Block et al. (1) have written a critique of the vibrational theory of olfaction that rests on two main points: (i) they report negative results (i.e., identical responses to normal and deuterated musk isotopomers) in a cultured human embryonic kidney cell derivative line expressing heterologous olfactory receptors; and (ii) they claim that our previous report (2) that humans can smell the difference between undeuterated and deuterated musk isotopomers is in error because of a contaminating impurity they suggest is responsible for the smell difference. We wish to answer these points.
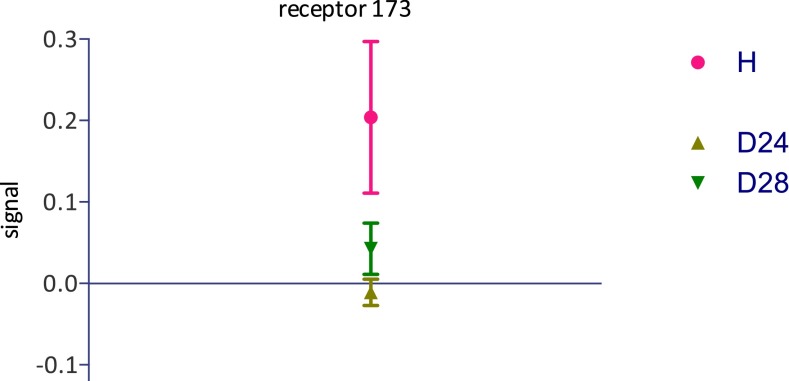
Block et al. (1) graciously made the primary data of the musk receptor screen (depicted in figure S3.1 of ref. 1) available to us. We ran an unpaired t test on the entire receptor repertoire and found two (296 and 173 in their numbering) that showed differences between H and D isotopomers. Receptor 296 is their OR51E1, which “was determined to be a nonresponsive OR [odorant receptor] in the follow-up confirmation experiments due to a high receptor background” (1). Its larger response to deuterated isotopomers was not mentioned. Receptor 173 was not described in Block et al.’s paper at all. Its response (Fig. 1) to D24 and D28 musks was smaller (P < 0.02) than to H and the response to D4 was intermediate. We urge Block et al. to publish the identity of receptor 173 and to reexamine these two receptors that potentially invalidate the main conclusion of their paper.
Fig. 1.
Response of Block et al.’s (1) receptor no. 173 in superfamily 5, to isotopomers of cyclopentadecanone. Numbers taken from the primary data plotted in figure S3.1 of ref. 1. The D4 point, omitted for clarity, is at 0.06 ± 0.04.
Block et al. (1) assert that an impurity may have affected the odor of our deuterated musks. The peak in our NMR spectra, which they point to, is likely to be caused by a small fraction of cyclopentadecane. Block et al. describe the same impurity in their synthesis, and it is visible in their NMR spectra (figure S2.6 of ref. 1) at 0.87. This impurity does not coelute with the musk and is shown and correctly labeled in figure 2 of ref. 2. Furthermore, our sham deuteration protocol controlled for this. We therefore remain entirely confident that the difference in smell between isotopomers revealed by our double-blind trials is because of the pure peak of deuterated musk.
We are struck by the omission of any description of odor character of the deuterated musks Block et al. (1) synthesized and tested. One assumes that a paper entitled “Implausibility of the vibrational theory of olfaction” would have made use of this information if the isotopes smelled identical.
Block et al. (1) assert that deuteration affects many physicochemical properties of odorants. This assertion should be contrasted with their report that in all 14 dose–response curves shown, the affinity of the receptors for deuterated odorants was indistinguishable from that of the hydrogen counterparts. Their results show convincingly that those properties of odorants that are involved in molecular recognition (and therefore in shape theories of olfaction) are left unaltered by deuteration. How then do flies (3), humans (2), and possibly their receptors 173 and 296 detect isotopes?
On balance, we feel that Block et al.’s (1) conclusion that vibrational theories are implausible is premature.
Footnotes
The authors declare no conflict of interest.
References
- 1.Block E, et al. Implausibility of the vibrational theory of olfaction. Proc Natl Acad Sci USA. 2015;112(21):E2766–E2774. doi: 10.1073/pnas.1503054112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gane S, et al. Molecular vibration-sensing component in human olfaction. PLoS ONE. 2013;8(1):e55780. doi: 10.1371/journal.pone.0055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco MI, Turin L, Mershin A, Skoulakis EM. Molecular vibration-sensing component in Drosophila melanogaster olfaction. Proc Natl Acad Sci USA. 2011;108(9):3797–3802. doi: 10.1073/pnas.1012293108. [DOI] [PMC free article] [PubMed] [Google Scholar]