Abstract
Intimal hyperplasia is an impediment to patency in both arteries after percutaneous angioplasty (PTA) and veingraft. It is well known that migration and proliferation of vascular smooth muscle cells (SMCs) influence the vascular remodeling process, there are no therapies to prevent intimal hyperplasia of post-PTA arteries and vein grafts. Girdin (girders of actin filaments), also known as Gα-interacting vesicle associated protein (GIV) is a novel actin-binding Akt substrate.Girdin is highly expressed in limited types of cells such as smooth muscle cells, neuroblasts, and cancer cells. Girdin is involved in the cell migration, proliferation and remodeling of actin filaments.
This study revealed that Girdin is involved with intimal hyperplasia in carotid arteries after balloon injury and vein grafts and vascular SMCs migration and proliferation. There are suggestions that Girdin has pivotal roles in migration and proliferation of vascular SMCs and that gene therapy targeting Girdin could be a novel therapeutic strategy for restenosis after PTA and vein graft failure.
Keywords: intimal hyperplasia, Girdin, gene therapy
Introduction
Vascular injury caused by angioplasty induces deendothelialization, which promotes the deposition of platelets at the injured site and subsequent recruitment of leukocytes.
Growth factors and cytokines released from platelets, leukocytes and vascular smooth muscle cells (SMCs) stimulate the migration and proliferation of VSMCs, which results in neointima formation and restenosis.1–3) Moreover, late graft failure of autologous vein grafts is also associated with intimal hyperplasia resulting from the migration and proliferation of vascular smooth muscle cells.
The phosphatidylinositol 3-kinase (PI3K)-Akt pathway is a key regulator of several processes such as cell survival, proliferation and growth downstream of these humoral factors.4–6) Accumulating evidence suggests that the PI3K-Akt pathway and its downstream components also play essential roles in vascular remodeling.7–11) However, the underlying molecular mechanisms are not completely understood.
A search for Akt-binding proteins led to the identification of Girdin (girders of actin filament), also known as Akt phosphorylation enhancer (APE) and G-interacting vesicle associated protein (GIV).12–14) Girdin is a large 220-kDa protein with unique amino- and carboxyl terminal domains flanking a long coiled-coil region. Girdin forms oligomers through its amino-terminal domain and coiled-coil region. The carboxyl-terminal domain contains the actin-binding site and the phosphatidylinositol phosphate-binding motif located near the Akt phosphorylation site (serine 1416). Therefore, Girdin is postulated to crosslink actin filaments and to anchor them to the plasma membrane in quiescent cells. In response to growth factors, Akt phosphorylates Girdin at serine 1416, and the phosphorylated Girdin detaches from the plasma membrane with actin filaments. Subsequently, the phosphorylated Girdin accumulates in the lamellipodia of migrating cells, leading to the rearrangement of the actin cytoskeleton.12) To our knowledge, Girdin is the only actin-binding protein that is directly phosphorylated by Akt.
This paper reviews whether Girdin modulates the vascular remodeling process in vitro and in vivo, and the ability of Girdin to induce cell migration, proliferation and survival in VSMCs. In this paper we introduce the vector for gene delivery to the vasculature, and a novel therapeutic strategy for restenosis after percutaneous angioplasty (PTA) and vein graft failure.
Intimal Hyperplasia
The process of intimal hyperplasia is common to various forms of vascular diseases, such as atherosclerosis, restenosis, and transplant vasculopathy.15,16) In response to vascular injury, the medial smooth muscle cells proliferate and migrate into the intima, where they proliferate and secrete abundant extracellular matrix to form the neointima.15) In another way, various biological changes that lead to intimal hyperplasia occur when a vein graft is implanted in the arterial circulation. Those changes include the migration and invasion of inflammatory cells, a loss of endothelial cells, an increased sheer stress, and migration and proliferation of VSMCs of vein grafts.17,18)
Girdin and Intimal Hyperplasia
Girdin is expressed in VSMCs but not endothelial cells in large vessels with a thick smooth muscle layer, such as carotid and femoral arteries.19) Girdin is also localized in media of vein.
It is reported that Girdin is upregulated in developing neointima.20,21) Miyake et al. reported that as the neointima grew thicker, enhanced expression and phosphorylation of Girdin were observed in the neointima of rat balloon injury (BI) model with immunohistochemical assessment. Both the expression and the phosphorylation of Girdin in the neointima peaked at 14 days after BI, and persisted for at least 28 days. Then, the level dropped to the baseline by 42 days after BI. We previously reported Girdin expression in rabbit vein graft. Girdin was located in the neointima rather than in the media as the neointima thickened. By immunohistochemical analysis, Girdin was detected especially in neointima of vein grafts. The expression of Girdin was increased over time and peaked at 14 days after bypass grafting. Moreover, we compared expression of Girdin in vein grafts with poor and normal runoff in which the neointima was clearly thickened to determine whether Girdin affects intimal hyperplasia in vein grafts. Girdin expression significantly increased during neointima formation at 7 and 14 days after bypass grafting in poor runoff models compared with normal runoff models using Western blot analyses.
Girdin Localization
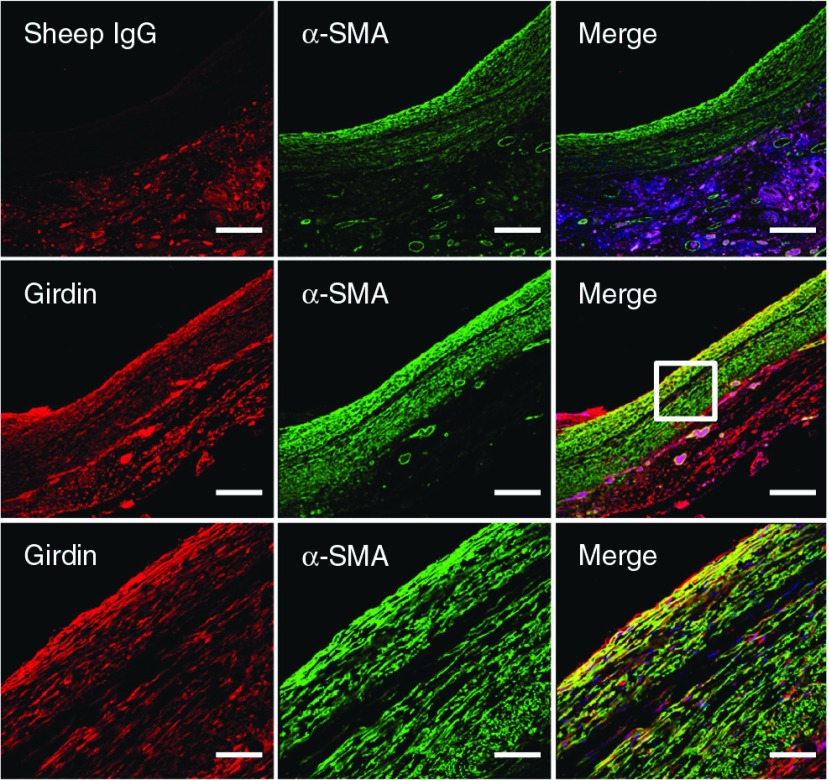
Miyake et al. reported that most of the cells constituting the neointima revealed positive staining for Girdin and α-smooth muscle actin (α-SMA) in rat BI model. In addition, Girdin localization was confirmed in 14-day-old vein grafts by immunofluorescence staining using anti-Girdin antibody and anti-α-SMA antibody (Fig. 1). Girdin localizes to α-SMA+ cells in the media and neointima. Cells that were both Girdin+ and α-SMA+ existed mainly in the vein graft neointima at 14 days after bypass grafting. This finding suggests that Girdin is upregulated in the developing neointima.
Fig. 1.
Vein grafts at 14 days post-operation were subjected to immunofluorescence staining using sheep IgG as a negative control or anti-Girdin antibody (red), and anti-α-SMA antibody (green). Cell nuclei were labeled with DAPI. Representative photos at low magnifications (upper and middle panel) are presented. The boxed area is magnified (lower panel). Bars: 200 µm (upper and middle); 50 µm (lower). α-SMA: α-smooth muscle actin
Gene Transfer and Inhibition of Intimal Hyperplasia
To further analyze the involvement of Girdin in vascular remodeling, an adenoviral vector encoding shRNA directed against Girdin was transferred into balloon-injured arteries to selectively inhibit the expression of endogenous Girdin. The introduction of an shRNA against Girdin attenuated the neointima formation without affecting reendothelialization. On the other hand, siRNA that targets Girdin was mixed with atelocollagen, which stabilizes and releases nucleic acid reagents slowly, and the mixture was applied perivascularly to the vein grafts during surgery. Girdin knockdown via perivascular application of siRNA using atelocollagen markedly reduced intimal thickening after the grafting operation. So, Girdin is essential for intimal hyperplasia in vivo.
Girdin Affects Cell Proliferation But Not Cell Survival During Intimal Hyperplasia
Cell migration, cell proliferation, and apoptosis of vascular SMCs are major steps in intimal hyperplasia.22,23) Miyake et al. examined the cell proliferation and apoptosis in balloon injured arteries using immunostaining for proliferating-cell nuclear antigen (PCNA) and TUNEL staining, respectively. The knockdown of Girdin significantly reduced the PCNA labeling indices in the neointima, but had no apparent effect on TUNEL labeling indices. We also examined cell proliferation and apoptosis in vein grafts using immunostaining, and the results suggest that Girdin affects cell proliferation but not cell survival.
Girdin Involves in Rearrangement of Actin Cytoskeleton of VSMCs
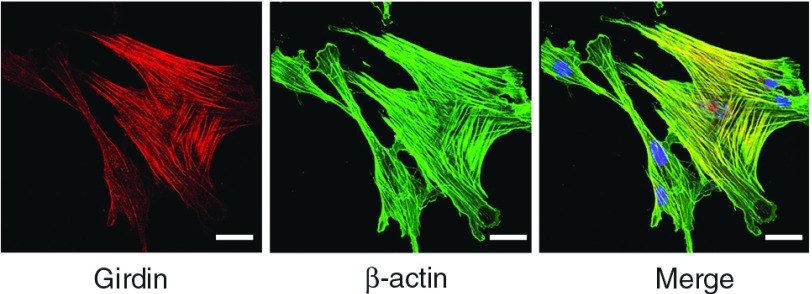
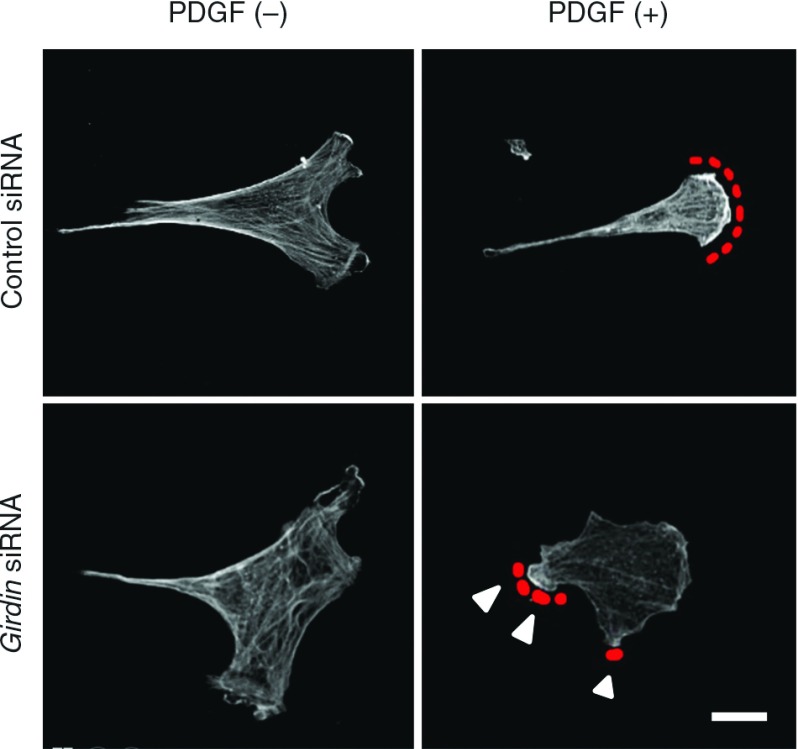
Immunocytochemical analysis revealed Girdin localized on actin stress fibers in primary SMCs (Fig. 2). When Girdin-depleted SMCs are stimulated with serum, the formation of thick stress fibers significantly decreased compared with control SMCs. The stimulation with PDGF-BB in the Girdin-depleted cells results in attenuation of the wide extension of lamellipodia at the leading-edge making membrane protrusions smaller (Fig. 3).
Fig. 2.
Venous SMCs were stained using anti-Girdin antibody and anti-β-actin antibody. Girdin localizes to actin stress fibers. Bar: 20 µm. SMCs: smooth muscle cells
Fig. 3.
Venous SMCs were stimulated with PDGF-BB (20 ng/ml) for 10 minutes and stained with anti-β-actin antibody. Red dotted lines denote lamellipodia at the leading edge. Arrowheads denote lesspolarized and less extended membrane protrusions comparedwith the control cells. Bar: 20 µm. SMCs: smooth muscle cells
These results show that actin remodeling of lamellipodia in migrating SMCs require Girdin.
Girdin Depletion Inhibits VSMCs Migration and Proliferation In Vitro
The function of Girdin on the migration and proliferation of human arterial VSMC was examined by Miyake et al. The involvement of Girdin in cell migration was examined using wound-healing assays. The number of cells that migrated into the wounded area and the migration velocity were significantly reduced in the Girdin-depleted cells as compared to the control cells. Moreover, to assess the effect of Girdin on hVSMC proliferation, MTS[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, innersalt] colorimetric assays were performed. On day 5 after the transfection of siRNAs, the proliferation of the Girdin-depleted cells was markedly suppressed.
We also inquired whether Girdin depletion supressed primary venous SMCs migration by wound healing assays. Girdin depletion showed markedly reduced migration distance. We observed fewer cells migrating into the wounded area as seen in arterial SMCs. Furthermore, water-soluble tetrazolium salt (WST)-1 assays showed that proliferation was significantly suppressed using Girdin-depleted SMCs. These findings show that Girdin depletion inhibits migration and proliferation of SMCs in vitro.
Conclusion
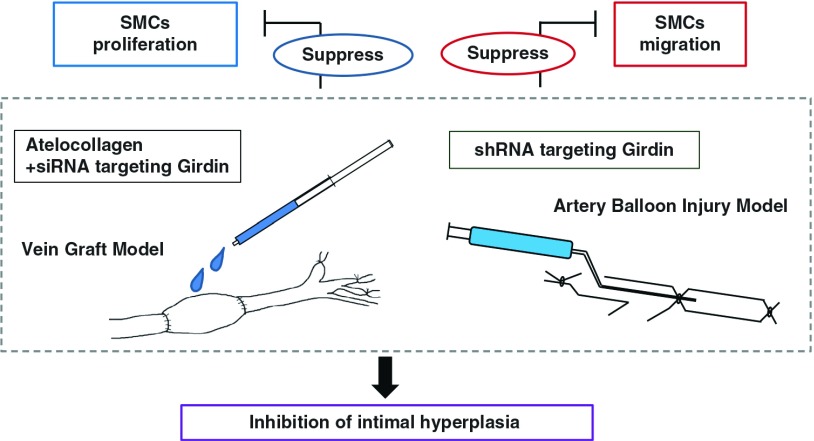
These studies demonstrated that Girdin regulates the remodeling of the actin cytoskeleton, as well as the migration and proliferation of both arterial and venous SMCs. Transduction of shRNA directed against Girdin into the rat artery suppressed intimal hyperplasia after balloon injury. In addition, atelocollagen-delivered siRNA targeting Girdin reduced intimal thickening after the grafting operation. This was accompanied by decreased cell migration and proliferation of VSMCs (Fig. 4).
Fig. 4.
The shRNA and atelocollagen-delivered siRNA targeting Girdin decreased cell proliferation and migration and led to inhibit the intimal hyperplasia. SMCs: smooth muscle cells
In conclusion, Girdin is essential for the rearrangement of the actin cytoskeleton, as well as for the migration and proliferation of both arterial and venous SMCs, which is vital for intimal hyperplasia. The Akt/Girdin signaling pathway might have functions as a crucial regulator of vascular remodeling. Therefore, Girdin may be an important therapeutic target for vascular proliferative diseases such as restenosis and atherosclerosis and late graft failure of autologous vein grafts.
Disclosure Statement
The authors declare no conflict of interest.
References
- Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation 2005; 111: 2257-73. [DOI] [PubMed] [Google Scholar]
- Mitra AK, Agrawal DK. In stent restenosis: bane of the stent era. J Clin Pathol 2006; 59: 232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007; 115: 1051-8. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell 2002; 111: 293-303. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 1999; 13: 2905-27. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489-501. [DOI] [PubMed] [Google Scholar]
- Stabile E, Zhou YF, Saji M, et al. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying G1/S exit. Circ Res 2003; 93: 1059-65. [DOI] [PubMed] [Google Scholar]
- Shigematsu K, Koyama H, Olson NE, et al. Phosphatidylinositol 3-kinase signaling is important for smooth muscle cell replication after arterial injury. Arterioscler Thromb Vasc Biol 2000; 20: 2373-8. [DOI] [PubMed] [Google Scholar]
- Lee HY, Chung JW, Youn SW, et al. Forkhead transcription factor FOXO3A is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res 2007; 100: 372-80. [DOI] [PubMed] [Google Scholar]
- Park KW, Yang HM, Youn SW, et al. Constitutively active glycogen synthase kinase-3beta gene transfer sustains apoptosis, inhibits proliferation of vascular smooth muscle cells, and reduces neointima formation after balloon injury in rats. Arterioscler Thromb Vasc Biol 2003; 23: 1364-9. [DOI] [PubMed] [Google Scholar]
- Huang J, Niu XL, Pippen AM, et al. Adenovirus-mediated intraarterial delivery of PTEN inhibits neointimal hyperplasia. Arterioscler Thromb Vasc Biol 2005; 25: 354-8. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Murakami H, Asai N, et al. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell 2005; 9: 389-402. [DOI] [PubMed] [Google Scholar]
- Anai M, Shojima N, Katagiri H, et al. A novel protein kinase B (PKB)/AKT-binding protein enhances PKB kinase activity and regulates DNA synthesis. J Biol Chem 2005; 280: 18525-35. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Niesman I, Fischer T, et al. Identification and characterization of GIV, a novel Galpha i/s-interacting protein found on COPI, endoplasmic reticulum-Golgi transport vesicles. J Biol Chem 2005; 280: 22012-20. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84: 767-801. [DOI] [PubMed] [Google Scholar]
- Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev 2004; 15: 237-54. [DOI] [PubMed] [Google Scholar]
- Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg 1994; 81: 1254-69. [DOI] [PubMed] [Google Scholar]
- Ishida M, Komori K, Yonemitsu Y, et al. Immunohistochemical phenotypic alterations of rabbit autologous vein grafts implanted under arterial circulation with or without poor distal runoff—implications of vein graft remodeling. Atherosclerosis 2001; 154: 345-54. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Asai N, Enomoto A, et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol 2008; 10: 329-37. [DOI] [PubMed] [Google Scholar]
- Miyake H, Maeda K, Asai N, et al. The actin-binding protein Girdin and its Akt-mediated phosphorylation regulate neointima formation after vascular injury. Circ Res 2011; 108: 1170-9. [DOI] [PubMed] [Google Scholar]
- Miyachi H, Mii S, Enomoto A, et al. Role of Girdin in intimal hyperplasia in vein grafts and efficacy of atelocollagen-mediated application of small interfering RNA for vein graft failure. J Vasc Surg 2013; August 12 pii: S0741-5214(13)01275-5. doi: 10.1016/j.jvs.2013.06.080 [DOI] [PubMed] [Google Scholar]
- Clowes AW, Schwartz SM. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res 1985; 56: 139-45. [DOI] [PubMed] [Google Scholar]
- Bochaton-Piallat ML, Gabbiani F, Redard M, et al. Apoptosis participates in cellularity regulation during rat aortic intimal thickening. Am J Pathol 1995; 146: 1059-64. [PMC free article] [PubMed] [Google Scholar]