Abstract
INTRODUCTION:
The 2011 national guidelines for the management of childhood community-acquired pneumonia (CAP) recommended narrow-spectrum antibiotics (eg, ampicillin) for most children hospitalized with CAP. We assessed the impact of these guidelines on antibiotic prescribing at 3 children’s hospitals.
METHODS:
Children hospitalized with clinical and radiographic CAP were enrolled from January 1, 2010, through June 30, 2012, at 3 hospitals in Tennessee and Utah as part of the Centers for Disease Control and Prevention Etiology of Pneumonia in the Community study. Antibiotic selection was determined by the treating provider. The impact of the guidelines and hospital-level implementation efforts was determined by assessing the monthly percentage of enrolled children receiving third-generation cephalosporins or penicillin/ampicillin. Segmented linear regression was used to compare observed antibiotic selection in the postguideline period with expected antibiotic use projected from preguideline months.
RESULTS:
Overall, 2121 children were included. During the preguideline period, 52.8% (interquartile range 47.8–56.6) of children with CAP received third-generation cephalosporins, whereas 2.7% (2.1, 7.0) received penicillin/ampicillin. By 9 months postguidelines, third-generation cephalosporin use declined (absolute difference −12.4% [95% confidence interval −19.8% to −5.1%]), whereas penicillin/ampicillin use increased (absolute difference 11.3% [4.3%–18.3%]). The most substantial changes were noted at those institutions that implemented guideline-related dissemination activities.
CONCLUSIONS:
After publication of national guidelines, third-generation cephalosporin use declined and penicillin/ampicillin use increased among children hospitalized with CAP. Changes were more apparent among those institutions that proactively disseminated the guidelines, suggesting that targeted, hospital-based efforts are important for timely implementation of guideline recommendations.
What’s Known on This Subject:
The 2011 national guidelines for the management of pediatric community-acquired pneumonia recommended narrow-spectrum antibiotic therapy (eg, ampicillin) for most children hospitalized with pneumonia. Before the release of the guidelines, the use of broader-spectrum antibiotics (eg, third-generation cephalosporins) was much more common.
What This Study Adds:
After release of the guidelines, third-generation cephalosporin use declined and penicillin/ampicillin use increased among children hospitalized with pneumonia. Changes were most apparent among institutions that proactively disseminated the guidelines, underscoring the importance of local efforts for timely guideline implementation.
To assist clinicians in caring for children with community-acquired pneumonia (CAP), a committee convened by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) comprehensively reviewed best available evidence for the management of childhood CAP, culminating in the release of the first national consensus management guidelines in August 2011.1 Antibiotic selection was 1 of several major areas targeted by the committee. Recommendations emphasized the use of a single, narrow-spectrum antibiotic (ie, penicillin/ampicillin) for vaccinated children hospitalized with uncomplicated CAP. Evidence cited in support of this recommendation included the finding that Streptococcus pneumoniae was the most common bacterial pathogen causing pediatric CAP,2–4 observed declines in penicillin-resistant pneumococcal disease after conjugate vaccine introduction,5–8 and demonstrated effectiveness of high-dose penicillin therapy for relatively resistant pneumococcal infections outside the central nervous system.9,10
Despite the strength of evidence supporting narrow-spectrum therapy, broader-spectrum antibiotics were much more commonly used before the release of the guidelines. A recent study demonstrated that third-generation cephalosporins accounted for nearly 90% of prescribing for CAP at 29 U.S. children’s hospitals between 2005 and 2010.11 Thus, the recommendation for narrow-spectrum antibiotic therapy advocated a major shift in practice.
This study sought to assess the impact of the 2011 PIDS/IDSA guidelines on antibiotic prescribing for children hospitalized with CAP at 3 U.S. children’s hospitals. Because hospital-level efforts have been shown to be important for guideline implementation and altering prescribing practices,12–16 we also explored the impact of guideline-related, individual hospital-based initiatives.
Methods
Study Population
This study was nested within the Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study, a prospective, population-based, active surveillance of CAP hospitalizations among children (<18 years old) conducted between January 1, 2010, and June 30, 2012, in 3 US hospitals (Le Bonheur Children’s Hospital, Memphis, Tennessee; Primary Children’s Medical Center, Salt Lake City, Utah; and Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tennessee).17 Informed consent was obtained before enrollment. The study protocol was approved by the institutional review board of each study hospital and the CDC.
Children were enrolled in the EPIC study if they resided in a predefined catchment area and were hospitalized at a study hospital with (1) signs or symptoms of acute infectious illness (eg, abnormal temperature); (2) acute respiratory signs or symptoms (eg, cough); and (3) radiographic evidence of pneumonia.17 Children with recent hospitalization, significant immunosuppression, cystic fibrosis, and tracheostomy were excluded. Those with a clear alternative diagnosis (eg, acute pulmonary embolism) were also excluded. To standardize the determination of radiographic CAP, enrollment films were independently reviewed by a study radiologist at each site. For this study, the EPIC cohort was further restricted by excluding children <3 months of age and those not receiving antibiotics. Child and/or caregiver interviews (collecting sociodemographic characteristics, medical history, and history of present illness) and detailed medical record reviews (capturing medical history and hospitalization course and outcomes, in-hospital antibiotic usage, and laboratory data) were conducted for all enrolled children.
Outcomes
The primary outcome was the monthly percentage of children treated with a third-generation cephalosporin, including ceftriaxone, cefotaxime, or cefdinir. For each calendar month, this percentage was calculated by dividing the number of children receiving a third-generation cephalosporin with or without a macrolide during the first 2 days of hospitalization by the total number of enrolled children with CAP. Because the guidelines recommended the use of parenteral penicillin or ampicillin (or oral amoxicillin), we also evaluated the monthly percentage of children receiving these agents with or without a macrolide during the first 2 hospital days as a secondary outcome. We also examined trends in concurrent use of macrolides. Children receiving both a third-generation cephalosporin and penicillin/ampicillin and those receiving other antibiotics during the first 2 days of hospitalization were included for descriptive purposes only, because use of these agents would likely be reserved for circumstances that fell outside the scope of our study.
Exposure
The study exposure was time from the release of the PIDS/IDSA guidelines, expressed in months. Electronic publication of the guideline occurred on August 30, 2011, followed by print publication on October 1, 2011. EPIC enrollment encompassed 30 months from January 2010 through June 2012. The study period was divided into preguideline (20 months before September 1, 2011) and postguideline (9 months starting on October 1, 2011) periods. September 2011 was considered a transition period and excluded from analyses.
Statistical Analysis
We assessed the impact of the PIDS/IDSA CAP guidelines on empirical antibiotic selection using an interrupted time-series analysis, the reference standard for evaluating the impact of policies or programs.18,19 Segmented linear regression models were used to determine trends in the monthly percentages of children receiving third-generation cephalosporins or penicillin/ampicillin during both the pre- and postguideline periods. Autoregressive integrated moving average models were used to account for first-order autocorrelation in the error terms of consecutive observations. Because observations may be clustered at the hospital level, we used robust estimators of variance. We also seasonally adjusted the estimates because of known increases in pneumonia incidence during winter months.20 This same approach was used for our exploration of trends in concurrent macrolide use.
We anticipated a gradual uptake of the PIDS/IDSA guideline recommendations. Therefore, to quantify the cumulative impact of the guidelines and hospital-based efforts on empirical antibiotic selection, we compared the observed antibiotic prescribing by the end of the study period (9 months postguideline) with the expected antibiotic prescribing by the end of the study period had the guideline not been released (projected trend from the preguideline period).18,19
Sensitivity analyses to assess the robustness of our findings included restricting the cohort to children with independent radiographic confirmation of pneumonia (all children enrolled in the EPIC study had a chest radiograph consistent with pneumonia, although 10% lacked independent radiographic confirmation by a study radiologist), as well as analyses adjusting for temporal changes (aggregated by unit of analysis) in the proportion of children younger than 5 years, with comorbidities, direct admission to intensive care, presence of effusion on admission chest radiograph, and median length of hospital stay.
Subgroup analyses by hospital were also conducted to evaluate the potential differential impact of the PIDS/IDSA guidelines through individual hospital-level guideline-related initiatives. To assess for hospital-level efforts in response to publication of the PIDS/IDSA guidelines, we queried investigators from each study site (randomly designated as Hospitals A, B, and C) regarding the nature and timing of organized hospital-level activities pertaining to dissemination of the guidelines (eg, education campaigns) as well as other general activities related to antibiotic use (eg, antimicrobial stewardship program, local CAP clinical practice guidelines, or CAP order sets).
Results
Characteristics of the Study Population
There were 2628 children with CAP enrolled in the EPIC study. We excluded 507 children (19%) who did not receive antibiotics or were <3 months of age; 2121 children constituted the final study population. The median number of children enrolled in each month of the study was 65 (interquartile range [IQR] 47–93). Median age was 2 years (IQR 1–6); 55% were male; 56% were white; and 18% were Hispanic. Fifty-two percent of children had ≥1 comorbidities (asthma [34%] was most common). The baseline characteristics of enrolled children were similar before and after the release of the guidelines, with only minor differences noted between the periods (Table 1). Characteristics of the study population at each of the 3 study hospitals are shown in Table 2.
TABLE 1.
Characteristics of Participating Children Hospitalized With CAP, January 2010 to June 2012
| Characteristic | Preguidelines | Postguidelines |
|---|---|---|
| n | 1303 | 772 |
| No. enrolled per mo | 61 (44, 78) | 93 (65, 108) |
| Demographics | ||
| Age, yrs | 2 (1, 6) | 3 (1, 7) |
| Male gender | 55 | 53 |
| White race | 57 | 56 |
| Hispanic ethnicity | 18 | 19 |
| Clinical | ||
| Any comorbiditya | 52 | 51 |
| Asthma | 35 | 34 |
| Prematurity | 9 | 8 |
| Congenital heart disease | 7 | 7 |
| Seizure disorder | 6 | 6 |
| Other | 8 | 7 |
| Direct ICU admission | 12 | 14 |
| Pleural effusion | 15 | 12 |
| Length of stay, h | 68 (43, 112) | 61 (42, 105) |
Values are expressed as median (IQR) or %. Children enrolled in September 2011 were excluded (n = 46).
Groups are not mutually exclusive; “other” includes individual comorbidities present in <5% of the cohort.
TABLE 2.
Characteristics of Participating Children Hospitalized With CAP by Hospital, January 2010 to June 2012
| Characteristic | Hospital A | Hospital B | Hospital C |
|---|---|---|---|
| No. enrolled | 823 | 666 | 632 |
| No. admissions/mo | 27 (17, 34) | 17 (13, 30) | 18 (11, 30) |
| Demographics | |||
| Age, yrs | 2 (1, 5) | 3 (1, 7) | 2 (1, 7) |
| Male gender | 57 | 53 | 52 |
| White race | 29 | 65 | 84 |
| Hispanic ethnicity | 8 | 19 | 30 |
| Clinical | |||
| Any comorbidity | 58 | 57 | 38 |
| Direct ICU admission | 9 | 17 | 13 |
| Pleural effusion | 9 | 12 | 23 |
| Length of stay, h | 67 (45, 112) | 55 (38, 95) | 71 (42, 125) |
| Antibiotic therapy preguidelinesa | |||
| Third-generation cephalosporins (without/with macrolide) | |||
| Without macrolide | 45 | 37 | 33 |
| With macrolide | 15 | 20 | 9 |
| Penicillin/ampicillin (without/with macrolide) | |||
| Without macrolide | 1 | 4 | 9 |
| With macrolide | <1 | <1 | <1 |
| Other β-lactams | 5 | 11 | 13 |
| Vancomycin/clindamycinb | 27 | 23 | 24 |
| Other | 6 | 5 | 12 |
| Guideline-related hospital activities | |||
| Educational conferences | No | January 18, 2012 | November 10, 2011 |
| Antibiotic recommendations disseminated to faculty | No | April 12, 2012 | No |
| Other hospital activities | |||
| Antimicrobial stewardship program | Yes, July 2011 | Yes, continuous | Yes, continuous |
| CAP clinical practice guideline | No | No | No |
| CAP electronic order set | Yesc | Yesc | No |
Values are expressed as median (IQR) or %.
Median monthly percentage of use during the period preguidelines (January 2010 to August 2011).
Monotherapy or in combination with other agents.
Order sets at both institutions recommended third-generation cephalosporins as first-line therapy and did not change during the study period.
Antibiotic Prescribing in the Preguideline Period
In the preguideline period, third-generation cephalosporins were the most commonly prescribed antibiotic class for children at all 3 hospitals (range 43%–61%), whereas penicillin/ampicillin was much less commonly prescribed (range 1%–9%); concurrent macrolide use was more common among those receiving third-generation cephalosporins (Table 2). Overall, Hospital A had the highest proportion of children receiving third-generation cephalosporins and the lowest proportion of children receiving penicillin/ampicillin.
Changes in Antibiotic Prescribing after Publication of PIDS/IDSA Guidelines
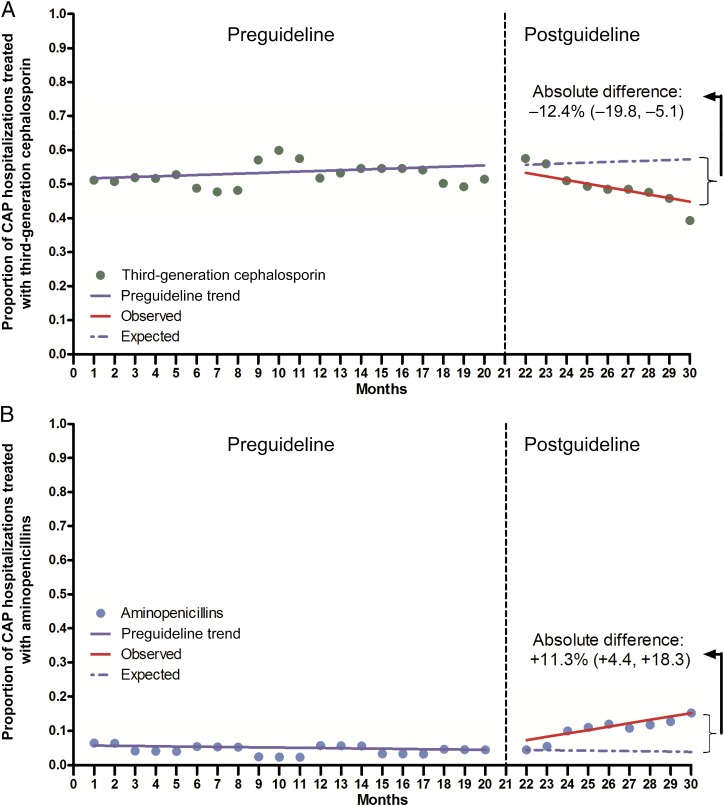
During the preguideline period, use of third-generation cephalosporins and penicillin/ampicillin was stable, with monthly median proportions of 52.8% (IQR 47.8%–56.6%) and 2.7% (IQR 2.1%–7.0%), respectively (Fig 1). During the postguideline period, the proportion of children who received empirical treatment with third-generation cephalosporins progressively declined, whereas penicillin/ampicillin use increased. By the end of the study period, and compared with the expected use estimated from the preguidelines trend, we noted an absolute decrease of −12.4% (95% confidence interval [CI] −19.8% to −5.1%) for third-generation cephalosporin use and an absolute increase of 11.3% (95% CI 4.4% to 18.3%) for penicillin/ampicillin use (Table 3). Results from more complex models and sensitivity analyses were essentially identical to our main findings (Table 4). An exploration of concurrent macrolide use with either a third-generation cephalosporin or penicillin/ampicillin demonstrated an increasing trend in the preguideline period and a decreasing trend in the postguideline period, resulting in an absolute decrease of −14.4% (95% CI −23.6% to −5.2%) by the end of the study period (Supplemental Fig 2). Macrolide monotherapy was uncommon in both the preguideline (3.5%) and postguideline (4.4%) periods. Prescribing of other antibiotic agents was also relatively stable (data not shown).
FIGURE 1.
Impact of national guidelines on prescribing of third-generation cephalosporins (A) and penicillin/ampicillin (B) for pediatric CAP, January 2010 to June 2012. Seasonally adjusted estimates of third-generation cephalosporin (A, green) and penicillin/ampicillin (B, blue) use among children hospitalized with CAP at 3 US children’s hospitals before and after the release of national guidelines; the vertical dashed line indicates the month the guidelines were first released (August 30, 2011); the pre- and postguideline periods included January 2010 to August 2011 and October 2011 to June 2012, respectively; absolute difference (%) was calculated by comparing the observed antibiotic prescribing patterns by the end of the study period (estimated from the postguideline regression model) with the expected antibiotic prescribing by the end of the study period had the guideline not been released (estimated from the preguideline trend).
TABLE 3.
Changes in Empirical Antibiotic Selection for Children Hospitalized With CAP at 3 US Children’s Hospitals After Release of National Consensus Guidelines, January 2010 to June 2012
| Site | Empirical Antibiotic Selection | |||||
|---|---|---|---|---|---|---|
| Third-Generation Cephalosporin | Penicillin/Ampicillin | |||||
| Observed Use, % | Expected Use, % | Absolute Difference, % (95% CI) | Observed Use, % | Expected Use, % | Absolute Difference, % (95% CI) | |
| Hospital A | 60.0 | 68.2 | −8.2 (−23.6 to 7.2) | 2.6 | 0.6 | 2.0 (−2.0 to 6.0) |
| Hospital B | 21.4 | 49.1 | −27.6 (−43.6 to −11.7) | 23.7 | 3.3 | 20.4 (10.6 to 30.3) |
| Hospital C | 30.6 | 48.0 | −17.3 (−29.2 to −5.4) | 29.5 | 5.4 | 24.1 (−6.2 to 54.5) |
| All sites | 44.8 | 57.3 | −12.4 (−19.8 to −5.1) | 15.2 | 3.9 | 11.3 (4.4 to 18.3) |
Absolute difference was calculated by comparing the observed antibiotic prescribing patterns by the end of the study period (observed use estimated from the postguideline regression model) with the expected antibiotic prescribing by the end of the study period had the guideline not been released (expected use estimated from the preguideline trend).
TABLE 4.
Sensitivity Analyses: Reduction in Selection of Third-generation Cephalosporins for Empirical Treatment of Children With CAP After Release of National Guidelines
| Original and Sensitivity Analyses | Absolute Difference, % (95% CI) |
|---|---|
| Original estimation | −12.4 (−19.8 to −5.1) |
| Estimated from last month of preintervention | −10.6 (−16.3 to −5.0) |
| Restricted to independently confirmed radiographic pneumoniaa | −16.2 (−25.7 to −6.6) |
| Adjusted for proportion <5 yrs old | −13.6 (−27.0 to −0.2) |
| Adjusted for total no. of hospitalizations | −13.7 (−21.8 to −5.7) |
| Adjusted for proportion with comorbidity | −12.6 (−20.4 to −4.8) |
| Adjusted for pleural effusion | −12.6 (−20.3 to −4.9) |
| Adjusted for median length of stay | −11.2 (−22.2 to −2.5) |
| Adjusted for direct admission to intensive care | −12.7 (−21.8 to −3.7) |
Absolute difference was calculated by comparing the observed antibiotic prescribing patterns by the end of the study period (estimated from the postguideline regression model) with the expected antibiotic prescribing by the end of the study period had the guideline not been released (estimated from the preguideline trend).
All children enrolled in the EPIC study had a chest radiograph consistent with pneumonia, although 10% lacked independent radiographic confirmation by a study radiologist.
PIDS/IDSA Guidelines Dissemination and Subgroup Analyses by Hospital
Within 4 months of their release, the PIDS/IDSA prescribing recommendations were highlighted during 1 or more pediatric departmental educational conferences at hospitals B and C (Table 2). In addition, at Hospital B, the recommendation for penicillin/ampicillin use was endorsed by the Infectious Diseases Division and was disseminated to all pediatric faculty by E-mail. Hospital A did not implement formal dissemination efforts after the release of the guidelines during the study period. All 3 hospitals reported the presence of an antimicrobial stewardship program, although none specifically targeted CAP or restricted the use of third-generation cephalosporins, aminopenicillins, or macrolides. Hospitals B and C reported the presence of a stewardship program continuously during the EPIC study period, whereas Hospital A’s program was established in July 2011. Hospitals A and B reported the presence of an electronic order set for CAP that recommended third-generation cephalosporin therapy for the duration of the EPIC study period; Hospital C did not have a CAP order set. None of the hospitals had a local CAP practice guideline during the study period.
By the end of the EPIC study, and compared with the expected antibiotic use from the preguideline period, all study hospitals showed declining trends in the use of third-generation cephalosporins. However, the declines were statistically significant only in the 2 hospitals that implemented efforts to disseminate the PIDS/IDSA guidelines (Hospital B absolute difference −27.6% [95% CI −43.6% to −11.7%]; Hospital C absolute difference −17.3% [95% CI −29.2% to −5.4%]), whereas in Hospital A the decline in third-generation cephalosporin use was modest and not statistically significant (absolute difference −8.2% [95% CI −23.6% to 7.2%]). Hospital B demonstrated a significant increase in penicillin/ampicillin use (absolute difference 20.4% [95% CI 10.6% to 30.3%]), whereas hospitals A and C showed increases that were not statistically significant (absolute difference 2.0% [95% CI −2.0% to 6.0%] and 24.1% [95% CI −6.2% to 54.5%], respectively) (Table 3). We also noted declines in concurrent macrolide use in each of the 3 hospitals, although declines were not statistically significant (Supplemental Table 5).
Discussion
Our study demonstrates changes in antibiotic selection among children hospitalized with CAP at 3 U.S. institutions after publication of the PIDS/IDSA guidelines for the management of childhood CAP. Overall, use of third-generation cephalosporins declined significantly after release of the guidelines, whereas penicillin/ampicillin use increased. We noted consistent trends across study sites, although changes were most apparent in institutions that conducted active hospital-based educational efforts to disseminate the PIDS/IDSA guidelines.
Third-generation cephalosporins were the most commonly used antibiotics before release of the PIDS/IDSA guidelines, accounting for approximately half of antibiotic prescribing, with stable rates, during the preguideline period of the study. Their use declined in the postguideline period, accounting for 44.8% of prescribing by the end of the EPIC study. The use of penicillin/ampicillin increased significantly from a baseline of 2.7% in the preguideline period to 15.2% by the end of the EPIC study. Thus, the observed changes were temporally associated with the publication of the PIDS/IDSA guidelines and consistent with the recommendation of penicillin/ampicillin instead of broader-spectrum third-generation cephalosporins for most children hospitalized with pneumonia. Concurrent macrolide use also declined; macrolides were more commonly combined with third-generation cephalosporins than with penicillin/ampicillin. Although macrolides are often used for presumed atypical CAP, several studies have raised concerns regarding increasing macrolide use and development of antibiotic resistance.21–23 Whereas adult guidelines recommend macrolide/cephalosporin combination therapy for CAP,24 the PIDS/IDSA guidelines do not recommend routine macrolide combination therapy for children. Rather, the guidelines suggest consideration of macrolide combination therapy only in specific circumstances, although this recommendation was rated as weak and based on moderate-quality evidence. Therefore, this decline in concurrent macrolide use may also reflect practice changes as a result of publication of the PIDS/IDSA guidelines.
Changes in antibiotic selection after release of the guidelines appear to vary by hospital. Although we could not assess the penetration of formal initiatives or characterize informal activities at each hospital, the 2 institutions that incorporated active, hospital-based educational efforts demonstrated the largest reductions in third-generation cephalosporin use along with the largest increases in penicillin/ampicillin use. At both hospitals, penicillin/ampicillin use was equivalent to third-generation cephalosporin use by the end of the study period. Dissemination efforts at these 2 hospitals were similar and included departmental educational activities shortly after release of the guidelines. In contrast, the hospital without active educational efforts showed more modest declines in prescribing of third-generation cephalosporins and increases in penicillin/ampicillin use that did not reach statistical significance. These findings suggest that active hospital-based efforts are important for rapid implementation of guideline recommendations. However, room for improvement remains. In spite of the observed declines, third-generation cephalosporins were still prescribed for 44.8% of children by the end of the study period (although in some circumstances broader-spectrum therapy was appropriate, eg, critical illness).
Previous studies document wide institutional variability in the management of children hospitalized with CAP,11,25 a phenomenon also observed in our study. Such variation has the potential to contribute to disparities in outcomes, quality of care, and hospitalization costs. The development of consensus national guidelines is a key step toward reducing variation and facilitating best practices. Nevertheless, national recommendations may be insufficient to induce rapid practice change. A retrospective study of 38 US children’s hospitals demonstrated only modest increases in PIDS/IDSA guideline-concordant prescribing for CAP ≤18 months postguidelines.25 Reasons might include lack of awareness and/or agreement with content, poor evidence quality or ambiguous recommendations, clinical inertia (prior practice), organizational barriers, and absence of consequences for discordant management strategies.26,27 These barriers must be addressed to ensure widespread and effective implementation of guidelines in local environments.
Local, hospital-based activities, such as management guidelines and order sets, may improve adherence to national recommendations. A 2012 study of 41 US children’s hospitals reported that less than one-third had a CAP practice guideline.13 Among those hospitals with a guideline recommending penicillin-based therapy (n = 7), 46% of children received penicillin/ampicillin compared with 24% of children at hospitals without a practice guideline.13 Experiences at individual hospitals have been similar.12,28 Multilevel interventions may be most effective for influencing physician prescribing behavior.29,30 Ambroggio et al demonstrated that the development of a hospital-based practice guideline, accompanied with an educational campaign and modification of an existing CAP order set, increased guideline-concordant antibiotic prescribing for children hospitalized with CAP from 30% to 100% within 6 months.16 Importantly, none of the participating hospitals in our study had a hospital-based CAP management guideline during the study period. In addition, existing order sets in hospitals A and B were not modified after release of the PIDS/IDSA guidelines and continued to recommend third-generation cephalosporins. It is likely that additional efforts, such as the introduction of local practice guidelines and revised order sets at the study hospitals, would have prompted more substantial changes in antibiotic prescribing during the study period.
Our findings must be interpreted in light of several limitations. First, this is an ecologic study that evaluated the impact of national guidelines on antibiotic selection for children hospitalized with CAP in real-world settings. We did not randomize hospitals to different interventions and did not analyze individual provider practices to ascertain awareness of the guidelines’ recommendations or individual preferences for antibiotic prescribing. Instead, our analyses used the strongest quasi-experimental design for program/policy evaluations18 and analyzed data that were prospectively collected and aggregated by month to assess the impact of the guidelines. The EPIC study period allowed us to evaluate early changes up to 9 months after release of the guidelines. Although we documented encouraging and rapid initial changes in antibiotic selection, whether the observed changes were sustained beyond the study period is unknown. We observed a significant change in antibiotic selection closely related to release of the guidelines and in coordination with local efforts; however, the impact of other concurrent interventions cannot be ruled out. Finally, our study is restricted to 3 large academic children’s hospitals that participated in a prospective CDC-sponsored project; our results may not be generalizable to other settings. Nevertheless, our study complements and expands on previous retrospective assessments using administrative data, by applying rigorous selection criteria to prospectively identify CAP hospitalizations and collecting detailed hospital-level information of dissemination efforts.
Conclusions
After publication of national consensus guidelines for management of CAP in children, use of third-generation cephalosporins declined and penicillin/ampicillin use increased at 3 large pediatric hospitals, consistent with the guidelines’ recommendations. However, the magnitude and speed of changes in prescribing varied by hospital and were more substantial at those institutions that proactively disseminated the recommendations promoted by the guidelines. Additional studies in a variety of settings are needed to monitor the spread and long-term sustainability of these initial encouraging observations and to identify the most effective hospital-based strategies to facilitate rapid implementation of national guidelines.
Supplementary Material
Acknowledgments
The authors thank the children and families who graciously consented to participate in the EPIC study. We also thank Shanda Phillips, RN, BSN, Chris Stockmann, MSc, Jody Cockcroft, BS, CCRP, Lyn Finelli, DrPH, MS, and all of the members of the EPIC team, as well as LeBonheur Children’s Hospital, Memphis, Tennessee; Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tennessee; and Primary Children’s Medical Center, Salt Lake City, Utah.
Glossary
- CAP
community-acquired pneumonia
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- EPIC
Etiology of Pneumonia in the Community
- IDSA
Infectious Diseases Society of America
- IQR
interquartile range
- PIDS
Pediatric Infectious Diseases Society
Footnotes
Drs Williams, Zhu, and Grijalva participated in data analysis; Drs Williams and Grijalva drafted the initial manuscript; and all authors participated in conceptualization and study design, interpretation of results, critical review and manuscript revision, and review and approval of final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award K23AI104779 to Dr Williams and the Agency for HealthCare Research and Quality under Award 1R03HS022342 to Dr Grijalva. The Etiology of Pneumonia in the Community (EPIC) study was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases at the Centers for Disease Control and Prevention through cooperative agreements with each study site and was based on a competitive research funding opportunity. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bradley JS, Byington CL, Shah SS, et al. Pediatric Infectious Diseases Society and the Infectious Diseases Society of America . The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113(4). Available at: www.pediatrics.org/cgi/content/full/113/4/e701 [DOI] [PubMed] [Google Scholar]
- 3.Juvén T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19(4):293–298 [DOI] [PubMed] [Google Scholar]
- 4.Heiskanen-Kosma T, Korppi M, Jokinen C, et al. Etiology of childhood pneumonia: serologic results of a prospective, population-based study. Pediatr Infect Dis J. 1998;17(11):986–991 [DOI] [PubMed] [Google Scholar]
- 5.Pilishvili T, Lexau C, Farley MM, et al. Active Bacterial Core Surveillance/Emerging Infections Program Network . Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41 [DOI] [PubMed] [Google Scholar]
- 6.Kyaw MH, Lynfield R, Schaffner W, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network . Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455–1463 [DOI] [PubMed] [Google Scholar]
- 7.CDC. Effects of new penicillin susceptibility breakpoints for Streptococcus pneumoniae—United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2008;57(50):1353–1355 [PubMed]
- 8.Hampton LM, Farley MM, Schaffner W, et al. Prevention of antibiotic-nonsusceptible Streptococcus pneumoniae with conjugate vaccines. J Infect Dis. 2012;205(3):401–411 [DOI] [PubMed] [Google Scholar]
- 9.Dagan R, Hoberman A, Johnson C, et al. Bacteriologic and clinical efficacy of high dose amoxicillin/clavulanate in children with acute otitis media. Pediatr Infect Dis J. 2001;20(9):829–837 [DOI] [PubMed] [Google Scholar]
- 10.Weinstein MP, Klugman KP, Jones RN. Rationale for revised penicillin susceptibility breakpoints versus Streptococcus pneumoniae: coping with antimicrobial susceptibility in an era of resistance. Clin Infect Dis. 2009;48(11):1596–1600 [DOI] [PubMed] [Google Scholar]
- 11.Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3). Available at: www.pediatrics.org/cgi/content/full/129/3/e597 [DOI] [PubMed] [Google Scholar]
- 13.Neuman MI, Hall M, Hersh AL, et al. Influence of hospital guidelines on management of children hospitalized with pneumonia. Pediatrics. 2012;130(5). Available at: www.pediatrics.org/cgi/content/full/130/5/e823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntosh KA, Maxwell DJ, Pulver LK, et al. A quality improvement initiative to improve adherence to national guidelines for empiric management of community-acquired pneumonia in emergency departments. Int J Qual Health Care. 2011;23(2):142–150 [DOI] [PubMed]
- 15.Evans CT, Weaver FM, Rogers TJ, et al. Guideline-recommended management of community-acquired pneumonia in veterans with spinal cord injury. Top Spinal Cord Inj Rehabil. 2012;18(4):300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambroggio L, Thomson J, Murtagh Kurowski E, et al. Quality improvement methods increase appropriate antibiotic prescribing for childhood pneumonia. Pediatrics 2013;131(5). Available at: www.pediatrics.org/cgi/content/full/131/5/e1623 [DOI] [PMC free article] [PubMed]
- 17.Jain S, Williams DJ, Arnold SR, et al. CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309 [DOI] [PubMed] [Google Scholar]
- 19.Self WH, Speroff T, Grijalva CG, et al. Reducing blood culture contamination in the emergency department: an interrupted time series quality improvement study. Acad Emerg Med. 2013;20(1):89–97 [DOI] [PMC free article] [PubMed]
- 20.Kurian BT, Ray WA, Arbogast PG, Fuchs DC, Dudley JA, Cooper WO. Effect of regulatory warnings on antidepressant prescribing for children and adolescents. Arch Pediatr Adolesc Med. 2007;161(7):690–696 [DOI] [PubMed] [Google Scholar]
- 21.Kronman MP, Hersh AL, Feng R, Huang YS, Lee GE, Shah SS. Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994-2007. Pediatrics. 2011;127(3). Available at: www.pediatrics.org/cgi/content/full/127/3/e411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6). Available at: www.pediatrics.org/cgi/content/full/128/6/e1053 [DOI] [PubMed] [Google Scholar]
- 23.Hicks LA, Chien YW, Taylor TH, Jr, Haber M, Klugman KP, Active Bacterial Core Surveillance (ABCs) Team . Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996-2003. Clin Infect Dis. 2011;53(7):631–639 [DOI] [PubMed] [Google Scholar]
- 24.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America. American Thoracic Society . Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross RK, Hersh AL, Kronman MP, et al. Impact of Infectious Diseases Society of America/Pediatric Infectious Diseases Society guidelines on treatment of community-acquired pneumonia in hospitalized children. Clin Infect Dis. 2014;58(6):834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lugtenberg M, Zegers-van Schaick JM, Westert GP, Burgers JS. Why don’t physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465 [DOI] [PubMed] [Google Scholar]
- 28.Smith MJ, Kong M, Cambon A, Woods CR. Effectiveness of antimicrobial guidelines for community-acquired pneumonia in children. Pediatrics. 2012;129(5). Available at: www.pediatrics.org/cgi/content/full/129/5/e1326 [DOI] [PubMed] [Google Scholar]
- 29.Francke AL, Smit MC, de Veer AJ, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boaz A, Baeza J, Fraser A, European Implementation Score Collaborative Group (EIS) . Effective implementation of research into practice: an overview of systematic reviews of the health literature. BMC Res Notes. 2011;4:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.