Significance
Arguments for an abiotic origin of organic compounds in deep-sea hot springs are compelling because of their potential role in the origin of life and sustaining microbial communities. Theory predicts that warm H2-rich fluids circulating through serpentinizing systems create a favorable thermodynamic drive for inorganic carbon reduction to organic compounds. We show that abiotic synthesis proceeds by two spatially and temporally distinct mechanisms. Abundant dissolved CH4 and higher hydrocarbons are likely formed in H2-rich fluid inclusions over geologic timescales. Conversely, formate production by ΣCO2 reduction occurs rapidly during subsurface mixing, which may support anaerobic methanogenesis. We confirm models for abiotic metastable organic compound formation and argue that alkanes in all ultramafic-influenced vents may form independently of actively circulating serpentinizing fluids.
Keywords: abiotic organic synthesis, hydrothermal systems, methane, formate, fluid–vapor inclusions
Abstract
Arguments for an abiotic origin of low-molecular weight organic compounds in deep-sea hot springs are compelling owing to implications for the sustenance of deep biosphere microbial communities and their potential role in the origin of life. Theory predicts that warm H2-rich fluids, like those emanating from serpentinizing hydrothermal systems, create a favorable thermodynamic drive for the abiotic generation of organic compounds from inorganic precursors. Here, we constrain two distinct reaction pathways for abiotic organic synthesis in the natural environment at the Von Damm hydrothermal field and delineate spatially where inorganic carbon is converted into bioavailable reduced carbon. We reveal that carbon transformation reactions in a single system can progress over hours, days, and up to thousands of years. Previous studies have suggested that CH4 and higher hydrocarbons in ultramafic hydrothermal systems were dependent on H2 generation during active serpentinization. Rather, our results indicate that CH4 found in vent fluids is formed in H2-rich fluid inclusions, and higher n-alkanes may likely be derived from the same source. This finding implies that, in contrast with current paradigms, these compounds may form independently of actively circulating serpentinizing fluids in ultramafic-influenced systems. Conversely, widespread production of formate by ΣCO2 reduction at Von Damm occurs rapidly during shallow subsurface mixing of the same fluids, which may support anaerobic methanogenesis. Our finding of abiogenic formate in deep-sea hot springs has significant implications for microbial life strategies in the present-day deep biosphere as well as early life on Earth and beyond.
Seawater-derived hydrothermal fluids venting at oceanic spreading centers are a net source for dissolved carbon to the deep sea, with vent fluid carbon contents directly tied to the sustenance of the subseafloor biosphere (1). Highly reducing fluids rich in dissolved H2, such as those discharging from serpentinizing hydrothermal systems, are of particular interest because of the potential for abiotic reduction of dissolved inorganic carbon to organic compounds (2–6) and their potential role as precursor compounds for prebiotic chemistry associated with the origin of life (7). Although there is increasing evidence that supports an abiotic origin for CH4 and other low-molecular weight organic compounds in ultramafic-hosted hydrothermal systems (8–10), the physical conditions, reaction pathways, and timescales that control abiotic organic synthesis at oceanic spreading centers remain elusive. Working models for the formation of abiotic CH4 and other hydrocarbons observed in vent fluids involve reduction of ΣCO2 and/or CO through Fischer–Tropsch-type processes during active circulation of seawater-derived hydrothermal fluids that are highly enriched in dissolved H2 because of serpentinization of host rocks; however, this mechanism has not been conclusively shown in natural systems. Others have suggested that leaching of CH4 and low-molecular weight hydrocarbons from magmatic fluid inclusions hosted in plutonic rocks may contribute at some level to the inventory of organic compounds observed in axial hot-spring fluids (1, 11, 12). The relative influence of these processes has important implications for the total flux and real-time concentrations of aqueous organic compounds delivered to the oceans by ridge-crest hydrothermal activity. Here, we use multiple lines of evidence to preclude abiotic reduction of ΣCO2 to CH4 during active fluid circulation but show that it is reduced to the metastable intermediate species formate instead.
Results and Discussion
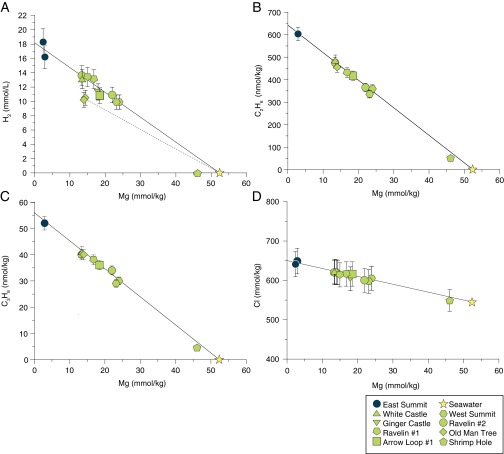
Located at 2,350-m depth on the Mid-Cayman Rise (13, 14), hydrothermal fluids emanate from the Von Damm vent field at temperatures as high as 226 °C (Fig. S1). Ultramafic, gabbroic, and basaltic rocks are associated with the Mount Dent oceanic core complex that hosts this site (15–17). The highest temperature fluids venting at East Summit are characterized by high-dissolved H2 (18.2 mmol/L), CH4 (2.81 mmol/L), elevated C2+ hydrocarbons, low-dissolved metals, near-neutral pH (5.6), and near-zero concentrations of dissolved Mg (Fig. 1A, Table 1, and Fig. S2 A–C). Relative to seawater, dissolved Cl and ΣCO2 abundances in the near-endmember East Summit fluids are slightly enriched, with concentrations of 651 and 2.80 mmol/kg, respectively (SI Text, section 1). Lower temperature fluids that contain substantial concentrations of Mg are also observed at the summit and around the flanks of the Von Damm mound. Aqueous concentrations of Cl, CH4, ethane (C2H6), and propane (C3H8) in these fluids define single conservative mixing lines when each species is plotted against dissolved Mg, suggesting that these fluids have formed by subsurface mixing of cold Mg-rich ambient seawater with the same near-zero Mg fluids (18) sampled at the East Summit (Fig. 1A and Fig. S2 B–D).
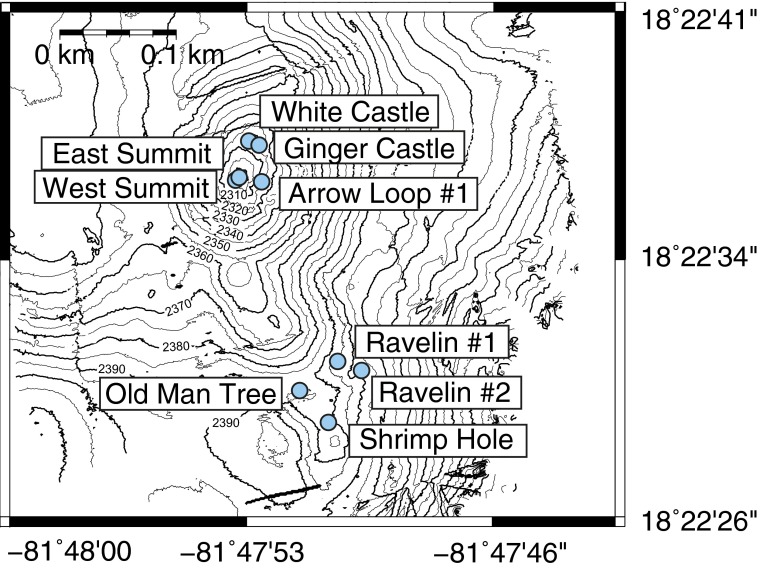
Fig. S1.
Bathymetry of the Von Damm hydrothermal field located on the western flank of the Mid-Cayman Rise, with locations of fluid sampling indicated with circles.
Fig. 1.

Plots of measured Mg vs. (A) CH4, (B) ΣCO2, (C) ΣHCOOH, and (D) ΣCO2 + ΣHCOOH concentrations for Von Damm vent fluids. Mg content is used as an indicator for seawater mixing; solid lines denote conservative dilution of the near-endmember composition (blue circles) with seawater (yellow stars), whereas dashed lines indicate species concentrations that result from nonconservative mixing in elevated Mg fluids (green symbols). Select δ13CCO2 values are plotted in B near corresponding samples. Uncertainties (2σ) not shown are smaller than symbols.
Table 1.
Measured and calculated abundance and stable isotope data for Von Damm vent fluids
| Vent | Sample | T (°C) | Mg (mm) | pH* | Cl (mm) | H2 (mM) | ΣHCOOH (μm) | ΣCO2 (mm) | CH4 (mM) | C2H6 (nm) | C3H8 (nm) | δ13CCO2 (‰) | δ13CCH4 (‰) | δ13CC2H6 (‰) | δ13CC3H8 (‰) |
| East Summit | Endmember | — | 0 | 5.56 | 651 | 18.2 | 88.2 | 2.80 | 2.81 | 639 | 56 | NA | NA | NA | NA |
| East Summit | J2-612-IGT2 | 226 | 2.93 | 5.65 | 649 | 16.2 | 82.0 | 2.79 | 2.62 | 603 | 52 | 0.8 | −15.6 | −12.9 | −9.8 |
| East Summit | J2-616-IGT8 | 226 | 2.43 | 5.56 | 641 | 18.3 | 85.6 | 2.75 | 2.72 | — | — | 0.9 | −15.3 | −12.3 | — |
| White Castle | J2-616-IGT1 | 151 | 13.5 | 5.77 | 622 | 13.1 | 352† | 2.51 | 2.08 | 485 | 41 | 1.5 | −15.6 | — | — |
| Ginger Castle | J2-617-IGT4 | 125 | 18.0 | 6.06 | 604 | 11.3 | 337† | 2.35 | 1.88 | — | — | 2.2 | −15.8 | −13.2 | −10.8 |
| Ravelin 1 | J2-617-IGT6 | 145 | 15.0 | 5.83 | 614 | 13.4 | 147† | 2.52 | 2.02 | — | — | 1.9 | −15.6 | — | — |
| Ravelin 1 | J2-617-IGT2 | 131 | 16.8 | 5.93 | 616 | 13.1 | 132 | 2.40 | 1.96 | 431 | 38 | 1.4 | −15.1 | — | — |
| Arrow Loop 1 | J2-616-IGT6 | 134 | 18.5 | 5.86 | 616 | 10.8 | 274† | 2.27 | 1.74 | 417 | 36 | 1.9 | −15.7 | −12.5 | — |
| West Summit | J2-621-IGT1 | 123 | 24.0 | 6.00 | 605 | 9.94 | 428 | 2.08 | 1.64 | 359 | 30 | 3.3 | −15.6 | −12.6 | — |
| West Summit | J2-621-IGT4 | 123 | 23.2 | 6.01 | 597 | 9.94 | 428† | 2.07 | 1.67 | 335 | 29 | 3.6 | −15.1 | — | — |
| Ravelin 2 | J2-621-IGT2 | 116 | 13.4 | 5.88 | 620 | 13.6 | — | 1.98 | 2.10 | 475 | 40 | 3.8 | −15.1 | −12.9 | −9.7 |
| Ravelin 2 | J2-621-IGT8 | 115 | 22.0 | 6.12 | 600 | 10.9 | 474† | 1.88 | 1.73 | 365 | 34 | 3.3 | −15.4 | −12.7 | — |
| Old Man Tree | J2-612-IGT6 | 115 | 14.4 | 5.81 | 620 | 10.5 | 663 | 1.80 | 1.97 | — | — | 2.6 | −15.2 | — | — |
| Old Man Tree | J2-612-IGT8 | 114 | 14.0 | 5.89 | 621 | 10.2 | 669† | 2.03 | 1.92 | 455 | 40 | 2.9 | −15.0 | −12.6 | −11.6 |
| Shrimp Hole | J2-617-IGT1 | 21 | 46.1 | 7.73 | 549 | 0.01 | BD | 2.01 | 0.29 | 51.8 | 4.6 | 1.1 | −15.1 | — | — |
| Bottom SW | ∼5 | 52.4 | ∼8 | 545 | 0 | ∼1 | 2.25 | 0 | 0 | 0 | 1.1 | NA | NA | NA |
Analytical uncertainties (2σ) are ±2 °C for T; ±3% for Mg and Cl; ±5% for H2, ΣHCOOH, ΣCO2, CH4, C2H6, and C3H8; ±0.05 units for pH; ±0.3‰ for δ13CCO2; ±0.8‰ for δ13CCH4; ±0.4‰ for δ13CC2H6; and ±0.7‰ for δ13CC3H8. Values that were not determined are indicated by —. BD, below detection (1.0 μm for ΣHCOOH); IGT, isobaric gas tight; mm, mmol/kg; mM, mmol/L; μm, μmol/kg; NA, not applicable; nm, nmol/kg; SW, seawater; T, temperature.
Shipboard pH is reported (25 °C and 1 atm).
Sample used to calculate measured affinities in Fig. 2.
Fig. S2.
Plot of measured Mg vs. measured (A) H2, (B) C2H6, (C) C3H8, and (D) Cl. Conservative behavior in Cl, C2H6, and C3H8 as well as CH4 in mixed fluids (green symbols; in the text) during mixing between near-endmember fluid (blue circles) and seawater (yellow stars) suggests that the Von Damm vent field is fed by a single-source fluid originating in the high-temperature reaction zone below the seafloor. Nonconservative H2 behavior occurs at two mixed fluid vents: Old Man Tree (115 °C) and Shrimp Hole (21 °C). Uncertainties (2σ) not shown are smaller than symbols.
Elevated concentrations of dissolved H2, CH4, and low-molecular weight hydrocarbons are remarkably similar to abundances in other ultramafic-influenced hydrothermal systems (8–10, 19), consistent with a strong influence of serpentinization reactions in subseafloor reaction zones on the composition of Von Damm vent fluids. The carbon isotopic composition of dissolved CH4 is uniform across the Von Damm vent field, with a δ13C value of −15.4‰ (Table 1). This value is significantly heavier than those typically associated with thermogenic CH4 generation (−25‰ to −50‰) or microbial production of CH4 from ΣCO2 (−30‰ to −70‰) (20, 21), providing compelling evidence for an abiotic origin for Von Damm CH4. An abiotic origin for CH4 has been invoked for other ultramafic-influenced systems at Rainbow, Logatchev, and Lost City hydrothermal fields, where δ13C values for CH4 range from −9‰ to −16‰ (8–10, 19).
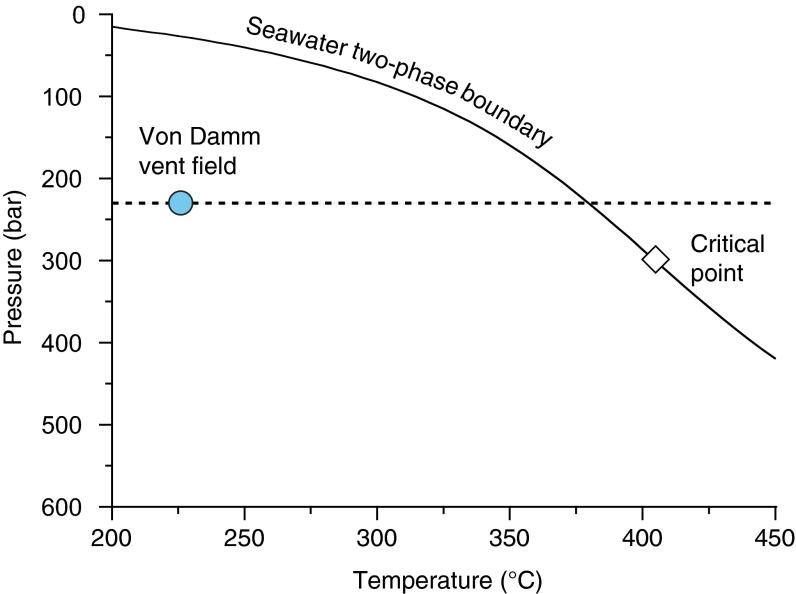
The abundance and isotopic composition of aqueous carbon species in the Von Damm endmember fluids place important constraints on deep-seated processes responsible for the production of CH4. Maximum fluid temperatures at Von Damm are at least 150 °C cooler than the predicted two-phase boundary for seawater at in situ seafloor pressures (22), suggesting that the minor Cl enrichment is not the result of subsurface phase separation (Fig. S3). Instead, the 19% enrichment in Von Damm endmember fluid Cl content (Fig. S2D) likely reflects the removal of water from seawater-derived fluids during serpentinization hydration reactions at low fluid to rock mass ratio (23). Applying a 19% correction to local bottom seawater ΣCO2 concentrations (2.25 ± 0.11 mmol/kg) yields a predicted fluid ΣCO2 abundance of 2.69 mmol/kg that matches, within analytical error, the observed endmember ΣCO2 (2.80 ± 0.14 mmol/kg). Because the corrected ΣCO2 abundance of the endmember fluid is nearly identical to that of ambient bottom seawater, we infer that no significant amounts of ΣCO2 are added to or removed from the fluids during deep convective circulation before mixing in near-seafloor upflow zones. This argument is further supported by the δ13C isotopic composition of the endmember ΣCO2 (0.9‰ ± 0.3‰), which is identical, within error, to that of local bottom seawater (1.1‰ ± 0.3‰). This conservation of ΣCO2 during circulation through the crust has profound implications for the origin of the CH4 in Von Damm vent fluids. With the addition of 2.81 mmol/kg CH4, the endmember fluids contain approximately double the total carbon content of ambient seawater. Because the ΣCO2 in the endmember fluids cannot provide the source of this carbon, this finding implies that CH4 formation from reduction of inorganic sources is not occurring during active fluid circulation at the Von Damm site. Because CH4 is the dominant product expected during abiogenic n-alkane synthesis (11), we infer that C2+ hydrocarbon formation is also not occurring during active fluid circulation.
Fig. S3.
Plot of maximum sampled temperature and seafloor pressure conditions at the Von Damm vent field (blue circle). The curve represents the two-phase boundary of seawater (22). To attain measured temperatures, Von Damm fluids would have cooled by at least 150 °C after phase separation.
Radiocarbon analysis provides additional confirmation that Von Damm CH4 is not derived from fluid ΣCO2 contents. The four Von Damm CH4 samples measured, including the East Summit fluid, all reveal 14C contents near the detectable limit [fraction modern (Fm) = 0.0025] (Table S1). In contrast, corresponding ΣCO2 samples contain detectable modern 14C contents (Fm = 0.0236–0.0373) that would be transferred to CH4 if it were generated by ΣCO2 reduction occurring during fluid circulation (Table S1). Thus, the model postulated for the formation of abundant CH4 at the Lost City vent field involving the leaching of radiocarbon-dead ΣCO2 from fluid inclusions hosted in plutonic rocks and its subsequent reduction to CH4 during hydrothermal fluid circulation (8) cannot account for the occurrence of CH4 at Von Damm.
Table S1.
Radiocarbon data for the Von Damm vent fluids
| Vent | Sample | Measured CH4 (Fm) | Measured ΣCO2 (Fm) | Corrected ΣCO2 (Fm) | Accession nos. |
| East Summit | J2-612-IGT2 | 0.0064 ± 0.0013 | 0.0660 ± 0.0015 | 0.0251 ± 0.0046 | OS-104460 (CH4), OS-105946 (ΣCO2) |
| East Summit | J2-616-IGT8 | 0.0056 ± 0.0013 | 0.0712 ± 0.0050 | 0.0373 ± 0.0069 | OS-104461 (CH4), OS-104700 (ΣCO2) |
| Ravelin 2 | J2-621-IGT2 | 0.0051 ± 0.0013 | — | — | OS-104462 (CH4) |
| Ravelin 2 | J2-621-IGT8 | 0.0074 ± 0.0023 | 0.3573 ± 0.0029 | 0.0236 ± 0.0395 | OS-104706 (CH4), OS-104344 (ΣCO2) |
IGT, isobaric gas tight.
In contrast, we suggest that CH4 and C2+ hydrocarbons in the Von Damm vent fluids are derived from leaching of carbon-rich fluid inclusions at depth. We postulate that the abundant CH4, C2H6, and C3H8 in Von Damm vent fluids were formed when magmatic volatiles trapped in plutonic rocks reequilibrated during cooling to temperatures <400 °C, generating hydrocarbon-rich and ΣCO2-poor fluid–vapor inclusions as described for CH4-rich Southwest Indian Ridge gabbros (12, 24). We propose that, at Von Damm, these hydrocarbons are subsequently liberated during hydrothermal alteration of the Mount Dent oceanic core complex host rocks (15–17). CH4 observed in Southwest Indian Ridge plutonic rock fluid inclusions is characterized by δ13C values of −10‰ to −30‰ (1, 25), a range that matches the values for not just the Von Damm field (−15.4‰) but also, all previously studied ultramafic-influenced submarine hydrothermal fields (8–10). This observation, again, supports our arguments that the processes that we reveal here may be directly relevant to all such systems.
Another line of evidence supporting a magmatic volatile-rich fluid inclusion input is the isotopic composition of He in the Von Damm vent fluids, which indicates R/Ra values of 8.0–8.2 that are consistent with a mantle source (26) (Table S2). Measured CH4/3He ratios (∼2.4 × 108) that are just below the average value of ΣCO2/3He measured in mantle rocks (1 × 109) (26) also support a mantle-derived (fluid inclusion) source for the hydrocarbons. This CH4/3He ratio suggests a conversion of ∼24% of mantle-derived carbon to CH4. Formation of graphite, which can precipitate on cooling of plutonic fluid inclusions (12), may account for the remainder of the carbon.
Table S2.
He isotope data for the Von Damm vent fluids
| Vent | Sample | Measured 3He/4He (R/Ra) | Measured 4He (mL STP/g) | Measured 4He (μmol/kg) | Calculated 3He* (μmol/kg) | Measured CH4 (μmol/kg) | Measured CH4/3He | Predicted C† (μmol/kg) | Measured CH4/predicted C (% converted) |
| East Summit | J2-616-IGT8 | 8.209 ± 0.131 | 2.477E−05 | 1.01 | 1.147E−05 | 2,715 | 2.367E+08 | 11,473 | 0.24 |
| Ravelin 1 | J2-617-IGT6 | 8.265 ± 0.133 | 1.871E−05 | 0.76 | 8.721E−06 | 2,020 | 2.316E+08 | 8,721 | 0.23 |
| Ginger Castle | J2-617-IGT4 | 7.956 ± 0.130 | 1.785E−05 | 0.73 | 8.012E−06 | 1,875 | 2.340E+08 | 8,012 | 0.23 |
IGT, isobaric gas tight; STP, standard temperature and pressure.
Calculated from 4He and R/Ra.
Predicted from 3He and the standard magmatic CO2/3He value of 1 × 109.
Thermodynamic models for the abiotic synthesis of aqueous organic compounds under hydrothermal conditions have postulated that kinetic barriers to the formation of CH4 preclude stable equilibrium in the C-H-O chemical system, thereby creating a thermodynamic drive for the formation of metastable organic species in submarine hot springs (3, 4). The abundance of aqueous carbon species more oxidized than CH4 in Von Damm vent fluids supports such a model. For example, in contrast to dissolved CH4 concentrations, concentrations of ΣCO2 in lower temperature mixed fluids at Von Damm are depleted by as much as 25% relative to a conservative mixing assumption. These depletions are accompanied by 13C enrichment of the residual ΣCO2 compared with the endmember vent fluids and seawater (Fig. 1B and Table 1) and significantly enriched formate species (ΣHCOOH = HCOOH + HCOO−) abundances of 73–605 μmol/kg relative to conservative mixing (Fig. 1C). This result suggests that abiotic ΣHCOOH formation represents a sink for vent fluid ΣCO2 in subsurface mixing zones. Consistent with this interpretation, the amount of carbon present as ΣHCOOH and ΣCO2 in the endmember fluid at East Summit remains constant during mixing in the cooler fluids (Fig. 1D). Despite a strong thermodynamic drive, CH4 production from ΣCO2 does not occur because of well-established kinetic limitations, permitting the formation of metastable intermediate ΣHCOOH species. Reduction of ΣCO2 by H2 in mixed fluids (CO2 + H2 = HCOOH) is consistent with thermodynamic predictions in the absence of CH4 production and laboratory experiments that have shown rapid reaction kinetics over hour to day timescales (2, 6) and isotopic enrichment of the residual ΣCO2.
Thermodynamic Evaluation of ΣHCOOH Abundances.
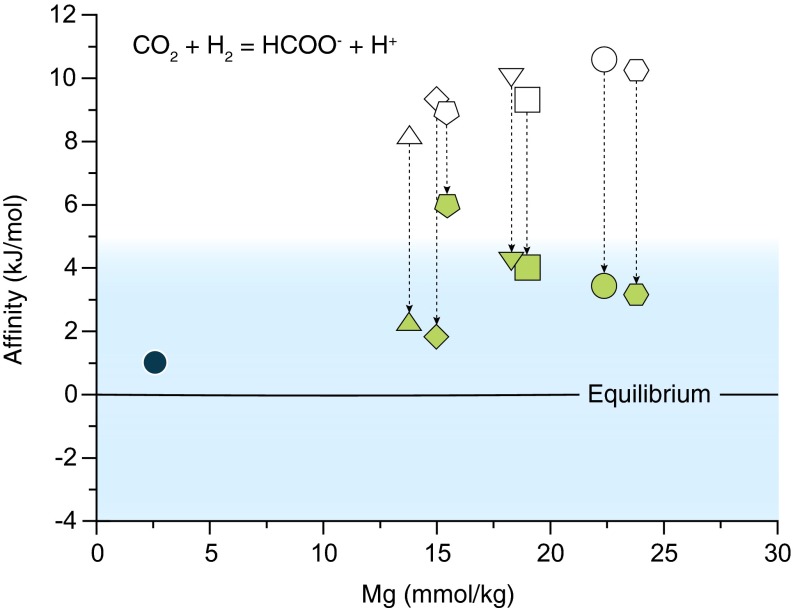
Fluid compositions are consistent with metastable thermodynamic equilibrium between ΣCO2, ΣHCOOH, and H2 in Von Damm mixed fluids, providing additional support for an abiotic origin. Formation of ΣHCOOH on mixing represents a move to a near-equilibrium condition as indicated by decreasing chemical affinities that reach values below 5 kJ/mol for most of the sampled fluids (Fig. 2 and SI Text, section 2). Thus, unlike CH4, the absence of kinetic barriers allows for abiotic synthesis of ΣHCOOH in subsurface mixing zones during active circulation of submarine hydrothermal fluids.
Fig. 2.
Chemical affinity for the production of HCOO− from ΣCO2 and H2 in Von Damm mixed fluids. White symbols indicate a thermodynamic drive for reaction (positive affinity) as written based on conservative dilution of the near-endmember ΣHCOOH composition (blue circle). Green symbols denote affinity calculated with actual mixed fluid ΣHCOOH contents. Thermodynamic equilibrium is defined as affinity = 0 ± 5 kJ/mol (light blue shading). Symbol shapes correspond to those in Fig. 1.
Because of kinetic inhibition of CH4 formation, there is a thermodynamic drive for the abiotic production of other metastable low-molecular weight organic species in addition to ΣHCOOH. Although methanol (CH3OH) production from ΣCO2 and H2 is thermodynamically favorable at Von Damm, the predicted metastable equilibrium CH3OH abundances in mixed fluids would be greater than predicted ΣCO2 abundances, exceeding 2 mmol/kg. Our observation that the amounts of carbon present as ΣHCOOH and ΣCO2 remain constant during mixing (Fig. 1D), therefore, precludes equilibrium CH3OH formation. Similarly, abiotic production of other carboxylic acids (e.g., acetic, propanoic, and butanoic) is likely kinetically inhibited, because these species are below detection (< 1 μmol/kg). The organosulfur compound methanethiol (CH3SH) is present in Von Damm fluids at abundances that do not reflect metastable equilibrium with ΣCO2 and has been attributed to thermal alteration of microbial biomass or other sources of preexisting organic matter (27). The presence of other potential metastable species, such as amino acids, was not investigated; however, if they formed on mixing, it is likely that their concentrations are below the precision of ΣCO2 analysis (∼100 μmol/kg).
Implications.
Vent microorganisms inhabit environments dominated by mixed hydrothermal fluids, where ΣHCOOH can be used as an energy or fixed carbon source by methanogenesis. With abundances approaching those of ΣCO2, ΣHCOOH-based methanogenesis could be a viable metabolic strategy at Von Damm, and abiotic ΣHCOOH may represent an important substrate for microorganisms in high-H2 hydrothermal fluids, which was hypothesized previously for Lost City (28, 29). Formate is the first intermediate species formed in the acetyl-CoA pathway (30), and its abiotic production can reduce the energetic demand for an organism, while also serving as the first step toward forming reduced carbon species that were central to primitive biochemical pathways on early Earth (31). Our demonstration of abiogenic production of CH4 and likely, C2+ n-alkanes in deep-sea hydrothermal systems is also relevant to understanding metabolic options on early Earth environments as well as life strategies in modern systems.
Fluid circulation at Von Damm integrates abiotic organic species formed on long as well as short timescales. Hydrothermal fluids are rich in CH4 leached from ancient magmatic volatile fluid inclusions hosted in plutonic rocks, where it is formed over geologic timescales, and ΣHCOOH, which is formed actively during shallow subsurface mixing over hours to days. These findings represent a fundamental advance in our understanding of processes leading to abiotic organic synthesis in modern and ancient systems on Earth as well as other planetary bodies (7, 31, 32). Furthermore, the demonstration of ongoing ΣHCOOH synthesis is important for microbial communities in the present-day oceanic crust, with exciting implications for microbial metabolisms and life strategies in any warm high-H2 natural waters.
SI Text
1. Calculation of Endmember Compositions.
An endmember composition is calculated for the 226 °C East Summit fluid following the established practice in hydrothermal chemistry of regressing individual chemical species to zero Mg content based on studies that show near-quantitative Mg removal in high-temperature fluids (Table 1) (18). For some species (e.g., ΣCO2 and ΣHCOOH), lower temperature (21–151 °C) mixed fluids exhibit nonconservative behavior that is the result of processes occurring during mixing between an endmember fluid and seawater in the subsurface before their expression at the seafloor. Because extrapolation to zero-Mg endmembers would not be meaningful for nonconservative species, their concentrations are considered in terms of measured abundances only.
2. Assessment of HCOOH Metastable Equilibrium Using Chemical Affinity.
The equilibrium state of the reaction
| [S1] |
in Von Damm mixed fluids can be assessed by calculating the chemical affinity (A) defined by the relationship
| [S2] |
where R is the universal gas constant, T is measured fluid temperature (Kelvin), Qr is the reaction quotient, and Keq is the equilibrium constant at T and seafloor pressure (230 bar). In situ pH values ranging from 5.7 to 5.9 were calculated at seafloor pressure and sampled maximum temperature using thermodynamic data compiled in the SUPCRT92 database (41, 42) and the EQ3/6 software package (43, 44). Activity coefficients were calculated in EQ3/6 for HCOO− and assumed to be unity for neutral dissolved species.
Materials and Methods
Vent fluid samples were collected using 150-mL titanium isobaric gas-tight samplers (33) deployed by the remotely operated vehicle Jason II aboard the R/V Atlantis (Cruise AT18-16) in January of 2012. Thermocouples were calibrated with a National Institute of Standards and Technology temperature calibrator, and the maximum measured temperature for each sample is reported (Table 1). Samples were extracted and processed within 24 h of sampler recovery. Immediately after withdrawing the fluid aliquot from the isobaric gas-tight sampler, pH (25 °C and 1 atm) was measured by potentiometry using an Ag/AgCl reference electrode. Aliquots were collected in Optima HCl-cleaned high-density polyethylene bottles for shore-based analysis of Mg, Cl, and total formate species (ΣHCOOH = HCOOH + HCOO−) in samples stored frozen. Shipboard measurement of dissolved H2 and CH4 was accomplished by molecular-sieve gas chromatography (GC) with thermal conductivity detection after a headspace extraction (3, 34). Aliquots for shore-based total dissolved inorganic carbon (ΣCO2 = CO32− + HCO3− + H2CO3) abundance and stable and radiocarbon isotope analysis of CH4 and ΣCO2 were transferred to evacuated 25-mL serum vials poisoned with Hg2Cl and sealed with butyl rubber stoppers that were preboiled in NaOH to remove trace hydrocarbons (35). Dissolved He was extracted from fluid samples on board the ship using a portable vacuum line and transferred to evacuated aluminosilicate glass break-seal tubes for shore-based He isotope analysis. Fluid aliquots were transferred into sealed glass tubes fitted with Teflon and stainless steel valves for shore-based C2H6 and C3H8 analysis using a purge and trap device interfaced to molecular-sieve GC with flame ionization detection (34). Dissolved Cl and ΣHCOOH abundances were determined by ion chromatography (36, 37). Dissolved Mg concentrations were determined on a ThermoElectron Element2 inductively coupled plasma mass spectrometer (MS) (36, 38). Dissolved ΣCO2 abundances were determined by headspace gas GC injection with thermal conductivity detection (34, 36). Stable carbon isotopes (δ13CΣCO2 and δ13CCH4) were measured by isotope ratio monitoring MS using a Finnigan DeltaPlusXL Mass Spectrometer coupled to an Agilent 6890 GC (1,150 °C combustion temperature). Stable carbon isotope data are reported in standard δ-notation (δ13C) expressed as
| [1] |
where Rsamp and Rstd are the isotope ratios (13C/12C) of the sample and the standard, respectively. Carbon stable isotopes are reported relative to the Vienna Pee Dee Belemnite Scale. Because of variable entrainment of ambient seawater that contains 2.25 mmol/kg CO2 with a δ13CΣCO2 value of 1.1‰, reported sample δ13CΣCO2 values have been calculated from measured values using isotope mass balance (34). Analytical uncertainties (2σ) in abundance and isotopic analyses are listed in Table 1. Radiocarbon (14CΣCO2 and 14CCH4) analysis was conducted at the Woods Hole Oceanographic Institution National Ocean Sciences Accelerator Mass Spectrometry Facility (Table S1). Results are expressed in terms of Fm, representing the deviation of the sample relative to the modern National Bureau of Standards Oxalic Acid I standard (NIST-SRM-4990; A.D. 1950) (39). Correction of ΣCO2 radiocarbon measurements (Table S1) removes the effects of entrainment of ambient seawater through an isotopic mass balance approach that is analogous to the approach for δ13CΣCO2. [For example, this calculation uses vent fluid [Mg] as measured, East Summit fluid [ΣCO2] as measured, Ravelin 2 fluid [ΣCO2] assuming conservative endmember–seawater mixing (i.e., before ΣHCOOH formed), seawater [Mg] = 52.4 mmol/kg, seawater [ΣCO2] = 2.25 mmol/kg, and seawater Fm = 0.9300 (∼580 y, estimated from 2,500-m depth; World Ocean Circulation Experiment Caribbean line A22, 1997).] Measured analytical uncertainties are listed in Table S1. Corrected ΣCO2 uncertainties are conservative estimates calculated by error propagation of independent variables (e.g., also taking into account the effects of [Mg] and [ΣCO2] analytical uncertainties). He abundance and isotope compositions were determined at the Isotope Geochemistry Facility at Woods Hole Oceanographic Institution (Table S2). Helium was cryogenically separated from the other noble gases (40), and analyzed as described in the work by German et al. (13). Uncertainties for 4He abundances are approximately ±5% because of splitting procedures (Table S2).
Supplementary Material
Acknowledgments
We thank the captain and crew of the R/V Atlantis and the ROV Jason II team for their dedication, expertise, and assistance with sample collection. We also thank M. D. Kurz and J. M. Curtice for the isotopic analysis of helium and J. C. Kinsey for generating the bathymetric map. Financial support for this research was provided by National Aeronautics and Space Administration Award NNX-327 09AB75G, National Science Foundation Award OCE-1061863, and the Woods Hole Oceanographic Institution Ocean Ridge Initiative.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506295112/-/DCSupplemental.
References
- 1.Kelley DS, Baross JA, Delaney JR. Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu Rev Earth Planet Sci. 2002;30(1):385–491. [Google Scholar]
- 2.Seewald JS, Zolotov M, McCollom TM. Experimental investigation of single carbon compounds under hydrothermal conditions. Geochim Cosmochim Acta. 2006;70(2):446–460. [Google Scholar]
- 3.Shock EL. Geochemical constraints on the origin of organic compounds in hydrothermal systems. Orig Life Evol Biosph. 1990;20(3-4):331–367. [Google Scholar]
- 4.Shock EL. Chemical environments in submarine hydrothermal systems. In: Holm NG, editor. Marine Hydrothermal Systems and the Origin of Life. Springer; Dordrecht, The Netherlands: 1992. pp. 67–107. [DOI] [PubMed] [Google Scholar]
- 5.Shock EL, Schulte MD. Organic synthesis during fluid mixing in hydrothermal systems. J Geophys Res. 1998;103(E12):28513–28527. [Google Scholar]
- 6.McCollom TM. Experimental constraints on the hydrothermal reactivity of organic acids and acid anions: I. Formic acid and formate. Geochim Cosmochim Acta. 2003;67(19):3625–3644. [Google Scholar]
- 7.Martin W, Baross J, Kelley D, Russell MJ. Hydrothermal vents and the origin of life. Nat Rev Microbiol. 2008;6(11):805–814. doi: 10.1038/nrmicro1991. [DOI] [PubMed] [Google Scholar]
- 8.Proskurowski G, et al. Abiogenic hydrocarbon production at lost city hydrothermal field. Science. 2008;319(5863):604–607. doi: 10.1126/science.1151194. [DOI] [PubMed] [Google Scholar]
- 9.Charlou JL, Donval JP, Fouquet Y, Jean-Baptiste P, Holm NG. Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36°14'N, MAR) Chem Geol. 2002;191(4):345–359. [Google Scholar]
- 10.Charlou JL, et al. High production and fluxes of H2 and CH4 and evidence of abiotic hydrocarbon synthesis by serpentinization in ultramafic-hosted hydrothermal systems on the Mid-Atlantic Ridge. In: Rona PA, Devey CW, Dyment J, Murton BJ, editors. Diversity of Hydrothermal Systems on Slow Spreading Ocean Ridges. American Geophysical Union; Washington, DC: 2010. pp. 265–296. [Google Scholar]
- 11.McCollom TM, Seewald JS. Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem Rev. 2007;107(2):382–401. doi: 10.1021/cr0503660. [DOI] [PubMed] [Google Scholar]
- 12.Kelley DS, Früh-Green GL. Abiogenic methane in deep-seated mid-ocean ridge environments: Insights from stable isotope analyses. J Geophys Res. 1999;104(B5):10439–10460. [Google Scholar]
- 13.German CR, et al. Diverse styles of submarine venting on the ultraslow spreading Mid-Cayman Rise. Proc Natl Acad Sci USA. 2010;107(32):14020–14025. doi: 10.1073/pnas.1009205107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connelly DP, et al. Hydrothermal vent fields and chemosynthetic biota on the world’s deepest seafloor spreading centre. Nat Commun. 2012;3:620. doi: 10.1038/ncomms1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballard RD, et al. Geological and geophysical investigation of the Mid-Cayman Rise Spreading Center: Initial results and observations. In: Talwani M, Harrison CG, Hayes DE, editors. Deep Drilling Results in the Atlantic Ocean: Ocean Crust. American Geophysical Union; Washington, DC: 1979. pp. 66–93. [Google Scholar]
- 16.Stroup J, Fox P. Geologic investigations in the Cayman Trough: Evidence for thin oceanic crust along the Mid-Cayman Rise. J Geol. 1981;89(4):395–420. [Google Scholar]
- 17.Hayman NW, et al. Oceanic core complex development at the ultraslow spreading Mid-Cayman Spreading Center. Geochem Geophys Geosyst. 2011 doi: 10.1029/2010GC003240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bischoff J, Dickson F. Seawater-basalt interaction at 200°C and 500 bars: Implications for the origin of seafloor heavy metal deposits and regulation of seawater chemistry. Earth Planet Sci Lett. 1975;25(3):385–397. [Google Scholar]
- 19.Schmidt K, Koschinsky A, Garbe-Schönberg D, de Carvalho L, Seifert R. Geochemistry of hydrothermal fluids from the ultramafic-hosted Logatchev hydrothermal field, 15°N on the Mid-Atlantic Ridge: Temporal and spatial investigation. Chem Geol. 2007;242(1-2):1–21. [Google Scholar]
- 20.Valentine DL, Chidthaisong A, Rice A, Reeburgh WS, Tyler SC. Carbon and hydrogen isotope fractionation by moderately thermophilic methanogens. Geochim Cosmochim Acta. 2004;68(7):1571–1590. [Google Scholar]
- 21.Schoell M. The hydrogen and carbon isotopic composition of methane from natural gases of various origins. Geochim Cosmochim Acta. 1980;44(5):649–661. [Google Scholar]
- 22.Bischoff JL, Rosenbauer RJ. An empirical equation of state for hydrothermal seawater (3.2 percent NaCl) Am J Sci. 1985;285(8):725–763. [Google Scholar]
- 23.Allen DE, Seyfried WE., Jr Serpentinization and heat generation: Constraints from Lost City and Rainbow hydrothermal systems. Geochim Cosmochim Acta. 2004;68(6):1347–1354. [Google Scholar]
- 24.Kelley DS. Methane-rich fluids in the oceanic crust. J Geophys Res. 1996;101(B2):2943–2962. [Google Scholar]
- 25.Kelley DS, Früh-Green GL. Volatile lines of descent in submarine plutonic environments: Insights from stable isotope and fluid inclusion analyses. Geochim Cosmochim Acta. 2001;65(19):3325–3346. [Google Scholar]
- 26.Marty B, Tolstikhin IN. CO2 fluxes from mid-ocean ridges, arcs and plumes. Chem Geol. 1998;145(3-4):233–248. [Google Scholar]
- 27.Reeves EP, McDermott JM, Seewald JS. The origin of methanethiol in midocean ridge hydrothermal fluids. Proc Natl Acad Sci USA. 2014;111(15):5474–5479. doi: 10.1073/pnas.1400643111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang SQ, Butterfield DA, Schulte M, Kelley DS, Lilley MD. Elevated concentrations of formate, acetate and dissolved organic carbon found at the Lost City hydrothermal field. Geochim Cosmochim Acta. 2010;74(3):941–952. [Google Scholar]
- 29.Lang SQ, et al. Microbial utilization of abiogenic carbon and hydrogen in a serpentinite-hosted system. Geochim Cosmochim Acta. 2012;92(1):82–99. [Google Scholar]
- 30.Fuchs G. CO2 fixation in acetogenic bacteria: Variations on a theme. FEMS Microbiol Rev. 1986;39(3):181–213. [Google Scholar]
- 31.Martin W, Russell MJ. On the origin of biochemistry at an alkaline hydrothermal vent. Philos Trans R Soc Lond B Biol Sci. 2007;362(1486):1887–1925. doi: 10.1098/rstb.2006.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell MJ, Hall AJ, Martin W. Serpentinization as a source of energy at the origin of life. Geobiology. 2010;8(5):355–371. doi: 10.1111/j.1472-4669.2010.00249.x. [DOI] [PubMed] [Google Scholar]
- 33.Seewald JS, Doherty K, Hammar T, Liberatore S. A new gas-tight isobaric sampler for hydrothermal fluids. Deep Sea Res Part 1 Oceanogr Res Pap. 2002;49(1):189–196. [Google Scholar]
- 34.Cruse A, Seewald JS. Geochemistry of low-molecular weight hydrocarbons in hydrothermal fluids from Middle Valley, northern Juan de Fuca Ridge. Geochim Cosmochim Acta. 2006;70(8):2073–2092. [Google Scholar]
- 35.Oremland RS, Des Marais DJ. Distribution, abundance and carbon isotopic composition of gaseous hydrocarbons in Big Soda Lake, Nevada: An alkaline, meromictic lake. Geochim Cosmochim Acta. 1983;47(12):2107–2114. [Google Scholar]
- 36.Reeves EP, et al. Geochemistry of hydrothermal fluids from the PACMANUS, Northeast Pual and Vienna Woods hydrothermal fields, Manus Basin, Papua New Guinea. Geochim Cosmochim Acta. 2011;75(4):1088–1123. [Google Scholar]
- 37.McCollom TM, Seewald JS. A reassessment of the potential for reduction of dissolved CO2 to hydrocarbons during serpentinization of olivine. Geochim Cosmochim Acta. 2001;65(21):3769–3778. [Google Scholar]
- 38.Craddock PR, et al. Rare earth element abundances in hydrothermal fluids from the Manus Basin, Papua New Guinea: Indicators of sub-seafloor hydrothermal processes in back-arc basins. Geochim Cosmochim Acta. 2010;74(19):5494–5513. [Google Scholar]
- 39.Olsson I. 1970. The use of oxalic acid as a standard. Proceedings of the Radiocarbon Variations and Absolute Chronology Nobel Symposium, ed Olsson I (Wiley, Chichester, United Kingdom), p 17.
- 40.Lott DE. Improvements in noble gas separation methodology: A nude cryogenic trap. Geochem Geophys Geosyst. 2001 doi: 10.1029/2001GC000202. [DOI] [Google Scholar]
- 41.Johnson J, Oelkers E, Helgesen H. SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C. Comput Geosci. 1992;18(7):899–947. [Google Scholar]
- 42.Shock EL. Organic acids in hydrothermal solutions: Standard molal thermodynamic properties of carboxylic acids and estimates of dissociation constants at high temperatures and pressures. Am J Sci. 1995;295(5):496–580. doi: 10.2475/ajs.295.5.496. [DOI] [PubMed] [Google Scholar]
- 43.Wolery TJ. EQ3NR, A Computer Program for Geochemical Aqueous Speciation-Solubility Calculations: Theoretical Manual, User's Guide, and Related Documentation (Version 7.0) Lawrence Livermore National Lab; Oak Ridge, TN: 1992. [Google Scholar]
- 44.Wolery TJ, Daveler SA. EQ6, A Computer Program for Reaction Path Modeling of Aqueous Geochemical Systems: Theoretical Manual, User's Guide, and Related Documents. Lawrence Livermore National Lab; Oak Ridge, TN: 1992. [Google Scholar]