions. Reprod Sci 19: 1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnampalam AP, Weston GC, Trajstman AC, Susil B, Rogers PA. 2004. Molecular classification of human endometrial cycle stages by transcriptional profiling. Mol Hum Reprod 10: 879–893. [DOI] [PubMed] [Google Scholar]
- Punyadeera C, Dassen H, Klomp J, Dunselman G, Kamps R, Dijcks F, Ederveen A, de Goeij A, Groothuis P. 2005. Oestrogen-modulated gene expression in the human endometrium. Cell Mol Life Sci 62: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid NA, Lalitkumar S, Lalitkumar PG, Gemzell-Danielsson K. 2011. Endometrial receptivity and human embryo implantation. Am J Reprod Immunol 66: 23–30. [DOI] [PubMed] [Google Scholar]
- Reis-Filho JS. 2009. Next-generation sequencing. Breast Cancer Res 11: S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel A, Achache H, Stevens J, Smith Y, Reich R. 2011. MicroRNAs are associated with human embryo implantation defects. Hum Reprod 26: 2830–2840. [DOI] [PubMed] [Google Scholar]
- Riesewijk A, Martin J, Van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simón C. 2003. Gene expression profiling of human endometrial receptivity on days LH +2 versus LH +7 by microarray technology. Mol Hum Reprod 9: 253–264. [DOI] [PubMed] [Google Scholar]
- Ruíz-Alonso M, Blesa D, Simón C. 2012. The genomics of the human endometrium. Biochim Biophys Acta 1822: 1931–1942. [DOI] [PubMed] [Google Scholar]
- Ruíz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simón C. 2013. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 100: 818–824. [DOI] [PubMed] [Google Scholar]
- Ruíz-Alonso M, Gómez E, Díaz-Gimeno P, Rincón-Bertolín A, Simón C. 2014. Clinical application of the endometrial receptivity array. Médecine de la Reproduction, Gynécologie Endocrinologie 16: 120–126. [Google Scholar]
- Salamonsen LA, Hannan NJ, Dimitriadis E. 2007. Cytokines and chemokines during human embryo implantation: Roles in implantation and early placentation. Semin Reprod Med 25: 437–444. [DOI] [PubMed] [Google Scholar]
- Simmen FA, Simmen RC. 2006. Orchestrating the menstrual cycle: Discerning the music from the noise. Endocrinology 147: 1094–1096. [DOI] [PubMed] [Google Scholar]
- Simon C, Gimeno MJ, Mercader A, O’Connor JE, Remohi J, Polan ML, Pellicer A. 1997. Embryonic regulation of integrins β3, α4, and α1 in human endometrial epithelial cells in vitro. J Clin Endocrinol Metab 82: 2607–2616. [DOI] [PubMed] [Google Scholar]
- Simon C, Oberye J, Bellver J, Vidal C, Bosch E, Horcajadas JA, Murphy C, Adams S, Riesewijk A, Mannaerts B, et al. 2005. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod 20: 3318–3327. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. 2006. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147: 1097–1121. [DOI] [PubMed] [Google Scholar]
- Tapia A, Gangi LM, Zegers-Hochschild F, Balmaceda J, Pommer R, Trejo L, Pacheco IM, Salvatierra AM, Henriquez S, Quezada M, et al. 2008. Differences in the endometrial transcript profile during the receptive period between women who were refractory to implantation and those who achieved pregnancy. Hum Reprod 23: 340–351. [DOI] [PubMed] [Google Scholar]
- Tseng LH, Chen I, Chen MY, Yan H, Wang CN, Lee CL. 2010. Genome-based expression profiling as a single standardized microarray platform for the diagnosis of endometrial disorder: An array of 126-gene model. Fertil Steril 94: 114–119. [DOI] [PubMed] [Google Scholar]
- Ulbrich SE, Groebner AE, Bauersachs S. 2013. Transcriptional profiling to address molecular determinants of endometrial receptivity–Lessons from studies in livestock species. Methods 59: 108–115. [DOI] [PubMed] [Google Scholar]
- Van Vaerenbergh I, McIntire R, Van Lommel L, Devroey P, Giudice L, Bourgain C. 2010. Gene expression during successful implantation in a natural cycle. Fertil Steril 93: 268.e15–268.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vaerenbergh I, Fatemi HM, Blockeel C, Van Lommel L, In’t Veld P, Schuit F, Kolibianakis EM, Devroey P, Bourgain C. 2011. Progesterone rise on HCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online 22: 263–271. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, et al. 2001. The sequence of the human genome. Science 291: 1304–1351. [DOI] [PubMed] [Google Scholar]
- Wang B, Sheng JZ, He RH, Qian YL, Jin F, Huang HF. 2008. High expression of l-selectin ligand in secretory endometrium is associated with better endometrial receptivity and facilitates embryo implantation in human being. Am J Reprod Immunol 60: 127–134. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. 2009. RNA-Seq: A revolutionary tool for transcriptomics. Nat Rev Genet 10: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaihara A, Otsuka Y, Iwasaki S, Aida T, Tachikawa T, Irie T, Okai T. 2005. Differences in gene expression in the proliferative human endometrium. Fertil Steril 83: 1206–1215. [DOI] [PubMed] [Google Scholar]