Significance
The development of renewable liquid fuels and bioproducts is critical to reducing global reliance on petroleum and mitigating climate change, particularly for applications where few low-carbon alternatives exist. We combine chemical catalysis with life-cycle greenhouse gas (GHG) modeling to create a new platform for producing biobased aviation fuel and automotive lubricant base oils. The recyclable catalysts we developed are capable of converting sugar and biomass-derived alkyl methyl ketones into cyclic enones via condensation reactions. These products can subsequently be hydrodeoxygenated to create a new class of aviation fuel and lubricant candidates with superior cold flow properties, density, and viscosity that substantially reduce GHG emissions relative to conventional petroleum.
Keywords: biofuels, lubricants, life cycle assessment, methyl ketones, greenhouse gases
Abstract
Decarbonizing the transportation sector is critical to achieving global climate change mitigation. Although biofuels will play an important role in conventional gasoline and diesel applications, bioderived solutions are particularly important in jet fuels and lubricants, for which no other viable renewable alternatives exist. Producing compounds for jet fuel and lubricant base oil applications often requires upgrading fermentation products, such as alcohols and ketones, to reach the appropriate molecular-weight range. Ketones possess both electrophilic and nucleophilic functionality, which allows them to be used as building blocks similar to alkenes and aromatics in a petroleum refining complex. Here, we develop a method for selectively upgrading biomass-derived alkyl methyl ketones with >95% yields into trimer condensates, which can then be hydrodeoxygenated in near-quantitative yields to give a new class of cycloalkane compounds. The basic chemistry developed here can be tailored for aviation fuels as well as lubricants by changing the production strategy. We also demonstrate that a sugarcane biorefinery could use natural synergies between various routes to produce a mixture of lubricant base oils and jet fuels that achieve net life-cycle greenhouse gas savings of up to 80%.
Countries around the world are enacting legislation to curb greenhouse gas (GHG) emissions. Strategies for decarbonizing road transportation include an array of options from improving engine efficiency and blending bioethanol/biodiesel with gasoline/diesel to using plug-in electric vehicles (1–3). Aviation fuels pose a unique problem because stringent specifications require oxygen-free compounds, limiting the options available (4). Biofuel solutions such as farnesane have been proposed; however, these offer only modest GHG reduction benefits (SI Appendix) and the wide boiling range requirement for jet fuels sets a limit on the amount of single-component renewable fuels that may be blended. At the other end of the spectrum are automotive lubricant base oils where a narrow range of compounds is highly desirable. Poly-α-olefins (PAOs) containing 30 carbon atoms obtained from oligomerization of fossil-derived 1-decene are considered as the benchmark of superior performance for crankcase oils and have a high demand (5). Importantly, the GHG footprint associated with PAO base oils can be higher on a per-mass basis than petroleum-derived fuels if even a fraction of the lubricant is repurposed as fuel at its end of life (6).
The goal of our work was to develop a strategy for the flexible production of jet fuels and lubricant base oils in a Brazilian sugarcane refinery designed to achieve a meaningful reduction in life-cycle GHG emissions. Our approach involves conversion of sugars in sugarcane-derived sucrose and hemicellulose to ketones using a combination of chemical and biocatalytic processes. For example, 2-butanone, can be obtained by the dehydration of fermentation-derived 2,3-butanediol (7, 8) or via chemical/biochemical (9, 10) decarboxylation of levulinic acid (11). The fermentation of various biomass-derived sugars using Clostridia strains produces a mixture of acetone, butanol, and ethanol (ABE), which can be used to synthesize a mixture of monoalkylated/dialkylated ketones (12), specifically 2-pentanone and 2-heptanone. Additional synthons may be produced from bioalcohol-derived olefins (13) or biomass-derived furanic platform molecules, such as 2,5-dimethylfuran and 2-methylfuran, via hydrogenolysis to produce 2-hexanone and 2-pentanone, respectively, with as high as 98% selectivity (14). The biomass-derived methyl ketones are then catalytically self- and cross-condensed to produce C12‒C45 condensates, which serve as potential jet fuels (C12‒C21) and synthetic lubricants (C33+) after hydrodeoxygenation. We show that our strategy gives a new class of compounds that can be incorporated into production schemes that result in up to 81% reductions in GHG emissions, exceeding even those of conventional biofuels such as sugarcane ethanol.
Results and Discussion
We begin by identifying heterogeneous catalysts and appropriate reaction conditions for the self-condensation of ketones (1) to produce dimer/trimer condensates 2‒4 in high overall yield (Table 1). Table 1 entries 1‒6 demonstrate that whereas ketones containing methyl functionality at one end undergo selective trimerization to produce isomers of 3 and 4 in the presence of base (MgAlO) and acid (Nb2O5) catalysts, respectively, hindered internal ketones condense to form mostly dimers (2) in the presence of acid catalysts (entries 7‒9). The formation of 2‒5 involves aldol-type condensations and Michael additions of ketone enolates or enols. Though the selectivity to 3 and 4 depends on the nature of the catalyst (acid/base), the pathway to 3 and 4 proceeds via 2 as an observable intermediate. Importantly, the reactivity of 1 also depends on the degree of steric hindrance near the reactive centers. Hence, the condensation of less-hindered methyl ketones proceeds smoothly at mild conditions (Table 1, entries 1‒3, 5, and 6). The condensates (2‒5) are finally hydrogenated to remove olefinic and carbonyl functionalities, as illustrated schematically in Table 1; this is accomplished in the liquid phase at 160 °C with 3.5 MPa of H2 using Pt/NbOPO4 (15) for 5‒12 h to produce saturated alkanes 6‒9 in yields as high as 99% (SI Appendix). For all of the condensation experiments, solvents such as toluene or cyclohexane are used as diluents to facilitate better understanding of the reaction. Practical application of such a process would use a reactive distillation setup and solvent-free conditions. Experiments conducted under such solvent-free conditions demonstrated that yields and selectivity comparable to those in Table 1 could be easily achieved through an optimization study (SI Appendix).
Table 1.
Self-condensation of ketones in the presence of solid acid/base catalysts
| Entry | Ketone (1) | Catalyst† | T, °C | Time, h | Conv.1, % | Yields of condensates, %* | |||||
| Cn | R1 | R2 | 2 (C2n) | 3 (C3n) | 4 (C3n) | 5 | |||||
| 1 | C4 | Me | H | MgAlO | 170 | 3 | 100 | 0 | 95 | 0 | 4 |
| 2 | C5‒C7 | Et/nPr/ nBu | H | MgAlO | 150 | 3 | 100 | 0 | 95‒98 | 0 | 0 |
| 3‡ | C6 | nPr | H | MgAlO | 150 | 3 | 99 | 0 | 97 | 0 | 0 |
| 4 | C8‒C15 | nPentyl‒ ndodecyl | H | MgAlO | 180 | 12 | 100 | 0 | 96‒99 | 0 | 0 |
| 5 | C5 | Et | H | Nb2O5 | 180 | 6 | 99 | 1 | 8 | 73 | 0 |
| 6 | C7 | nBu | H | Nb2O5 | 180 | 6 | 98 | 2 | 8 | 78 | 0 |
| 7 | C7 | Et | Et | Nb2O5 | 180 | 20 | 86 | 73 | 0 | 0 | 0 |
| 8 | C9 | nBu | Et | Nb2O5 | 180 | 20 | 79 | 69 | 0 | 0 | 0 |
| 9 | C11 | nBu | nBu | Nb2O5 | 180 | 20 | 61 | 54 | 0 | 0 | 0 |
Ketone (1, 2 mmol), catalyst (200 mg), and toluene (3 mL) was heated in a sealed Q-tube reactor. Conv.1, Conversion of compound 1.
Mixture of positional and stereoisomers.
Catalyst MgAlO represents calcined Mg/Al-hydrotalcite (Mg/Al = 3:1) and Nb2O5 represents calcined niobic acid.
Cyclohexane (3 mL) was used as solvent.
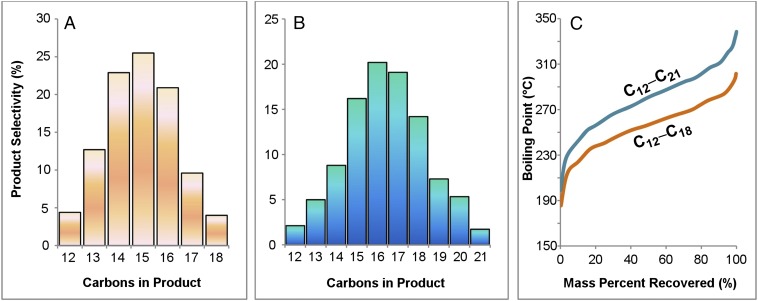
Next, we demonstrate the feasibility of producing jet fuel by cross-condensation of these methyl ketones to replicate the multicomponent mixtures of typical fuel blends. Reactions involving multiple synthons, such as a mixture of C4‒C6 or C4‒C7 methyl ketones, produced an array of trimer condensates (3), including those of intermediate carbon numbers (C12‒C18 or C12‒C21) resulting from all possible self-/cross-condensations (Fig. 1 and SI Appendix, Fig. S1). Encouraged by this finding, we evaluated key jet fuel properties of alkanes (7) derived from equimolar C4‒C6 ketone synthons (SI Appendix, Fig. S2). The results (SI Appendix, Table S1) confirmed that (i) the cloud, pour, and freezing points are lower than −54, −46, and −100 °C, respectively. These measurements exceeded the equipment measurement limits and are far superior to currently available aviation biofuels. (ii) The volumetric energy density is 38.3 MJ/L and is also superior to typical aviation fuels. (iii) The boiling range is 198‒302 °C, comparable to that of conventional jet fuel (148‒280 °C). Therefore, by adjusting the concentrations of ketones, it is possible to produce mixtures of alkanes that mimic conventional jet fuel.
Fig. 1.
MgAlO-catalyzed cross-condensations of mixed ketone synthons and product distributions. Reaction conditions: a mixture of ketones (1, 2 mmol in total), catalyst (200 mg), and toluene (3 mL) was heated to 160 °C in a sealed Q-tube reactor for 5 h. (A) 2-butanone, 2-pentanone, and 2-hexanone (1.1:1.1:1.0). (B) 2-butanone, 2-pentanone, 2-hexanone, and 2-heptanone (1.0:1.0:1.1:1.1). (C) Simulated distillation curve for alkanes (C12‒C18 and C12‒C21) resulting from hydrodeoxygenation of condensates shown in A and B.
Petroleum refineries generate a variety of supplementary products vital to the transportation sector, including base oils used as lubricant blendstocks. The demand for synthetic lubricants (PAOs), produced from fossil-derived ethylene, also continues to grow due to their superior mechanical properties compared with conventional base oils, making them the benchmark for automobile lubricants (5); this inspired us to synthesize C30- to C45-range molecules through self-condensation of ketones (1a‒c) (SI Appendix, Table S1). Synthon 1a was obtained via monoalkylation of acetone with 2-ethylhexanol derived from Guerbet coupling of ethanol/1-butanol. Similarly, 1b was synthesized via monoalkylation of acetone with 1-octanol, which may further be produced from furfural and acetone (16). Finally, biomass-derived 2-methylfuran gave 1c (17). Ketones 1a‒c underwent self-condensations to deliver the respective trimers (3a‒c), which were then subjected to hydrodeoxygenation (SI Appendix) to obtain C33 and C45 alkanes (7a‒c) in >97% isolated yields.
Our lubricant base oil molecules contain a cyclohexane ring and a quaternary carbon atom, and consequently represent a class of compounds that have not yet been tested as lubricants. We characterized these compounds by measuring their viscosity index (VI) and pour point and comparing them to those of conventional base oils and PAOs (SI Appendix, Table S2). All of the molecules synthesized showed excellent pour points (−51 to −69 °C) comparable to PAOs and far exceeding petroleum base oils, which have pour points in the −10 to −20 °C range. Thus, for most weather conditions, the compounds that we report here would be suitable in lubricant formulations. VI is a much more important property, a high VI indicating that the viscosity of the base oil does not change strongly with temperature, which is a desirable property for lubricant base oils and a strong motivation for using PAOs in formulations. Our studies show that the VI of 7b was very similar to that of PAOs (123 vs. 124) (18), whereas VIs of 7a and 7c compared well with those of mineral oil blendstocks (66 and 94 vs. 80‒120). These test cases show only a glimpse of the possibilities for additional tribology research for lubricant applications, and developing an understanding between molecular structure and properties could unlock a new class of lubricants.
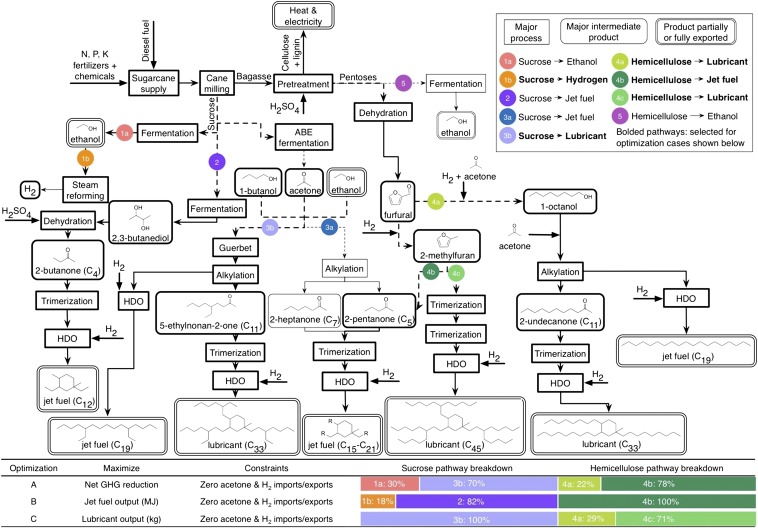
To quantify the carbon intensity of our lubricant and jet fuel production platform, we developed a model to simulate the process steps in a Brazilian sugarcane biorefinery and determine the life-cycle GHG footprint of potential products relative to their conventional petroleum counterparts. We modeled various pathways for producing drop-in jet fuel, lubricants, and ethanol using sucrose and bagasse-derived hemicellulose, with cellulose and lignin combusted for heat and power (Fig. 2). Through an integration of linear programming, process simulations, and life-cycle GHG modeling, we determined optimal allocations of sucrose and hemicellulose subject to different objective functions. We also explored the sensitivity of our results to uncertain variables (SI Appendix). In each case, we constrained biorefinery acetone and hydrogen imports/exports to zero to prevent the facility from relying on fossil inputs and eliminated the need to find local markets for excess acetone and hydrogen coproducts, which can be challenging in rural Brazil. We hypothesized that, although most individual pathways shown in Fig. 2 do not satisfy this constraint, furanic and fermentative pathways could be combined in a single facility to balance processes that produce excess hydrogen and acetone with those deficient in them.
Fig. 2.
Process flow diagram for alkane production options for jet/lubricant applications from Brazilian sugarcane.
Maximizing the total reduction in annual GHG emissions achieved by the biorefinery (optimization A), assuming ethanol displaces gasoline, bioderived jet fuel displaces petroleum jet fuel, and bioderived lubricants displace PAO base oil, results in a product mix comprised of ∼40% lubricants, 40% ethanol, and 20% jet fuel by volume (Fig. 3). In this case, 70% of sucrose is routed through a lubricant pathway via ABE fermentation, with a small jet fuel coproduct (pathway 3b) and the remaining 30% is used to produce ethanol via fermentation (pathway 1a); the ethanol coproduct of ABE fermentation is also sold as fuel and the hydrogen coproduct is used on-site. All hemicellulose is converted to furfural, 78% of which is routed to jet fuel via 2-methylfuran (pathway 4b) and 22% of which is converted to lubricants with a minor jet fuel coproduct via 1-octanol (pathway 4a).
Fig. 3.
Life-cycle greenhouse gas results for selected optimization runs (detailed in Fig. 2).
Maximizing jet fuel production (optimization B) is the only case in which on-site dedicated hydrogen production is required. We chose steam reforming of ethanol to model this step; however, many other options are also viable, e.g., reforming of biogas derived from anaerobic digestion of vinasse, aqueous-phase reforming of sugar, or direct gasification of bagasse (19). Our model suggests that 18% of sucrose is used to produce hydrogen via ethanol (pathway 1b), the remaining 82% is converted to jet fuel via 2,3-butanediol (pathway 2), and all hemicellulose is converted to jet fuel via 2-methylfuran (pathway 4b); the final output is 100% jet fuel. An attempt to maximize the lubricant base oil production (optimization C) results in an allocation similar to optimization A: all sucrose is processed through pathway 3b (lubricant via ABE fermentation) and 29% of hemicellulose is routed through pathway 4a. The remaining 71% of hemicellulose, is converted to 2-methylfuran, but unlike optimization A, is ultimately converted to C45 lubricant via pathway 4c.
In each optimization, the GHG-intensity reductions relative to conventional petroleum products are substantial, ranging from 57% to 81%. Fig. 3 shows GHG emissions broken down by sectors directly responsible for emissions, including error bars to capture uncertainty associated with power offset credits for the biorefinery. For example, GHG emissions released at fertilizer manufacturing facilities are attributed to chemicals and fertilizers, emissions associated with grid electricity supplied to the facility are represented in upstream electricity use, and N2O emissions from sugarcane fields resulting from nitrogenous fertilizer application are attributed to sugarcane cultivation and harvesting. Further details on the life-cycle GHG inventory are provided in SI Appendix. Optimization B results in greater emissions related to chemical production than A or C because of the sulfuric acid required for dehydration of 2,3-butanediol, and subsequent sodium hydroxide needed to neutralize the acid for disposal. The results for optimizations A and C are most favorable, but are sensitive to the uncertain fate of lubricants at their end of life.
Because automotive lubricant recycling practices vary geographically and reliable data are scarce, we make assumptions based on available literature and conduct a sensitivity analysis. Our model assumes that 10% of lubricants are reused, 10% of used lubricants are repurposed as a component in asphalt, and the remaining oil is leaked, oxidized, improperly disposed of, or combusted as fuel (20). We assume all carbon not sequestered in asphalt is ultimately oxidized to CO2. Because this assumption impacts the GHG footprint of both biolubricants and conventional PAO base oil, the pathways selected for each optimization remain unchanged if we assume no carbon sequestration in asphalt. Our sensitivity analysis also shows that the GHG benefits may be increased if ABE fermentation can be tuned to produce a more favorable product mixture; a modest 10% increase in acetone yield can reduce the overall GHG footprint of the products by 10%. However, even given product yields currently achievable in the laboratory, our results indicate that catalytic pathways can produce more desirable fuels and lubricants while achieving net GHG emissions comparable or reduced relative to conventional ethanol pathways. Additionally, we find that increases in furfural yield from C5 sugars can alter the optimization results. Choosing a resource-intensive acid catalyst such as HCl will significantly increase the net GHG footprint of all nonethanol hemicellulose pathways, even if it produces higher product yields than competing acid catalysts.
By integrating various ketone synthons from biomass via self-/cross-condensations, we have shown that a range of cyclic alkanes with desired composition, exceptional cold-flow properties, higher volumetric energy density, and appropriate boiling distributions can be produced for jet fuel applications. The condensation described here is catalyzed by inexpensive, heterogeneous mixed/pure oxides and is suitable for large-scale fuel production. In addition, ketone condensation can also produce a new class of biolubricants with properties comparable to fossil-derived lubricants. Guided by life cycle analysis (LCA) combined with linear programming, integrated sugarcane biorefineries for producing jet fuels and lubricants could be built to minimize the overall GHG impact or maximize total energy output through novel combinations of furan and fermentation pathways. Increasing furan precursor yields and improving the ability to tune ABE fermentation product ratios will make such hybrid biorefineries even more efficient, offering dramatic GHG reductions relative to petroleum products. Needless to say, the commercial implementation of this technology would include financial implications that extend beyond GHG reductions; however, we hope that research such as that presented here will allow policymakers to create appropriate incentives to encourage optimal investments.
Supplementary Material
Acknowledgments
Funding for this work was provided by the Energy Biosciences Institute and National Science Foundation Graduate Research Fellowship Grant DGE 1106400 (to E.R.S.). This work was carried out in part at the Lawrence Berkeley National Laboratory, which is operated for the US Department of Energy under Contract Grant DE-AC02-05CH11231.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508274112/-/DCSupplemental.
References
- 1. Anonymous (2013) International Energy Outlook 2013 (US Energy Information Administration, Washington, DC)
- 2.Huber GW, Iborra S, Corma A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem Rev. 2006;106(9):4044–4098. doi: 10.1021/cr068360d. [DOI] [PubMed] [Google Scholar]
- 3.Alonso DM, Bond JQ, Dumesic JA. Catalytic conversion of biomass to biofuels. Green Chem. 2010;12(9):1493–1513. [Google Scholar]
- 4.Edenhofer O, et al. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change; Cambridge, UK: 2014. [Google Scholar]
- 5.Ray S, Rao PVC, Choudary NV. Poly-α-olefin-based synthetic lubricants: A short review on various synthetic routes. Lubrication Science. 2012;24(1):23–44. [Google Scholar]
- 6.Raimondi A, Girotti G, Blengini G, Fino D. LCA of petroleum-based lubricants: State of art and inclusion of additives. Int J Life Cycle Assess. 2012;17(8):987–996. [Google Scholar]
- 7.Multer A, McGraw N, Hohn K, Vadlani P. Production of methyl ethyl ketone from biomass using a hybrid biochemical/catalytic approach. Ind Eng Chem Res. 2013;52(1):56–60. [Google Scholar]
- 8.Ji X-J, Huang H, Ouyang P-K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol Adv. 2011;29(3):351–364. doi: 10.1016/j.biotechadv.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Gong Y, Lin L, Yan ZP. Catalytic hydrogenation and oxidation of biomass-derived levulinic acid. Bioresources. 2011;6(1):686–699. [Google Scholar]
- 10.Min K, et al. Conversion of levulinic acid to 2-butanone by acetoacetate decarboxylase from Clostridium acetobutylicum. Appl Microbiol Biotechnol. 2013;97(12):5627–5634. doi: 10.1007/s00253-013-4879-9. [DOI] [PubMed] [Google Scholar]
- 11.Rackemann DW, Doherty WOS. The conversion of lignocellulosics to levulinic acid. Biofuels. Bioproducts and Biorefining. 2011;5(2):198–214. [Google Scholar]
- 12.Anbarasan P, et al. Integration of chemical catalysis with extractive fermentation to produce fuels. Nature. 2012;491(7423):235–239. doi: 10.1038/nature11594. [DOI] [PubMed] [Google Scholar]
- 13.Mo F, Dong G. C-H bond activation. Regioselective ketone α-alkylation with simple olefins via dual activation. Science. 2014;345(6192):68–72. doi: 10.1126/science.1254465. [DOI] [PubMed] [Google Scholar]
- 14.Corma A, de la Torre O, Renz M. Production of high quality diesel from cellulose and hemicellulose by the Sylvan process: Catalysts and process variables. Energy & Environmental Science. 2012;5(4):6328–6344. [Google Scholar]
- 15.Xu W, et al. Effective production of octane from biomass derivatives under mild conditions. ChemSusChem. 2011;4(12):1758–1761. doi: 10.1002/cssc.201100361. [DOI] [PubMed] [Google Scholar]
- 16.Julis J, Leitner W. Synthesis of 1-octanol and 1,1-dioctyl ether from biomass-derived platform chemicals. Angew Chem Int Ed Engl. 2012;51(34):8615–8619. doi: 10.1002/anie.201203669. [DOI] [PubMed] [Google Scholar]
- 17.Corma A, de la Torre O, Renz M, Villandier N. Production of high-quality diesel from biomass waste products. Angew Chem Int Ed Engl. 2011;50(10):2375–2378. doi: 10.1002/anie.201007508. [DOI] [PubMed] [Google Scholar]
- 18.Wasserscheid P, Grimm S, Köhn Randolf D, Haufe M. Synthesis of synthetic lubricants by trimerization of 1-decene and 1-dodecene with homogeneous chromium catalysts. Adv Synth Catal. 2001;343(8):814–818. [Google Scholar]
- 19.Haryanto A, Fernando S, Murali N, Adhikari S. Current status of hydrogen production techniques by steam reforming of ethanol: A review. Energy Fuels. 2005;19(5):2098–2106. [Google Scholar]
- 20.Johnson MR, Reynolds JG, Love AH. Improving Used Oil Recycling in California. California Integrated Waste Management Board; Sacramento, CA: 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.