Abstract
Objective: Conventional open repair for proximal subclavian artery aneurysms (SCAAs) requires cardiopulmonary bypass. However, patients with proximal SCAA can be treated with hybrid repair.
Methods: Between 2007 and 2012, we performed hybrid repair to treat six consecutive patients with proximal SCAA (three left SCAAs, one right aberrant SCAA, two right SCAAs). Their median age was 73.5 [70–87] years, and the size of their aneurysm was 33.5 [30–45] mm. Thoracic endovascular aneurysm repair (TEVAR) only was used for one patient with left SCAA, TEVAR and supra-aortic bypass for two with left SCAA and one with right aberrant SCAA, and endovascular repair with reconstruction of the vertebral artery using the saphenous vein graft (SVG) for two with right SCAA.
Results: The follow-up duration was 3.7 [0.2–6.8] years. There was no 30-day mortality and only one early complication consisting of a minor stroke after TEVAR for shaggy aorta. Two late deaths occurred, one caused by cerebral infarction due to occlusion of SVG to the dominant vertebral artery 2 months after the operation and the other by aortic dissection 5 years postoperatively.
Conclusions: Hybrid repair can be a less-invasive alternative for proximal SCAA. Revascularization of neck vessels and TEVAR should be performed very carefully to prevent neurologic complications.
Keywords: subclavian artery, aneurysm, endovascular, vertebral artery
Introduction
Subclavian artery aneurysms (SCAAs) are relatively uncommon aneurysms of the peripheral arteries. The major location of SCAA is a proximal segment of the subclavian artery. The most common etiology of proximal SCAAs is atherosclerosis.1) Even though they are peripheral arterial aneurysms, conventional surgical treatment requires median sternotomy or thoracotomy to access and cardiopulmonary bypass to repair the aneurysm.2–4) To avoid such excessive invasiveness for the treatment of peripheral aneurysms, recent studies have focused on a less invasive procedure utilizing endografting.1,2,5) If SCAAs are close to the cerebral arteries including carotid arteries and vertebral arteries, they need to be revascularized before endografting to minimize the risk of neurologic events. This report concerns six patients who underwent such surgical treatments of proximal SCAAs associated with atherosclerosis.
Materials and Methods
Patients
Between 2007 and 2012, six consecutive patients with isolated proximal SCAA underwent hybrid repair in a single center. The demographics of the six patients examined in this study (median age: 73.5 [70–87] years old, five males) are summarized in Table 1. Proximal SCAAs were located in the left SCA of three patients, in the right aberrant SCA of one, and in the right SCA of two. In two patients, the vertebral artery was involved in right SCAAs. The median size of the aneurysm was 33.5 [30–45] mm. All patients were asymptomatic and their diagnosis was occasionally based on CT scan finding. Five patients had the history of repair of abdominal aortic aneurysm and two patients had coronary artery bypass grafting. Five patients had some comorbidities. One patient had right paresis due to cerebral infarction. In terms of neck vessels, two patients had stenosis, occlusion or calcification in the internal carotid arteries. In two patients with right SCAAs, the ipsilateral vertebral artery had antegrade flow or was the dominant vertebral artery. Four patients with a history of coronary artery disease required coronary artery bypass grafting by means of median sternotomy or percutaneous coronary intervention. One patient had left ventricle dysfunction (ejection fraction: 35%), two patients had moderate (grade III) echocardiographically detected aortic regurgitation. One patient had mild restrictive lung disease (estimated forced vital capacity: 74%). All patients showed good renal function with a serum creatinine level of less than 1.2 mg/dL. Two patients had shaggy and calcified aorta detected by means of CT scan.
Our indication for surgical treatment for proximal SCAA when asymptomatic is the size >30 mm. It was our policy to perform, before endografting, an extra-anatomical bypass to all branches with orifices that could be covered with stent-grafts. To ensure an adequate proximal landing zone of more than 2 cm in length, we aggressively chose zone 1 instead of zone 2. This policy was applied to all cases except for case 1. An 8-mm expanded polytetrafluoroethylene (ePTFE) graft (Goretex®; WL Gore & Associates, Inc., Flagstaff, AZ) was used for the bypass to the carotid, subclavian or axillary artery, and for the bypass to the vertebral artery, a saphenous vein graft was utilized.
The National Cerebral and Cardiovascular Center Institutional Review Board approved this retrospective study and waived the need to obtain patient consent. The follow-up was conducted at the outpatient clinic and was completed for all patients. Subclavian artery bypass graft patency at most recent follow-up was determined based on CT scan. The median clinical follow-up duration was 3.7 [0.2–6.8] years. The median follow-up of the last imaging was 1.9 [0.2–4.7] years. The continuous data for this study are expressed as median and range.
Surgical procedures
The details of the operative procedures are shown in Table 2.
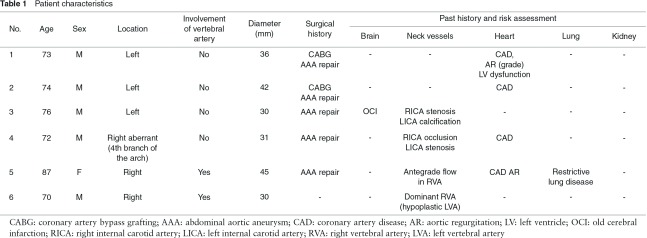
Left SCAA (cases 1, 2, 3). These three SCAAs did not involve the vertebral artery. The proximal landing site for the stent-graft was Zone 2 in case 1 and Zone 1 in cases 2 and 3. The left subclavian artery (LSCA) was not reconstructed in case 1 because the reconstruction was not considered to necessary for this high-risk patient at that time. The supra-aortic bypass was constructed with a branched-type 8-mm ePTFE graft from the right common carotid artery (RCCA) to the left common carotid artery (LCCA) and the LSCA in case 2 and from the right axillary artery (RAxA) to the LCCA and the left axillary artery (LAxA) in case 3 (Fig. 1).
Fig. 1.
Case 3 (76 y.o., male, left subclavian artery aneurysm) RAxA: right axillary artery; LCAA: left common carotid artery; LAxA: left axillary artery.
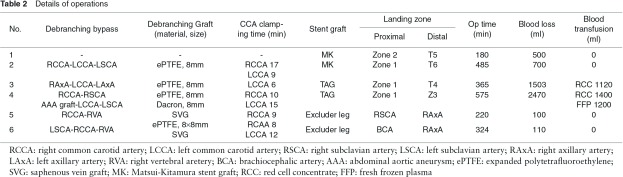
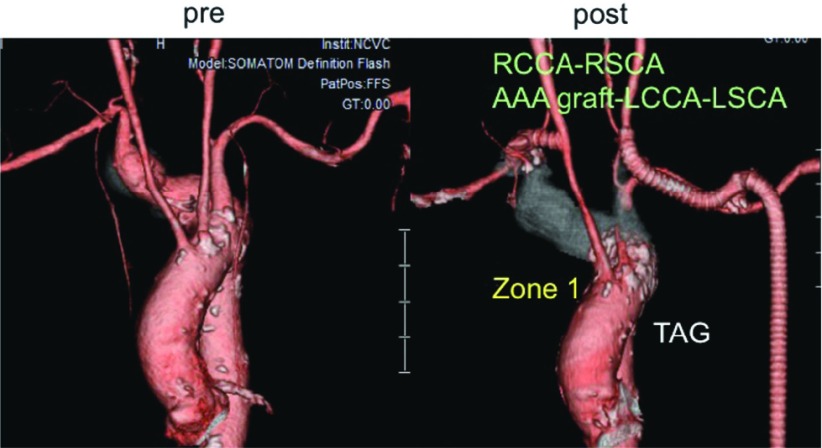
Right aberrant SCAA (Case 4: Fig. 2). The proximal landing site for stent-graft was Zone 1. As this particular patient had narrow RSCA and stenosis in right internal carotid artery, the supra-aortic bypass was made from RCCA to RAxA and also from AAA graft to LCCA and LAxA.
Fig. 2.
Case 4 (72 y.o., male, right aberrant subclavian artery aneurysm) RCCA: right common carotid artery, right subclavian artery; AAA: abdominal artery aneurysm; LCCA: left common carotid artery; LSCA: left subclavian artery.
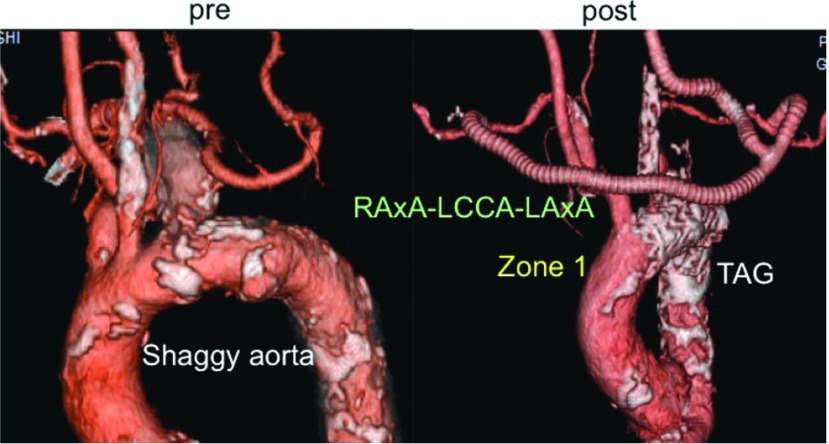
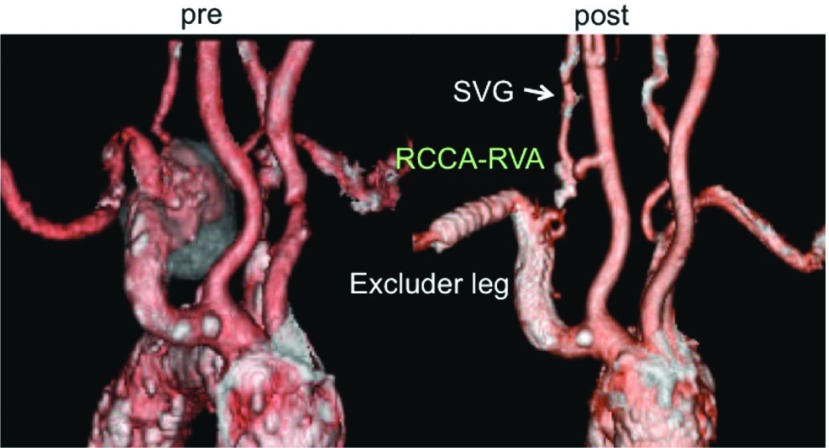
Right SCAA (cases 5, 6). The proximal landing site for the stent-graft was the right subclavian artery in case 5 (Fig. 3) and the right brachiocephalic artery with a bypass from LCCA to RCCA in case 6. The stent-grafts used were contralateral leg of Excluder of which size was 12 mm × 100 mm in case 5 and 16 mm × 100 mm in case 6. In both cases, the right vertebral artery was reconstructed with a saphenous vein graft (SVG). The inflow artery of the vertebral artery bypass was the right common carotid artery in case 5 and ePTFE graft in case 6.
Fig. 3.
Case 5 (87 y.o., female, right subclavian artery aneurysm) SVG: saphenous vein graft; RCAA: right common carotid artery; RVA: right vertebral artery.
The distal landing site of TEVAR was between Z3 and T6 in cases 1–4, and that of the stent-graft for the right SCAA in cases 5 and 6 was the axillary artery.
For prevention of type II endoleak, the SCA proximal to the vertebral artery and distal to SCAA was tied off in case 2, 3, and 4. The coil embolization of the aneurysm could not be performed because of coil instability due to intra-aneurysmal blood flow in case 1.
Median clamping time of the CCA was 9.5 [6–17] min.
A Matsui-Kitamura (MK) stent-graft (Kitamura Inc., Kanazawa, Japan) was used in cases 1 and 2, a Gore TAG (WL Gore) in cases 3 and 4 and the iliac extender of the Gore Excluder (WL Gore) in cases 5 and 6.
Basically, no anti-coagulative or anti-platelet agents were prescribed except for low-dose aspirin for two patients after the bypass to the vertebral artery.
Results
The initial success rate of the stent-graft was 100%. The median operating time including both bypass and TEVAR was 345 [180–575] min. Two patients (cases 3 and 4) required blood transfusion. The median durations of ICU stay and hospital stay were 1 [0–7] day and 18 [7–42] days, respectively.
No 30-day or in-hospital mortality occurred. Case 3 with a shaggy aorta (Fig. 3) suffered a minor stroke after TEVAR. This patient complained of visual disturbance one day after TEVAR due to posterior cerebral artery infarction, but the symptom resolved spontaneously within a week. In case 4, a hematoma formed around the neck wound was removed.
The patency of the stent-graft and all supra-aortic bypasses were confirmed by means of CT scan during follow-up period.
The SCAA without coil embolization of the distal artery in case 1 was thrombosed according to CT scan on postoperative day 21. During the follow-up period, a type II endoleak in two patients (cases 2 and 3) disappeared within 2 years, and a median aneurysm shrinkage of 4.7 [1.0–7.4] mm per 1 year was observed in 4 patients (67%).
There were two late deaths (cases 1 and 6). Case 1 suffered from acute type B aortic dissection 5 years after TEVAR. The entry tear was located at the proximal site of the MK stent-graft, which has a bare spring at the proximal landing site. The patient refused surgery because he also suffered from terminal stage liver cancer. Case 6 developed cerebral infarction due to occlusion of the SVG to the dominant right vertebral artery 2 months after the operation. In this particular patient, the right vertebral artery was 6 mm in diameter and it was suspected the resultant thin SVG or anastomotic stenosis was the cause of graft occlusion.
Discussion
SCAAs are reportedly extremely rare peripheral artery aneurysms. Dent et al. reported finding only two SCAAs when reviewing 1488 atherosclerotic arterial aneurysms.6) However, the actual prevalence may be higher than reported. A recent meta-analysis of 394 SCAAs2) found that the diagnosis is strongly related to the symptoms, which were present in 84% of the patients. None of the patients in our study, however, showed any symptoms and were diagnosed on the basis of CT scan results. Since patients with proximal SCAA tend to be asymptomatic, the meta-analysis found such patients were mostly diagnosed with a routine chest X-ray. Currently, the growing use of CT scan has made asymptomatic proximal SCAA easy to detect.
Of the six patients examined in this study, five (83%) had a history of abdominal aortic aneurysm and two (33%) had a shaggy and heavily calcified aorta, while four (67%) had coronary artery disease. SCAAs are more likely to occur in patients with other atherosclerotic diseases including aortic diseases. Nicholas et al. have suggested there is a strong association between SCAA and thoracic aortic disease.1) They reported that 53% (10/19) of SCAA patients had a history of prior aortic surgery, and 53% (10/19) underwent repair of concomitant thoracic aortic pathology at the time of SCAA repair. Patients with SCAA caused by atherosclerosis may thus be at high risk because of the overall progression of atherosclerosis.
In one meta-analysis,2) the threshold of rupture size could not be determined, but the SCAAs in this study were all ≥30 mm, which is the operative indication used by us for SCAA to prevent rupture, thrombosis, embolization and local compression. While proximal SCAA had a higher incidence of rupture than did distal aneurysm, only 9% of thrombo-embolic complications occurred in proximal SCAA.2) The risk of thrombo-embolic complications was found to be unrelated to the diameter of SCAAs and these complications can occur even in small aneurysms of only 12–25 mm. In view of the high rate of complications, Coselli and Crawford et al.3) recommended SCAA repair whenever feasible and regardless of size. Early intervention may be indicated to prevent complications, but SCAA repair requiring sternotomy or thoracotomy for access may be too invasive, while endovascular repair offers a less invasive treatment option.
The aforementioned meta-analysis found that the overall mortality and complication rates for conventional open SCAA repair were 8% and 26%, respectively, and those for endovascular repair 5% and 28%. Moreover, conventional open repair was associated with cardio-pulmonary complications, which may influence mortality. Endovascular repair can thus reduce mortality but it may entail the same range of morbidity rates as conventional repair. However, many complications of endovascular repair were minor ones including in-stent stenosis, stent fracture, thrombosis and pseudo-aneurysm formation, and these can be procedure-related. Early and late thrombosis and stenosis may be of concern for patients who were treated with a stent-graft placed in the subclavian artery, which is extremely mobile and exposed to rotational forces during arm movements.7–9) Basically, we indicate stent-graft placement according to the patient’s risk for surgery and the anatomical suitability. As exercise can increase the risk of stent-related complications, the indication of stent-graft placement for subclavian artery has been limited to the patients 70 years or older in this study.
Reacscularization of the subclavian artery during TEVAR for proximal SCAAs tends to be the treatment of choice.1,10–12) Five patients in our study underwent hybrid repair, that is, TEVAR and supra-aortic bypass for three patients, stent-graft placement and supra-aortic bypass with reconstruction of the vertebral artery for one patient, and stent-graft placement and reconstruction of the vertebral artery for the remaining patient. Byrne et al. analyzed 143 extra-anatomic procedures for carotid and subclavian reconstruction.13) Most bypass grafts were made of ePTFE and the 5-year patency rate was 92%, indicating that artificial bypass grafts can result in excellent patency rates. We also used ePTFE grafts for all patients, and no graft occlusion was encountered during the follow-up period, nor was any cerebral complication associated with clamping of the CCA for 9.5 [6–17] min for construction of the supra-aortic bypass. However, one aortic dissection and two neurologic complications were encountered in the early and late term. The retrograde aortic dissection occurred 5 years after TEVAR using the MK stentgraft with the proximal bare stent, but this late onset complication is not considered to be specific for the endovascular treatment of SCAA. One of the neurologic complications was a minor stroke caused by thrombo-embolism after TEVAR for the shaggy aorta. Among the 97 cases treated with TEVAR combined with supra-aortic bypass at our facility, only three similar minor strokes in posterior circulation have been encountered. As these minor strokes were suspected to have been caused by an embolus from the aorta near the orifice of the LSCA, we have been using balloon protection of LSCA during TEVAR. This complication is observed after TEVAR for aortic arch and is not specific for the treatment of SCAA. The other neurologic complication was a major stroke resulting from occlusion of the SVG to the dominant vertebral artery, which was presumably due to a procedure-related problem. When it comes to endovascular repair for proximal SCAA involving the vertebral artery with antegrade flow, we prefer reconstruction of the ipsilateral vertebral artery to simple ligation, since the former minimizes the risk of neurologic events. To avoid excessive exposure of the vertebral artery and simultaneous clamping of both vertebral and carotid arteries, the SVG was interposed between the vertebral artery and GoreTex graft. One method for reconstruction of the vertebral artery consists of direct anastomosis of the artery to the CCA.14) There have been three reports of hybrid repair involving reconstruction of the vertebral artery.15–17) In all three cases, endovascular repair for a right SCAA was performed with the common carotid-to-vertebral artery bypass using SVG without any occurrence of neurologic complications. Nevertheless, occlusion of the dominant vertebral artery can be fatal as seen in our study. To prevent postoperative neurologic complications, careful preoperative evaluation of the brain-supplying vessels, choice of a safe artery for crossclamping and for inflow of the bypass, meticulous anastomosis, and brief cross-clamping of the brain-supplying vessels are needed. These considerations are vital since in the long term, occlusion or thrombi associated with bypass grafting can lead to late neurologic complications.
Two limitations to this study are that it deals with a small number of cases at a single institution, is retrospective and has a short follow-up period. These limitations restrict comparisons of findings between procedures as well as with those of other published reports.
Conclusion
Endovascular and hybrid repair can be a less invasive alternative for patients with proximal SCAA and can reduce early mortality. However, revascularization of neck vessels and TEVAR should be performed very carefully to prevent neurologic complications because the patients might be at relatively high risk due to other advanced atherosclerotic diseases.
Acknowledgement
The authors acknowledge the Departments of Cardiovascular Surgery and Radiology.
Conflict of Interest Statement
The authors have declared that no Conflict of interest exists.
References
- Andersen ND, Barfield ME, Hanna JM, et al. Intrathoracic subclavian artery aneurysm repair in the thoracic endovascular aortic repair era. J Vasc Surg 2013; 57: 915-25. [DOI] [PubMed] [Google Scholar]
- Vierhout BP, Zeebregts CJ, van den Dungen JJ, et al. Changing profiles of diagnostic and treatment options in subclavian artery aneurysms. Eur J Vasc Endovasc Surg 2010; 40: 27-34. [DOI] [PubMed] [Google Scholar]
- Coselli JS, Crawford ES. Surgical treatment of aneurysms of the intrathoracic segment of the subclavian artery. CHEST J 1987; 91: 704-8. [DOI] [PubMed] [Google Scholar]
- Austin EH, Wolfe WG. Aneurysm of aberrant subclavian artery with a review of the literature. J Vasc Surg 1985; 2: 571-7. [DOI] [PubMed] [Google Scholar]
- Koseoglu K, Cildag B, Sen S, et al. Endovascular treatment of a mycotic subclavian artery aneurysm using stent-graft. EJVES Extra 2006; 11: 97-101. [Google Scholar]
- Dent TL, Lindenauer SM, Ernst CB, et al. Multiple arteriosclerotic arterial aneurysms. Arch Surg 1972; 105: 338-44. [DOI] [PubMed] [Google Scholar]
- Ewings EL, Wittgen CM, Paletta CE. Prolonged success with a covered endovascular stent after emergent use in radiation-induced subclavian artery blowout: a case report. Vasc Endovascular Surg 2008; 42: 187-91. [DOI] [PubMed] [Google Scholar]
- Kasirajan K, Matteson B, Marek JM, et al. Covered stents for true subclavian aneurysms in patients with degenerative connective. J Endovasc Ther 2003; 10: 647-52. [DOI] [PubMed] [Google Scholar]
- Szeimies U, Kueffer G, Stoeckelhuber B, et al. Successful exclusion of subclavian aneurysms with covered nitinol stents. Cardiovasc Intervent Radiol 1998; 21: 246-9. [DOI] [PubMed] [Google Scholar]
- Lamb KM, Moudgill N, Whisenhunt AK, et al. Hybrid endovascular treatment of an aberrant right subclavian artery with Kommerell aneurysm. Vascular 2014; 22: 458-63. [DOI] [PubMed] [Google Scholar]
- Ronchey S, Serrao E, Kasemi H, et al. Endovascular treatment of extracranial vertebral artery aneurysm and aberrant right subclavian artery aneurysm. J Cardiovasc Surg (Torino) 2014; 55: 265-9. [PubMed] [Google Scholar]
- Brown HA, Aruny JE, Elefteriades JA, et al. Subclavian aneurysm presenting with massive hemoptysis: a case report and review of the literature. Int J Angiol Off Publ Int Coll Angiol Inc 2013; 22: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J, Darling RC, Roddy SP, et al. Long term outcome for extra-anatomic arch reconstruction. An analysis of 143 procedures. Eur J Vasc Endovasc Surg 2007; 34: 444-50. [DOI] [PubMed] [Google Scholar]
- Berguer R, Flynn LM, Kline RA. Surgical reconstruction of the extracranial vertebral artery: management and outcome. J Vasc Surg 2000; 31: 9-18. [DOI] [PubMed] [Google Scholar]
- Resch TA, Lyden SP, Gavin TJ, et al. Combined open and endovascular treatment of a right subclavian artery aneurysm: a case report. J Vasc Surg 2005; 42: 1206-9. [DOI] [PubMed] [Google Scholar]
- Harding GE, Kribs SW, Forbes TL. Hybrid open and endovascular therapy for a proximal subclavian artery aneurysm. Vascular 2008; 16: 236-8. [DOI] [PubMed] [Google Scholar]
- Roh Y, Park K, Do Y, et al. A hybrid operation in a patient with complex right subclavian artery aneurysm. J Korean Surg Soc 2012; 82: 195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]