Significance
We demonstrate a novel and unexpected role of the transcription factor ThPOK as a potent oncogene in mice. During normal T-cell development, T-helper-inducing POZ/Krueppel-like factor (ThPOK) is selectively expressed in thymocytes developing to the CD4 lineage and is necessary for their differentiation. However, when ThPOK is expressed indiscriminately in all thymocytes, it causes highly penetrant thymic lymphoma. Strong T-cell receptor (TCR) signal rescues thymocytes from ThPOK-dependent lymphoma development, whereas weak signals promote lymphomagenesis. These results demonstrate a novel correlation between ThPOK, TCR signal strength, and lymphomagenesis. We also present evidence that ectopic ThPOK expression gives rise to a preleukemic and self-perpetuating precursor population, akin to a tumor stem cell.
Keywords: ThPOK, lymphoma, thymus, development, TCR
Abstract
The transcription factor T-helper-inducing POZ/Krueppel-like factor (ThPOK, encoded by the Zbtb7b gene) plays widespread and critical roles in T-cell development, particularly as the master regulator of CD4 commitment. Here we show that mice expressing a constitutive T-cell–specific ThPOK transgene (ThPOKconst mice) develop thymic lymphomas. These tumors resemble human T-cell acute lymphoblastic leukemia (T-ALL), in that they predominantly exhibit activating Notch1 mutations. Lymphomagenesis is prevented if thymocyte development is arrested at the DN3 stage by recombination-activating gene (RAG) deficiency, but restored by introduction of a T-cell receptor (TCR) transgene or by a single injection of anti-αβTCR antibody into ThPOKconst RAG-deficient mice, which promotes development to the CD4+8+ (DP) stage. Hence, TCR signals and/or traversal of the DN (double negative) > DP (double positive) checkpoint are required for ThPOK-mediated lymphomagenesis. These results demonstrate a novel link between ThPOK, TCR signaling, and lymphomagenesis. Finally, we present evidence that ectopic ThPOK expression gives rise to a preleukemic and self-perpetuating DN4 lymphoma precursor population. Our results collectively define a novel role for ThPOK as an oncogene and precisely map the stage in thymopoiesis susceptible to ThPOK-dependent tumor initiation.
Hematological malignancies remain a major cause of death, leading to one fatality every 10 min in the United States (www.lls.org). T-cell leukemia is historically linked with a poor prognosis (www.lls.org). The search for novel molecular drug targets remains an important goal of current research efforts, which requires a thorough understanding of the underlying molecular mechanisms.
The thymus is populated by progenitor cells from the bone marrow. The earliest T-cell precursors in the thymus exhibit the double negative 1 (DN1) phenotype, i.e., CD4−CD8lowCD25−CD44+, and express high levels of cKit. Subsequently they downmodulate cKit and traverse the DN2 (CD4−CD8−CD25+CD44+), DN3 (CD4−CD8−CD25+CD44−) and DN4 (CD4−CD8−CD25−CD44+) stages. Cells adopting the αβ T-cell lineage develop further to the double positive CD4+CD8+ (DP) stage, where αβ TCR complex is first expressed on the surface, allowing engagement by intrathymic peptide/MHC ligands. Negative selection at this stage leads to death by apoptosis, whereas positive selection leads to thymocyte activation and differentiation into single positive (SP) CD4+ or CD8+ T cells. Alternate commitment to either the CD4 or CD8 lineages is controlled by the Zn finger transcription factor T-helper-inducing POZ/Krueppel-like factor (ThPOK), whose expression is necessary and sufficient to direct development to the CD4 lineage (1–4). Strong antibody-mediated stimulation can induce ThPOK in developing thymocytes, indicating that ThPOK expression is controlled by TCR signaling (5, 6). A loss-of-function mutation of ThPOK does not affect the efficiency of positive or negative selection (4). Therefore, ThPOK plays a highly specific role in mediating CD4 commitment, and its expression is accordingly precisely controlled in immature thymocyte precursors (5).
ThPOK belongs to the POK family of transcription factors, which includes other factors that mediate important roles in hematopoiesis, i.e., Bcl6, PLZF, and LRF (7–12). Disregulated expression of POK factors is associated with various hematological malignancies, including PLZF in AML (13), Bcl6 in B-cell lymphoma (14, 15), and LRF/Pokemon in T-cell lymphoma and lung cancer (16). However, an oncogenic capacity for ThPOK has not so far been reported. In the present study, we show that ThPOK acts as a potent oncogene when expressed constitutively during mouse thymopoiesis. Most lymphomas from ThPOKconst mice exhibit activating mutations of Notch1, a major contributor to development of T-cell acute lymphoblastic leukemia (T-ALL) in humans. We further show that lymphomagenesis is blocked on a recombination-activating gene (RAG)-deficient background, but does not require RAG-mediated recombination per se, but instead depends on the DN > DP developmental transition. Finally, gene expression and sequencing analysis demonstrate similarities in gene expression programs between lymphomas induced by constitutive ThPOK and dominant negative Ikaros, suggesting that they affect a common pathway(s).
Results
Constitutive T-Cell–Specific ThPOK Expression Causes High Incidence of T-Cell Lymphoma.
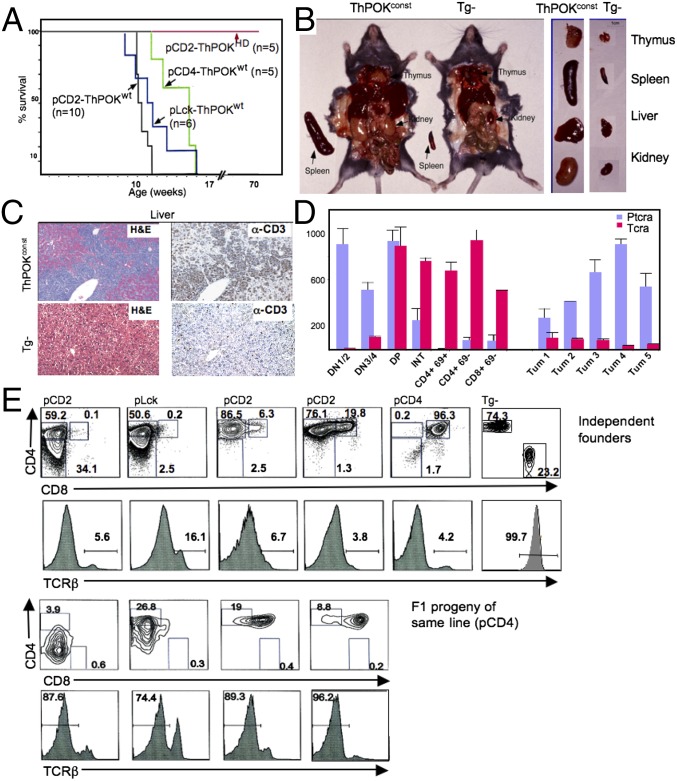
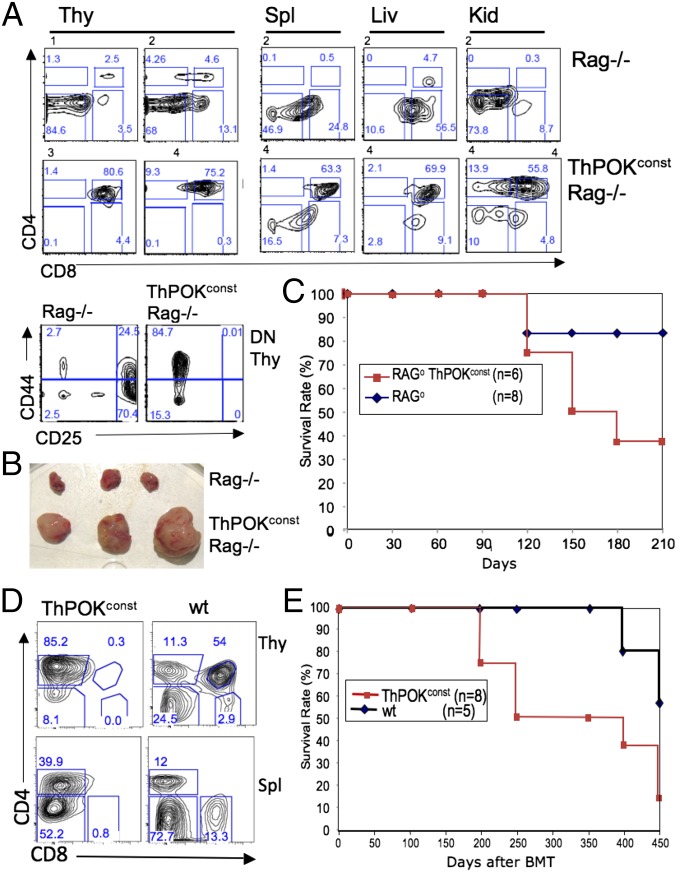
We developed several ThPOK transgenic lines that express WT murine ThPOK constitutively in the T-cell lineage, using either mouse CD4 (2), human CD2 (2), or mouse proximal Lck promoters (Methods). Constitutive ThPOK expression directs all positively selected thymocytes to the CD4 lineage (2). Interestingly, before 4 mo of age all constitutive ThPOK transgenic founders became visibly sick, exhibiting labored breathing, lethargy, and weight loss and rapidly died (Fig. 1A). Sick mice exhibited massive thymic hypertrophy and frequent hyperplasia of peripheral organs, due to infiltration by T-lymphoid cells, as revealed by CD3ε expression (Fig. 1 B and C). Infiltrating cells expressed the T-cell surface marker Thy1 but lacked surface TCR and were larger than normal T cells according to forward scatter measurements (Fig. 1 D and E and Fig. S1A). Such large Thy1+ TCRlo cells were first detected in the blood at 2- to 3 mo of age, indicating the approximate time of conversion of preleukemic thymocytes into aggressive metatstatic lymphomas. Adoptive transfer of peripheral Thy1+ cells from diseased 4-mo-old mCD4-ThPOK transgenic mice into immunodeficient hosts resulted in rapid infiltration of both lymphoid and nonlymphoid organs by Thy1+ TCRlo cells followed by sickness and death, confirming the malignant nature of the Thy1+ population (Fig. S1B). Importantly, 21 independent ThPOK transgenic founders exhibited the same disease symptoms, indicating that disease was a general consequence of constitutive ThPOK expression, rather than a specific consequence of transgene integration site (Fig. 1A). We could generate a heritable line from founder 198 in which ThPOK is controlled by the mouse CD4 promoter.
Fig. 1.
Constitutive T-cell–specific ThPOK expression induces thymic lymphoma. (A) Survival plots of transgenic mice expressing ThPOK under the control of hCD2, mCD4, or mProxLck promoter vectors. Mice are all independent founders. (B) Dissection of representative 3-mo-old mCD4-ThPOK mouse and nontransgenic littermate showing enlarged lymphoid and nonlymphoid organs in the former. (C) Liver section of 3-mo-old mCD4-ThPOK and WT control mice stained for intracellular CD3 (Right). (D) RT-PCR analysis of TCRα and pTα expression in five independent mCD4-ThPOK tumors and sorted normal thymocyte subsets. (E) FACS analyses of Thy1+ peripheral blood lymphocytes (PBL) of representative 3-mo-old constitutive T-cell–specific ThPOK transgenic mice stained for surface expression of CD4, CD8, and TCRβ. Top shows independent founders controlled by hCD2, mCD4, or mProxLck promoters; Bottom shows F1 progeny of one mCD4-driven line.
ThPOK-Mediated T-Cell Lymphomas Are Clonal in Origin.
Lymphomas from different ThPOK transgenic mice displayed distinct CD4 and CD8 expression patterns, and exhibited a single TCR Dβ2-Jβ2.7 rearrangement, implying that tumors from the same mouse arise from a single precursor (note that our PCR analysis used the whole tumor sample from each ThPOKconst transgenic mouse, even if it included cells with different CD4/CD8 surface phenotypes) (Fig. 1D and Fig. S2A). Although most lymphomas resemble early thymic precursors in that they show low TCR surface expression and express pre-TCR rather than TCRα (Fig. 1D), a few lymphomas expressed high surface TCR, allowing FACS analysis of surface Vβ/Vα expression. One such TCRhi lymphoma showed exclusive surface expression of Vβ8.1/8.2, whereas another lacked expression of Vβ8 entirely, implying that it expressed a different unknown Vβ chain. In contrast to restricted Vβ use, surface Vα use appeared diverse, because a particular Vα chain, Vα11, was expressed by only 4–15% of tumor cells, similar to its frequency among normal T cells (Fig. S2B). These findings indicate that TCRα rearrangement continues after development of a clonal TCRβ+ lymphoma precursor population, and therefore that lymphomagenesis is initiated at an immature stage when RAG proteins, required for TCRα rearrangement, are still expressed. Comparative genomic hybridization (CGH) array analysis of five different ThPOKconst lymphomas showed that RAG-mediated TCRα and -β deletions vary in size between tumors, further supporting their clonal origin (Fig. S2C; note tumor 3 has a much smaller TCRα deletion than other samples). Lack of surface TCR expression by most ThPOKconst lymphomas suggests that most Vα rearrangements are unproductive and/or are unable to pair efficiently with rearranged TCRβ. Finally, CGH array analysis of tumors revealed clonal deletions at two known tumor suppressor loci, Ikaros and Pten (Fig. S2 C and D). Collectively, the above observations demonstrate that constitutive expression of ThPOK in the T-cell lineage induces highly penetrant lymphomas of clonal origin. A representative line in which ThPOK is expressed by the mouse CD4 promoter (line 198) was selected for further breeding and analysis (referred to henceforth as ThPOKconst mice).
Notch Signaling Pathway Is Activated in ThPOKconst Lymphomas.
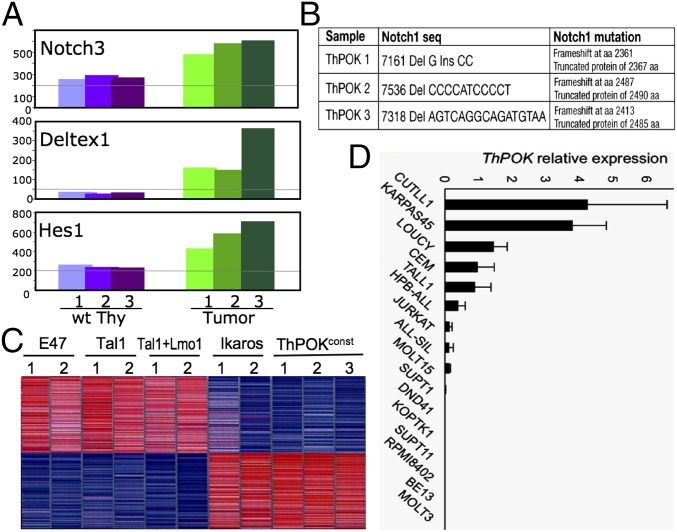
Given that the Notch signaling pathway is frequently activated in T-cell lymphomas of humans and mice (17), we assessed induction of known Notch target genes in ThPOKconst lymphomas. Indeed, several known Notch targets (Hes1, Deltex1, and Notch3) are induced in all tumors examined (Fig. 2A). All tumors exhibited frameshift mutations of Notch1, either upstream or within the PEST domain (Fig. 2B). Lack of a functional PEST domain causes increased stability of active Notch1, explaining induction of Notch target genes, and similar activating Notch1 mutations are observed in human TALL (18). Notch target genes are not induced in ThPOKconst thymocytes before overt lymphomagenesis, so that ThPOK transgene expression does not directly activate the Notch signaling pathway (Fig. S3). To further characterize lymphomas arising in the ThPOKconst model, we carried out microarray-based gene expression analysis of ThPOKconst lymphomas, together with tumors from four other mouse lymphoma models, i.e., Ikaros1 dominant negative plastic mutant, E2A knockout, TAL1 transgenic and TAL1+LMO1 double transgenic models (19–27). In this comparison, ThPOKconst tumors were clearly distinct from those resulting from E2A inactivation or TAL1 induction and more closely resembled tumors from Ikaros plastic mice (Fig. 2C and Fig. S4). Whereas ThPOK and Ikaros lymphomas share a partly related gene expression signature (Fig. 2C), supervised analysis also shows significant differences (Fig. S5). To evaluate the potential role of ThPOK in human oncogenesis, we analyzed ThPOK expression in a panel of human T-ALL lymphoma lines by real-time RT-PCR. Significantly, ThPOK expression is detected in a substantial fraction of these lines (Fig. 2D). Because all of these lymphomas are immature in phenotype (28, 29), and ThPOK is not expressed in immature human thymocytes under physiological conditions (30), aberrant induction of ThPOK in early stages of T-cell development may contribute significantly to lymphoma initiation/development in human T-ALL.
Fig. 2.
ThPOKconst lymphomas show Notch induction and resemble dominant-negative (DN) Ikaros lymphomas. (A) RT-PCR analysis of Hes1, Deltex1, and Notch3 mRNA expression in total thymocytes from three different 2- to 3-mo-old ThPOKconst mice, typed as “lymphoma” positive, according to enlarged thymus size and altered thymocyte subset distribution (green columns). Expression of the same genes is shown for total thymocytes from three WT control mice (purple columns). (B) Notch1 mutations from three independent ThPOKconst lymphomas. (C) Oligonucleotide microarray gene expression comparison of ThPOKconst lymphomas with other mouse T-cell lymphomas. Unsupervised hierarchical clustering indicates closest similarity to Ikaros DN lymphomas. (D) Comparison of human ThPOK RNA expression levels by RT-PCR (normalized for GAPDH), in panel of human T-ALL cell lines.
ThPOK-Mediated Lymphomagenesis Requires DN-to-DP Developmental Transition.
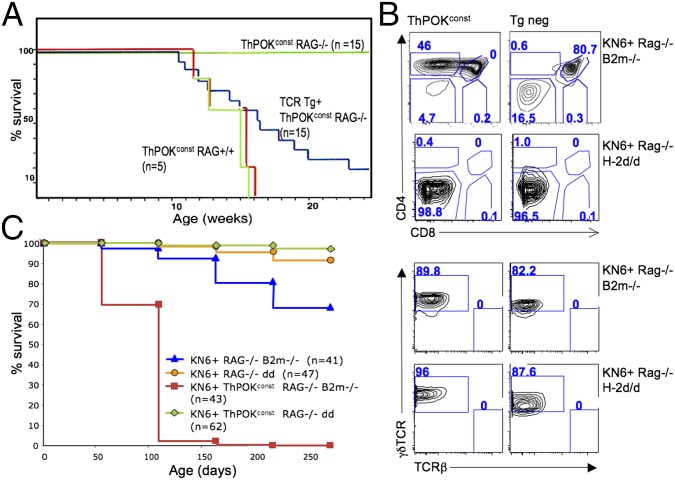
The fact that most tumors from ThPOKconst transgenic RAG+ mice show low surface TCR expression and transcribe pTα suggests an immature developmental origin (Fig. 1 D and E and Fig. S1B). To precisely define the developmental stage at which lymphomagenesis occurs, we introduced the ThPOKconst transgene onto a RAG−/− background, which blocks development at the DN3 stage. RT-PCR analysis of sorted thymocyte subsets from ThPOKconst RAG−/− mice reveals that transgene expression begins at least by the DN2 stage (Fig. S6B; note that endogenous ThPOK is expressed in some DN1 thymocytes, but not DN2 cells). Significantly, ThPOKconst RAG−/− mice fail to develop lymphoma up to at least 2 y of age (Fig. 3A), indicating a requirement for (i) RAG-mediated recombination itself, (ii) pre-TCR/TCR expression/signaling, and/or (iii) development beyond the DN3 stage. To distinguish these possibilities, we introduced an αβTCR transgene (AND) (31) onto the ThPOKconst RAG−/− background, which restores development to the DP and SP CD4 stages. In the presence of the αβTCR transgene, ThPOKconst-mediated lymphomagenesis is largely restored, indicating that tumor development depends on progression beyond the DN3 stage and/or on TCR/pre-TCR expression/signaling, but does not require RAG-mediated DNA cleavage (Fig. 3A). To distinguish whether TCR expression/signaling and/or development beyond the DN3 stage are required, we crossed ThPOKconst RAG−/− mice to animals expressing the transgenic KN6 γδTCR, which recognizes the nonclassical MHC class I products T22 and T10 (32, 33). On a β2m+/+ background, which supports ligand expression, KN6 thymocytes undergo development to the γδ T-cell lineage, as evidenced by progression to the DN4 stage and loss of expression of CD24, a marker of immaturity, whereas on a β2m−/− background, that lacks ligand expression, they undergo development to the DP stage, indicative of adoption of the αβ T-cell lineage (34, 35). Development to the DP stage involves massive proliferation, whereas development to the γδ lineage does not. ThPOKconst KN6+ RAG−/− thymocytes similarly undergo alternate development to the γδ or αβ lineages in the presence or absence of ligand, respectively (Fig. 3B). Interestingly, ThPOKconst KN6+ mice expressing the MHC ligand for KN6 did not develop lymphoma, although the ThPOK transgene was expressed in γδTCR+ DN4 cells that arise in these mice (Fig. S6B). In contrast, ThPOKconst KN6+ β2m−/− Rag−/− thymocytes, which lack ligand for the KN6 TCR and hence do not receive strong TCR-mediated signals, but undergo efficient progression to the DP stage, did develop lymphomas (Fig. 3 B and C and Fig. S7). This finding indicates that TCR expression/signaling is not sufficient for ThPOK-mediated lymphomagenesis, but that development beyond the DN3 stage is required. Collectively, these observations demonstrate that constitutive expression of ThPOK in the T-cell lineage induces lymphomagenesis at the transition between the DN3 and DP stages and is dependent on expression of a TCR isoform that promotes this transition, but not on Rag-mediated recombination.
Fig. 3.
ThPOK-mediated lymphomagenesis requires DN-to-DP developmental transition. (A) Survival plots of ThPOKconst mice crossed to Rag2+/+, Rag2−/−, or αβTCR Tg+ Rag−/− backgrounds, as indicated. (B) FACS analysis of γδTCR Tg+ (KN6+) Rag2−/− β2m−/− and γδTCR Tg+ (KN6+) Rag2−/− H-2d/d thymocytes in presence or absence of ThPOKconst transgene, showing CD4, CD8, γδTCR, and TCRβ expression. Note that due to RAG deficiency there is no rearrangement of endogenous TCR genes in these mice, so that TCRβ expression is lacking. (C) Survival plots of ThPOKconst mice crossed to KN6+ RAG−/− B2m−/− (ligand negative) or KN6+ RAG−/− H-2d/d (ligand positive) backgrounds, as indicated. Note that all ThPOKconst KN6+ RAG−/− B2m−/− mice died by 200 d, and those examined showed clear thymic lymphoma, whereas no mice from other groups showed evidence of lymphoma. (However, a few of these also died, likely from infection.)
ThPOK Transgene Promotes Changes in Gene Expression in Immature Thymocytes.
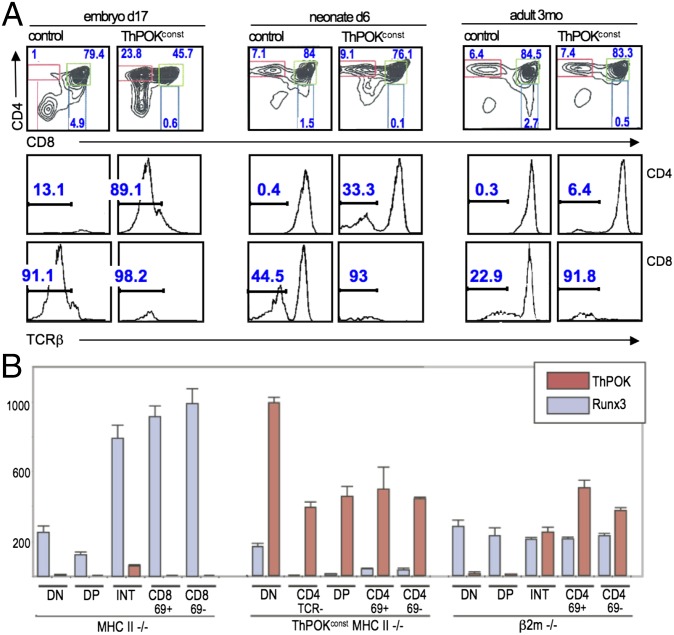
Despite the apparent immature origin of ThPOKconst lymphomas, many tumors exhibit a mature SP CD4 phenotype, although combined with low TCR surface expression (Fig. 1E and Fig. S1B). Similar TCRlo SP CD4 cells are found in the thymus of 3-mo-old preleukemic ThPOKconst mice, but not WT control mice (Fig. 4A). TCRlo SP CD4 thymocytes could arise from DP precursors by down-modulation of CD8 or from immature DN thymocytes by up-modulation of CD4. To distinguish these possibilities, we examined embryonic day 17 (e17) mice, when SP CD4 cells have not yet developed. Whereas e17 WT mice lack SP CD4 cells, ThPOKconst mice possess >20% SP CD4 (Fig. 4A, Left). Given the early stage in ontogeny, we infer that TCRlo SP CD4 cells do not arise from DP precursors, at least not by positive selection. Instead, SP CD4 TCRlo cells appear to arise from premature CD4 expression and/or delayed CD8 up-modulation at the DN > DP transition. Consistent with premature CD4 induction, ThPOKconst RAG−/− thymocytes, in which development is blocked at the DN3 stage, exhibit significant CD4 up-modulation (Fig. S6A). The fact that SP CD4 TCRlo cells diminish in adults (<0.5% by 3 mo) (Fig. 4A, Right), suggests that the embryonic microenvironment may favor generation of these cells. Of note, the CD8+ ISP (immature single positive) subset is absent in ThPOKconst mice (Fig. 4A) and may be replaced by SP CD4 TCRlo cells. Because of the known role of Runx factors in repressing CD4 transcription (36), we tested whether Runx expression was impaired in thymocytes from ThPOKconst mice. Indeed, Runx3 transcripts were decreased almost to background levels in all thymic subsets from ThPOKconst mice, indicating a profound repressive effect of the ThPOK transgene on Runx3 transcription (Fig. 4B). Thus, CD4 derepression in ThPOKconst thymocytes may reflect direct binding to the CD4 silencer by ThPOK (37) and/or repression of Runx3 expression.
Fig. 4.
Altered early thymocyte development in ThPOKconst mice. (A) FACS analysis of thymocytes from ThPOKconst and control non-Tg mice at different stages in ontogeny, as indicated, showing expression of CD4, CD8, and TCRβ. Note prominent TCRβ− SP CD4 subset in embryonic ThPOKconst mice, which diminishes progressively in neonates and adults. Also, note that the CD8+ immature single positive (ISP) subset is absent in ThPOKconst mice at e17 (0.6% SP CD8 cells, compared with 4.9% in WT control). (B) RT-PCR analysis of ThPOK and Runx3 mRNA expression in indicated sorted thymic subsets from ThPOKconst MHC class II−/− mice, compared with MHC class II−/− and β2m−/− mice. Note that ThPOK is expressed constitutively in all subsets from ThPOKconst MHC class II−/− mice, whereas Runx3 is severely repressed in these mice. INT refers to CD4+8low subset intermediate between DP and SP stages.
A Single Wave of DP Development Is Sufficient for Lymphomagenesis.
To elucidate the timing of ThPOK-mediated lymphomagenesis, we used a system in which the DN > DP transition can be transiently induced in RAG−/− mice by injecting anti-CD3ε antibody (38). This system allows us to ask whether a single wave of thymocyte proliferation can cause lymphomagenesis. Whereas untreated RAG−/− and ThPOKconst RAG−/− mice are arrested at the DN3 stage, antibody treatment triggers differentiation to the DP stage and proliferation. In RAG−/− animals, DP thymocytes declined after a few months, and very few T cells were found in the periphery (Fig. 5 A and B). In contrast, in ThPOKconst Rag−/− mice, DP thymocytes persisted and expanded for at least 7 mo after antibody stimulation, causing massive thymic hypertrophy, sickness, and eventual death (Fig. 5C). Several mice showed T-cell infiltration of peripheral organs (Fig. 5A). The i.p. transfer of 2 × 106 thymic lymphoma cells from sick antibody-treated ThPOKconst Rag−/− mice into Rag2−/− hosts resulted in tumor development within 20 d in multiple organs in all hosts (n = 6). These results indicate that a single wave of thymocyte proliferation in adult ThPOKconst mice is sufficient to initiate the molecular cascade leading to lymphomagenesis. The latency period of tumor development in this system is considerably longer compared with ThPOKconst RAG+ mice, i.e., almost half the mice are still alive at 4 mo (Fig. 5C). Longer latency might be due to: (i) the adult microenvironment, which may somehow render thymocytes less susceptible to ThPOK-mediated transformation than the embryonic one. (ii) ThPOKconst Rag−/− thymocytes fail to express pre-TCR or experience pre–TCR-mediated signaling, which could accelerate lymphomagenesis. (iii) Multiple waves of TCR signaling may enhance survival and or proliferation of lymphoma precursors.
Fig. 5.
Single wave of thymopoiesis supports ThPOK-mediated lymphomagenesis. (A) FACS analysis of thymus, spleen, liver, and kidney cells. (B) Relative size of thymus and spleen of ThPOKconst Rag−/− mice 7 mo after anti-CD3ε antibody stimulation (numbers above panels in A refer to individual mice). (C) Survival plot for antibody-stimulated ThPOKconst Rag−/− and control Rag−/− mice. Note that the single RAG−/− mouse that died during the study period showed no evidence of lymphoma and probably died from bacterial infection. (D) ThPOKconst bone marrow transfer into Rag2−/− recipients. FACS plot of thymocytes and splenocytes from Rag2−/− recipients 3 mo after transfer of 106 T-cell–depleted bone marrow cells from ThPOKconst mice. Note aberrant thymocyte subset distribution indicative of onset of lymphomagenesis. (E) Survival plot of Rag2−/− mice receiving either ThPOKconst or wild-type bone marrow cells.
To distinguish these possibilities, we transferred T-cell–depleted ThPOKconst Rag+ bone marrow (BM) cells into adult Rag2−/− recipients, so that thymocyte expansion is restricted to the adult environment, but normal pre-TCR signaling is preserved. At 6 wk after bone marrow transplantation (BMT), all recipients exhibited substantial peripheral CD4+ T cells but lacked CD8+ cells (n = 9), similar to donor ThPOKconst mice (Fig. 5D). All recipients of ThPOKconst BM went on to develop lymphoma (as evidenced by thymic hypertrophy, sickness, and death). However, the latency was greatly extended compared with ThPOKconst mice, such that many recipients were still alive >1 y after BM reconstitution, consistent with the notion that the fetal microenvironment may predispose to lymphoma development (Fig. 5E).
Identification of Putative Tumor Precursor Cells in ThPOKconst Thymus.
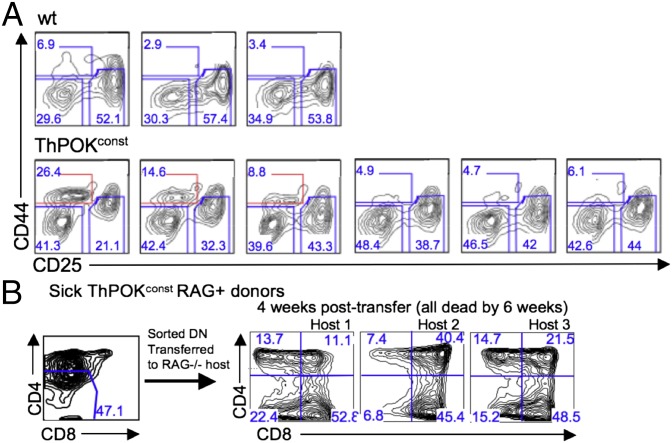
Given the latency between antibody stimulation and lymphoma development in antibody-injected ThPOKconst RAG−/− mice, a long-lived or self-renewing lymphoma progenitor population must persist for several months after antibody injection. Presumably, similar tumor progenitor cells also arise in ThPOKconst RAG+ mice upon normal pre-TCR signaling of DN thymocytes. To identify such lymphoma progenitor cells, we used FACS and cell transfer approaches. Significantly, in both antibody-injected ThPOKconst RAG−/− mice and ThPOKconst RAG+ mice, we detected an accumulation of DN4 (CD44− CD25−) cells (Figs. 5A, Lower Left and 6A). In the case of antibody-treated ThPOKconst RAG−/− mice, it is particularly clear that DN4 thymocytes are long-term persisting or self-renewing cells, because they are absent before antibody treatment. In addition, an unusual DN1-like population often appears in ThPOKconst RAG+ mice (Fig. 6A, Bottom three panels; note subset highlighted in red), which could also represent an intermediate in lymphoma development. To test their lymphomagenic potential, sorted ThPOKconst RAG+ DN thymocytes were transferred into sublethally irradiated RAG−/− recipients. Strikingly, all host mice that received total DN thymocytes from tumor-bearing mice developed aggressive lymphoma. As few as 25,000 DN4 cells caused lymphoma induction, whereas DN1 cells failed to cause lymphoma. This finding indicates that DN4 but not DN1 cells are fully transformed. Transfer of total DN cells gave rise to lymphoma populations with multiple different coreceptor surface phenotypes (DN, ISP, DP, and SP CD4) in the same adoptive host. Hence DN thymocytes from lymphoma-bearing ThPOKconst mice maintain potential for further differentiation.
Fig. 6.
Characterization of DN tumor precursor cells in ThPOKconst mice. (A) FACS analysis of gated TCR− DN (CD4−CD8−) thymocytes from 5-wk-old ThPOKconst mice and WT littermates. Note the relative increase in the DN4 (CD44−CD25−) fraction in all ThPOKconst mice, and the appearance of CD44INT cells, which also express variable levels of CD25, in a subset of ThPOKconst animals (Bottom row; CD44INT population is outlined in red). (B) A total of 25–100 × 103 sorted DN thymocytes from indicated mice were transferred into sublethally irradiated Rag2−/− recipient mice, and PBLs were analyzed by FACS for CD4 and CD8 expression at indicated times.
Discussion
The study of the molecular mechanisms that control T-cell development and T-cell leukemia indicates that oncogenes and oncogenic pathways often function as essential regulators of physiological T-cell development. In the context of conventional T-cell development, we previously provided the first evidence to our knowledge that ThPOK acts as a master regulator of the CD4/CD8 decision point, whose presence or absence dictates development to the CD4 or CD8 lineages, respectively (2, 4). Later we found that ThPOK also plays a widespread and critical role in development of nonconventional T-cell subsets like iNKT cells and selected γδ T-cell subsets (6, 39, 40). In all of these mature T-cell lineages, ThPOK is expressed at more-or-less high levels under normal physiological conditions. In contrast, ThPOK is not or hardly expressed in immature precursor thymocytes that precede these different mature lineages, i.e., in immature DN and DP thymocytes. Herein, we report for the first time to our knowledge that constitutive expression of ThPOK in immature thymocytes leads to aggressive and highly penetrant thymic lymphoma, implicating ThPOK as a potent oncogene.
Thus, ThPOKconst mice exhibited 100% incidence of lymphoma by 4 mo of age in multiple independent lines. Phenotypically, ThPOKconst lymphoma cells are immature in origin, which closely mimics human T-ALL. Furthermore, we report elevated ThPOK expression in 40% of immature human T-ALL lines. Given that ThPOK is normally silenced in immature DN and DP thymocyte stages of human, as in mouse, aberrant induction of ThPOK at these stages may contribute to lymphoma initiation/development in human T-ALL. Tumor cells isolated from different ThPOKconst lines showed activating Notch mutations, also similar to human T-ALL. One ThPOKconst lymphoma sample displayed deletion of Ikaros, and gene expression microarray analysis data revealed a close resemblance between ThPOKconst tumors and thymic lymphomas from mice carrying a dominant negative mutation of Ikaros. Hence, ThPOK gain-of-function and Ikaros loss-of-function mutants may in part affect the same intracellular pathways.
We have used genetic approaches to precisely delineate the developmental requirements for ThPOK-mediated transformation. We show that immature thymocytes up to and including the DN3 stage are insensitive to ThPOK-mediated transformation, since ThPOKconst RAG−/− mice fail to develop lymphomas. This finding could indicate a requirement for: (i) RAG-mediated recombination itself, (ii) pre-TCR/TCR expression/signaling, or (iii) differentiation to the DN4 stage or beyond. The first possibility is excluded by the fact that an αβTCR transgene restores lymphomagenesis in ThPOKconst RAG−/− mice. Regarding the second possibility, TCR signaling by itself is insufficient for lymphomagenesis, because strong TCR signaling through the KN6 γδTCR does not promote lymphomagenesis. Collectively, our results therefore support a model whereby development up to or beyond the DN4 stage is necessary for lymphomagenesis. It remains to be clarified whether TCR signaling is necessary only to promote development or whether it also directly synergizes with ThPOK to initiate lymphomagenesis. Regarding our KN6 TCR transgenic analysis, it is possible that strong ligand-mediated TCR signals that drive development to the γδ lineage and weaker TCR signals that drive development to the αβ lineage may trigger different intracellular pathways and that only weaker signals synergize with ThPOK at the DN > DP transition to generate seed populations of preleukemic cells, which eventually give rise to overt thymic lymphoma. Because ThPOK is normally expressed in a subset of γδ DN thymocytes (NKT γδ cells), and is in fact necessary for their development, a physiological mechanism seems to have evolved to prevent ThPOK-mediated lymphomagenesis in the γδ lineage. However, cells developing to the αβ lineage do not require such a mechanism, because ThPOK is not normally expressed in these cells.
In terms of ontogeny, we show that ThPOK can induce lymphomas at either fetal or adult stages, but that penetrance and rate of onset seem to be enhanced at the fetal stage. There are at least two explanations: (i) In our adult, antibody-induced, lymphoma model, there is only a single wave of thymopoiesis, so that the cumulative number of tumor progenitor cells is limited in comparison with mice undergoing continuous thymopoiesis. (ii) Embryonic thymocytes may actually be more susceptible to lymphomagenesis compared with adult thymocytes. Based on the fact that transferred bone marrow cells from ThPOKconst RAG+ mice also show delayed lymphoma onset, we favor the notion that embryonic thymocytes are more susceptible.
We postulate that the lymphoma-promoting capacity of ThPOK reflects a capacity to partly arrest development between the DN3 and DP stages. This hypothesis is based on results of antibody-mediated CD3ε stimulation of ThPOKconst RAG−/− thymocytes. The key result is the long (4 mo) time lag between antibody stimulation and lymphoma development and persistence of DN4 cells during this period. This result indicates that a progenitor population can survive and/or propagate itself during this lag period, allowing for accumulation of secondary mutations, like activating Notch1 mutations, that result in fully developed lymphomas. We postulate that progenitor cells are derived from DN4 cells, because as few as 25,000 DN4 cells from 4-wk-old ThPOKconst mice can transfer aggressive lymphoma to adoptive hosts. Transferred cells partly maintain the DN4 phenotype, but also diverge to multiple more mature subsets, suggesting that DN4 progenitor cells are capable of both self-renewal and differentiation. Consistent with the idea that ThPOK may impart self-renewing capacity to DN4 thymocytes, we have found that cultured DN4 like ThPOKconst lymphoma cells partly show up-modulation of HSC markers Sca1 and ckit.
Methods
Mice.
All experimentation involving animals was approved by Institutional Animal Care and Use Committee (IACUC), Fox Chase Cancer Center. The ThPOKconst transgene (line 198) has been described previously (2).
PCR Assays.
Primers for PCR are given in SI Methods.
Microarray Analysis.
Gene expression profiling of mouse tumors was carried out using Affymetrix Mouse Genome 430A 2.0 Array, as detailed in SI Methods.
CGH Array Analysis.
CGH array analysis was carried out as reported (41). Further details can be viewed in SI Methods.
Supplementary Material
Acknowledgments
We acknowledge the assistance of Jim Oesterling for flow cytometry, Phil Czyzewicz for animal husbandry, Jianming Pei of the Genomics Facility for CGH array analysis, and the Cell Culture Facility for culturing lymphoma lines. This work was supported by National Institutes of Health R01 CA153260 and P30 CA006927, and a William J. Avery Postdoctoral Fellowship (to J.M.-B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424104112/-/DCSupplemental.
References
- 1.Dave VP, et al. CD3 delta deficiency arrests development of the alpha beta but not the gamma delta T cell lineage. EMBO J. 1997;16(6):1360–1370. doi: 10.1093/emboj/16.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He X, et al. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433(7028):826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 3.Kappes DJ, He X, He X. Role of the transcription factor Th-POK in CD4:CD8 lineage commitment. Immunol Rev. 2006;209:237–252. doi: 10.1111/j.0105-2896.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 4.Keefe R, Dave V, Allman D, Wiest D, Kappes DJ. Regulation of lineage commitment distinct from positive selection. Science. 1999;286(5442):1149–1153. doi: 10.1126/science.286.5442.1149. [DOI] [PubMed] [Google Scholar]
- 5.He X, et al. CD4-CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity. 2008;28(3):346–358. doi: 10.1016/j.immuni.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Park K, et al. TCR-mediated ThPOK induction promotes development of mature (CD24-) gammadelta thymocytes. EMBO J. 2010;29(14):2329–2341. doi: 10.1038/emboj.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duy C, et al. BCL6 is critical for the development of a diverse primary B cell repertoire. J Exp Med. 2010;207(6):1209–1221. doi: 10.1084/jem.20091299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovalovsky D, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9(9):1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SU, et al. LRF-mediated Dll4 repression in erythroblasts is necessary for hematopoietic stem cell maintenance. Blood. 2013;121(6):918–929. doi: 10.1182/blood-2012-03-418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda T, et al. LRF is an essential downstream target of GATA1 in erythroid development and regulates BIM-dependent apoptosis. Dev Cell. 2009;17(4):527–540. doi: 10.1016/j.devcel.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranuncolo SM, et al. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nat Immunol. 2007;8(7):705–714. doi: 10.1038/ni1478. [DOI] [PubMed] [Google Scholar]
- 12.Savage AK, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29(3):391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, et al. Fusion between a novel Krüppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 1993;12(3):1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerckaert JP, et al. LAZ3, a novel zinc-finger encoding gene, is disrupted by recurring chromosome 3q27 translocations in human lymphomas. Nat Genet. 1993;5(1):66–70. doi: 10.1038/ng0993-66. [DOI] [PubMed] [Google Scholar]
- 15.Ye BH, et al. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993;262(5134):747–750. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- 16.Maeda T, et al. Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature. 2005;433(7023):278–285. doi: 10.1038/nature03203. [DOI] [PubMed] [Google Scholar]
- 17.Ferrando AF. 2009. The role of NOTCH1 signaling in T-ALL. ASH Education Book, vol. 2009, no. 1, pp 353–361.
- 18.Weng AP, et al. 2004. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306(5694): 269–271.
- 19.Aplan PD, et al. An scl gene product lacking the transactivation domain induces bony abnormalities and cooperates with LMO1 to generate T-cell malignancies in transgenic mice. EMBO J. 1997;16(9):2408–2419. doi: 10.1093/emboj/16.9.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bain G, et al. E2A deficiency leads to abnormalities in alphabeta T-cell development and to rapid development of T-cell lymphomas. Mol Cell Biol. 1997;17(8):4782–4791. doi: 10.1128/mcb.17.8.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Condorelli GL, et al. T-cell-directed TAL-1 expression induces T-cell malignancies in transgenic mice. Cancer Res. 1996;56(22):5113–5119. [PubMed] [Google Scholar]
- 22.Fisch P, et al. T-cell acute lymphoblastic lymphoma induced in transgenic mice by the RBTN1 and RBTN2 LIM-domain genes. Oncogene. 1992;7(12):2389–2397. [PubMed] [Google Scholar]
- 23.Kelliher MA, Seldin DC, Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIalpha. EMBO J. 1996;15(19):5160–5166. [PMC free article] [PubMed] [Google Scholar]
- 24.Larson RC, et al. Protein dimerization between Lmo2 (Rbtn2) and Tal1 alters thymocyte development and potentiates T cell tumorigenesis in transgenic mice. EMBO J. 1996;15(5):1021–1027. [PMC free article] [PubMed] [Google Scholar]
- 25.McGuire EA, Rintoul CE, Sclar GM, Korsmeyer SJ. Thymic overexpression of Ttg-1 in transgenic mice results in T-cell acute lymphoblastic leukemia/lymphoma. Mol Cell Biol. 1992;12(9):4186–4196. doi: 10.1128/mcb.12.9.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83(2):289–299. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 27.Yan W, et al. High incidence of T-cell tumors in E2A-null mice and E2A/Id1 double-knockout mice. Mol Cell Biol. 1997;17(12):7317–7327. doi: 10.1128/mcb.17.12.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palomero T, et al. CUTLL1, a novel human T-cell lymphoma cell line with t(7;9) rearrangement, aberrant NOTCH1 activation and high sensitivity to gamma-secretase inhibitors. Leukemia. 2006;20(7):1279–1287. doi: 10.1038/sj.leu.2404258. [DOI] [PubMed] [Google Scholar]
- 29.Drexler HG. The Leukemia-Lymphoma Cell Line Factsbook. Academic; London: 2000. [Google Scholar]
- 30.McClory S, et al. Evidence for a stepwise program of extrathymic T cell development within the human tonsil. J Clin Invest. 2012;122(4):1403–1415. doi: 10.1172/JCI46125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaye J, et al. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341(6244):746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 32.Bonneville M, et al. Recognition of a self major histocompatibility complex TL region product by gamma delta T-cell receptors. Proc Natl Acad Sci USA. 1989;86(15):5928–5932. doi: 10.1073/pnas.86.15.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito K, et al. Recognition of the product of a novel MHC TL region gene (27b) by a mouse gamma delta T cell receptor. Cell. 1990;62(3):549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- 34.Haks MC, et al. Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity. 2005;22(5):595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Pereira P, et al. Blockade of transgenic gamma delta T cell development in beta 2-microglobulin deficient mice. EMBO J. 1992;11(1):25–31. doi: 10.1002/j.1460-2075.1992.tb05023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Telfer JC, Hedblom EE, Anderson MK, Laurent MN, Rothenberg EV. Localization of the domains in Runx transcription factors required for the repression of CD4 in thymocytes. J Immunol. 2004;172(7):4359–4370. doi: 10.4049/jimmunol.172.7.4359. [DOI] [PubMed] [Google Scholar]
- 37.Muroi S, et al. Cascading suppression of transcriptional silencers by ThPOK seals helper T cell fate. Nat Immunol. 2008;9(10):1113–1121. doi: 10.1038/ni.1650. [DOI] [PubMed] [Google Scholar]
- 38.Levelt CN, Mombaerts P, Iglesias A, Tonegawa S, Eichmann K. Restoration of early thymocyte differentiation in T-cell receptor beta-chain-deficient mutant mice by transmembrane signaling through CD3 epsilon. Proc Natl Acad Sci USA. 1993;90(23):11401–11405. doi: 10.1073/pnas.90.23.11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engel I, et al. Co-receptor choice by V alpha14i NKT cells is driven by Th-POK expression rather than avoidance of CD8-mediated negative selection. J Exp Med. 2010;207(5):1015–1029. doi: 10.1084/jem.20090557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engel I, Zhao M, Kappes D, Taniuchi I, Kronenberg M. The transcription factor Th-POK negatively regulates Th17 differentiation in Vα14i NKT cells. Blood. 2012;120(23):4524–4532. doi: 10.1182/blood-2012-01-406280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timakhov RA, et al. Recurrent chromosomal rearrangements implicate oncogenes contributing to T-cell lymphomagenesis in Lck-MyrAkt2 transgenic mice. Genes Chromosomes Cancer. 2009;48(9):786–794. doi: 10.1002/gcc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.