Significance
It has long been known that perception is atypical in autism. The mechanisms underlying these atypicalities, however, are far from being well understood. Here, we test the integrity of one candidate mechanism, adaptation, in children with and without autism by assessing their susceptibility to number adaptation. We show that adaptation to numerosity is significantly attenuated in children with autism, with the size of their aftereffects only one-third of those of typical children. These results extend existing findings of reduced adaptation to high-level social stimuli and critically suggest that atypicalities in adaptive mechanisms may be pervasive in autism, at least at higher levels. These results fit well with recent Bayesian theories of autism, which propose fundamental problems with prediction.
Keywords: autism, adaptation, prediction, Bayesian, number
Abstract
Autism is known to be associated with major perceptual atypicalities. We have recently proposed a general model to account for these atypicalities in Bayesian terms, suggesting that autistic individuals underuse predictive information or priors. We tested this idea by measuring adaptation to numerosity stimuli in children diagnosed with autism spectrum disorder (ASD). After exposure to large numbers of items, stimuli with fewer items appear to be less numerous (and vice versa). We found that children with ASD adapted much less to numerosity than typically developing children, although their precision for numerosity discrimination was similar to that of the typical group. This result reinforces recent findings showing reduced adaptation to facial identity in ASD and goes on to show that reduced adaptation is not unique to faces (social stimuli with special significance in autism), but occurs more generally, for both parietal and temporal functions, probably reflecting inefficiencies in the adaptive interpretation of sensory signals. These results provide strong support for the Bayesian theories of autism.
Autism is a heritable, lifelong neurodevelopmental condition with striking effects on social communication. The condition, however, is also associated with a range of nonsocial symptoms, including both hypersensitivity and hyposensitivity to perceptual stimuli, and sensory-seeking behaviors such as attraction to light, intense looking at objects, and fascination with brightly colored objects. These sensory atypicalities, which now form part of the diagnostic criteria for autism (1), can have debilitating effects on the lives of autistic people (2) and their families (3).
We have recently proposed a Bayesian account of autism (4), suggesting that it is not sensory processing itself that is disrupted in individuals with autism, but the interpretation of the sensory input. The Bayesian class of theories, including predictive coding and other generative models (5–7), assumes that perception is an optimized combination of external sensory data (the likelihood) and an internal model (the prior). We suggested that this process may be atypical in autism, in that the internal priors are underweighted and less used than in typical individuals. Our theory has been followed by several others along similar lines (8–11).
The suggestion of underutilization of priors leads to several specific predictions. One strong prediction is that autistic individuals should show reduced adaptation aftereffects. Adaptation, which occurs throughout sensory systems, represents a form of experience-dependent plasticity in which our current sensory experience is intimately affected by how we viewed the world only moments before. It is widely held to pose numerous functional advantages (12–16), including serving to autocalibrate perceptual systems to their environment by dynamically tuning its responses to match the distribution of stimuli to make maximal use of the limited working range of the system (12–16). It achieves this by reducing the transmission of redundant information and maximizing sensitivity to relevant information.
Some evidence exists to suggest that certain forms of adaptation are reduced in autism. Adaptation to faces, which normally biases perception away from the adapted identity (17, 18), is significantly attenuated in children with autism relative to typical children (19). This has been confirmed by several subsequent studies demonstrating diminished adaptation to faces in children with autism for facial configuration (20), emotion (21), and diminished adaptation to apparent eye-gaze direction (22). Relatives of autistic children also show reduced face adaptation (23).
These effects thus far have been demonstrated with high-level social information (faces or facial attributes). There has been little evidence showing that adaptation to other, nonsocial stimuli is reduced in autism—at least at lower levels of the visual hierarchy. For example, preliminary measurements in our laboratory suggest that the motion aftereffect, one of the most robust and most studied forms of perceptual adaptation, is as strong in autistic children as in age- and ability-matched typical children. Autistic children also showed the same amount of adaptation (24) to a more complex combination of moving stimuli, purportedly reflecting perceptual “causality” (25): Two colliding objects can appear to bounce off (“causing” the other to reverse direction) or slide over each another. Adapting to an unambiguous bounce biases observers in favor of the sliding interpretation. Although this would appear to be a form of high-level adaptation (causality), there is some controversy, with evidence that it actually operates at a lower level, biasing the interpretations of shape (26).
Faces are important social stimuli—particularly so for individuals with autism. Autistic children show atypical gaze patterns to faces, especially to the eyes, even at a very early age (27, 28). It is therefore possible that the atypical adaptation to faces may be specific to this emotionally charged, high-level social stimulus, in line with some prominent theoretical models of autism that posit autism as a disorder of social information processing (28–30). Alternatively, it is possible that diminished adaptation is a more general atypicality of autistic perception, confined to coding high-level stimulus dimensions. This is plausible because the adaptability of neurons is thought to increase as one progresses along the visual hierarchy, enabling higher visual areas to stay alert to novel, salient stimuli (16). High-level attributes could therefore be at greater risk of atypicality.
In this study, we therefore sought to examine the extent and nature of diminished adaptation in children with autism by measuring adaptation to numerosity. We chose numerosity specifically for three reasons. First, it is a relatively high-level perceptual attribute, quite distinct from other visual attributes such as texture (31, 32), and elaborated at relatively high levels of analysis, including intraparietal sulcus (IPS) and prefrontal cortex (33, 34). Second, numerosity is both functionally and neurally distinct from face stimuli. Indeed, although faces are processed in temporal cortex (35), number is clearly a parietal function (33, 34). Third, unlike face recognition skills, number skills are often reported anecdotally as a relative strength for individuals with autism (for example, in the popular film “Rain Man”). Although there is evidence for superior number skills in some autistic individuals (36), it is currently unclear whether such superiorities are manifest more broadly (37, 38).
Like most perceptual systems, numerosity is susceptible to adaptation: Prolonged exposure to a more numerous visual stimulus makes a subsequent stimulus appear less numerous and vice versa (39). As mounting evidence suggests that numerosity adaptation occurs at a high level, across modalities and types of presentation (32, 40), it seemed an ideal candidate to test whether reduced adaptation in autism is a general phenomenon affecting much of the perceptual brain or restricted to socially relevant visual stimuli, such as faces.
Results
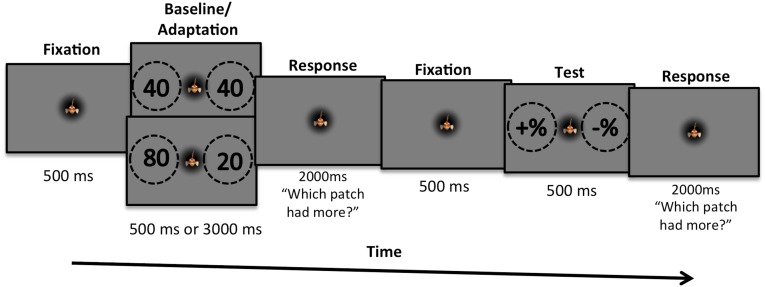
We measured adaptation with a child-friendly computer game in which the children were asked to help an animated fish (“Freddy”) shown in center screen to find food (Fig. 1). Trials started with participants fixating the animated fish, and then two dot sequences were presented sequentially: the adaptor pair followed by the test pair. After each pair, participants were asked to indicate which of the two patches of dots were more numerous (“contained more food”). To maintain concentration and to avoid confusion, children were asked to respond after both sets, but only the response to the test pair was recorded. In the baseline condition the adaptor pair was neutral with 40 dots (the standard number) on each side; in the adaptation condition it comprised 80 dots on the left and 20 dots on the right.
Fig. 1.
The paradigm used to measure the numerosity effect in children. In the baseline condition, “adaptation” was to a neutral numerosity (40 dots each side, the average number in the test) and lasted 500 ms. In the adaptation condition (shown here) the adaptation stimuli comprised 80 dots on the left and 20 dots on the right and lasted for 3,000 ms (with dot positions randomized every 500 ms). Participants were told to respond after each pair was presented (to prevent confusion of when to respond) but only responses following the test pair were of interest.
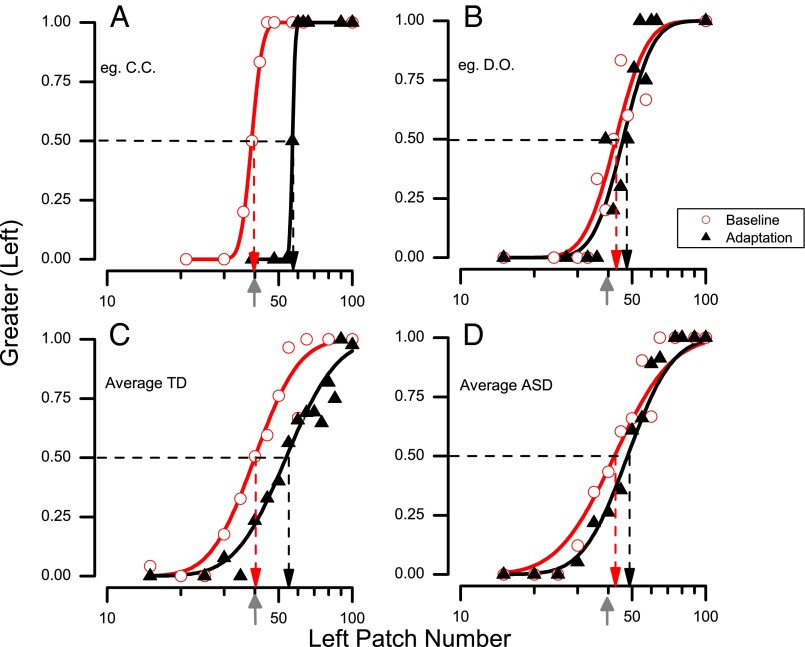
For each participant and condition we plotted psychometric functions like those of Fig. 2, plotting the proportion of responses where the patch at left appeared more numerous than the patch at right, against the number of dots in the left test patch. The number of dots on the right varied inversely to that on the left, by the same proportion, so the geometric mean of both patches was always 40. The data were fitted with cumulative Gaussian functions, whose median (50% point) estimates the point of subjective equality (PSE), the point where the two patches are judged equally numerous. Fig. 2A shows representative psychometric functions for a typical child and Fig. 2B shows those for a child with autism. In the baseline condition, the PSE for both children was near 40, where left and right patches were equally numerous, indicating that perception was unbiased. After adaptation, however, the curve of the typical child moved to the right, yielding a PSE of 53. This means that for the dot clouds to appear perceptually equal, the side adapted to high numbers needed to contain 54 dots and the other side 27 dots. Adaptation also occurred with the child with autism spectrum disorder (ASD), but to a much lesser extent, with a PSE of 47. The lower panel (Fig. 2 C and D) show similar functions pooled over all participants. The pooled data show the same trend as those of the individual participants, with the group of typical children showing a much stronger adaptation effect than the group of children with ASD.
Fig. 2.
Example of psychometric functions, plotting the proportion of trials participants reported the left side as appearing more numerous, as a function of numerosity of the left side (the right side varied inversely by the same proportion, so the geometric mean of the two stimuli equaled the standard 40 dots, indicated by the gray arrows on the abscissae). The vertical dashed lines point to the estimates of the points of subjective equality (PSE), given by the median of the fitted cumulative Gaussian functions. Data in red refer to baseline conditions and those in black to adaptation conditions. (A) Data for a representative typically developing child. (B) Data for a representative child with ASD. (C) All data for the typical group pooled (n = 18). (D) All data for the ASD group pooled (n = 16).
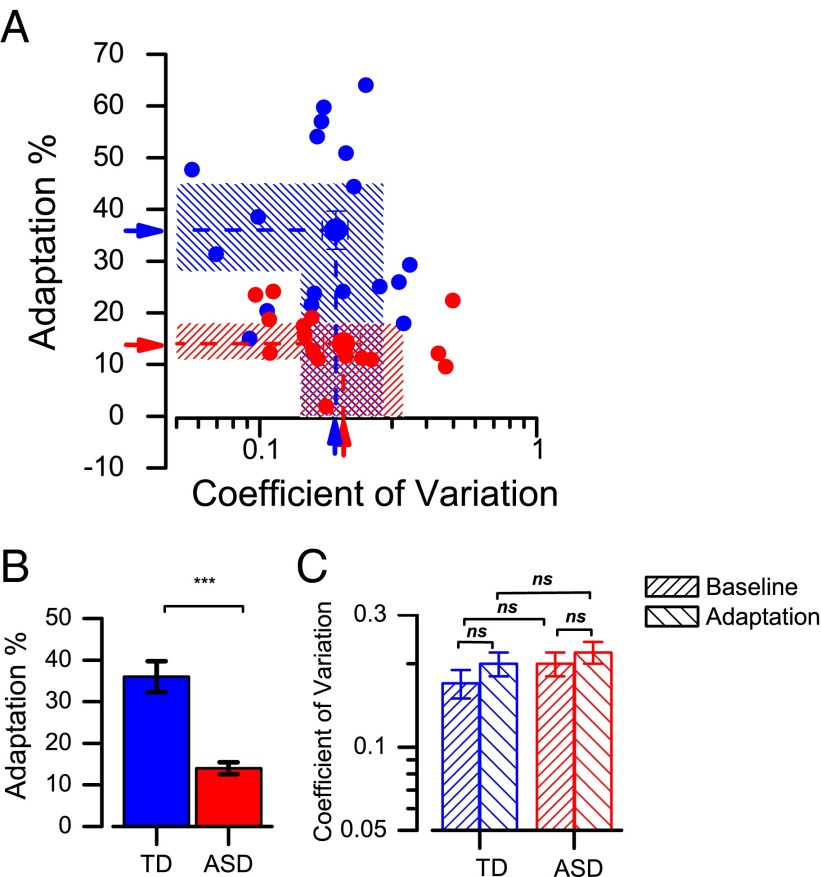
Pooling raw data over participants is not strictly justified, as each one may have individual biases that, when averaged, give a misleadingly broad function (indeed, the pooled functions of Fig. 2 are broader than the individual ones). Also, any outliers would bias the data without being observable or excludable. A more robust approach is to consider the adaptation effect of the individual participants. We defined adaptation magnitude as the percentage of increase or decrease in PSE (Eq. 1) and plotted this on the ordinate of Fig. 3A, separately for children with ASD (red symbols) and typical children (blue symbols). The difference is clearly systematic, with most autistic children showing far less adaptation than typical children. Adaptation effects for the autistic children are on average three times less than those for the typical children, and not one single autistic child has adaptation levels that encroach on the 95% confidence intervals for the typical group. This is also clear from the plot of Fig. 3B, plotting the same data as a bar graph. The difference between the two groups is significant at P < 0.0001.
Fig. 3.
(A) Scatterplot of adaptation magnitude against the coefficient of variation for all participants (children with ASD, red symbols; typical children, blue symbols). The arrows indicate the mean of the two groups and shaded areas 95% confidence intervals. Coefficients of variation are similar between typically developing comparison children and children with ASD, whereas the size of the adaptation is different between the two groups. (B) Bar graphs showing the size of the aftereffect for the two groups, with symbols showing individual data. Error bars correspond to ±1 SEM. (C) Mean coefficient of variation for discriminating numerosity in the baseline and adaptation conditions for the two groups.
The abscissa of Fig. 3A reports the coefficient of variation of the participants (averaged pre- and postadaptation), given by the SD of the best-fitting Gaussians to the psychometric functions, normalized by the average physical quantity, 40 dots. Although the average of the ASD group is slightly higher, the confidence intervals show there is no significant separation. This is also clear from the bar graphs of Fig. 3C, separating the coefficient of variation for the baseline and adaptation conditions, suggesting similar discrimination precision between the groups. A mixed-design ANOVA, with condition (baseline, adaptation) as a repeated-measures factor and group (autism and typical) as a between-participants factor on the coefficient of variation, revealed no main effect of condition, F(1,32) = 2.35, P = 0.14; no significant main effect of group, F(1,32) = 0.37, P = 0.55; and no significant condition-by-group interaction, F(1,32) = 0.39, P = 0.54.
We also examined the relationship between the size of the aftereffect and measures of symptomatology. No significant correlations were found, either with the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) (41) total (r = 0.28, P = 0.35) or with subscale scores (social affect, r = −0.26, P = 0.45; restricted, repetitive behavior, r = 0.24, P = 0.50). Nor did the magnitude of the aftereffect correlate significantly with chronological age or verbal or nonverbal ability in either group of children (P > 0.18 in all cases). The coefficient of variation, however, correlated negatively with age (r = −0.41, P = 0.015, pooling the two groups together), as might be expected.
Discussion
The results clearly show that children with autism adapt to numerosity much less than typical children, by only one-third as much. Discrimination precision, however, was similar in both groups of children, showing that the difference in adaptation does not reflect inattention or some other more generic difficulty with judging number in these children. This result is similar to the face identity aftereffect findings, where adaptation was significantly reduced relative to that of typical children, but the ability to discriminate between faces was not (19).
Reduced numerosity adaptation in autism is consistent with previous research showing reduced levels of face adaptation (19–23) and suggests that adaptive mechanisms may be in general atypical in autism, not only for social stimuli, but also for other high-level extractions such as number perception. Although both face and number perception are generally considered to be “high-level” processes, the neural substrates underlying each are quite distinct: Faces are processed through the inferotemporal cortex (35), whereas number involves intraparietal and prefrontal cortex (33, 34)—a different processing stream, thought to have very different functional roles (42). Thus, reduced adaptation in autism seems to generalize throughout the perceptual brain, for both social and nonsocial stimuli.
It is unclear why other lower-level functions that have been tested, such as simple and complex motion tasks, do not show reduced adaptation (24). Adaptation effects occur at multiple levels of visual processing, from photoreceptors to complex perceptual systems, but the mechanisms may be quite distinct. Some forms of adaptation, particularly at low neural levels, may simply reflect reduced neural responsiveness after extensive stimulation (15). However, many theories take the view that adaptation is not simply neural fatigue, but is functionally beneficial—an active process that serves to maximize efficient use of neural mechanisms (12–16). It may be only this latter class of adaptation that is reduced in autism. Perhaps adaptation to motion stimuli, including those purported to reflect causality [but may in fact reflect deformation of objects (26)], acts at lower levels of processing by processes akin to “neural fatigue,” and these more automatic processes are unaffected in individuals with autism. Indeed there is good evidence that the motion aftereffect is low level, occurring before the combination of luminance and color information (43) and in retinotopic coordinates (44). Adaptation to so-called causality is also in retinotopic coordinates (25), suggesting that it, too, is quite low level. On the other hand, adaptation to numerosity occurs in spatiotopic coordinates (40). Nevertheless, more work is needed to understand better why some, but not all, types of perceptual function are affected in individuals on the autism spectrum.
Two prominent theories of autistic perception are the weak central coherence theory, which suggests difficulties in the integration of local sensory signals, which compromise the formation of global percepts in autism (45), and the enhanced perceptual functioning account (46), which posits that a local-processing bias leads to strengths in the processing of simple stimuli and to weaknesses in the processing of more complex stimuli. It is not immediately obvious how either theory can fully account for the results here, as the overall performance in number discrimination—both before and after adaptation—was not reduced in autism, as may be expected if there were difficulties in integrating the distributed information (the dots) to yield an estimate of number. In the more general sense, however, reduced adaptation is broadly consistent with both theories, as they each suggest that autism is characterized by reduced influence of top–down contextual information, which could include the adaptor.
These accounts, however, are unable to specify the underlying (altered) computational mechanisms in reduced adaptation. These mechanisms may be more readily explained by the recent Bayesian models of autism (4, 8–11), which clearly predict that individuals with autism should give less weighting to prior or predictive information, such as the consequences of previous stimulation. Critically, in this study, number perception of autistic children postadaptation was more accurate, in that the target patch of dots corresponded better to physical reality than to expectations. Although the mechanisms of adaptation may not be fully understood, adaptation is one of the clearest examples of transient neural plasticity, where the output of perceptual processes depends not only on the current stimuli, but also on the immediate history. Many models link adaptation effects to Bayesian prediction (47, 48), suggesting that priors may serve as standards for self-calibration, which is the function of adaptation. Atypicalities in the prior—either in its construction or in use as a calibration standard—should impact the magnitude of adaptation.
More generally, we believe our results fit well with the notion that autism is associated with atypicalities in flexible perceptual processing and, in particular, in prediction (11). Fundamental difficulties in the ability to predict the forthcoming sensory environment will result in less adaptation and habituation (as observed here), and this reduced adaptation may underlie some of the symptoms of sensory overload that can have catastrophic influences on the lives of autistic people (2). Problems in effectively adapting to and calibrating against observed sensory evidence could lead to both hypersensitivities and hyposensitivities in perception, which can be very disturbing and stressful to people with autism. Future work should examine both the precise neural underpinnings of attenuated adaptation in autism and ways of addressing it to enable autistic people to perceive and experience the world around them with less distress.
Methods
Participants.
We tested 16 children diagnosed with an ASD aged 7–14 y (mean 10.3 y, SD 2.1) and 18 typically developing children (mean 11.0 y, SD 2.1). All children with autism met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (1) for autism, according to an independent clinician, and met criteria for an ASD on the ADOS-2 (41) (Table 1). The comparison group of typically developing children was individually matched with children with autism in terms of chronological age, t(32) = 1.54, P = 0.132, and full-scale IQ, t(32) = 1.49, P = 0.151 (independent samples t test, two tailed), as measured by the Wechsler Abbreviated Scales of Intelligence (WASI) (49) (Table 1). All children had a total IQ score above 80 and were thus considered “cognitively able.” No child had a medical or developmental disorder other than ASD, as reported by parents, or was on medication. Also, no typically developing child had a current or past medical or psychiatric diagnosis, as reported by parents. All children showed normal visual acuity. Participants were tested individually in a quiet room either at home or at the Stella Maris Research Hospital. The study was approved by the regional pediatrics ethics committee at the Azienda Ospedaliero-Universitaria Meyer.
Table 1.
Descriptive statistics for developmental variables for children with autism and typically developing children
Stimuli and Procedure.
Stimuli were generated with the Psychophysics Toolbox (50) and presented at a viewing distance of 57 cm on a 23” LCD Acer monitor (resolution = 1,920 × 1,080 pixels, refresh rate = 60 Hz, mean luminance = 60 cd/m2), run on a Macintosh laptop. All stimuli were patches of dots of 10° diameter, filled with nonoverlapping dots of diameter 10 arcmin, half white and half black, at 90% contrast. To encourage participants to attend to the central fixation point, an animation of a fish jumping, bouncing, sliding, or rolling was continually displayed at screen center. Fixation was monitored by the experimenters (two were present throughout all trials).
We used a child-friendly computer game in which children earned points by helping the centrally displayed animated fish (Freddy) to find the most food. Each trial comprised two separate presentations of two stimuli, both pairs of dot patches: The first pair was the adaptor pair and the second pair was the test pair. After 500 ms fixation on the animated fish, the adaptor pair was displayed and participants were asked to indicate (by button press) which cloud of dots was more numerous (“which patch has more food”). In the baseline condition, the adaptor pair had a neutral numerosity (40 dots on each side, like the standard) and was displayed for only 0.5 s (to reduce testing time, as the adaptation was neutral). In the adaptation condition the adaptor pair comprised 80 dots at the left location (twice the standard) and 20 dots at the right location (half the standard). In this condition the adaptor pair was displayed for 3 s and refreshed six times with new random samples (always 80 at left, 20 at right).
After 2,500 ms, during which the participant had 2,000 ms to respond and 500 ms of fixation, the probe pair was displayed. The number of dots in the test pair was varied by the QUEST adaptive algorithm (51), which computed a Gaussian distribution centered at the PSE with SD of 0.15 log units. The stimuli were varied symmetrically, so that if the stimulus on the right was increased, that on the left was decreased by the same proportion, leaving the geometric average at 40: For example, if the right stimulus was 60 (40 × 1.5), the left would be 27 (40 ÷ 1.5). Participants were then given 2,000 ms to respond to which patch had more food. To prevent the children from becoming confused over to which pair to respond, the adaptor pair or the test pair, they were instructed to respond to both, although only responses to the probe pair were analyzed here.
Data were plotted as the proportion of responses where the left side appeared greater than the right, as a function of the number of the left patch, and fitted with a Gaussian error function. The median of this function yields the PSE and the SD yields the precision threshold (just-noticeable difference), which, divided by the tested number, estimates the coefficient of variation. Overall, 50 trials were presented to all children (25 each in baseline and adaptation conditions), all in one testing session. Experimenters monitored gaze at all times to ensure they were fixating center screen.
We defined the magnitude of adaptation (A) as the percentage of increase in dot number of the left-hand side compared with baseline condition,
| [1] |
where NAdapt and NBase refer, respectively, to the number of dots at the PSE in the adaptation and baseline conditions.
Acknowledgments
We thank all of the children and families who kindly gave up their time to take part in this study. This work was supported by a grant from the United Kingdom’s Medical Research Council (MR/J013145/1) and European Union Grants Framework 7 - European Research Council (FP7-ERC) “Space Time and Number in The Brain (STANIB)” and “Early cortical sensory plasticity and adaptability in human adults (ESCPLAIN).” Research at the Centre for Research in Autism and Education is also supported by The Clothworkers’ Foundation and Pears Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th Ed American Psychiatric Association, Washington DC; 2013. [Google Scholar]
- 2.Williams D. Somebody Somewhere: Breaking free from the World of Autism. Times Books; New York: 1994. [Google Scholar]
- 3.Bagby MS, Dickie VA, Baranek GT. How sensory experiences of children with and without autism affect family occupations. Am J Occup Ther. 2012;66(1):78–86. doi: 10.5014/ajot.2012.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellicano E, Burr D. When the world becomes ‘too real’: A Bayesian explanation of autistic perception. Trends Cogn Sci. 2012;16(10):504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Kersten D, Mamassian P, Yuille A. Object perception as Bayesian inference. Annu Rev Psychol. 2004;55:271–304. doi: 10.1146/annurev.psych.55.090902.142005. [DOI] [PubMed] [Google Scholar]
- 6.Knill DC, Pouget A. The Bayesian brain: The role of uncertainty in neural coding and computation. Trends Neurosci. 2004;27(12):712–719. doi: 10.1016/j.tins.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Mamassian P, Landy M, Maloney L. Bayesian modelling of visual perception. In: Rao R, Olshausen B, Lewicki M, editors. Probabilistic Models of the Brain: Perception and Neural Function. Bradford Books; Cambridge, MA: 2002. [Google Scholar]
- 8.Friston KJ, Lawson R, Frith CD. On hyperpriors and hypopriors: Comment on Pellicano and Burr. Trends Cogn Sci. 2013;17(1):1. doi: 10.1016/j.tics.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Lawson RP, Rees G, Friston KJ. An aberrant precision account of autism. Front Hum Neurosci. 2014;8:302. doi: 10.3389/fnhum.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van de Cruys S, de-Wit L, Evers K, Boets B, Wagemans J. Weak priors versus overfitting of predictions in autism: Reply to Pellicano and Burr (TICS, 2012) Iperception. 2013;4(2):95–97. doi: 10.1068/i0580ic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha P, et al. Autism as a disorder of prediction. Proc Natl Acad Sci USA. 2014;111(42):15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow HB, Foldiak P. 1989. Adaptation and decorrelation in the cortex. The Computing Neuron, ed Durbin R, Miall C, Mitchison G (Addison-Wesley, Wokingham, England), pp 54–72.
- 13.Clifford CWG, Rhodes G. Fitting the Mind to the World: Adaptation and After-Effects in High-Level Vision. Oxford Univ Press; Oxford: 2005. [Google Scholar]
- 14.Kohn A. Visual adaptation: Physiology, mechanisms, and functional benefits. J Neurophysiol. 2007;97(5):3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- 15.Solomon SG, Kohn A. Moving sensory adaptation beyond suppressive effects in single neurons. Curr Biol. 2014;24(20):R1012–R1022. doi: 10.1016/j.cub.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boynton GM. Adaptation and attentional selection. Nat Neurosci. 2004;7(1):8–10. doi: 10.1038/nn0104-8. [DOI] [PubMed] [Google Scholar]
- 17.Leopold DA, O’Toole AJ, Vetter T, Blanz V. Prototype-referenced shape encoding revealed by high-level aftereffects. Nat Neurosci. 2001;4(1):89–94. doi: 10.1038/82947. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes G, Jeffery L. Adaptive norm-based coding of facial identity. Vision Res. 2006;46(18):2977–2987. doi: 10.1016/j.visres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Pellicano E, Jeffery L, Burr D, Rhodes G. Abnormal adaptive face-coding mechanisms in children with autism spectrum disorder. Curr Biol. 2007;17(17):1508–1512. doi: 10.1016/j.cub.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 20.Ewing L, Pellicano E, Rhodes G. Atypical updating of face representations with experience in children with autism. Dev Sci. 2013;16(1):116–123. doi: 10.1111/desc.12007. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford MD, Troubridge EK, Walsh J. Visual afterimages of emotional faces in high functioning autism. J Autism Dev Disord. 2012;42(2):221–229. doi: 10.1007/s10803-011-1233-x. [DOI] [PubMed] [Google Scholar]
- 22.Pellicano E, Rhodes G, Calder AJ. Reduced gaze aftereffects are related to difficulties categorising gaze direction in children with autism. Neuropsychologia. 2013;51(8):1504–1509. doi: 10.1016/j.neuropsychologia.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiorentini C, Gray L, Rhodes G, Jeffery L, Pellicano E. Reduced face identity aftereffects in relatives of children with autism. Neuropsychologia. 2012;50(12):2926–2932. doi: 10.1016/j.neuropsychologia.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Karaminis T, et al. Atypicalities in perceptual adaptation in autism do not extend to perceptual causality. PLoS ONE. 2015;10(3):e0120439. doi: 10.1371/journal.pone.0120439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolfs M, Dambacher M, Cavanagh P. Visual adaptation of the perception of causality. Curr Biol. 2013;23(3):250–254. doi: 10.1016/j.cub.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Arnold D, Petrie K, Gallagher R, Yarrow K. A squishiness visual aftereffect – Not causality adaptation. J Vis. 2014;14(10):1332. doi: 10.1167/15.9.4. [DOI] [PubMed] [Google Scholar]
- 27.Elsabbagh M, et al. BASIS Team Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Curr Biol. 2012;22(4):338–342. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones W, Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Dev Neuropsychol. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- 30.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 31.Anobile G, Cicchini GM, Burr DC. Separate mechanisms for perception of numerosity and density. Psychol Sci. 2014;25(1):265–270. doi: 10.1177/0956797613501520. [DOI] [PubMed] [Google Scholar]
- 32. Anobile G, Cicchini GM, Burr D Mechanisms for perceiving numerosity and texture: A review. Perception, in press.
- 33.Nieder A. Counting on neurons: The neurobiology of numerical competence. Nat Rev Neurosci. 2005;6(3):177–190. doi: 10.1038/nrn1626. [DOI] [PubMed] [Google Scholar]
- 34.Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004;44(3):547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Perrett DI, Mistlin AJ, Chitty AJ. Visual neurones responsive to faces. Trends Neurosci. 1987;10(9):358–364. [Google Scholar]
- 36.Iuculano T, et al. Brain organization underlying superior mathematical abilities in children with autism. Biol Psychiatry. 2014;75(3):223–230. doi: 10.1016/j.biopsych.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aagten-Murphy D, et al. 2015. Numerical estimation in children with autism. Autism Res, 10.1002/aur.1482.
- 38.Jones CR, et al. Reading and arithmetic in adolescents with autism spectrum disorders: Peaks and dips in attainment. Neuropsychology. 2009;23(6):718–728. doi: 10.1037/a0016360. [DOI] [PubMed] [Google Scholar]
- 39.Burr D, Ross J. A visual sense of number. Curr Biol. 2008;18(6):425–428. doi: 10.1016/j.cub.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 40.Arrighi R, Togoli I, Burr DC. 2014. A generalized sense of number. Proc R Soc Lond B Biol Sci 281:20141791. [DOI] [PMC free article] [PubMed]
- 41.Lord C, et al. 2012. Autism Diagnostic Observation Schedule, (ADOS-2) (Western Psychological Services, Torrance, CA), 2nd Ed.
- 42.Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: Two cortical pathways. Trends Neurosci. 1983;6(0):414–417. [Google Scholar]
- 43.McKeefry DJ, Laviers EG, McGraw PV. The segregation and integration of colour in motion processing revealed by motion after-effects. Proc Biol Sci. 2006;273(1582):91–99. doi: 10.1098/rspb.2005.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turi M, Burr D. 2012. Spatiotopic perceptual maps in humans: Evidence from motion adaptation. Proc R Soc Lond B Biol Sci 279(1740):3091–3097.
- 45.Happé F, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 46.Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- 47.Chopin A, Mamassian P. Predictive properties of visual adaptation. Curr Biol. 2012;22(7):622–626. doi: 10.1016/j.cub.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 48.Stocker AA, Simoncelli EP. Sensory adaptation within a Bayesian framework for perception. In: Weiss Y, Schoelkopf B, Platt J, editors. Advances in Neural Information Processing Systems. MIT Press, Cambridge, MA: 2006. pp. 1291–1298. [Google Scholar]
- 49.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation, San Antonio: 1999. [Google Scholar]
- 50.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 51.Watson AB, Pelli DG. QUEST: A Bayesian adaptive psychometric method. Percept Psychophys. 1983;33(2):113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]