Significance
Preexisting secretory IgA (S-IgA) antibodies can provide immediate immunity via their unique capability to eliminate a pathogen before it passes the mucosal barrier. Several clinical trials have reported that S-IgA against influenza virus was induced by intranasal administration of an inactivated influenza vaccine. S-IgA in mucosa consists predominantly of dimers rather than tetramers, as revealed by ultracentrifugation almost 50 years ago. However, direct evidence concerning the quaternary architectures and the physiological function of polymers larger than dimers has not been reported in healthy humans. The present study revealed, for the first time to our knowledge, the existence of large polymeric IgA in the healthy human upper respiratory mucosa, as well as the physiological functions of these molecules in protecting against viral infection in humans.
Keywords: secretory IgA, influenza virus, intranasal inactivated influenza vaccine, high-speed atomic force microscopy
Abstract
Secretory IgA (S-IgA) antibodies, the major contributors to humoral mucosal immunity to influenza virus infection, are polymeric Igs present in many external secretions. In the present study, the quaternary structures of human S-IgA induced in nasal mucosa after administration of intranasal inactivated influenza vaccines were characterized in relation to neutralization potency against influenza A viruses. Human nasal IgA antibodies have been shown to contain at least five quaternary structures. Direct and real-time visualization of S-IgA using high-speed atomic force microscopy (AFM) demonstrated that trimeric and tetrameric S-IgA had six and eight antigen-binding sites, respectively, and that these structures exhibited large-scale asynchronous conformational changes while capturing influenza HA antigens in solution. Furthermore, trimeric, tetrameric, and larger polymeric structures, which are minor fractions in human nasal IgA, displayed increased neutralizing potency against influenza A viruses compared with dimeric S-IgA, suggesting that the larger polymeric than dimeric forms of S-IgA play some important roles in protection against influenza A virus infection in the human upper respiratory tract.
Antibodies in respiratory mucosa are primary mediators of protective immunity against influenza. Notably, preexisting secretory IgA (S-IgA) antibodies can provide immediate immunity by eliminating a pathogen before the virus passes the mucosal barrier (1–3). Parenteral vaccination induces serum IgG but not S-IgA, so vaccine efficacy is limited. In contrast, intranasal administration of an inactivated influenza vaccine elicits both S-IgA and IgG responses, thus improving the protective efficacy of current vaccination procedures (4–8).
IgA in human serum exists predominantly in the form of monomers, whereas the majority of IgA in external secretions is present in the form of polymers. These polymeric IgA forms are associated with the extracellular portion of the polymeric Ig receptor, generating a complex (receptor + polymeric IgA) called S-IgA (9). S-IgA primarily corresponds to dimeric IgA, although low levels of some larger polymeric forms, particularly tetramers, are also present (9–15). Polymeric S-IgA has been shown (both in vitro and in experimental animal models) to be more effective than monomeric IgA or IgG for the neutralization of influenza viruses (16–19). However, little is known of the quaternary structures and neutralizing potencies in viral infection of the various forms of polymeric S-IgA in the human nasal mucosa. In this study, the quaternary structures and neutralizing potencies of nasal antibodies against influenza virus were examined using nasal wash samples from healthy adults who had received intranasally administered inactivated influenza vaccines. These nasal wash samples, containing variously sized Igs, were separated by gel filtration chromatography (GFC) and assessed for neutralization activity against influenza virus. The quaternary structures of the nasal IgA induced by intranasally administered inactivated influenza vaccines then were determined using biochemical techniques and high-speed atomic force microscopy (AFM). We found that human nasal IgA comprised at least five quaternary structures, including monomer, dimer, trimer, and tetramer structures, as well as a polymeric form larger than the tetramer structure. Among these forms, the polymeric structure demonstrated higher neutralizing potency against seasonal influenza viruses (H3N2) and highly pathogenic avian influenza virus (H5N1) compared with the dimeric form, suggesting that large polymeric S-IgA antibodies play crucial roles in protective immunity against influenza virus infection of the human upper respiratory tract.
Results
Influenza Virus Neutralizing Potency of Each GFC Fractionation of Human Nasal Wash Samples.
Nasal wash samples from five healthy adult volunteers, who were vaccinated five times intranasally with inactivated A/Victoria/210/2009 (H3N2; Victoria)-like virus whole-virion vaccines, were collected as described in Materials and Methods (also Fig. S1). It was confirmed that these volunteers showed relatively high serum neutralizing antibody responses against Victoria virus after the second vaccination.
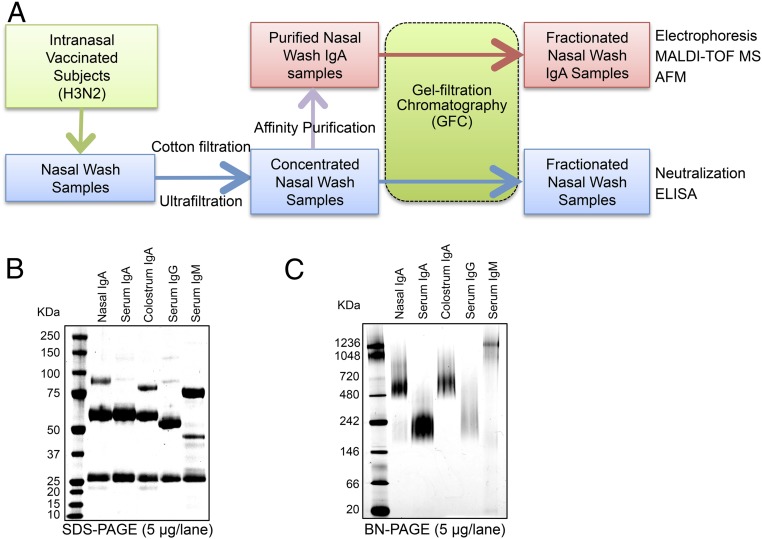
Fig. S1.
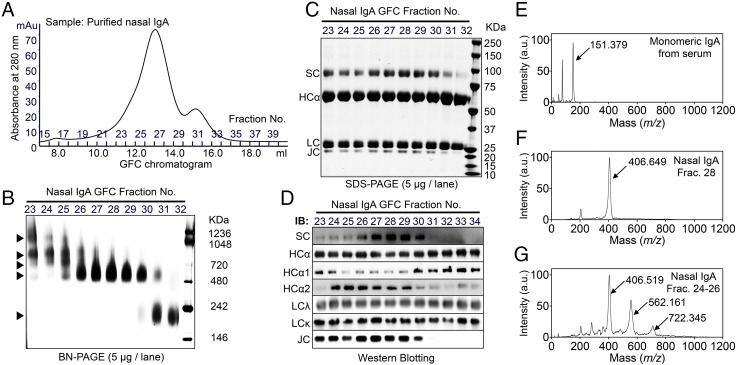
Schematic representation of the preparation and analysis of nasal wash samples from intranasally vaccinated healthy volunteers. (A) Nasal wash samples were concentrated using centrifugal concentrators. The proportions of IgM, IgA, and IgG antibodies to total antibody after concentration were ∼1.5 (±0.2)%, 73.6 (±3.6)%, and 24.9 (±3.6)%, respectively (mean ± SEM). The concentrated nasal wash samples were used as an initial source in various assays to analyze the properties of nasal IgA antibodies. Specifically, the concentrated nasal wash samples were used to examine the molecular size distribution of each antibody and to determine the neutralizing potency and the binding potency to HA molecules of each fraction after separation through GFC. In addition, the IgA antibody fractions, purified from the pooled nasal wash samples by affinity column chromatography and then separated by GFC, were analyzed using several biochemical methods, and the structure and dynamics of IgA molecules in physiological solutions were evaluated by high-speed AFM. The neutralizing potency and the binding potency to HA molecules of each fraction after separation by GFC were analyzed in relation to the differences in molecular size. (B) SDS/PAGE analysis of purified nasal IgA, serum IgA, colostrum IgA, serum IgG, and serum IgM under reducing conditions. Purified nasal IgA was composed of an α-heavy chain (HCα), light chain (LC), SC, and JC. (C) Blue native (BN)/PAGE analysis of purified nasal IgA, serum IgA, colostrum IgA, serum IgG, and serum IgM. Purified nasal IgA was not homogeneous and included at least two distinct oligomeric structures, with approximate molecular masses of ≥500 kDa and >720 kDa.
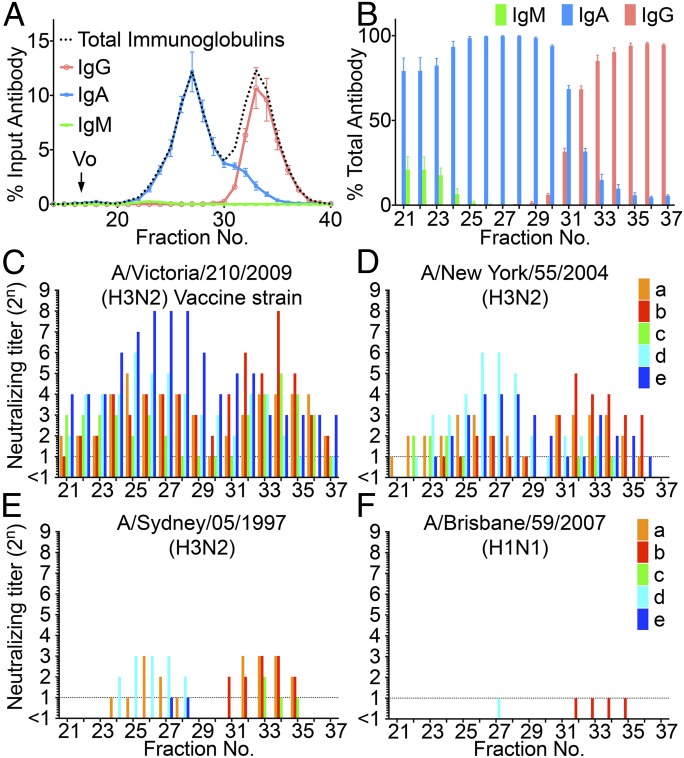
Samples were separated by GFC, and the quantities of IgA, IgG, and IgM in each fraction were estimated by ELISA. The ELISA showed that IgM and IgG were detected primarily in fractions 21–24 and 30–38, respectively, whereas IgA were detected in a wider range of fractions (21–36) (Fig. 1 A and B). These observations suggested that nasal IgA possessed striking size heterogeneity.
Fig. 1.
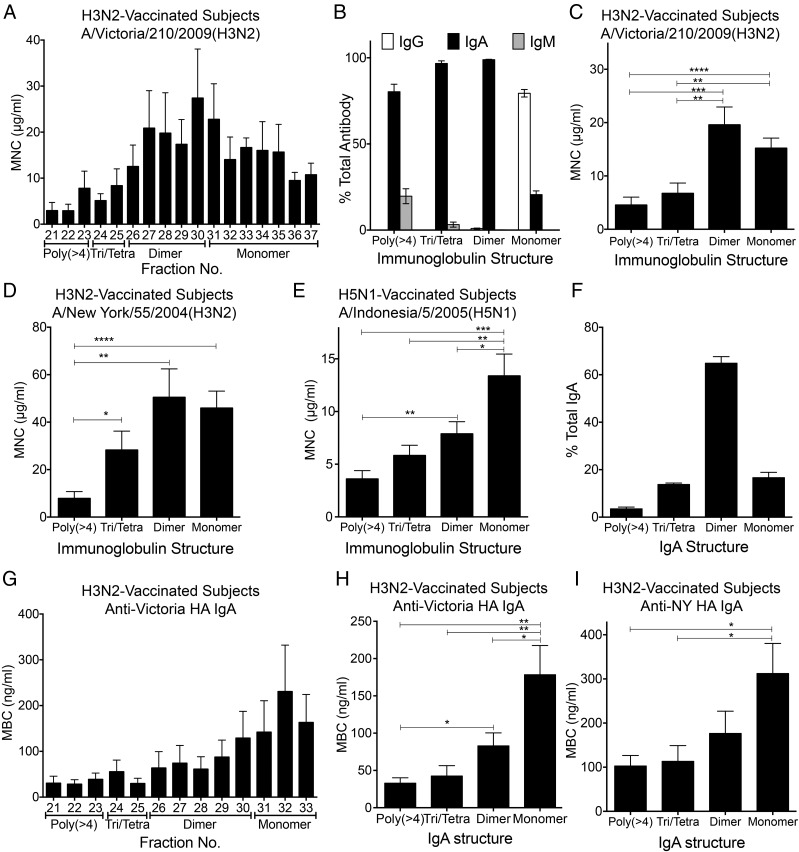
Size distribution of Igs in the nasal wash samples separated by GFC and estimation of the neutralizing potency of each fraction. (A) Fractionation pattern of nasal wash samples on Superose 6 GFC. The amounts of IgM, IgG, and IgA (wt/wt) in each fraction were estimated using ELISA. Values plotted are means, and error bars represent SEM. Vo, void volume. (B) Proportions of the amounts of IgM, IgG, and IgA (wt/wt) to the total amounts of antibodies in each fraction (IgM + IgG + IgA, weight) were estimated. Values plotted are means, and error bars represent SEM. (C) Neutralization titer against the vaccine strain Victoria virus was determined in each fraction separated by GFC of nasal wash samples collected from five participants (a, b, c, d, and e aged 33, 23, 27, 24, and 34 years, respectively, at the time of sample collection). (D–F) Neutralization titer of each fraction against NY virus, Sydney virus, and Brisbane virus also was determined for the same nasal samples collected from the five participants (a–e).
Each fraction also was assayed for neutralization potency against the Victoria virus (which had been used for vaccination) and against other influenza viruses, including A/New York/55/2004 (H3N2; NY) virus (HA of 96.6% amino acid similarity to Victoria HA), A/Sydney/05/1997 (H3N2; Sydney) virus (HA of 92.9% amino acid similarity to Victoria HA), and A/Brisbane/59/2007 (H1N1; Brisbane) virus (HA of 41.8% amino acid similarity to Victoria HA) (Fig. 1 C–F). Profiles of neutralization titers against Victoria virus from the five volunteers showed two distinct peaks of neutralization, comprising fractions 21–30 (which contained mainly polymeric IgA) and fractions 31–37 (which contained mainly IgG).
The peak titers of neutralization activity were found to be attenuated when tested using genetically divergent viruses, and the degree of attenuation was consistent with the degree of divergence. Specifically, apparent titers were ranked as Victoria > NY > Sydney > Brisbane viruses, an order that corresponds to decreasing HA amino acid similarity among these strains. This observation suggested that the neutralization activities in fractionated nasal wash samples reflected the presence of virus-specific antibodies (notably, anti-HA antibodies), rather than nonspecific antiviral activities in the nasal mucus (Fig. 1 C–F). In addition, the profiles varied slightly among volunteers (Fig. 1 C–F). The profiles against NY virus for nasal wash samples from some volunteers (Fig. 1D, a and b) showed higher neutralization titers in IgG fractions and lower neutralization titers in polymeric IgA fractions, whereas the profiles from other volunteers (Fig. 1D, c–e) showed lower neutralization titers in IgG fractions and higher neutralization titers in polymeric IgA fractions. The profile against Sydney virus showed that the nasal wash sample from one volunteer provided neutralization titers only in the polymeric IgA fractions (Fig. 1E, d). Meanwhile, neutralization activity against Brisbane virus was not detected in any of the volunteers (Fig. 1F). The higher neutralization titers seen in polymeric IgA fractions (compared with IgG fractions) suggested greater cross-reactivity of polymeric IgA to variant H3N2 viruses than of IgG. The higher neutralizing titers of polymeric IgA fractions against influenza virus were further confirmed in additional studies using H5N1 intranasally vaccinated subjects and H5N1 viruses (Fig. S2).
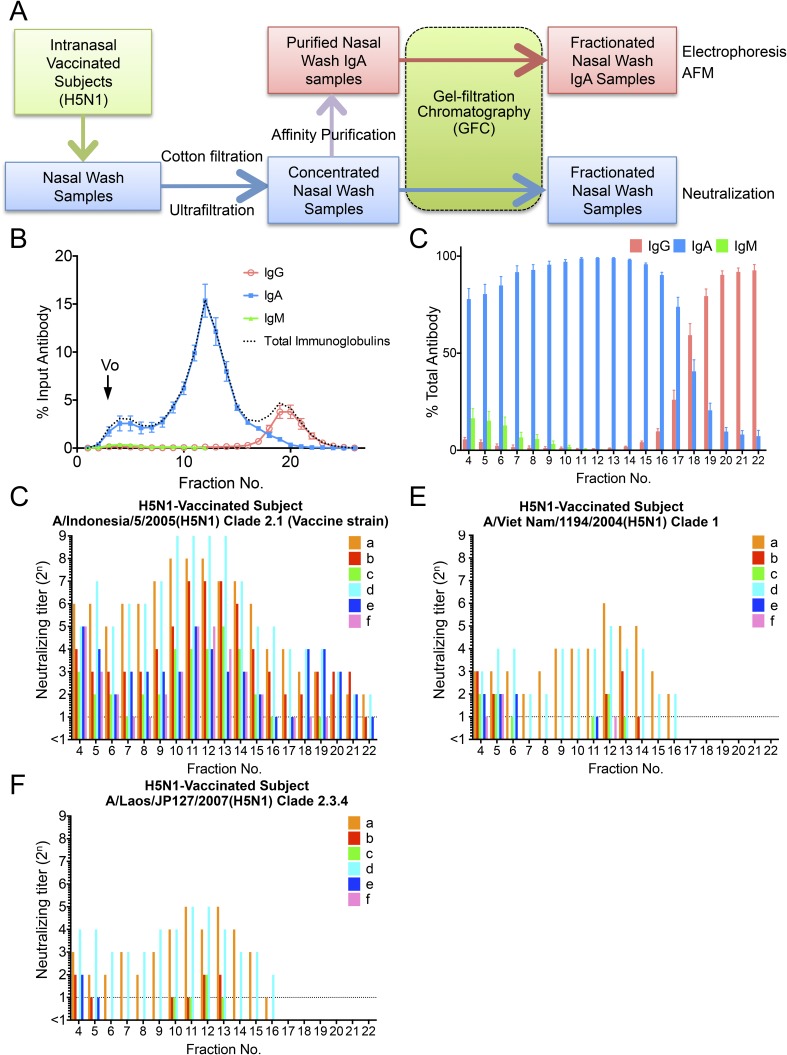
Fig. S2.
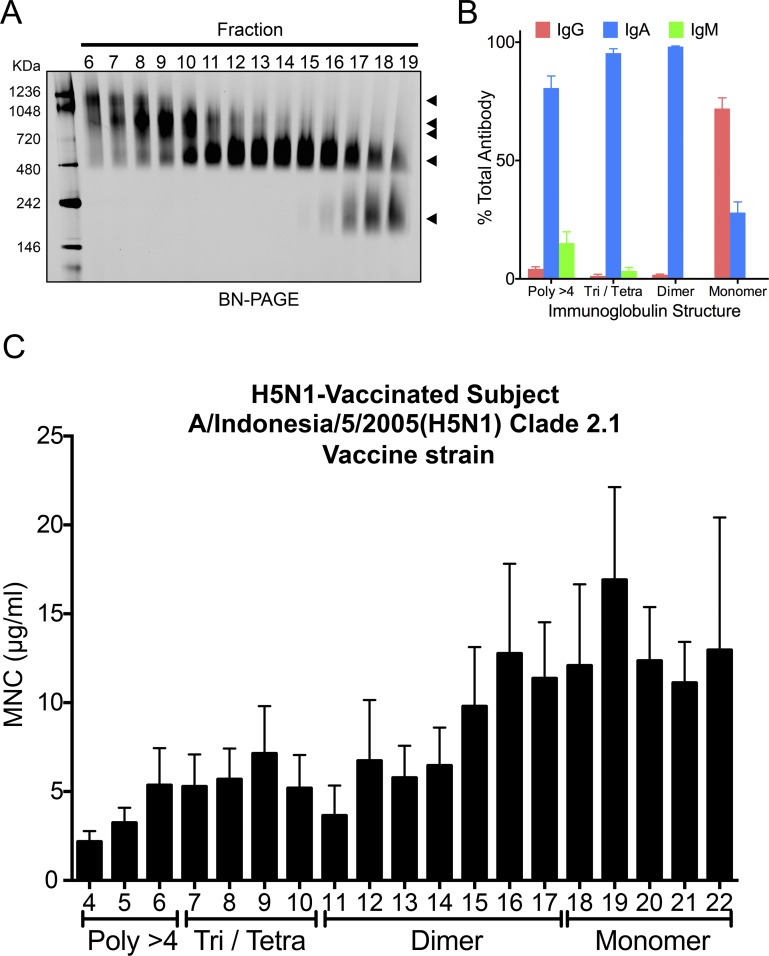
Nasal wash samples from six volunteers vaccinated intranasally with an inactivated whole-virion H5N1 virus vaccine were separated by GFC, and neutralizing potency was estimated for each fraction. (A) Schematic presentation of preparation and analysis of nasal wash samples from intranasally vaccinated healthy volunteers. (B) Fractionation pattern of nasal wash samples on Superose 6 GFC. The amount of IgM, IgG, and IgA (wt/wt) in each fraction was estimated using ELISA. Values plotted are means, and error bars represent SEM. Vo, void volume. (C) Proportion of the amount of IgM, IgG, and IgA (wt/wt) to the total amount of antibodies in each fraction (IgM + IgG + IgA, weight) was estimated. Values plotted are means, and error bars represent SEM. (D) Neutralization titer against the vaccine strain A/Indonesia/5/2005 (H5N1) virus was determined in each fraction separated by GFC of nasal wash samples collected from six participants (a–f). (E and F) Neutralization titer of each fraction against A/Viet Nam/1194/2004 (H5N1) virus and A/Laos/JP127/2007 (H5N1) virus also was determined with the same nasal samples collected from six participants (a–f). The profiles against A/Indonesia/5/2005 (H5N1) virus showed higher neutralization titers in polymeric IgA fractions and lower neutralization titers in IgG fractions. Furthermore, the profiles against A/Viet Nam/1194/2004 (H5N1) virus and A/Laos/JP127/2007 (H5N1) virus for nasal wash samples from all of the volunteers showed no neutralization titers in IgG fractions despite detection of neutralization titers in polymeric IgA fractions. These observations suggested that greater cross-reactivity of polymeric IgA to H5N1 viruses than of IgG to H5N1 viruses and polymeric IgA appeared to play a pivotal role in protecting the human nasal mucosa from infection of H5N1 viruses.
Binding Activity of Antibodies to Influenza Virus HA in Each GFC Fraction.
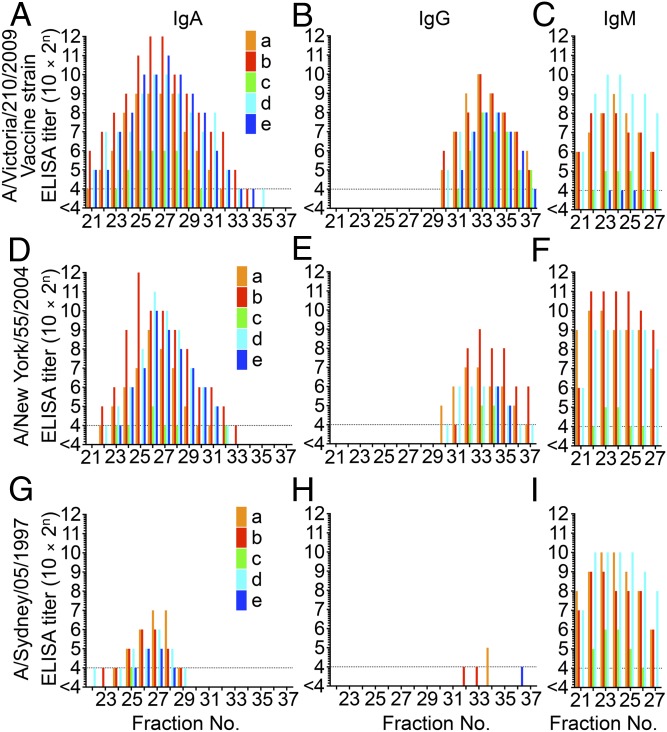
To determine the isotypes of the Igs responsible for neutralization activity in nasal mucus, HA-specific IgM, IgA, and IgG responses for Victoria HA, NY HA, and Sydney HA were examined using ELISA. HA-specific IgA titers were detected in a broad range of fractions and peaked in fractions 21–30, which corresponded to one of the peaks of neutralization activity (Fig. 2 A, D, and G). HA-specific IgG titers peaked in fractions 31–37, which corresponded to the second peak of neutralization activity (Fig. 2 B, E, and H). Furthermore, reduction of the peak titers of HA-specific IgA and IgG against NY HA or Sydney HA coincided with the decrease of neutralization titers in each peak fraction (and, as noted above, correlated with genetic divergence from Victoria HA). Notably, HA-specific antibodies that were cross-reactive to Sydney HA were detected in the polymeric IgA fractions, but not in the IgG fractions (Fig. 2 G and H). This observation suggested that polymeric IgA possess greater cross-reactivity to variant H3N2 viruses than do IgG antibodies. In contrast, the reduction of the peak titer of HA-specific IgM (correlating with genetic divergence from Victoria HA) was not observed regardless of the detected level of HA-specific IgM (Fig. 2 C, F, and I). These observations demonstrated that IgA and IgG contributed to the neutralization activities detected in the range of fractions 21–30 and fractions 31–37, respectively; IgM did not appear to contribute to these neutralization activities.
Fig. 2.
Determination of binding activity of antibodies to HA molecules in each GFC fraction by ELISA. The IgA ELISA titer (A), IgG ELISA titer (B), and IgM ELISA titer (C) against HA molecules of Victoria virus (H3N2) were determined in each fraction separated by GFC of nasal wash samples collected from five participants (a, b, c, d, and e aged 33, 23, 27, 24, and 34 years, respectively, at the time of sample collection). HA-specific IgA titers were detected in a broad range of fractions and peaked at fractions 21–30, which corresponded to the first of two peaks of neutralization activity. HA-specific IgG titers peaked at fractions 31–37, which corresponded to the second of two peaks of neutralization activity. The IgA ELISA titer (D), IgG ELISA titer (E), and IgM ELISA titer (F) against HA molecules of NY virus (H3N2) were determined in each fraction separated by GFC of nasal wash samples collected from five participants (a–e). The IgA ELISA titer (G), IgG ELISA titer (H), and IgM ELISA titer (I) against HA molecules of Sydney virus (H3N2) were determined in each fraction separated by GFC of nasal wash samples collected from five participants (a–e). Reduction of the peak titers of HA-specific IgA and IgG antibodies against NY HA (which has 96.6% amino acid similarity to Victoria HA) or Sydney HA (which has 92.9% amino acid similarity to Victoria HA) correlated with decreased sequence similarity to Victoria HA.
Quaternary Structures of Nasal IgA.
To determine the quaternary structures of human nasal IgA, purified nasal IgA was obtained by affinity chromatography using pooled (among the five volunteers) nasal wash samples, and the purified IgA molecules were then applied to GFC fractionation (Fig. 3A). Blue native PAGE of fractionated samples showed that nasal IgA antibodies were composed of at least five distinct quaternary structures (Fig. 3B). SDS/PAGE revealed that the IgA antibodies in fractions 23–30 were composed of α-heavy chains, light chains, secretory component (SC), and J chain (JC) (Fig. 3C). In contrast, the IgA in fraction 32 lacked an SC or JC, suggesting that the fraction 32 IgA was monomeric (Fig. 3C). Western blotting using several antibodies revealed that the subclass proportions (of IgA1 and IgA2) varied with the quaternary structure (Fig. 3D). To determine more accurately the molecular size of these antibodies, high-mass MALDI-TOF MS was used. For analysis of monomeric IgA, serum IgA was used. Serum IgA yielded one major peak with MH+ = 151.379 ± 0.174 kDa (mean ± SD), a size that is consistent with monomeric IgA (Fig. 3E); fraction 28 of nasal IgA yielded one major peak with MH+ = 406.649 ± 0.389 kDa, a size that corresponds to dimeric S-IgA (two monomers, one SC, and one JC) (Fig. 3F). Meanwhile, three major peaks with MH+ = 406.519 ± 0.422 kDa, MH+ = 562.161 ± 0.576 kDa, and MH+ = 722.342 ± 0.844 kDa were detected in the mixture of fractions 24–26 of nasal IgA, indicating that fractions 24–26 consisted of dimeric, trimeric (three monomers, one SC, and one JC), and tetrameric (four monomers, one SC, and one or two JCs) S-IgA (Fig. 3G). These results suggested that nasal IgA comprised a total of at least five quaternary structures: monomer, dimer, trimer, and tetramer structures, as well as a polymer larger than the tetramer.
Fig. 3.
Biochemical characterization of the quaternary structures of nasal IgA. (A) Purified nasal IgA was subjected to GFC separation according to quaternary structures. (B) Blue native (BN)/PAGE analysis of each fraction of gel-filtered nasal IgA. Nasal IgA samples were composed of at least five distinct quaternary structures (arrowheads). (C) SDS/PAGE analysis of each fraction of gel-filtered nasal IgA under reducing conditions. (D) Western blotting analysis of each fraction of gel-filtered nasal IgA using anti-SC, anti-human IgA α-heavy chain (HCα), anti-human IgA1 subclass-specific (HCα1), anti-human IgA2 subclass-specific (HCα2), anti-human light chain λ (LCλ), anti-human light chain κ (LCκ), and anti-JC antibodies. IB, immunoblot. High-mass MALDI-TOF analysis of serum IgA (E), fraction 28 of nasal IgA (F), and fractions 24–26 of nasal IgA (G). (E) With serum IgA, one major peak (arrow) was detected. (F) With fraction 28 (Frac.) of nasal IgA, one major peak (arrow) was detected. (G) With mixtures of fractions 24, 25, and 26 of nasal IgA antibodies, three major peaks (arrows) were detected. a.u., arbitrary unit; Frac., fraction.
Visualized Quaternary Structures and Dynamics of Nasal IgA.
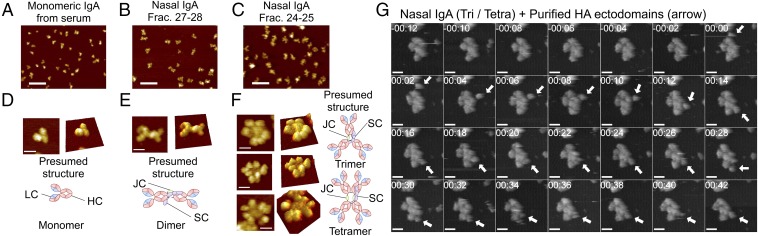
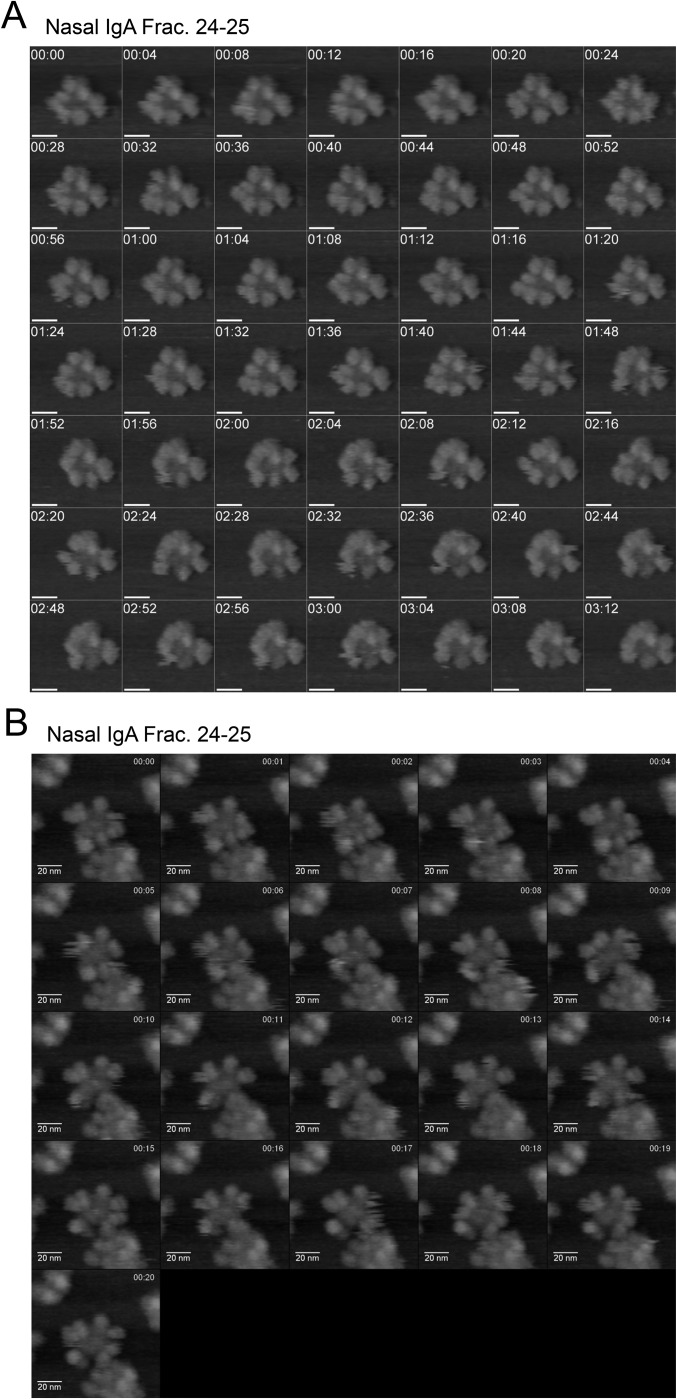
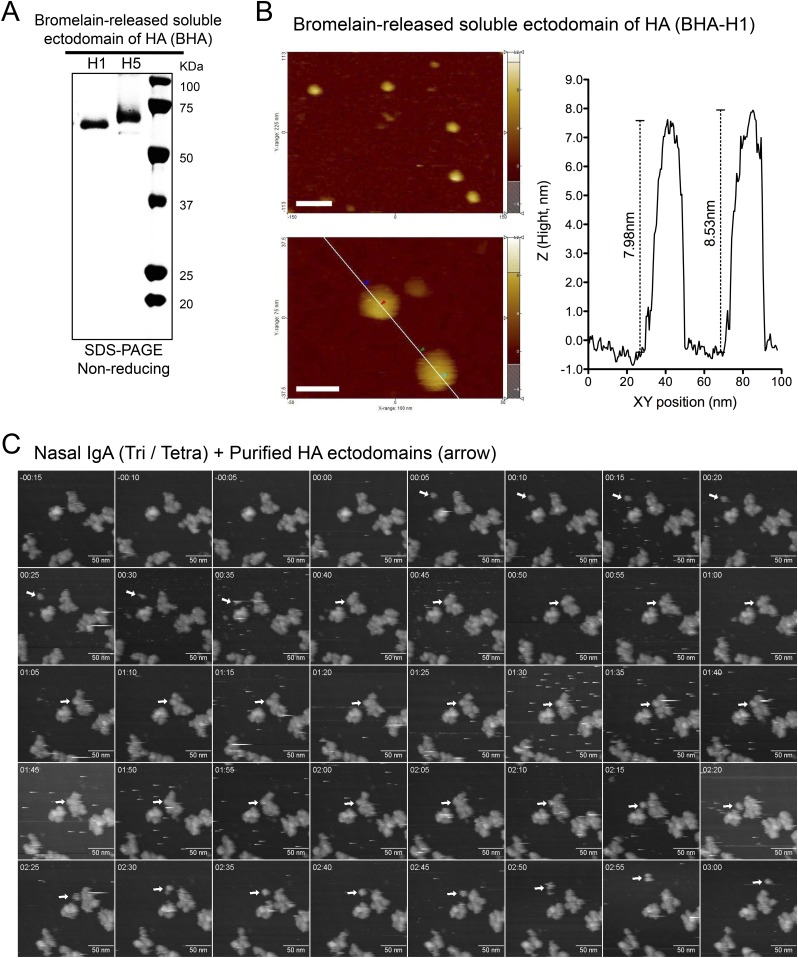
To analyze the quaternary architectures of these oligomeric complexes, nasal IgA was visualized using high-speed AFM. Separate preparations of nasal and serum IgA were subjected to AFM imaging under liquid conditions. Each IgA population comprised a homogeneous mix of particle sizes (Fig. 4 A–C). However, nasal IgA (of both fractions 27–28 and fractions 24–25) particles were significantly larger (P < 0.0001) than particles of serum monomeric IgA (678.6 ± 328.9 nm3, mean ± SD). Furthermore, nasal IgA particles in fractions 24–25 (2,456 ± 865.1 nm3) were also significantly larger (P < 0.0001) than particles in fractions 27–28 (1,738 ± 431.4 nm3). High-resolution observations revealed that molecules of serum IgA, which were monomeric, were nearly all triangular with acute angles, apparently consisting of two Fab regions and one Fc region (Fig. 4D). Furthermore, molecules of nasal IgA in fractions 27–28 appeared to consist of two IgA monomers arranged end-to-end (Fig. 4E). In addition, nasal IgA molecules in fractions 24–25, which were composed of trimers and tetramers, appeared as asterisk- or four-leaf clover–shaped complexes (Fig. 4F). To observe the structural dynamics of tetrameric IgA, successive images were captured. We observed that each radial arm rocked asynchronously and appeared to bend out of the plane defined by the central portion of the complex (Fig. S3 A and B and Movies S1 and S2). To address whether these radial arms were capable of capturing antigens, specific HA antigens were added during the AFM observations. Imaging revealed that the round-shaped HA antigens (Fig. S4 A and B) associated with the radial regions of nasal IgA and moved discontinuously along with the edge of the IgA, suggesting that the radial arms of trimeric and tetrameric IgA were reversibly binding to the antigens (Fig. 4G, Fig. S4C, and Movie S3).
Fig. 4.
AFM revealed the quaternary molecular structures of nasal IgA. AFM images of serum IgA (A), fractions 27–28 of nasal IgA (B), and fractions 24–25 of nasal IgA (C) antibodies at low resolution. (Scale bars, 100 nm.) AFM images of serum IgA (D), fractions 27–28 of nasal IgA (E), and fractions 24–25 of nasal IgA (F) at the single particle level. (Scale bars, 20 nm.) Schematic diagrams next to or under the AFM images show presumed structures of monomeric, dimeric, trimeric, and tetrameric IgA. (G) Successive AFM images of the interaction between nasal trimeric/tetrameric IgA and HA ectodomains of influenza virus (arrows). The time stamp is given as min:sec. The frame rate is 0.5 frames per second. (Scale bars, 20 nm.) (Also Movie S3.) Tetra, tetramer; Tri, trimer.
Fig. S3.
AFM observation of dynamics of nasal tetrameric IgA. (A and B) Time-lapse AFM imaging of nasal tetrameric IgA reveals that the radial arms of the four-leaf clover–shaped complex were rocked asynchronously and bent out of the plane defined by the central portion of the complex. The frame rate in A is 0.25 frame per second, and the frame rate in B is one frame per second. (Scale bars, 20 nm.) The time stamp is given as min:sec. (Also Movies S1 and S2.) Frac., fraction.
Fig. S4.
Time-lapse AFM imaging of the interaction between an HA molecule and nasal polymeric IgA. (A) Ectodomains of H1 HA and H5 HA were prepared by digestion of the purified virions with bromelain in the absence of reducing agents; the digest products were analyzed by SDS/PAGE under nonreducing conditions. (B) AFM images of bromelain-released soluble ectodomain of H1 HA at low resolution (Upper Left) and high resolution (Lower Left). (Scale bars: Upper Left, 50 nm; Lower Left, 20 nm.) The bar graph (Right) indicates the height (Z section) along the white line (Lower Left). The height of each HA molecule was about 8 nm, consistent with data from the X-ray crystal structure. (C) Successive AFM images of the interaction between nasal trimeric/tetrameric IgA and soluble ectodomain of H5 HA. The round-edged HA ectodomain (arrow) was trapped by the radial regions of the nasal IgA and moved along with the edge of the IgA. The frame rate is 0.2 frames per second. The time stamp is given as min:sec. (Scale bars, 50 nm.) Tetra, tetramer; Tri, trimer.
Relationship Between Ig Structure and Neutralizing Potency.
To determine whether there were molecular size-related differences in neutralizing potency, the minimum neutralizing concentration (MNC) of antibody was calculated for each GFC fraction of the nasal wash sample in each subject. For this purpose, the neutralization titer of each fraction was defined as the lowest concentration at which neutralization was detected. The MNC then was expressed as the ratio of total antibody concentration to neutralization titer. MNCs were compared between groups as classified by the quaternary molecular architectures of the Igs. The MNC of antibody (against Victoria virus) in each fraction progressively decreased as molecular size increased (Fig. 5A). To estimate quaternary structure-related differences in neutralizing activity, each GFC fraction was classified according to the quaternary structure of the Igs. The antibodies in each fraction consisted of various proportions of Ig isotypes, including IgM, IgG, and IgA. Monomer fractions consisted mainly of IgG, with a minor proportion of IgA. In contrast, the polymeric forms larger than the tetramer fraction were composed primarily of IgA, and the remaining portion was composed of IgM. Furthermore, dimer and trimer/tetramer fractions were composed of IgA exclusively (Fig. 5B), suggesting that polymeric Igs in nasal mucus consist primarily of IgA. The MNCs of each Ig structure also were determined. The MNCs of trimeric/tetrameric and larger polymeric Igs against Victoria virus were significantly decreased compared with the MNCs of the monomeric and dimeric Igs (Fig. 5C). Furthermore, the MNCs of the larger polymeric Igs against NY virus were significantly decreased compared with the MNCs of the monomeric, dimeric, and trimeric/tetrameric Igs (Fig. 5D). These results indicated that polymeric nasal mucus Igs (including the trimer, tetramer, and larger Igs) had significantly higher neutralizing activity against H3N2 influenza virus than the monomeric and dimeric nasal mucus Igs. The higher neutralizing potencies of larger polymeric Igs against influenza virus were further confirmed in additional studies using H5N1 intranasally vaccinated subjects and H5N1 viruses (Fig. 5E and Fig. S5).
Fig. 5.
Comparison of neutralizing potency between Igs with different quaternary structures. (A) Neutralizing potency of various-sized antibodies against Victoria virus was defined as the MNC of antibodies (IgM + IgG + IgA) in each fraction. (B) Proportions of IgM, IgG, and IgA quantity (wt/wt) in each quaternary structure to total antibody quantity in each quaternary structure (IgM + IgG + IgA, weight). The mean MNC of each quaternary structure of antibody against Victoria virus (C) and NY virus (D) is shown. (E) Mean MNC of each quaternary structure of antibody against Indonesia virus (H5N1) from subjects vaccinated with H5N1 vaccines. (F) Proportion of IgA quantity (wt/wt) in each quaternary structure to total nasal IgA (weight). (G) Binding activities of IgA with various quaternary structures specific for HA of Victoria virus were defined as the MBC of IgA in each fraction. The mean MBC of each quaternary structure of IgA specific for HA of Victoria virus (H) and NY virus (I) is shown. All error bars are SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Poly, polymer; Tetra, tetramer; Tri, trimer.
Fig. S5.
Comparison of neutralizing potency against H5N1 virus between Igs with different quaternary structures. (A) BN/PAGE analysis of each fraction of gel-filtered nasal IgA. Five micrograms of Ig was applied in each lane. Bands were visualized by Coomassie staining. (B) Proportion of IgM, IgG, or IgA quantity (wt/wt) in each quaternary structure to quantity of total antibodies in each quaternary structure (IgM + IgG + IgA, weight). The number of subjects is six. Error bars represent SEM. (C) Neutralizing potencies of variously sized antibodies against A/Indonesia/5/2005 (H5N1) virus were defined as MNCs of antibodies (IgM + IgG + IgA) in each fraction. The bar graph indicates the mean MNCs of antibodies in each individual. The number of subjects is six. Error bars represent SEM. The MNC of antibody against A/Indonesia/5/2005 (H5N1) virus (vaccination strain) in each fraction progressively decreased as molecular size increased. Poly, polymer; Tetra, tetramer; Tri, trimer.
Nasal IgA antibodies appeared to be composed of 60% dimer, 20% monomer, 15% trimer/tetramer, and 5% larger polymers (Fig. 5F). To confirm whether these quaternary structure-related differences in IgA antibody size were evident, the minimum binding concentration (MBC) of Victoria HA-specific IgA antibodies in each GFC fraction of the nasal wash sample also was estimated as the lowest concentration at which binding was detectable at or above a minimum binding threshold. The MBC of Victoria HA-specific IgA in each fraction progressively decreased as molecular size increased (Fig. 5G), and the MBCs of Victoria HA-specific dimeric, trimeric/tetrameric, and larger polymeric IgA antibodies were significantly decreased in comparison to the MBC of monomeric IgA antibodies (Fig. 5H). Furthermore, the MBC of Victoria HA-specific larger polymeric IgA antibodies also was significantly decreased in comparison to the MBC of dimeric IgA antibodies (Fig. 5H). In addition, the MBCs of trimeric/tetrameric and larger polymeric IgA antibodies against NY HA were significantly decreased in comparison to the MBC of monomeric IgA antibodies (Fig. 5I). These observations suggested that polymeric IgA antibodies, including the dimer, trimer, tetramer, and larger forms, contained significantly higher amounts and affinities of HA-specific antibodies.
Discussion
In this study, quaternary structures and influenza virus neutralization potency of S-IgA in nasal wash samples were characterized. These nasal washes were obtained from healthy adults immunized intranasally with inactivated influenza vaccine.
Previous studies on polymeric IgA have been performed using samples obtained from the sera of patients with multiple myeloma (20) or external secretions, such as colostrum and saliva (10–12). IgA myeloma sera have been shown to contain monomeric, dimeric, trimeric, and tetrameric IgA in various proportions (20). In contrast, IgA antibodies in physiological external secretions are represented by S-IgA and consist predominantly of dimers (sedimentation constant 11 S) rather than tetramers (15.5 S), as revealed by ultracentrifugation (9, 13–15). However, reliable information concerning the presence of polymers larger than tetramers in nasal mucus has not been reported. The present study using nasal wash samples revealed, for the first time to our knowledge, the existence of larger polymer IgA in the healthy human upper respiratory tract, as well as the physiological functions of these molecules in protecting against viral infection.
As we have shown here, the relationship between influenza virus neutralization potency and the quaternary structures of Igs demonstrated that multiple antigen-binding sites on each Ig polymer contributed to effective neutralization of influenza virus. These polymeric antibodies contained two isotypes, IgA and IgM. However, HA-specific ELISA experiments demonstrated that HA-specific IgM titers were not correlated with neutralization titers, indicating that polymeric IgA (not IgM) had the highest neutralization potencies. HA-specific IgA ELISA also demonstrated that the MBCs of HA-specific polymeric IgA were significantly lower than the MBCs of monomeric IgA. We propose three possible reasons as to why polymeric IgA is involved in the efficient neutralization of influenza virus in the respiratory mucosa. The first is that the proportion of HA-specific antibodies in polymeric IgA is higher than the proportion of HA-specific antibodies in monomeric IgA. The second is that the avidity of polymeric IgA increases with the increased number of antigen-binding sites (compared with the avidity of monomeric IgA). The third is that HA-bound polymeric antibodies contribute to the inhibition of infection due to their large molecular size. In this regard, we note our finding (using nasal wash samples from volunteers vaccinated intranasally with an inactivated whole-virion H5N1 virus vaccine; Figs. S2 and S5) that the neutralizing potency of polymeric Igs against influenza virus was higher than the neutralizing potency of monomeric Igs.
Our results are in agreement with results obtained in previous experiments using mouse mAbs, in which the polymeric IgA mAb had an enhanced antivirus immune response (17–19). In the present work, we demonstrated (for polyclonal antibodies) that polymeric IgA has significantly greater neutralization potency than either dimeric or monomeric IgA; this difference was consistent with the absolute neutralization activity in nasal mucus. These results suggest that polymeric IgA has a critical in vivo role in protection against influenza A virus infection.
In conclusion, we have demonstrated that large polymeric S-IgA has a critical in vivo role in protection from influenza virus infection in humans, and intranasal administration of inactivated influenza vaccines resulted in the induction of multiple molecular forms of neutralizing antibodies in the human nasal mucosa. Moreover, the characteristic flower-shaped quaternary structure of the polymeric complex appears to play a pivotal role in protecting the human upper respiratory tract from viral infection.
Materials and Methods
Detailed materials and methods are provided in SI Materials and Methods.
Human Participants, Vaccines, and Nasal Wash Sample Collection.
For human studies, the protocol and other relevant study documentation were reviewed and approved by the Ethics Committee of the National Institute of Infectious Diseases. Written informed consent was obtained from each participant using an ethics committee-approved form. All vaccines were supplied by the Research Foundation for Microbial Disease of Osaka University (Kanonji, Kagawa, Japan). Nasal wash samples were collected as described by Ainai et al. (5).
Purification of IgA and Analysis of Purified Igs.
Nasal IgA antibodies were purified from pooled nasal wash samples by affinity chromatography using CaptureSelect IgA (Invitrogen) according to the manufacturer’s instructions.
High-Speed AFM.
The AFM experiments were performed using high-speed AFM (Nano Live Vision and Nano Explorer, Research Institute of Biomolecule Metrology Co., Ltd.).
SI Materials and Methods
Human Participants, Vaccination Protocol, and Nasal Wash Sample Collection.
For human studies, the protocol and other relevant study documentation were reviewed and approved by the Ethics Committee of the National Institute of Infectious Diseases. Written informed consent was obtained from each participant using an ethics committee-approved form. None of the subjects had to be excluded due to a history of allergy to eggs, past or current neurological conditions, or respiratory illness or fever at the time of vaccination. Each participant was immunized with a total of five doses of either H3N2 (five subjects) or H5N1 (six subjects) inactivated whole-virus influenza vaccine administered at 3-wk intervals (as described below). The five H3N2-vaccinated volunteers were a subgroup of the H3N2 study described by Ainai et al. (6). The H5N1-vaccinated volunteers were six healthy male subjects who were participants in another clinical study (examining intranasal H5N1 vaccination). All of these individuals were shown to exhibit high serum neutralizing antibody responses to H3N2 or H5N1 after two or three (respectively) previous intranasal vaccinations with the respective virus. These volunteers then were administered a further three or two (respectively) intranasal vaccinations with the respective virus. The H3N2 vaccine contained 45 μg of HA per dose of a Victoria-like virus. The H5N1 vaccine contained 45 μg of HA per dose of an A/Indonesia/05/2005 (H5N1)-like virus. The vaccines were prepared from the purified viruses, which had been sedimented through a linear sucrose gradient and treated with formalin using the method of Davenport et al. (21). All vaccines were supplied by the Research Foundation for Microbial Disease of Osaka University (Kanonji, Kagawa, Japan). Nasal wash samples were collected as described by Ainai et al. (5). Nasal wash samples were collected by washing the nasal cavity several times with a nose irrigation device (Hananoa; Kobayashi Pharmaceutical) according to the manufacturer’s instructions. Serial nasal washes from each subject were performed once daily for 10 d. The pooled nasal wash samples from each subject were filtered using a 0.22-μm pore-sized bottle-top filter, including a membrane-covering cotton-mat “prefilter” to remove mucopolysaccharides and other debris. These pooled cleaned nasal wash samples were then concentrated to a final volume of ∼1 mL using a Vivacell centrifugal concentrator (Vivacell 100, with a molecular weight cutoff (MWCO) of 30,000; Sartorius Stedim Biotech). The concentrated nasal wash samples were stored at −80 °C until use. The protein level in the concentrated nasal wash was measured using a BCA Protein Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The levels of human IgA, IgG, and IgM antibodies in the nasal wash samples were estimated using (respectively) human IgA, IgG, and IgM ELISA kits (Bethyl Laboratories).
Purification of IgA and Analysis of Purified Igs.
Nasal IgA antibodies were purified from pooled [about 10% (vol/vol) of each enriched nasal wash sample from the five volunteers for H3N2 subjects or the six volunteers for H5N1 subjects were mixed, respectively] nasal wash samples by affinity chromatography using CaptureSelect IgA (Invitrogen) according to the manufacturer’s instructions. Human colostrum IgA, human serum IgG, and human serum IgM were purchased from Sigma–Aldrich. Human serum IgA was purchased from SCIPAC. For preparation of monomeric IgA, parallel fractionation with GFC was performed on serum IgA. The peak fraction (fraction 32) of this GFC fractionation was collected to obtain monomeric IgA. Nasal IgA, serum IgA, colostrum IgA, serum IgG, or serum IgM was analyzed by blue native (BN) PAGE using NativePAGE 4–16% (wt/vol) Bis-Tris gels and by SDS/PAGE using NuPAGE 4–12% (wt/vol) Bis-Tris gels (Invitrogen). NativeMark (Invitrogen) was used as a molecular weight standard in BN/PAGE, and Precision Plus Protein All Blue standards (Bio-Rad Laboratories, Inc.) were used in SDS/PAGE. Gels were stained with SimplyBlue SafeStain (Invitrogen). For Western blot analysis, antibodies were fractionated by SDS/PAGE, and the separated proteins were transferred to a PVDF filter (Millipore). The filter was incubated with primary antibodies, and immune complexes then were detected with HRP-conjugated secondary antibodies and Immobilon Western Chemiluminescent HRP Substrate (Millipore). For primary antibodies, mouse antisecretory component glycoprotein (SC-05) mAb was purchased from Abcam. HRP-conjugated goat anti-human κ-light chain and HRP-conjugated goat anti-human λ-light chain antibodies were purchased from Bethyl. Mouse anti-human IgA isotype-specific mAb, mouse anti-human IgA1 subclass-specific mAb, and mouse anti-human IgA2 subclass-specific mAb were purchased from Nordic Immunology. Rabbit anti-human IgJ polypeptide antibody was purchased from Lifespan Biosciences. For secondary antibodies, HRP-conjugated goat anti-mouse IgG-FC and anti-rabbit IgG H&L preadsorbed antibodies were purchased from Abcam.
High-Speed AFM.
The high-speed AFM experiments were performed using a Nano Live Vision High-Speed atomic force microscope (Research Institute of Biomolecule Metrology Co., Ltd.) or Nano Explorer High-Speed atomic force microscope (Research Institute of Biomolecule Metrology Co., Ltd.) with a silicon nitride cantilever (BL-AC10EGS or BL-AC10FS-A2; Olympus). To obtain images of IgA antibodies, serum IgA or nasal IgA [1–10 μg/mL, 2 μL in 10 mM phosphate buffer (pH 7.4)] was adsorbed on a mica surface (Ted Pella, Inc.), incubated for 5 min, washed with double-distilled water (DDW), and then subjected to time-lapse imaging in DDW. Images containing 192 × 144 pixels were obtained at a scan rate of 1 or 0.5 frames per second (fps). To observe the real-time interaction between IgA and HA antigen, timer and tetramer fractions of nasal IgA [10 μg/mL, 2 μL in 10 mM phosphate buffer (pH 7.4)] were adsorbed on the mica surface, incubated for 5 min, washed with DDW, and subjected to time-lapse imaging in DDW; HA (0.5 mg/mL, 10 μL in PBS) then was added in the imaging chamber. Images containing 192 × 144 pixels were obtained at a scan rate of 0.2 or 0.5 fps. Images were analyzed by SPIP software (Image Metrology A/S) and MetaMorph Imaging software (Molecular Devices, Inc.).
High-Mass MALDI-TOF MS Analysis.
All sample measurements were performed using a Bruker Ultraflex III MALDI-TOF/TOF mass spectrometer equipped with CovalX’s HM3 interaction module. CovalX’s interaction module contains a special detecting system designed to optimize protein interaction detection up to 2 MDa with nanomolar sensitivity. The MALDI-TOF MS analysis was performed using CovalX’s HM3 interaction module with a standard nitrogen laser and focusing on different mass ranges from 0 to 1,500 kDa. Aliquots (20 μL) of each protein sample were pipetted to prepare 10-fold dilutions of the samples with a final volume of 10 μL. Aliquots (1 μL) of each obtained dilution were mixed with 1 μL of a matrix composed of a recrystallized sinapinic acid matrix (10 mg/mL) in acetonitrile/water (1:1 vol/vol) and 0.1% TFA (K200 MALDI Kit; CovalX). After mixing, 1 μL of each sample was spotted on the MALDI plate (SCOUT 384; Bruker). After crystallization at room temperature, the plate was introduced into the MALDI mass spectrometer and analyzed immediately. The analysis was repeated in triplicate. For the analysis, the following parameters were applied: mass spectrometer, linear and positive mode; ion source 1, 20 kV; ion source 2, 17 kV; lens, 12 kV; pulse ion extraction, 150 ns; HM3 gain voltage, 3.14 kV; and HM3 acceleration voltage: 20 kV. To calibrate the instrument, an external calibration with clusters of BSA and IgG was used. For each sample, three spots were analyzed (300 laser shots per spot). The presented spectrum corresponds to the sum of 300 laser shots. The MS data were analyzed using CovalX’s Complex Tracker analysis software, version 2.0.
Fractionation of Nasal Wash Samples.
Concentrated nasal wash samples (500 μL) were fractionated on a Superose 6 10/300 GL gel filtration column in PBS (H3N2 subjects) or 10 mM phosphate buffer, pH 7.4 (pH5N1 subjects) using an FPLC AKTA chromatography system (all from GE Healthcare). Fractions (500 μL each) were collected in PBS (H3N2 study subjects) or 10 mM phosphate buffer, pH 7.4 (pH5N1 study subjects) at a flow rate of 0.5 mL⋅min−1.
Viruses.
Influenza viruses included Victoria, NY, Sydney, Brisbane, A/Indonesia/5/2005 (H5N1; Indonesia), A/Viet Nam/1194/2004 (H5N1; Vietnam), and A/Laos/JP085/2007 (H5N1; Laos) strains. The viruses were obtained from National Institute of Infectious Diseases or National Institute of Health Research and Development, propagated in the allantoic cavity of 10-d-old embryonated chicken eggs, and purified from the allantoic fluid. The 50% infectious dose in tissue culture (TCID50) of the virus was estimated using previously described methods (22). In brief, 10-fold serial dilutions of allantoic fluid containing the virus were inoculated onto Madin–Darby canine kidney (MDCK) cells (American Type Culture Collection no. CCL-34) in a 96-well culture plate and incubated for 3 d at 37 °C in a humidified 5% CO2 atmosphere. The cytopathic effect observed in the virus-containing wells was evaluated under a microscope, and the TCID50 was calculated using the Reed–Muench method (23).
Neutralization Assays.
Neutralizing antibody titers were examined using microneutralization assays as previously described, with minor modifications (22). Briefly, twofold serial dilutions of separated nasal wash samples were mixed with an equal volume of diluent containing an influenza virus equivalent of 100 TCID50 and added to the wells of a 96-well plate containing a monolayer culture of MDCK cells. Four control wells containing virus or diluent alone were included on each plate. The plates were incubated for 3 or 4 d at 37 °C in a humidified 5% CO2 atmosphere. All wells were observed for the presence or absence of cytopathic effects and then fixed with 10% (vol/vol) formalin phosphate buffer for more than 5 min at room temperature and stained with Naphthol blue black. The titers were recorded as the reciprocal of the highest dilution without cytopathic effect. The MNC was determined for individual fractions in each subject [sum of antibody concentrations (IgM + IgG + IgA)/(neutralization titer)] and then compared among the groups classified by quaternary molecular structures of the Igs.
Determination of HA-Specific IgM, IgG, and IgA Titers.
The titers of IgM, IgG, and IgA specific for the HA molecules of the Victoria virus, NY virus, and Sydney virus (HA-specific IgM antibody titer, HA-specific IgG antibody titer, and HA-specific IgA titer, respectively) in the gel filtration-fractionated nasal wash samples were determined by ELISA. The ELISAs were performed in microtiter plates (Costar) using the following procedure. First, wells of microtiter plates were coated with HA molecules purified from the three individual viruses according to the procedure of Phelan et al. (24). Second, the HA molecules were incubated with twofold serial dilutions of serum or standardized nasal wash samples followed by detection with goat anti-human IgM (μ-chain–specific; Bethyl Laboratories), goat anti-human IgG (γ-chain–specific; Bethyl Laboratories), or goat anti-human IgA (α-chain–specific; Bethyl Laboratories) conjugated to alkaline phosphatase. Third, the enzymatic reaction was initiated by the addition of 1 mg/mL p-nitrophenyl-phosphate as a substrate. Color development was measured at 405 nm using a microplate reader (Model 680; Bio-Rad Laboratories). The antibody titer for a given sample was calculated as the reciprocal of the highest dilution of the test sample that gave an A405 greater than a cutoff value equal to the mean A405 + 2 SD of 10 twofold serial dilutions (starting at 1:160 and ending at 1:81,920) of the negative control samples, which were (respectively) normal human IgM from human serum, IgG from human serum, and IgA from human colostrum (Sigma–Aldrich). The MBCs of nasal IgA were determined for individual fractions in each subject as (IgA concentration)/(ELISA titer), and were then compared between the groups classified by the quaternary molecular structures of IgA. The ELISA titer was defined as the highest dilution at which a specific binding for HA molecules of influenza virus was detected by ELISA.
HA Ectodomain (Bromelain Cleaved HA) Purification.
HA ectodomains were prepared by digestion of the purified virions with bromelain in the absence of reducing agents (25). Influenza virions were incubated with bromelain (B5144; Sigma) in TE buffer [100 mM Tris⋅HCl, 1 mM EDTA (pH 7.2)] for 16 h at 35 °C using virus protein-to-enzyme protein ratios of 4:1 (0.25 mg/mL bromelain). The reaction was stopped by adding the protease inhibitor E- 64 [N-(transepoxysuccinyl)-l-leucine 4-guanidinobutylamide] at a final concentration of 10 μM in the case of bromelain. The subviral particles were pelleted at 100,000 × g for 1.5 h at 4 °C (Beckman SW-50.1 rotor). After pelleting of the bromelain-digested virions, the supernatant was concentrated through Vivaspin centrifugal concentrators (VIVASPIN 20 with a molecular weight cutoff of 30,000; Sartorius Stedim Biotech) at 4 °C. The concentrated supernatant then was fractionated on a Superose 12 10/300 GL gel filtration column in PBS using an FPLC AKTA chromatography system (all from GE Healthcare) and analyzed by SDS/PAGE [NuPAGE 10% (wt/vol) Bis-Tris gels] under nonreducing conditions.
Statistical Analysis.
Statistical analyses were performed using the Prism statistical software package (version 5.0c; GraphPad Software, Inc.) and consisted of two-tailed unpaired Student’s t tests. The threshold for statistical significance was set at 5% (P < 0.05).
Supplementary Material
Acknowledgments
We thank Dr. W. W. Hall for critical reading of the manuscript; Mr. T. Tanimoto, Dr. Y. Gomi, Dr. S. Manabe, T. Ishikawa, and Dr. Y. Okuno at the Research Foundation for Microbial Disease of Osaka University for supplying the inactivated whole-virus influenza vaccines; A. Kanegami and M. Shichiri at the Research Institute of Biomolecule Metrology Co., Ltd. for technical support of AFM analysis; Dr. A. Nazabal at CovalX AG for technical support of high-mass MALDI-TOF MS analysis; and T. Miyazaki and T. Kamishita at Toko Yakuhin Kogyo Co., Ltd. for valuable suggestions on vaccination procedures of intranasal vaccine. The work was supported by grants from the Japanese Ministry of Health, Labor, and Welfare.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.C.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503885112/-/DCSupplemental.
References
- 1.Murphy BR, Clements ML. The systemic and mucosal immune response of humans to influenza A virus. Curr Top Microbiol Immunol. 1989;146:107–116. doi: 10.1007/978-3-642-74529-4_12. [DOI] [PubMed] [Google Scholar]
- 2.BR M . Mucosal immunity to viruses. In: Ogra PLMJ, Lamm ME, Strober W, McGee JR, Bienenstock J, editors. Handbook of Mucosal Immunology. Academic; San Diego: 1994. pp. 333–343. [Google Scholar]
- 3.Asahi Y, et al. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J Immunol. 2002;168(6):2930–2938. doi: 10.4049/jimmunol.168.6.2930. [DOI] [PubMed] [Google Scholar]
- 4.Tamura S. Studies on the usefulness of intranasal inactivated influenza vaccines. Vaccine. 2010;28(38):6393–6397. doi: 10.1016/j.vaccine.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Ainai A, et al. Characterization of neutralizing antibodies in adults after intranasal vaccination with an inactivated influenza vaccine. J Med Virol. 2012;84(2):336–344. doi: 10.1002/jmv.22273. [DOI] [PubMed] [Google Scholar]
- 6.Ainai A, et al. Intranasal vaccination with an inactivated whole influenza virus vaccine induces strong antibody responses in serum and nasal mucus of healthy adults. Hum Vaccin Immunother. 2013;9(9):1962–1970. doi: 10.4161/hv.25458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Riet E, Ainai A, Suzuki T, Hasegawa H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine. 2012;30(40):5893–5900. doi: 10.1016/j.vaccine.2012.04.109. [DOI] [PubMed] [Google Scholar]
- 8.van Riet E, Ainai A, Suzuki T, Kersten G, Hasegawa H. Combatting infectious diseases; nanotechnology as a platform for rational vaccine design. Adv Drug Deliv Rev. 2014;74:28–34. doi: 10.1016/j.addr.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4(6):590–597. doi: 10.1038/mi.2011.39. [DOI] [PubMed] [Google Scholar]
- 10.Tomasi TB, Jr, Tan EM, Solomon A, Prendergast RA. Characteristics of an Immune System Common to Certain External Secretions. J Exp Med. 1965;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halpern MS, Koshland ME. The stoichiometry of J chain in human secretory IgA. J Immunol. 1973;111(6):1653–1660. [PubMed] [Google Scholar]
- 12.Mestecky J, Kilian M. Immunoglobulin A (IgA) Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- 13.Woof JM, Mestecky J. Mucosal immunoglobulins. Immunol Rev. 2005;206:64–82. doi: 10.1111/j.0105-2896.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 14.Mestecky J, Raska M, Novak J, Alexander RC, Moldoveanu Z. Antibody-mediated protection and the mucosal immune system of the genital tract: relevance to vaccine design. J Reprod Immunol. 2010;85(1):81–85. doi: 10.1016/j.jri.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mestecky J, Alexander RC, Wei Q, Moldoveanu Z. Methods for evaluation of humoral immune responses in human genital tract secretions. Am J Reprod Immunol. 2011;65(3):361–367. doi: 10.1111/j.1600-0897.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor HP, Dimmock NJ. Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J Exp Med. 1985;161(1):198–209. doi: 10.1084/jem.161.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173(3):1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 18.Renegar KB, Jackson GD, Mestecky J. In vitro comparison of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J Immunol. 1998;160(3):1219–1223. [PubMed] [Google Scholar]
- 19.Tanimoto T, et al. Comparison of the cross-reactive anti-influenza neutralizing activity of polymeric and monomeric IgA monoclonal antibodies. Viral Immunol. 2012;25(5):433–439. doi: 10.1089/vim.2012.0026. [DOI] [PubMed] [Google Scholar]
- 20.Vaerman JP, Langendries A, Vander Maelen C. Homogenous IgA monomers, dimers, trimers and tetramers from the same IgA myeloma serum. Immunol Invest. 1995;24(4):631–641. doi: 10.3109/08820139509066863. [DOI] [PubMed] [Google Scholar]
- 21.Davenport FM, et al. Comparisons of Serologic and Febrile Responses in Humans to Vaccination with Influenza a Viruses or Their Hemagglutinins. J Lab Clin Med. 1964;63:5–13. [PubMed] [Google Scholar]
- 22.Kadowaki S, et al. Protection against influenza virus infection in mice immunized by administration of hemagglutinin-expressing DNAs with electroporation. Vaccine. 2000;18(25):2779–2788. doi: 10.1016/s0264-410x(00)00087-6. [DOI] [PubMed] [Google Scholar]
- 23.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoint. Am J Hyg. 1938;27(3):493–497. [Google Scholar]
- 24.Phelan MA, Mayner RE, Bucher DJ, Ennis FA. Purification of influenza virus glycoproteins for the preparation and standardization of immunological potency testing reagents. J Biol Stand. 1980;8(3):233–242. doi: 10.1016/s0092-1157(80)80039-4. [DOI] [PubMed] [Google Scholar]
- 25.Kordyukova LV, et al. Linker and/or transmembrane regions of influenza A/Group-1, A/Group-2, and type B virus hemagglutinins are packed differently within trimers. Biochim Biophys Acta. 2011;1808(7):1843–1854. doi: 10.1016/j.bbamem.2011.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.