Significance
Treg cells suppress excessive and aberrant immune responses. Impaired function or homeostasis of Treg cells would induce severe autoimmune and inflammatory diseases. Forkhead box P3 (FOXP3), as a master regulator of Treg cells, forms a large complex with other binding factors to modulate Treg-cell function subtly in pathological and physiological conditions. We identified that Deleted in breast cancer 1 (DBC1) is an essential subunit of the FOXP3 complex in human CD4+ Treg cells. Our results show that the inflammatory cytokines TNF-α or IL-6 trigger FOXP3 degradation, whereas downregulation of DBC1 expression prevents FOXP3 degradation and maintains Treg-cell function under inflammatory stimuli in vitro and in vivo. These findings unveil a previously unidentified pathway for therapeutically modulating FOXP3+ Treg-cell stability under inflammation.
Keywords: regulatory T cells, FOXP3 complex, inflammation, Deleted in breast cancer 1
Abstract
Forkhead box P3 (FOXP3)-positive Treg cells are crucial for maintaining immune homeostasis. FOXP3 cooperates with its binding partners to elicit Treg cells’ signature and function, but the molecular mechanisms underlying the modulation of the FOXP3 complex remain unclear. Here we report that Deleted in breast cancer 1 (DBC1) is a key subunit of the FOXP3 complex. We found that DBC1 interacts physically with FOXP3, and depletion of DBC1 attenuates FOXP3 degradation in inflammatory conditions. Treg cells from Dbc1-deficient mice were more resistant to inflammation-mediated abrogation of Foxp3 expression and function and delayed the onset and severity of experimental autoimmune encephalomyelitis and colitis in mice. These findings establish a previously unidentified mechanism regulating FOXP3 stability during inflammation and reveal a pathway for potential therapeutic modulation and intervention in inflammatory diseases.
The CD4+CD25+FOXP3+ Treg cells are actively engaged in the prevention of autoimmunity and the mitigation of aberrant or excessive immune responses (1–3). The transcription factor Forkhead box P3 (denoted “FOXP3” in humans, and “Foxp3” in mice) is a well-characterized marker of Treg cells, and its expression typically is considered a requisite for Treg-cell differentiation and function (4, 5). FOXP3 deficiency leads to the scurfy phenotype in mice and to the immune dysregulation, polyendocrinopathy, and enteropathy, X-linked syndrome in humans (6). Moreover, Treg-cell function is impaired in several autoimmune and inflammatory diseases, including colitis, rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus (7). Thus, the manipulation of Treg-cell function might provide a practical approach to the treatment of autoimmune and inflammatory diseases.
Despite the central role of FOXP3 in Treg cells, many questions remain regarding the molecular mechanisms by which FOXP3 regulates Treg-cell function. Foxp3 protein is expressed transiently in CD4+CD25− effector T cells upon T-cell receptor (TCR) stimulation but does not generate T cells with suppressive activity (8, 9). It has become evident that Foxp3 alone is insufficient to reproduce completely the differentiation and functional characteristics of Treg cells (10–12). FOXP3 binds with its partners to form multiple positive and negative feedback loops to regulate Treg-cell function subtly (13). FOXP3 interacts with FOXP1 to form heterodimers that promote FOXP3-mediated repression of IL-2 production (14). FOXP3 also binds with several nuclear factors, such as GATA3 (11, 15), RORγt (16), Eos (17), and RUNX1 (18), to drive the Treg cells’ genetic program. FOXP3 function also is regulated at the posttranslational level. FOXP3 has been shown to interact with the acetyltransferase Tat-interaction protein 60 kDa (TIP60) to promote FOXP3 acetylation, which is required for Treg cells’ suppressive function (19). P300 also regulates FOXP3 acetylation and stability by preventing proteasome-mediated FOXP3 degradation (20). Moreover, the class III deacetylase SIRT1 decreases Foxp3 acetylation and down-regulates Foxp3 protein expression (21). A recent study used an unbiased proteomic approach to analyze the composition of Foxp3 complexes comprehensively and identified ∼360 partners of Foxp3 (11); therefore, many questions remain regarding the differential modulation of this complex and its effect on Treg-cell differentiation and function.
Inflammation is beneficial for pathogen clearance and protection against infection; therefore restricting the activity of FOXP3+ Treg cells may be necessary to allow effective immune responses to occur during inflammation (2, 22–26). However, the molecular basis by which FOXP3 protein stability and Treg-cell function are regulated during inflammation remains unclear. Previous studies have shown that FOXP3+ Treg cells are prone to shift toward a Th17-like effector phenotype in the presence of TGF-β and IL-6 (27). The inflammatory cytokine TNF-α also has been shown to down-regulate FOXP3 expression and Treg cells’ suppressive function (28); nonetheless, a recent study has shown that TNF-α down-modulates Treg-cell function by reducing FOXP3 phosphorylation but has no effect on FOXP3 protein expression (29). Other studies have shown that populations of Treg cells highly expressing CD25 are stable and do not lose Foxp3 upon their adoptive transfer into lymphopenic hosts (30). Despite these findings, the molecular mechanism by which FOXP3 mediates regulation of Treg-cell function, especially under inflammation, is still poorly understood. We thus aimed at purifying the human FOXP3 complex to identify previously unidentified interaction partners that may play a role in FOXP3 stability and function.
In this study, we show that Deleted in breast cancer 1 (DBC1, also called “p30 DBC” and cell cycle and apoptosis regulator 2, CCAR2) is a previously unidentified component of the FOXP3 complex. We found that DBC1 interacts physically with FOXP3, and DBC1 depletion maintains FOXP3 expression and Treg cells’ suppressive function during inflammatory insult. Moreover, DBC1-deficient mice showed alleviated clinical symptoms in both experimental autoimmune encephalomyelitis (EAE) and colitis models and had reduced production of IL-17a. Dbc1-deficient mice displayed increased Foxp3+ cell frequency, numbers, and superior suppressive activity. Inhibition of caspase 8 activity rescued FOXP3 expression during TNF-α treatment, indicating that DBC1 negatively regulates FOXP3 and Treg-cell function, probably through the caspase 8-mediated pathway. Therapeutic targeting of this DBC1 pathway in Treg cells thus may provide a novel approach for treating autoimmune and inflammatory diseases, especially during acute inflammation.
Results
Identification of DBC1 as a Previously Unidentified Subunit of the FOXP3 Complex.
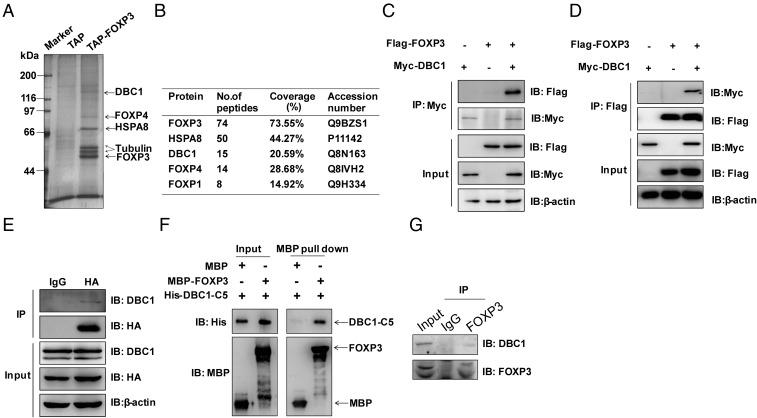
To purify previously unidentified FOXP3-interacting partners, a tandem affinity purification (TAP) tag, consisting of 2× Protein A and calmodulin-binding protein (CBP) linked by a tobacco etch virus (TEV) cleavage site was fused to the N terminus of FOXP3 (Fig. S1A), followed by lentiviral transduction into the human Jurkat T-cell line. We detected FOXP3 expression and its nuclear localization by Western blotting and immunofluorescence, respectively (Fig. S1 B and C). The results showed that TAP-tagged FOXP3 localized in the nucleus, as did endogenous FOXP3 in natural regulatory (nTreg) T cells (the GFP signal indicated that Jurkat cells had been transduced with the indicated gene). The FOXP3 complex was purified by TAP followed by mass spectrometry analysis; FOXP3 was the most abundant peptide (Fig. 1 A and B). FOXP1, a known binding partner of FOXP3 (14), also was detected using this protocol. Interestingly, we identified DBC1 as a major human FOXP3 complex-associated protein (Fig. 1 A and B).
Fig. 1.
FOXP3 interacts with DBC1. (A) The products from tandem affinity purification of Jurkat T cells stably expressing TAP or TAP-tagged FOXP3 were separated by SDS/PAGE and visualized by silver staining. (B) Identification of the proteins present in the FOXP3 complex by mass spectrometry. (C and D) Reciprocal immunoprecipitation of DBC1 and FOXP3. Flag-tagged FOXP3 and Myc-tagged DBC1 were cotransfected into HEK293T cells. Immunoprecipitation was performed with anti-Myc or anti-Flag antibodies plus Protein A/G beads. Protein blots were probed with antibodies as indicated. (E) Endogenous DBC1 interacts with FOXP3 in Jurkat cells stably expressing HA-tagged FOXP3. Interaction between overexpressed HA-tagged FOXP3 and endogenous DBC1 was performed by coimmunoprecipitation. Protein blots were probed with antibodies as indicated. (F) Direct interaction of His-DBC1-C5 and MBP-FOXP3 in an in vitro MBP pull-down assay detected by Western blotting. (G) Endogenous DBC1 and FOXP3 interact in primary human Treg cells. Immunoprecipitation was performed with anti-FOXP3 antibody or IgG plus Protein A/G beads. Protein blots were probed with antibodies as indicated.
We confirmed the interaction between FOXP3 and DBC1 by reciprocal coimmunoprecipitation of overexpressed FLAG-tagged FOXP3 and Myc-tagged DBC1 in HEK293T cells (Fig. 1 C and D). Consistent with the TAP result, we found that ectopically expressed DBC1 and FOXP3 could interact with each other. Furthermore, the interaction between HA-tagged FOXP3 and endogenous DBC1 was readily detected in Jurkat cells (Fig. 1E). To define the interaction domain between DBC1 and FOXP3, we generated truncated mutants of FOXP3 (Fig. S2A); an extra tag containing the 3× nuclear localization site (NLS) of SV40 large T antigen was added to the N terminus of the FOXP3 mutants that lacked the Forkhead domain to ensure their localization in the nucleus. The interaction between FOXP3 and DBC1 was abolished in the FOXP3 mutants (FOXP3-N2, -N3, and -N4) that lacked the linker region between the Leucine-Zipper and Forkhead domains (Fig. S2B). Similarly, we generated deletion mutants of DBC1 that maintained their own intact N-terminal NLS (Fig. S2C). We found that the deletion of the N-terminal 200 residues of DBC1 abolished the interaction between DBC1 and FOXP3 and that the C5 mutant that retained the 200 N-terminal residues of DBC1 was able to interact with FOXP3 (Fig. S2D). To test if DBC1 interacts directly with FOXP3, we performed the pull-down assay using purified maltose-binding protein (MBP)-tagged FOXP3 and His-tagged DBC1-C5 from Escherichia coli. Our results showed that FOXP3 could precipitate the DBC1-C5 mutant efficiently in vitro (Fig. 1F). Moreover, we sorted primary human Treg cells (CD4+CD25hiCD127lo) from human peripheral blood mononuclear cells (PBMCs) and observed by immunofluorescence that DBC1 colocalized with FOXP3 in the nucleus of primary human Treg cells (Fig. S3). Indeed, an anti-FOXP3 antibody coprecipitated endogenous FOXP3 with DBC1 from Treg cells expanded in vitro (Fig. 1G). Taken together, these results demonstrated that FOXP3 and DBC1 interact physically through the linker region of FOXP3 and the 200 N-terminal residues of DBC1. Previous studies have shown that DBC1 is a major inhibitor of SIRT1 in vitro (21) and in vivo (31), and DBC1 may play an important role in TNF-α–mediated apoptosis (32). However, it is unclear how DBC1 functions in Treg cells.
Analysis of Foxp3 Expression and Function in Dbc1-Deficient Treg Cells.
To characterize the role of Dbc1 in Treg-cell differentiation and homeostasis, we analyzed Foxp3 expression in WT and Dbc1-deficient mice. The spleens, peripheral lymph nodes, and mesenteric lymph nodes from Dbc1−/− mice were smaller than those from Dbc1+/+mice (Fig. S4A). The frequencies of CD4+CD25+Foxp3+ T cells in the thymi, lymph nodes, spleens, and blood also were examined by flow cytometry. We found that CD4+CD25+Foxp3+ T-cell percentages were increased significantly in the lymph nodes and spleens of Dbc1−/− mice compared with littermate WT controls, although the frequencies of these cells were similar in the thymi and peripheral blood of the two groups (Fig. S4 B and C). This result is not surprising, given that the total numbers of CD4+Foxp3+ Treg cells were increased significantly in the lymph nodes and spleens of Dbc1−/− mice as compared with WT mice (Fig. S4D).
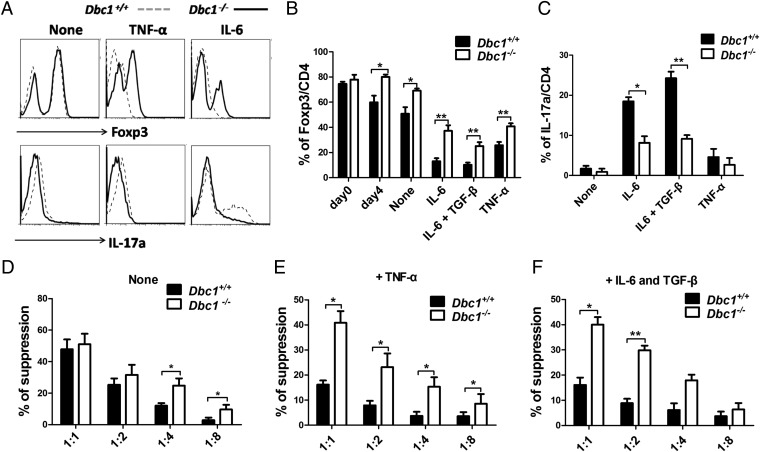
DBC1 undergoes caspase-dependent processing during TNF-α–induced cell death, during which the C-terminal region of DBC1 translocates to the mitochondria to augment apoptosis (32). The expression of the inflammatory cytokines TNF-α and IL-6 was reduced in Dbc1-deficient mice fed a high-fat diet (31), suggesting that DBC1 is involved in inflammation. Although the proinflammatory cytokines TNF-α and IL-6 have been shown to regulate Treg-mediated suppression negatively, the molecular mechanism underlying this observation remains unclear (33). Therefore, we investigated whether Dbc1 functions in controlling Foxp3 levels under stimulatory conditions. Indeed, we found that Dbc1+/+ Treg cells lost their Foxp3 expression dramatically after TNF-α treatment, but Dbc1−/− Treg cells maintained more stable Foxp3 expression (Fig. 2 A and B). Moreover, we tested whether Dbc1 could regulate Foxp3 stability in the presence of other proinflammatory cytokines. We found that Dbc1−/− Treg cells had more stable Foxp3 expression (Fig. 2 A and B) and produced less IL-17a during treatment with IL-6 or with IL-6 plus TGF-β (Fig. 2C). Taken together, these results suggest that Dbc1 may induce Foxp3 protein instability in Treg cells upon inflammatory insult.
Fig. 2.
Foxp3 from Dbc1−/− mice is more stable than Foxp3 from WT mice, and Treg cells from Dbc1−/− mice are more suppressive than Treg cells from WT mice, especially during inflammatory insult. (A) Treg cells isolated from thymi of C57BL/6 Foxp3-GFP mice were expanded for 4 d and were recultured in the presence of APCs with soluble anti-CD3 (1 μg/mL), anti-CD28 (1 μg/mL), anti–IL-4 (5 μg/mL), and anti–IFN-γ (5 μg/mL), with or without recombinant human (rh)-TGF-β (5 ng/mL), recombinant mouse (rm)-IL-6 (10 ng/mL), or rm-TNF-α (50 ng/mL). After 3 d these cells were harvested and stained for intracellular expression of Foxp3 and IL-17a. (B) The statistical percentages of Foxp3 expression among the different groups as indicated. (C) The statistical percentages of IL-17a expression among the different groups as indicated. “None” indicates no rm-TNF-α or rm-IL-6 treatment. (D–F) Suppressive activity of Treg cells from Dbc1−/− and Dbc1+/+ mice. Treg cells from Dbc1−/− and Dbc1+/+ mice without treatment (“None”) (D), pretreated with 50 ng/mL rm-TNF-α (E), or treated with 10 ng/mL rm-IL-6 and 5 ng/mL rh-TGF-β (F) were tested using an in vitro suppressive activity by indirectly measuring the proliferative rates of anti-CD3–activated T-responder cells (B6) labeled with CFSE. Cells were harvested on day 3 and stained with PE-conjugated anti-CD8 antibody. Samples were analyzed by flow cytometry and gated on the CD8+ population. The x axis shows the ratio of Treg cells to responder T cells. All data are representative of three independent experiments.
Next, we tested whether Dbc1 deficiency affects the suppressive function of Treg cells. Under normal conditions, CD4+CD25+ Treg cells from Dbc1−/− mice were more suppressive than those from Dbc1+/+mice (Fig. 2D); however, the suppressive function of TNF-α–treated CD4+CD25+ Treg cells from Dbc1−/− mice was significantly superior to that of Treg cells from Dbc1+/+mice (Fig. 2E). Similarly, Dbc1−/− Treg cells showed greater suppressive capacity than Dbc1+/+ Treg cells when the suppression assays were supplemented with IL-6 and TGF-β (Fig. 2F). Collectively, Dbc1 depletion not only stabilizes Foxp3 but also enhances the suppressive function of Treg cells, most noticeably during inflammatory stimuli.
DBC1 Deficiency Leads to the Functional Stability of Treg Cells During the Development of EAE and Colitis.
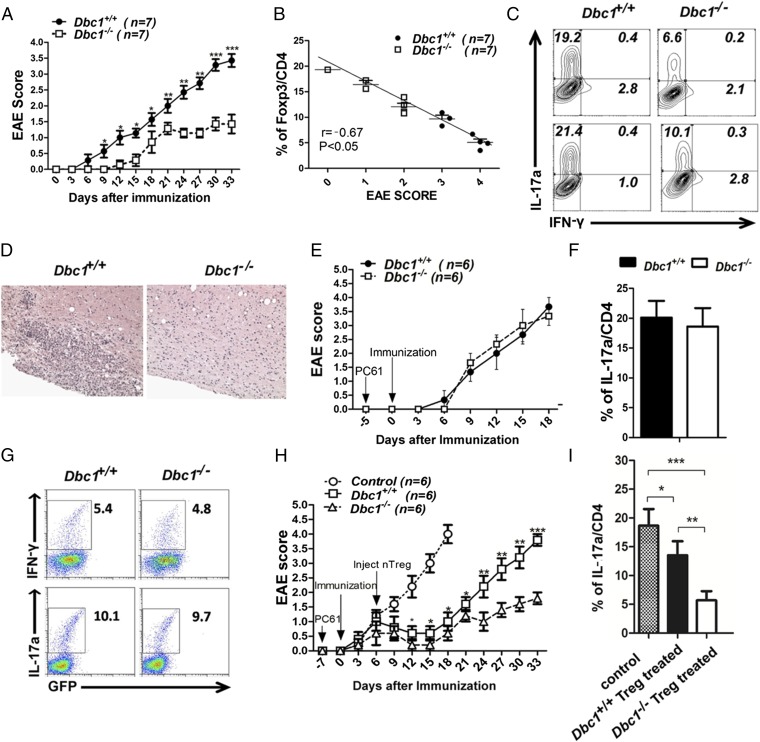
To determine the physiological relevance of DBC1 in Foxp3 stability during the course of an immune response in vivo, we also tested the effect of Dbc1 depletion in Foxp3-GFP mice induced with EAE. We found that Dbc1+/+ mice developed typical EAE, but in Dbc1−/− mice the onset of EAE was significantly delayed, and its severity was significantly reduced (Fig. 3 A and B). Consistent with reduced EAE severity, CD4+ cells from Dbc1−/− mice produced less IL-17a than CD4+ cells from Dbc1+/+mice (Fig. 3C). Histological analysis of the spinal cords also indicated that the infiltration of immune cells was restricted in Dbc1−/− mice (Fig. 3D).
Fig. 3.
Dbc1−/− mice develop less severe autoimmune disease symptoms during EAE induction. (A) EAE clinical scores for 8- to10-wk-old male Dbc1+/+ and Dbc1−/− mice (n = 7 in each group) were calculated on the indicated days after immunization with MOG35-55. (B) The statistical relationship between the percentage of Foxp3+ cells in the CD4+ population and EAE scores after EAE induction. (C) Representative flow cytometry data for IL-17a and IFN-γ expression in spleens and draining lymph nodes in each group. Mice were euthanized on day 33, and fresh cells taken from spleens were stained for Foxp3, IL-17a, and IFN-γ. (D) Representative microphotographs of spinal cord sections from mice in the Dbc1+/+ (n = 4) or Dbc1−/− (n = 1) group. (Original magnification: 200×.) Data are pooled from seven independent experiments. (E) EAE was induced in 8- to10-wk-old Dbc1+/+ and Dbc1−/− Foxp3-GFP mice (n = 4 mice in each group) following immunization with MOG35-55 as previously described. PC61, an anti-CD25 antibody, was injected 5 d before EAE induction. The curve shows the EAE clinical scores calculated in the different groups. (F and G) IL-17a and IFN-γ detected in E. The percentage of IL-17a+ cells in the CD4+ population in E (F) and the percentages of IL-17a+ and IFN-γ+ cells in E were analyzed by flow cytometry (G). (H) EAE was induced in B6 WT mice with MOG35-55. PC61 was injected to deplete Treg cells 7 d before EAE induction. Treg cells (2 × 106 for each mouse) from Dbc1+/+ or Dbc1−/− mice were injected into these mice via the tail vein 6 d after MOG35-55 immunization. PBS was injected as a control. EAE clinical scores were calculated at the indicated days after immunization. (I) The percentage of IL-17a+ cells in the CD4+ population in spinal cord analyzed by flow cytometry in H. IL-17a expression was detected 18 d after EAE induction in the control group and 33 d after EAE induction in the Dbc1+/+ and Dbc1−/− groups. *P < 0.05, **P < 0.01, ***P < 0.001.
To investigate whether the mitigation of EAE symptoms in Dbc1−/− mice was caused by the enhanced suppressive function of Treg cells, an anti-CD25 antibody isolated from the clone PC61 was used to deplete Treg cells before EAE induction. The loss of GFP indicated that Treg cells had been depleted in Foxp3-GFP mice after PC61 treatment (Fig. 3G), and this loss can last nearly 1 month. After Treg-cell depletion, Dbc1+/+ and Dbc1−/− mice developed EAE with similar severity (Fig. 3E) and produced comparable amounts of IL-17a and IFN-γ (Fig. 3 F and G). This result does not simply reflect the increased frequency of Treg cells in Dbc1-deficient mice, because Treg cells transferred from Dbc1-deficient mice controlled EAE development more effectively than similar doses of Treg cells from WT mice (Fig. 3H) and produced less IL-17a (Fig. 3I). Taken together, these results indicate that Dbc1 deficiency attenuates EAE progression by enhancing Treg cells’ stability and suppressive function and thus could provide clinical benefit in treating autoimmune diseases.
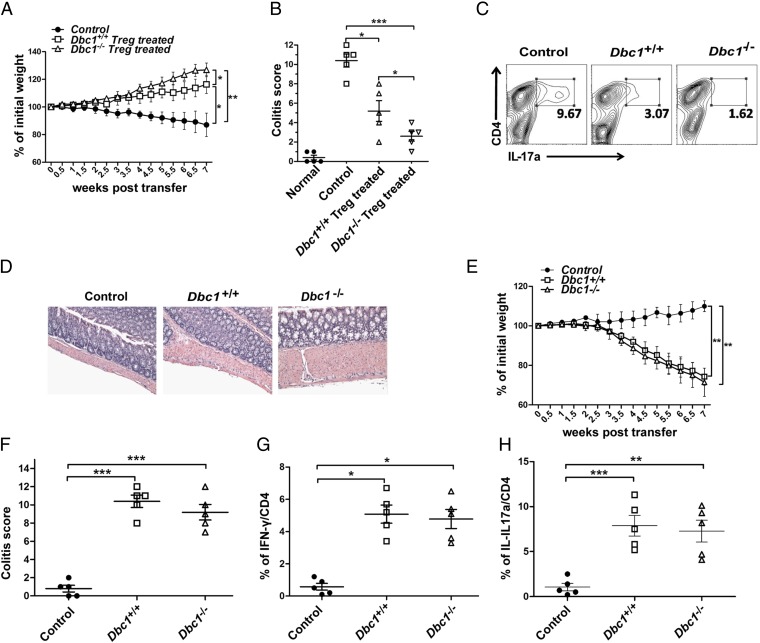
To delineate further the role of Dbc1 specifically in Treg cells in vivo, we carried out an adoptive transfer of WT CD4+CD45RBhi cells (colitogenic cells) into Rag2−/− mice to induce colitis (33) with or without the cotransfer of Treg cells from Dbc1+/+or Dbc1−/− mice. Although the cotransfer of Treg cells from Dbc1+/+mice was able to suppress colitis, we found that the Treg cells isolated from Dbc1−/−mice had superior therapeutic effects (Fig. 4 A and B). Although the cotransfer of Dbc1+/+ Treg cells significantly suppressed the generation of IL-17a+ T cells in the colitis model, IL-17a+ T cells were almost undetectable when Dbc1−/− Treg cells were used (Fig. 4C). Similarly, Dbc1−/− Treg cells provided more potent suppression of mucosal inflammation than Dbc1+/+ Treg cells (Fig. 4D). To characterize further the specific role of Treg cells from Dbc1−/− mice in the colitis model, we transferred CD4+CD45RBhi cells from Dbc1+/+ and Dbc1−/− mice into Rag2−/− mice to induce colitis, using PBS as control. Dbc1+/+ and Dbc1−/− CD4+CD45RBhi cells had similar capacity to induce colitis (Fig. 4 E and F) and produced comparable amounts of IL-17a and IFN-γ (Fig. 4 G and H). Combined with the data from adoptive transfer experiments produced in immunocompetent mice (Fig. 3H), these results further indicate that DBC1 deficiency specifically enhances the suppressive activity of Treg cells under conditions of inflammation in vivo.
Fig. 4.
Dbc1−/− Treg cells function profoundly in preventing colitis. (A) The changes in weight in the different groups in the colitis model. Treg cells (5 × 105) from the indicated mice were expanded for 4 d and then were cotransferred i.p. with 3 × 105 syngeneic CD4+CD45RBhi T cells into 8- to 10-wk-old Rag2−/− mice (n = 6 mice in each group). Rag2−/− mice receiving only CD4+CD45RBhi T cells were used as a control. Body weights were calculated twice a week. (B) Colitis scores were calculated according to pathological anatomy and histological expression in the different groups. Each data point represents an individual mouse. (C) The percentages of cells expressing IL-17a in the CD4+ population. Fresh cells taken from spleens of mice in the Dbc1−/−, Dbc1+/+, and control groups were stimulated with phorbol12-myristate13-acetate (PMA) and Ionomycin for 1 h and with brefeldin A (BFA) for 4 h (5 h total), followed by staining for IL-17a. (D) Representative photomicrographs of colonic sections from control recipients (n = 11) or recipients that received Dbc1+/+ (n = 7) or Dbc1−/− (n = 3) Treg cells. Data from six independent experiments were pooled. (Original magnification: 200×.) (E) The changes in weight in the different groups in the colitis model. Syngeneic CD4+CD45RBhi T cells (3 × 105) from Dbc1+/+ and Dbc1−/− mice were transplanted i.p. into 8- to 10-wk-old Rag2−/− mice (n = 4 mice in each group). Rag2−/− mice injected with PBS were used as the control group. Body weights were calculated twice a week. (F) Colitis scores were calculated according to the pathological anatomy and histological expression in the different groups. Each data point represents an individual mouse. (G and H) Mesenteric lymph nodes were isolated and stimulated with PMA plus Ionomycin and BFA. Cells were stained for IFN-γ/IL-17a/CD4 and then were analyzed by flow cytometry. *P < 0.05; **P < 0.02; ***P < 0.01.
Caspase 8-Mediated Degradation of FOXP3 by TNF-α.
Next, we investigated how DBC1 functions in controlling FOXP3 levels under stimulatory conditions. To test the role of TNF-α in FOXP3 protein stability rather than transcriptional regulation, we generated Jurkat cells stably expressing HA-tagged FOXP3 [Jurkat (HA-FOXP3) cells] in which FOXP3 expression is driven by a ubiquitin promoter. We treated Jurkat (HA-FOXP3) cells with TNF-α with and without the knockdown of DBC1 using shRNA (shDBC1) (Fig. S5 A and B) and found that both shRNAs (shDBC1-1 and shDBC1-2) targeting DBC1 prevented FOXP3 degradation after TNF-α treatment (Fig. S5C). The cleavage of poly(ADP-ribose) polymerase (PARP) indicated caspase activation by TNF-α (34). To understand further the kinetics and dynamic loss of FOXP3 protein after TNF-α treatment, we removed TNF-α by washing cells with fresh culture medium 12 h after TNF-α treatment, followed by collection at the indicated time points to check FOXP3 expression for immunoblotting. We found that TNF-α removal could rescue FOXP3 expression, indicating that this process is reversible (Fig. S5D). We performed similar experiments using human primary Treg cells, and results were consistent (Fig. 5 B and C). As shown in Fig. 5B, FOXP3 expression decreased markedly following TCR stimulation in the presence of IL-1β and IL-6 or TNF-α; however, when DBC1 expression was knocked down in human Treg cells, FOXP3 expression was largely sustained following TCR and TNF-α stimulation. Collectively, our results suggest that FOXP3 becomes unstable after TNF-α treatment in a DBC1-dependent manner; the effect of TNF-α is reversible; and the regulation of FOXP3 expression is dynamic at the protein level.
Fig. 5.
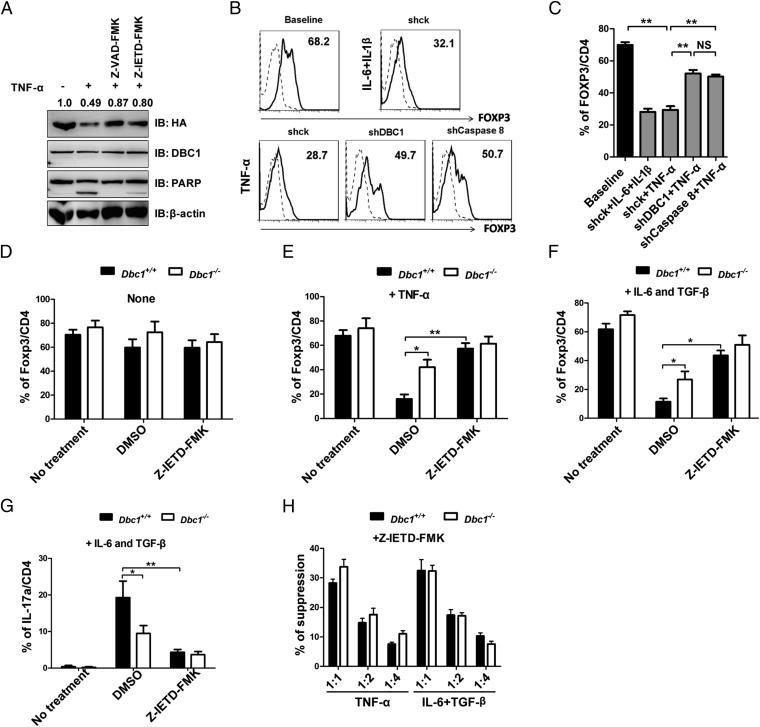
Caspase 8 mediates FOXP3 degradation during TNF-α stimulation. (A) Jurkat (HA-FOXP3) cells were stimulated with 5 ng/mL rh-TNF-α for 12 h. The caspase inhibitors Z-VAD-FMK (for pan-caspase) and Z-IETD-FMK (for caspase 8) (10 μM) were used as pretreatment for 1 h. Protein blots were probed with antibodies as indicated. (B) Human Treg cells expanded in vitro for 12 d were infected with lentivirus expressing pLKO.1-shCK-GFP, shDBC1-GFP, or shCaspase 8-GFP. Three days after infection, cells were treated with TCR or with TCR, rh-IL-6 (20 ng/mL) and rh-IL-1β (20 ng/mL) or with TCR and rh-TNF-α (100 ng/mL) for another 3 d. Then FOXP3 expression in the GFP+ population was analyzed by flow cytometry. Dashed lines represent Teff cells, and black lines represent Treg cells. (C) The percentage of FOXP3+ cells in CD4+ cells in the GFP+ population from B was analyzed by flow cytometry. (D) Treg cells obtained from Dbc1+/+ and Dbc1−/− mice were untreated (No treatment) or were pretreated with DMSO or the caspase 8 inhibitor Z-IETD-FMK for 1 h and then were cocultured with rh-IL-2 (200 U/mL) and anti-CD3/CD28 microbeads (1:2 ratio) for 4 d. Cells were harvested, stained for Foxp3/CD25/CD4, and analyzed by flow cytometry. (E) Treg cells were treated and cultured as described in D. Additional rm-TNF-α (100 ng/mL) was added in the culture system. Cells were harvested and stained for Foxp3/CD25/CD4 and analyzed by flow cytometry. (F and G) Treg cells were pretreated and cultured as described in D. rm-IL-6 (10 ng/mL) and rh-TGF-β (5 ng/mL) were added to the culture. Cells were harvested and stained for Foxp3/CD25/CD4 (F) or IL-17a/CD4 (G) and were analyzed by flow cytometry. “No treatment,” without treatment of inflammatory cytokines and inhibitors. (H) Suppressive activity of Treg cells from Dbc1−/− and Dbc1+/+ mice. Treg cells isolated from Dbc1−/− and Dbc1+/+ mice were pretreated with Z-IETD-FMK for 1 h and then were pretreated with 50 ng/mL rm-TNF-α or were treated with 10 ng/mL rm-IL-6 and 5 ng/mL rh-TGF-β. Treg cells’ suppressive function was tested as described in Methods. The x axis shows the ratio of Treg cells to responder T cells. All data are representative of three independent experiments.
To investigate the mechanisms underlying FOXP3 degradation by TNF-α, we treated cells with various inhibitors of different components of the protein-degradation machinery and then analyzed FOXP3 expression by immunoblotting. We found that the pan-caspase inhibitor Z-VAD-FMK could rescue the degradation of FOXP3, but the protein synthesis inhibitor cycloheximide (CHX), the proteasome inhibitor MG132, and the lysosomal enzyme inhibitor NH4Cl could not (Fig. S5E). We then tested different caspase-specific inhibitors to pinpoint further the specific caspase responsible for FOXP3 degradation. We found that only the caspase 8 inhibitor Z-IETD-FMK significantly prevented the degradation of FOXP3 (Fig. 5A and Fig. S5F). We further knocked down caspase 8 in human primary nTreg cells and observed that the reduction of caspase 8 in human nTreg cells significantly prevents FOXP3 degradation (Fig. 5 B and C). These data indicate that the degradation of FOXP3 upon TNF-α treatment is mediated by the activation of caspase 8.
Next, we tested the effects of Z-IETD-FMK (a caspase 8 inhibitor) on the expression of Foxp3 protein and on the suppressive function of Treg cells subjected to inflammatory treatment in mouse Treg cells. We found that Z-IETD-FMK alone did not have any effect on Foxp3 protein expression in Treg cells in steady-state cultures (Fig. 5D). However, although Treg cells from Dbc1+/+ mice lost Foxp3 protein expression during TNF-α stimulation, Foxp3 levels were rescued upon treatment with caspase 8 inhibitors at levels similar to those observed in Dbc1−/− Treg cells (Fig. 5E). Z-IETD-FMK–treated Dbc1+/+ Treg cells also restored Foxp3 expression when subjected to Th17-skewing conditions and produced less IL-17a (Fig. 5 F and G). Furthermore, we tested the suppressive function of Treg cells pretreated with Z-IETD-FMK under inflammatory conditions. We found that Z-IETD-FMK also could rescue Dbc1+/+ Treg cells’ suppressive function to the levels similar to those of Dbc1−/− Treg cells (Fig. 5H). Taken together, these findings show that the caspase 8 might play an important role in Dbc1-mediated Foxp3 degradation and suppressive function in Treg cells under inflammatory conditions.
Discussion
Foxp3 and its partners orchestrate the dynamic regulation of Treg-cell function to maintain immune homeostasis. However, research on the regulatory mechanisms of the FOXP3 complex under the influence of different environment cues has been lacking. Here we identified DBC1 as a previously unidentified subunit of the human FOXP3 complex by TAP in T cells. Moreover, we assessed the role of DBC1 in regulating FOXP3 expression and Treg-cell function under different inflammatory conditions and established a critical role for DBC1 in the course of the inflammatory immune response in vivo. We showed that DBC1 could interact with FOXP3 physically. Functionally, DBC1 significantly down-modulates FOXP3 expression and Treg-cell function under inflammatory cytokine stimuli including TNF-α, IL-6, or IL-6 plus TGF-β treatment as well as in disease models. The onset of EAE was delayed, and its severity was decreased in Dbc1−/− mice, an effect that was Treg-cell dependent, as shown by the depletion of Treg cells using anti-CD25 antibody (PC61). In addition, Dbc1 deficiency also partially protected mice from EAE. The role of Dbc1 may relate to Treg cells’ stability and function. Dbc1 does not affect the development of nTreg cells, because the frequency and total number of thymic Foxp3+ cells are similar in Dbc1−/− and WT mice. Conversely, Dbc1 mainly regulates the stability and function of Treg cells in the periphery. Adoptive transfer experiments using immunodeficient and immunocompetent mice revealed that the functional activity of Dbc1−/− Treg cells is strengthened in inflammatory diseases, providing the further evidence that Dbc1 modulates the functionality of Treg cells in inflammatory disease states. Furthermore, we also show that caspase 8 is involved in DBC1-mediated FOXP3 degradation under inflammatory conditions.
Accumulating evidence supports the notion that Treg cells harbor plasticity by sensing microenvironmental factors (35–39). Under inflammatory conditions, Treg cells down-modulate FOXP3 protein stability or Treg cells’ suppressive activity to facilitate pathogen clearance (24–26). The effect of the proinflammatory cytokine TNF-α on Treg cells remains controversial (28, 29, 40–42). Grinberg-Bleyer et al. (40) showed that TNF-α produced by T effector (Teff) cells boost Treg cells’ expansion, whereas Valencia et al. (28) reported that TNF-α down-modulated FOXP3 mRNA, FOXP3 protein, and their suppressive function. Recently, Nie et al (29) found that TNF-α induced protein phosphatase 1 and reduced phosphorylation of Ser418 on FOXP3, leading to impaired Treg-cell function. In our experimental system, Treg cells lost their Foxp3 expression and suppressive function after TNF-α stimulation. Although Dbc1−/− Treg cells harbored slightly higher Foxp3 expression and suppressive function at steady state, the differences were much more pronounced upon TNF-α treatment, in which WT Treg cells significantly lost Foxp3 expression and suppressive function. Dbc1−/− Treg cells also maintained Foxp3 expression and suppressive function after treatment with IL-6 or IL-6 plus TGF-β. Consistent with the high expression of Foxp3 in Dbc1−/− Treg cells under inflammatory conditions, Dbc1−/− CD4+ T cells produced less IL-17a. Our EAE and colitis models together confirmed that the effect of Dbc1 is Treg-cell specific and that Dbc1 deficiency could ameliorate EAE symptoms and mitigate mucosal inflammation in vivo. We also observed that Dbc1−/− Treg cells lost Foxp3 protein and suppressive function slightly after TNF-α, IL-6, or IL-6 plus TGF-β treatment, suggesting that DBC1 may not be the only factor that affects Foxp3 stability under inflammatory conditions.
To explore further the mechanisms of DBC1-mediated down-regulation of Treg-cell function, we found that the caspase 8 inhibitor Z-IETD-FMK could rescue FOXP3 degradation and Treg cells’ suppressive function under inflammatory conditions. This result indicates that the caspase 8-mediated pathway is responsible for FOXP3 degradation and Treg-cell function. This finding was validated further through a knockdown method to down-regulate the level of caspase 8 in human nTreg cells. Although TNF-α could trigger cell apoptosis, we found that Ac-DEVD-CHO and Z-LEHD-FMK (inhibitors of caspases 3/7 and 9, respectively) inhibited apoptosis but did not reverse FOXP3 degradation, suggesting that FOXP3 protein reduction is not caused solely by the cell apoptosis induced by TNF-α. However, the molecular mechanisms by which DBC1 affects the caspase 8-mediated pathway to regulate FOXP3 stability and Treg-cell function require further investigation.
In summary, our study has shown that DBC1 mediates the reduction of FOXP3 expression in Treg cells in the presence of inflammatory stimuli. Our data also unveiled a caspase 8-dependent FOXP3 destabilization pathway that potentially could be targeted to modulate Treg-cell function during inflammation. These results therefore may provide important applications as therapy in autoimmune diseases where overexuberant and destructive inflammatory responses require dampening.
Methods
Mice.
C57BL/6 (B6) and C57BL/6-Foxp3-GFP (B6 GFP+) mice were purchased from Jackson Laboratory. C57BL/6-Dbc1−/−mice were generated at the Mayo Clinic (31). Dbc1−/− mice and Dbc1−/−Foxp3-GFP mice were obtained by crossing Dbc1+/− with Dbc1+/− in B6 or B6 GFP+ mice. All mice were maintained in a single specific pathogen-free room in the animal facility at the Penn State Hershey College of Medicine. Sex-matched 6- to 12-wk-old mice were used in all experiments. All animals were treated according to National Institutes of Health guidelines for the use of experimental animals (43) with the approval of the Penn State Hershey College of Medicine Institutional Animal Care and Use Committee.
In Vitro Cell Proliferation and Suppression Assay.
Naive CD4+ T cells (CD4+CD25−CD62L+) were purified by positive selection using CD4-specific MACS beads (Miltenyi Biotec). Treg cells were sorted from the thymi and were expanded using anti-CD3/CD28–coated beads and recombinant human (rh)-IL-2 for the number of days indicated. For in vitro suppression assays, 3 × 105 CD4+CD25− responder T cells isolated from C57BL/6 mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and were mixed with varying amounts of Treg cells as indicated. Cell mixtures were cocultured in the presence of 3 × 105 irradiated antigen-presenting cells (APCs) and soluble anti-CD3 mAb (0.025 μg/mL) in RPMI 1640 culture medium for 3 d. Cells were harvested at day 3, stained for phycoerythrin (PE)-conjugated anti-CD8, and analyzed on an LSRII flow cytometry system.
Treg-Cell Conversion Experiment.
Treg cells expanded for 4 d were recultured in the presence of APCs with soluble anti-CD3 (1 μg/mL), anti-CD28 (1 μg/mL), anti–IL-4 (5 μg/mL), and anti–IFN-γ (5 μg/mL), with or without rh-TGF-β (5 ng/mL), recombinant mouse (rm)-IL-6 (10 ng/mL), or rm-TNF-α (50 ng/mL). These cells were harvested after 3 d and were stained for intracellular expression of Foxp3 and IL-17a.
Induction of EAE.
C57BL/6 WT and Dbc1−/− mice were injected s.c. with 100 mg of an emulsion of MOG35-55 peptide (CPC Scientific) in complete Freund’s adjuvant in the hind flank on day 0 and day 7. Pertussis toxin (List Biological Laboratories) at a dose of 250 μg per mouse was injected i.v. on days 0, 2, 7, and 9. Disease severity was evaluated on a 0–5 scale as previously described; the observers scoring the mice were blinded to the experiments.
Colitis Model.
Treg cells were obtained from C57BL/6 WT and Dbc1−/− mice as described above. Syngeneic CD4+CD45RBhi T cells (3 × 105) were coinjected i.p. with or without 5 × 105 Treg cells into Rag2−/− mice. Mice developed clinical signs of colitis within 3.5–4.5 wk (wk 4) after transfer. Mice were monitored daily and weighed twice a week. Any mice showing clinical signs of severe disease were killed according to animal regulation standards.
Human nTreg Cells.
Human FOXP3+CD4+CD25+ T cells were sorted from PBMCs on the gate of CD4+, CD25+, and CD127low. These cells were stimulated with anti-CD3/anti-CD28–coated beads (in a ratio of three beads to one cell) in the presence of 300 U/mL rh-IL-2 for 1–2 wk. These cells were harvested and infected with lentivirus expressing pLKO.1-shCK-GFP (control), pLKO.1-shDBC1-GFP, or pLKO.1-shCaspase 8-GFP for 3 d. These cells were harvested and restimulated with TCR or with TCR and rh-IL-6/IL-1β, or with TCR and rh-TNF-α for 3 d. FOXP3 expression was determined by flow cytometry assay. The informed consent for all human experiments were approved by the Institutional Review Boards of Penn State University and Institut Pasteur of Shanghai. Informed consent was obtained from recruited healthy volunteer.
Statistical Analysis.
Analysis of statistically significant differences between the groups of mice was performed by the Student’s t test and Wilcoxon test survival curves with the log rank test using GraphPad PRISM software (GraphPad). Data are presented as the mean ± SEM. Differences were considered significant when P values were <0.05.
Supplementary Material
Acknowledgments
We thank K. Lan for providing the FUGW (flap-Ub promoter-GFP-WRE), del8.9, and vesicular stomatitis virus G constructs. This work was supported by National Basic Research Program of China Grants 2014CB541803 and 2014CB541903; NIH Grants AR059103 and AI084359; National Natural Science Foundation of China Grants 31170825, 31200646, 31150110337, and 30972702; the American College of Rheumatology Within Our Reach Fund; the Arthritis Foundation; the Wright Foundation; the Novo Nordisk-Chinese Academy of Sciences Foundation; and National Science and Technology Major Project Grants 2012ZX10002007-003 and 2013ZX10003009-002. B.L. is a recipient of the Shanghai “Rising Star” Program 10QA1407900 and Chinese Academy of Sciences “100-Talent” Program. S.G.Z. is a recipient of a Tina C Foundation Lupus Research Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421463112/-/DCSupplemental.
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler SF. FOXP3: Of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavin MA, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103(17):6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37(1):129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 10.Fu W, et al. A multiply redundant genetic switch ‘locks in’ the transcriptional signature of regulatory T cells. Nat Immunol. 2012;13(10):972–980. doi: 10.1038/ni.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudra D, et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol. 2012;13(10):1010–1019. doi: 10.1038/ni.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38(3):414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Hori S. The Foxp3 interactome: A network perspective of T(reg) cells. Nat Immunol. 2012;13(10):943–945. doi: 10.1038/ni.2424. [DOI] [PubMed] [Google Scholar]
- 14.Li B, et al. FOXP3 is a homo-oligomer and a component of a supramolecular regulatory complex disabled in the human XLAAD/IPEX autoimmune disease. Int Immunol. 2007;19(7):825–835. doi: 10.1093/intimm/dxm043. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35(3):337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du J, Huang C, Zhou B, Ziegler SF. Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J Immunol. 2008;180(7):4785–4792. doi: 10.4049/jimmunol.180.7.4785. [DOI] [PubMed] [Google Scholar]
- 17.Pan F, et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325(5944):1142–1146. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono M, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446(7136):685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 19.Li B, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci USA. 2007;104(11):4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Loosdregt J, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115(5):965–974. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- 21.van Loosdregt J, et al. Rapid temporal control of Foxp3 protein degradation by sirtuin-1. PLoS ONE. 2011;6(4):e19047. doi: 10.1371/journal.pone.0019047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: Differentiation, specification, subphenotypes. Nat Immunol. 2009;10(7):689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 23.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Lu L, et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci USA. 2014;111(33):E3432–E3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity. 2013;39(2):272–285. doi: 10.1016/j.immuni.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, et al. PIM1 kinase phosphorylates the human transcription factor FOXP3 at serine 422 to negatively regulate its activity under inflammation. J Biol Chem. 2014;289(39):26872–26881. doi: 10.1074/jbc.M114.586651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valencia X, et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108(1):253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nie H, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19(3):322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 30.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329(5999):1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escande C, et al. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120(2):545–558. doi: 10.1172/JCI39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundararajan R, Chen G, Mukherjee C, White E. Caspase-dependent processing activates the proapoptotic activity of deleted in breast cancer-1 during tumor necrosis factor-alpha-mediated death signaling. Oncogene. 2005;24(31):4908–4920. doi: 10.1038/sj.onc.1208681. [DOI] [PubMed] [Google Scholar]
- 33.Lan Q, et al. Polyclonal CD4+Foxp3+ Treg cells induce TGFbeta-dependent tolerogenic dendritic cells that suppress murine lupus-like syndrome. J Mol Cell Biol. 2012;4(6):409–419. doi: 10.1093/jmcb/mjs040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abraham MC, Shaham S. Death without caspases, caspases without death. Trends Cell Biol. 2004;14(4):184–193. doi: 10.1016/j.tcb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Gao Y, et al. Molecular mechanisms underlying the regulation and functional plasticity of FOXP3(+) regulatory T cells. Genes Immun. 2012;13(1):1–13. doi: 10.1038/gene.2011.77. [DOI] [PubMed] [Google Scholar]
- 36.Koenen HJ, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112(6):2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 37.Miyao T, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36(2):262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31(5):772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wohlfert E, Belkaid Y. Plasticity of T reg at infected sites. Mucosal Immunol. 2010;3(3):213–215. doi: 10.1038/mi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grinberg-Bleyer Y, et al. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J Clin Invest. 2010;120(12):4558–4568. doi: 10.1172/JCI42945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Housley WJ, et al. Natural but not inducible regulatory T cells require TNF-alpha signaling for in vivo function. J Immunol. 2011;186(12):6779–6787. doi: 10.4049/jimmunol.1003868. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, et al. TNF-α impairs differentiation and function of TGF-β-induced Treg cells in autoimmune diseases through Akt and Smad3 signaling pathway. J Mol Cell Biol. 2013;5(2):85–98. doi: 10.1093/jmcb/mjs063. [DOI] [PubMed] [Google Scholar]
- 43. Institute of Laboratory Animal Resources (US). Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.