Significance
A major obstacle to the eradication of HIV-1 by combination antiretroviral therapy (cART) is the formation of cellular reservoirs in CD4+ T lymphocytes (carrying latently integrated provirus) and tissue macrophages. Infected macrophages assemble new virions in subcellular vacuoles known as virus-containing compartments (VCC), hiding them from the immune system and, in part, from antiretroviral agents. Here we report that extracellular ATP is capable of inducing the rapid release of virions accumulated in VCC via interaction with the P2X7 receptor and without inducing cell death, whereas the antidepressant agent Imipramine blocks the release. Thus, our study identifies two “druggable” targets affecting the release of stored virions from infected human macrophages that could bear relevance for purging HIV-1 reservoirs in individuals receiving cART.
Keywords: HIV, macrophage, ATP, virion-containing compartment, P2X7
Abstract
HIV type 1 (HIV-1) infects CD4+ T lymphocytes and tissue macrophages. Infected macrophages differ from T cells in terms of decreased to absent cytopathicity and for active accumulation of new progeny HIV-1 virions in virus-containing compartments (VCC). For these reasons, infected macrophages are believed to act as “Trojan horses” carrying infectious particles to be released on cell necrosis or functional stimulation. Here we explored the hypothesis that extracellular ATP (eATP) could represent a microenvironmental signal potentially affecting virion release from VCC of infected macrophages. Indeed, eATP triggered the rapid release of infectious HIV-1 from primary human monocyte-derived macrophages (MDM) acutely infected with the CCR5-dependent HIV-1 strain. A similar phenomenon was observed in chronically infected promonocytic U1 cells differentiated to macrophage-like cells (D-U1) by costimulation with phorbol esters and urokinase-type plasminogen activator. Worthy of note, eATP did not cause necrotic, apoptotic, or pyroptotic cell death, and its effect on HIV-1 release was suppressed by Imipramine (an antidepressant agent known to inhibit microvesicle formation by interfering with membrane-associated acid sphingomyelinase). Virion release was not triggered by oxidized ATP, whereas the effect of eATP was inhibited by a specific inhibitor of the P2X7 receptor (P2X7R). Thus, eATP triggered the discharge of virions actively accumulating in VCC of infected macrophages via interaction with the P2X7R in the absence of significant cytopathicity. These findings suggest that the microvesicle pathway and P2X7R could represent exploitable targets for interfering with the VCC-associated reservoir of infectious HIV-1 virions in tissue macrophages.
HIV type 1 (HIV-1) infects CD4+ T lymphocytes, myeloid dendritic cells, and monocyte-macrophages, cell types sharing the expression of the primary viral receptor (R) CD4 on their surface together with one or more chemokine R, usually CCR5 and/or CXCR4 (1, 2). The discovery of combination antiretroviral therapy (cART) in the mid 1990s has significantly impacted the natural history of the infection by virtually suppressing, in optimal conditions, the capacity of the virus to infect target cells, thereby resulting in a prolonged, near-normal life expectation of infected individuals. Therapy suspension almost inevitably results in the resumption of virus replication and disease progression in most individuals, owing to the existence of viral reservoirs of likely heterogeneous cellular origin that are unaffected by prolonged cART (3, 4). Consequently, complete eradication of HIV-1 from infected individuals is not currently achievable without targeting these cART-insensitive sources of infection. Latently infected CD4+ T cells with both “central memory” and “transitional effector memory” phenotypes have been well characterized as reservoirs of replication-competent HIV-1 (4, 5); in contrast, the contribution of non–T-cell subsets to the overall viral reservoir in cART-treated individuals has remained more elusive (6–8).
Concerning mononuclear phagocytes, whether bone marrow precursor cells and circulating monocytes are truly infected in vivo is a matter of debate, whereas a greater consensus exists on the role played by resident tissue macrophages of different organs. In particular, brain-associated macrophages or microglia, together with astrocytes, have been reported as the main targets and source of virus in the central nervous system (4, 5, 8, 9). In addition, infected mononuclear phagocytes are considered responsible for the slower second-phase decay of plasma viremia observed in patients starting cART (9).
Unlike CD4+ T cells, mononuclear phagocytes are depleted neither in vivo (at least in terms of circulating monocytes) nor in vitro on HIV-1 infection. In addition, macrophage infection, both in vitro and in vivo, has been described as characterized by budding and accumulation of HIV-1 particles in virus-containing compartments (VCC) of debated origin (10–13). These peculiar features of macrophage infection led to the “Trojan horse” hypothesis of a pathogenic role of mononuclear phagocytes as capable of accumulating virions in subcellular compartments invisible to immune recognition (14, 15) and relatively insensitive to antiretrovirals (15, 16). It is hypothesized that VCC-associated virions are released either as a consequence of cell death or by functional stimulation of the infected cells. In this regard, we have previously observed that IFN-γ stimulation of the chronically infected promonocytic U1 cell line differentiated to macrophage-like cells by phorbol-12, myristate, 13-acetate (PMA) caused a profound redirection of the major site of virion production from the plasma membrane to VCC (17). This observation was later extended to other stimulants of PMA-differentiated U1 cells, including urokinase-type plasminogen activator (uPA) and CD11b/CD18 (Mac-1) integrin ligands (18, 19). Of interest, a similar phenotype has been reported in infected primary human monocyte-derived macrophages (MDM) by interference with endogenously released CCL2/monocyte chemotactic protein-1 (MCP-1) (20).
Regarding the nature of VCC, Gould et al. (14) proposed that retroviruses exploit the exosome biogenesis pathway for both the formation and release of infectious particles as well as for the uptake of a R-independent, gp120 envelope (Env)-dependent infection. More recently, VCC are believed to represent intracellular sequestration of plasma membrane areas rich in a subset of tetraspanins (12). Likely because of their origin, VCC can be transiently (11) connected to the extracellular medium through microchannels or conduits (21, 22). Of interest, VCC-like compartments accessible by the extracellular medium preexist in uninfected macrophages and express similar markers, including CD9, CD81, CD63 (23), and, identified more recently, CD36 (24). Recently, it was demonstrated that on HIV-1 infection, Gag is recruited to these preexisting compartments in which virion assembly occurs, leading to their conversion into VCC (24, 25). Whether the release of virions is a regulated process, and the nature of the signal(s) that may induce virion discharge from VCC, remain largely unknown, however.
In the present study, we investigated the hypothesis that signals from an inflamed microenvironment may affect HIV-1 accumulation and release from macrophage-associated VCC. We focused on extracellular ATP (eATP), a molecule passively released during necrotic cell death (26), but also actively released on cell stimulation via innate immunity R, as reviewed previously (27). We indeed observed that eATP induced a rapid release of HIV-1 virions accumulated in VCC of both primary human MDM acutely infected with HIV-1 and chronically infected U1 cells differentiated to macrophage-like cells (D-U1 cells) (28). eATP-induced release of HIV-1 virions was not associated with gross cytopathic effects and was blocked by imipramine, an antidepressant agent known to inhibit the membrane-associated acid sphingomyelinase (aSMase), an enzyme involved in the formation of membrane microvesicles (29, 30). Finally, we found that eATP-dependent discharge of virions from VCC was attributed mainly to engagement of the P2X7R on the surface of infected macrophages.
Results
eATP Induces Release of Mature Infectious HIV-1 Virions from Acutely Infected Human Primary MDM.
MDM from nine donors were infected with the CCR5-dependent (R5) HIV-1BaL [multiplicity of infection (moi), 0.1] and were then cultured for up to 30 d, with supernatants collected every 2–3 d for testing for the presence of HIV-1 by Mg2+-dependent reverse transcriptase (RT) activity. As shown in Fig. S1A, MDM from all donors were productively infected with R5 HIV-1, with peak levels of virus replication typically observed between day 12 and day 18 postinfection (PI). Based on these kinetics, culture supernatants were removed from infected MDM at 15 d PI, and were replaced with fresh Complete Medium containing or not containing eATP (1 mM). The supernatants were collected after 10–30 min, and their RT activity content was quantified. eATP stimulation increased the activity of RT, a viral enzyme exclusively associated with virions (31), released in culture supernatants compared with unstimulated cells at all times tested (Fig. 1A); these results were independently confirmed by p24 Gag ELISA. Next, a concentration-dependent titration of eATP, between 5 and 0.1 mM, based on published evidence (32, 33), was conducted in HIV-1–infected MDM of eight independent donors. A significant increase in RT activity release in culture supernatants was observed when cells were stimulated with eATP concentrations ≥0.5 mM (Fig. 1B). For simplicity, the remaining experiments were conducted using 1 mM eATP, a concentration inducing maximal release of virions without evident cytopathicity, as addressed below.
Fig. 1.
eATP induces the release of HIV-1 virions in acutely infected primary human MDM. (A) Release of HIV-1 virion by MDM 15 d PI on stimulation with eATP (1 mM). The cell culture supernatant was removed and substituted with fresh culture medium before eATP stimulation. Significantly increased RT activity was observed on eATP stimulation at all time points tested (mean ± SE, n = 9; *P < 0.05, **P < 0.01, ***P < 0.001 by t test). (B) eATP in a range of concentrations from 5 to 0.1 mM was tested for its ability to induce virion release from MDM (n = 8). Concentrations equal or superior to 0.5 mM induced a significant release of RT activity in culture supernatants.
To evaluate whether the eATP-induced released was exclusively associated with CD4/Co-R–dependent infection, we carried out similar experiments in MDM infected with VSVg-pseudotyped NLAD8 (R5 virus), and found similar results (Fig. S2).
To determine whether the virions released from unstimulated and eATP-stimulated MDM were infectious or defective, we performed an infectivity assay using the TZM-bl reporter cell line that carries a Tat-sensitive promoter driving the expression of firefly luciferase (luc), thus reflecting the capacity of virions to infect, integrate, and express a functional Tat protein (34). The supernatants of MDM established from four independent donors and infected for 15 d were removed, and the cells were then resuspended in fresh medium and stimulated or not with eATP for an additional 30 min. The MDM supernatants were then analyzed for their RT activity content and, in parallel, incubated with TZM-bl cells; the luc levels were then evaluated after 24 h. As shown in Fig. 2A (Left), eATP stimulation resulted in comparable increases in both RT activity and infectivity of the MDM culture supernatants. Furthermore, when the same amount of virions (based on RT activity) was used to infect TZM-bl cells, comparable levels of infectivity were detected in supernatants of both unstimulated and eATP-stimulated MDM (Fig. 2A, Right), suggesting that viral particles released in basal and eATP-stimulated conditions originate from the same pool of virions.
Fig. 2.
eATP stimulation of infected MDM leads to the release of infectious virions. (A) Infectivity of virions released by unstimulated and eATP-stimulated MDM tested in TZM-bl cells. Either equal volumes of supernatants (Left) or equal cpm/µL counts (Right) from 15-d-old infected MDM were loaded onto TZM-bl cells for 30 min. Cells were washed, and fresh medium was added to the cell cultures; luc activity was determined after 24 h. The increased levels of RT activity present in the supernatants of eATP-stimulated MDM were associated with a similar increase in infectivity (n = 4, Left and n = 3, Right, respectively; mean ± SE; **P < 0.01, ***P < 0.001 by t test). (B) Supernatants derived from HIV-1–infected stimulated and unstimulated MDM were checked for RT activity content (Left) and transferred to autologous PHA-stimulated CD4+ T cells. After 30 min, the cells were washed and kept in culture for 12 d in medium enriched with IL-2. Every 3 d, the supernatants were collected for RT activity quantification and replaced with fresh medium containing IL-2 (Right; mean ± SD, n = 2).
We further tested the infectivity of the virions released from unstimulated and eATP-stimulated MDM in a more physiological context, on autologous CD4+ T cells. To this end, CD4+ T lymphocytes were isolated together with monocytes from the same healthy donors. Monocytes were differentiated to MDM and were infected, and CD4+ T cells were frozen. The cells were then thawed and activated by phytohemagglutinin (PHA) 3 d before incubation with the supernatants from 15-d-old infected MDM stimulated or not with eATP for 30 min (Fig. 2B, Left). Greater levels of virus replication were observed when activated autologous CD4+ T cells were incubated with the supernatants derived from eATP-stimulated MDM compared with those derived from unstimulated MDM (Fig. 2B, Right). Taken together, these findings indicate that eATP rapidly triggers the release of mature, infectious virions from HIV-1–infected macrophages.
We also analyzed whether VCC-associated virion release corresponds to an actual depletion of cell-associated viral protein content. First, we observed a reduction in the RT activity content on cell lysis after 30 min of stimulation with eATP (Fig. S1B). Next, we performed intracellular p24 Gag staining of HIV-1–infected MDM before and after 30 min of stimulation with eATP. A significant reduction of p24 Gag+ cells was observed on eATP stimulation (Fig. S1 C and D).
eATP Induces Cell Remodeling and Accumulation of Cell-Associated Viral Proteins in Proximity to the Plasma Membrane.
To analyze the process of eATP-induced virion release in a dynamic manner, we infected MDM with an HIV-1 Gag iGFP ∆Env VSVg-pseudotyped virus, which has been characterized previously (11, 15). In particular, the p55 Gag precursor of this virus is internally tagged with GFP, allowing its visualization in the cytosol of infected macrophages and the appearance of VCC as early as 2–3 d PI (11). The culture supernatants of HIV-1 Gag-iGFP–infected macrophages established from four independent donors were removed at 4 d PI and replaced with fresh Complete Medium supplemented with 20 mM Hepes. eATP was added to the culture supernatant at 5 min after live-imaging acquisition with an inverted confocal microscope equipped with a spinning disk.
As observed with cells from donor 9, eATP stimulation resulted in a profound remodeling of the plasma membrane. Nonetheless, after membrane reorganization and blebbing, the cells recovered their original shape (Fig. 3 and Movie S1). eATP stimulation of MDM from donor 15 showed a significant relocalization of VCC and a concentration of viral proteins in proximity of the plasma membrane (Fig. 3 and Movies S2 and S3). Taken together, this morphological evidence supports the concept that eATP-mediated release of HIV-1 virions is a transient phenomenon that involves reorganization of the whole cell surface.
Fig. 3.
Time-lapse accumulation of HIV-1 proteins in infected MDM stimulated with eATP. Human MDM were infected with a VSV-g Gag iGFP ΔEnv pseudotyped virus at an moi of 0.1. At 4 d after infection, the cells were washed and kept in complete medium supplemented with 20 mM Hepes during acquisition. The images were acquired every 30 s with an inverted spinning-disk confocal microscope maintained at 37 °C. The photogalleries were mounted by using the Fiji software that was also used for the related movies. (Scale bars: 10 µm.)
To better visualize the accumulation of virions in VCC, we performed an ultrastructural analysis of infected MDM before and after eATP stimulation. EM profiles clearly showed the presence of numerous VCC in unstimulated HIV-infected MDM at 15 d PI, whereas eATP stimulation resulted in their release proximal to the plasma membrane (Fig. 4 and Fig. S3). Taken together, these results indicate that eATP triggers a rapid release of HIV-1 virions accumulated mainly in VCC of the infected macrophages.
Fig. 4.
eATP induces the release of virions from MDM-associated VCC. Supernatants from 15-d-old infected MDM was removed and substituted with fresh culture medium. Cells were then either left unstimulated (Nil) or were stimulated with eATP (1 mM) for 30 min before fixation. TEM pictures of a single infection representative of three independently performed infections were shot with a magnification of 60,000×, and show a prominent accumulation of mature virions in VCC in unstimulated conditions (Nil, Upper) and their release in proximity of the plasma membrane after eATP stimulation (Lower); arrowheads indicate the plasma membrane.
eATP-Dependent Virion Release from MDM and D-U1 Cells Is Not Associated with Significant Cytopathicity.
It is well established that intracellular ATP is released by necrotic cells and represents a “danger signal” capable of triggering either cell necrosis or apoptosis (26, 35–37). Nonetheless, we observed no sign of necrotic cell death, as assessed by measurement of lactate dehydrogenase (LDH) release on infected or uninfected MDM exposed to eATP for 30 min (Fig. 5, Left). In addition, no obvious signs of cytopathicity were observed when infected MDM were first exposed to eATP and then maintained in culture for another 7 d after removal of the stimulant.
Fig. 5.

eATP-mediated release of VCC-associated virions is not caused by necrotic cell death. eATP stimulation of primary human MDM or D-U1 cells does not cause cell necrosis. Fifteen-day-old infected MDM or D-U1 cells were stimulated or not with eATP (1 mM) for 30 and 10 min, respectively, after which their supernatants were collected and analyzed for LDH content as indicator of necrotic cell damage. The supernatant of cells necrotized by repeated cycles of freezing and thawing (F/T) served as a positive control. The results indicate that eATP does not cause necrotic cell death in either cell type (mean ± SE, n = 5 for MDM and n = 3 for D-U1 cells).
Given that acute HIV-1 infection has been associated with the triggering of different cytopathicity pathways (38), we also investigated the effect of eATP in chronically infected monocytic cells carrying integrated HIV-1 proviruses. In this regard, we reported previously that distinct molecules, including IFN-γ, uPA, and ligation of CD11b/CD18 integrin, lead to significant expansion of the VCC in U1 cells differentiated by phorbol esters to become macrophage-like cells (here defined as D-U1 cells) (17, 39). As observed in acutely infected MDM, no evidence of necrotic cell death was observed in D-U1 cells stimulated with eATP (Fig. 5, Right).
We also investigated whether eATP exposure induced cleavage of caspase 3 and 1, key enzymes for inducing the apoptotic cascade and pyroptotic cascade, respectively (40). We found no evidence of cleavage of either caspase on short-term stimulation of infected MDM or D-U1 cells with eATP (Fig. S4).
Imipramine Blocks eATP-Induced Virion Release in MDM and D-U1 Cells.
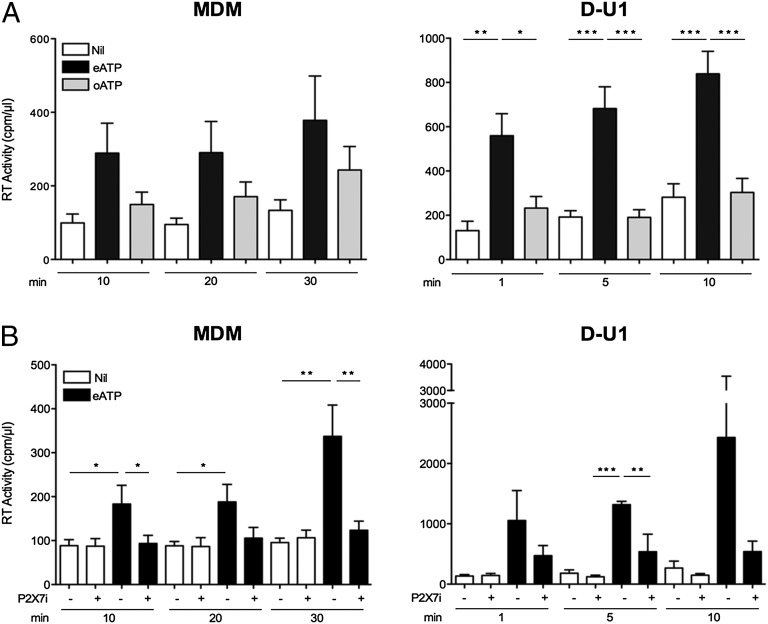
eATP can induce profound morphological changes, including membrane blebbing (Fig. 3, donor 9) and production of vesicles of different size, such as exosomes (<100 nm) and microvesicles (0.1–1 µm), containing mediators of intercellular communication such as functional R, IFNs, microRNAs, and danger signals (41). Of interest, the antidepressant agent imipramine has been shown to act as a potent inhibitor of aSMase, an enzyme responsible for the formation and shedding of microvesicles (30, 42). Thus, we tested whether this agent could interfere with the release of virions from VCC of acutely infected MDM and D-U1 cells. For this, MDM and D-U1 cells were preincubated or not with imipramine (10 µM) for 15 and 5 min, respectively; the pharmacologic agent was then removed, and the cells were incubated or not with eATP (1 mM). eATP induced virion release in D-U1 cells at levels comparable to those in acutely infected MDM and with faster kinetics, whereas imipramine blocked both basal and eATP-induced virion release in both MDM and D-U1 cells, although this effect was lost 30 min after eATP stimulation, at least in the case of infected MDM (Fig. 6). The foregoing results suggest a potential role of the microvesicle pathway in eATP-dependent release of HIV-1 virions from VCC of infected macrophages.
Fig. 6.
eATP-mediated release of VCC-associated virions is inhibited by Imipramine. The culture supernatants of 15-d-old infected MDM was replaced by fresh culture medium, preincubated or not with imipramine (10 µM) for 15 min, and then stimulated or not with eATP (1 mM). Supernatants were collected 10, 20, and 30 min after eATP stimulation to determine their RT activity content. D-U1 cells were preincubated for 5 min with Imipramine (10 µM) and stimulated for shorter times (1–10 min) with eATP, as indicated. Imipramine significantly reduced both basal and eATP-dependent release of HIV-1 virions in both MDM and D-U1 cells, except at the last time point (30 min) of MDM stimulation. The results are mean ± SE of seven independent experiments for MDM and three independent experiments for D-U1 cells. *P < 0.05, **P < 0.01, t test.
eATP-Dependent Virion Release from Infected MDM and D-U1 Cells Occurs via Interaction with P2X7R.
P2X7 is a purinergic R expressed by mononuclear phagocytes and known to be responsive to eATP stimulation at concentrations >500 µM (26). Indeed, we confirmed by Western blot analysis that MDM express P2X7R, and that this expression is unaffected by HIV-1 infection and/or cell exposure to eATP (Fig. S5A).
We next stimulated the cells with oxidized ATP (oATP), an eATP antagonist for P2X7R binding devoid of biological activity (33). As expected, eATP, but not oATP, induced virion release from infected MDM (Fig. 7A, Left). Because in addition to P2X7R, oATP has been reported to bind to P2X1R and P2X2R, we stimulated infected MDM with eATP after their short preincubation with A-438079, a selective inhibitor of P2X7R (43, 44), which prevented the eATP-dependent virion release from MDM (Fig. 7B, Left).
Fig. 7.
eATP-induced virion release from infected MDM and D-U1 cells is mediated by interaction with P2X7R. (A) Infected cells were either stimulated with eATP (1 mM) or oATP (1 mM) for 10–30 min (in the case of MDM) or for 1–10 min (for D-U1 cells) or left unstimulated, after removal of cell supernatants. eATP, but not oATP, induced the release of virions from both cell types. The results represent the mean ± SE of five independent experiments with MDM and three independent experiments with D-U1 cells. *P < 0.05, **P < 0.01, ***P < 0.001, t test. (B) Infected cells were preincubated or not with the selective P2X7R inhibitor A-438079 (100 µM) for 15 min for MDM or 5 min for D-U1 cells. The inhibitor was left in culture, and the cells were stimulated or not with eATP (1 mM) for 10–30 min for MDM or 1–10 min for D-U1 cells. The P2X7R inhibitor prevented most of eATP-induced production of HIV-1 virions in both cell types, particularly at 10 min poststimulation in the case of MDM (mean ± SE; n = 5). For D-U1 cells, three independent experiments were performed (mean ± SE; **P < 0.01, ***P < 0.001, t test).
In addition, we collected supernatants from D-U1 cells at different time points (from 1 to 10 min) and analyzed them for HIV-1 content by RT activity. As observed with acutely infected MDM, eATP rapidly induced the release of HIV-1 virions (Fig. 7A, Right), which coincided with a decrease in cell-associated viral proteins. Like MDM, D-U1 cells also expressed P2X7R, as determined by Western blot analysis (Fig. S5B). Consequently, oATP failed to induce virion release, and the selective P2X7R inhibitor A-438079 prevented the eATP-mediated release of virions from D-U1 cells (Fig. 7 A and B, Right). Moreover, a competition assay in D-U1 cells simultaneously exposed to both eATP and oATP resulted in a significant reduction in ATP-mediated release in the presence of oATP (Fig. S6). Thus, eATP induces HIV-1 release from infected MDM and D-U1 cells, at least in part through interaction with P2X7R.
Discussion
In the present study, we have demonstrated that eATP is a potent inducer of virion release from VCC, a functional subcellular compartment uniquely observed in macrophages. The effect of eATP was not the result of either passive (necrosis) or active (apoptosis) cytopathicity, and it was prevented by imipramine, an antidepressant agent reported to block the microvesicle release pathway. The inductive effect of eATP observed in acutely infected MDM was reproduced in chronically infected U1 cells carrying integrated proviruses and differentiated to macrophage-like cells by the combination of PMA and uPA. In both cell types, release of eATP-dependent virions was mediated, at least in major part, by interaction with purinergic P2X7R. Thus, eATP represents a physiological stimulus with the capacity to induce a rapid release of preformed HIV-1 virions accumulating in VCC of infected macrophages.
Along with CD4+ T cells, human mononuclear phagocytes are a main target of HIV-1 infection. Although whether bone marrow-associated precursor cells and circulating monocytes harbor HIV-1 in vivo is controversial (45), infection of resident tissue macrophages has been observed both in seropositive individuals and in macaques experimentally infected with simian immunodeficiency virus (SIV) (5). In particular, infected macrophages and microglia represent, together with astrocytes, the dominant productively infected cell types in the central nervous system (46–48), where they constitute an immunologic sanctuary poorly reached by antiretroviral agents (7, 13, 49).
Although HIV-1 infects both T lymphocytes and macrophages via the sequential interaction of gp120 Env with CD4 and CCR5 (or CXCR4), there are significant differences in infection outcomes in the two cell types. First, virus replication in T cells is strictly linked to their proliferative status, whereas productive infection of nonproliferating macrophages, including MDM, does not, reflecting a general feature distinguishing lentiviruses from other retroviruses (50). Second, unlike CD4+ T cells, neither circulating monocytes nor tissue macrophages are significantly depleted in infected individuals or SIV-infected macaques (15); also in vitro, infection of MDM does not lead to their cell death. Consequently, whereas most in vitro infected CD4+ T cells die, MDM can survive for months in culture if a sufficient nutrient supply is provided, where they become “viral factories,” constantly releasing virions (9, 15). In this regard, a prominent feature of macrophage infection, observed both in vivo and in vitro, is their capacity to actively synthesize and accumulate virions in subcellular compartments, the VCC (11).
Previously, VCC were ascribed to the multivesicular body secretory apparatus on the basis of the common expression of some markers, such as CD9, CD63, and CD81 (23). More recently, VCC have been considered to represent invaginations of the plasma membrane areas enriched in tetraspanins that are sequestered intracellularly, also expressing CD36 (24). Thus, VCC represent a unique feature of macrophage infection, and their dimension and virion load are highly sensitive to the state of macrophage activation consequent to extracellular signals present in the microenvironment. In this regard, we previously reported that stimulation of PMA-differentiated U1 cells with IFN-γ (D-U1 cells) leads to a significant redirection of the major site of virion budding from the plasma membrane to VCC (17). Later on, we observed the same phenomenon in both D-U1 cells and primary infected MDM when cells were stimulated with either uPA or ligands of the CD11b/CD18 (Mac-1) integrin (39). Of interest, Fantuzzi et al. (51) reported that blockade of the secretion of the endogenous chemokine CCL2, a biomarker of HIV-1 encephalitis and AIDS-dementia complex, leads to the accumulation of HIV-1 virions in primary MDM (20). In this present study, we found that eATP represents a microenvironmental stimulus capable of inducing rapid discharge of VCC-associated virions from infected macrophages. Given that at least one-third of VCC have been reported to be connected to the plasma membrane (11, 21), the gross morphological changes associated with eATP stimulation, resulting in cell contraction and membrane blebbing, could lead to an increased tension of the plasma membrane. VCC may then open up, bringing important amounts of membranes to the plasma membrane and thereby reducing the tension along with the release of the VCC content in the extracellular milieu.
In our experiments, eATP-induced virion release was not consequent to necrotic cell death, as determined by LDH release, or to apoptosis or pyroptosis (dependent on cleavage of caspase-3 or caspase-1, respectively), although we cannot exclude the possibility that other proinflammatory signals could be released by this experimental procedure.
Of interest is the fact that eATP-stimulated virion release was prevented by imipramine, an antidepressant agent. In this regard, in addition to its multiple effects on serotonine, dopamine, and other neuromediators, imipramine has been shown to inhibit aSMase, a membrane-associated enzyme that hydrolyzes sphingomyelin into ceramide, thereby triggering membrane reorganization with formation of microvesicles (30), also termed ectosomes (52). Thus, the microvesicle/ectosomal route could be a relevant eATP-inducible pathway leading to the release of VCC-associated virions. This hypothesis is reinforced by the recent observation that microvesicles and exosomes are involved in the spread of infection from infected MDM to uninfected cells as a potential strategy to avoid the immune recognition of infected cells (23).
ATP is a nucleoside triphosphate involved in multiple enzymatic intracellular reactions as a coenzyme and energy-transfer molecule (32). ATP synthesis occurs in the mitochondria and represents the major energy source for the cells. In addition, once actively or passively released in the extracellular environment, ATP (along with ADP and adenosine) acts as a potent signaling molecule acting via interaction with purinergic R, classically subdivided into P2Y and P2X R families (26). Whereas P2YR are seven-transmembrane metabotropic R coupled with GTP, the activation of which results in the induction of Ca2+ fluxes, P2XR are ionotropic, Ca2+-permeable ion channels that open on ligand binding (26). Cell stimulation by eATP can lead to cell death or to the activation of different secretory pathways such as exosomes, autophagolysosome fusion with the cell membrane, and microvesicle formation and release (35).
Concerning the early steps of HIV-1 infection, Perfettini et al. (53) have shown that exposure of CD4+ cells to HIV-1 leads to the release of ATP through pannexin-1 hemichannels. The released eATP then would act in an autocrine/paracrine fashion to activate purinergic R, in particular P2Y2, inducing phosphorylation/recruitment of the proline-rich tyrosine kinase-2 (Pyk2) (53). Indeed, pannexin-1, P2Y2, and Pyk2 have been shown to be recruited in the contact site between HIV-1 Env-expressing and CD4/CXCR4-containing membranes, suggesting that they act within the virologic synapse to enhance membrane–membrane fusion and facilitate cell-to-cell virus transmission (53, 54). Later, Hazleton and Eugenin (55) showed that antagonists of P2X1R, P2X7R, and P2Y1R blocked virus replication in MDM, demonstrating that eATP binds to and activates P2X1R, causing Ca2+ fluxes that facilitate HIV entry. According to those authors, sufficient ATP accumulates over time and activates P2X7R, leading to further Ca2+ fluxes and downstream signaling events. Finally, ATP would be converted to ADP, activating a P2Y1R-dependent cascade that facilitates later stages in the HIV life cycle (55). Of note, both groups demonstrated that HIV-1 triggered the release of ATP within 5–15 min after cell exposure, and that the endogenously released eATP facilitated the first steps of infection through an autocrine activation of purinergic Rs.
As mentioned earlier, P2X7R is known to require high concentrations (> 500 µM) of eATP for its activation, which otherwise occurs in a few minutes (32, 35). P2X7R activation leads to the opening of pannexin-1 channels, formation of membrane blebs, and expression of phosphatidyl-serine on the cell surface. If R stimulation lasts for only a short time (i.e., minutes), then this process, also termed “pseudoapoptosis,” is characterized by the opening of membrane pores, release of exosomes, microvesicles, and other particles (32, 33, 56). In this regard, we have demonstrated that oATP, the inactive form of ATP, does not induce the release of virions from infected cells and is an antagonist of eATP, at least in D-U1 cells. We also have shown that preincubation of either MDM or D-U1 cells with A-438079, a selective inhibitor of P2X7R, impairs the release of eATP-mediated HIV-1 virions. Of note, different P2X7R antagonists are currently being studied in clinical trials for treatment of rheumatoid arthritis (57).
In summary, our study adds evidence about the potential role of eATP in HIV-1 infection. In addition to favoring the initial entry phase of infection, as reported by independent investigators, it can induce the release of virions from primary human MDM, an accepted surrogate model of tissue macrophages. Our study suggests that the use of impramine may influence the dynamics of the macrophage-associated virion reservoir, whereas triggering of P2X7R may lead to the rapid release of infectious HIV-1 from macrophages. We believe that these observations have relevance for the potential elimination of infected macrophages by either pharmacologic or immune-mediated effectors.
Materials and Methods
Reagents.
Ficoll-Hypaque was purchased from Amersham Biosciences Europe, and Percoll was purchased from GE Healthcare. DMEM, PBS, FBS, normal human serum (NHS), penicillin, streptomycin, and glutamine were purchased from Lonza. IL-2 was purchased from Novartis. Ruthenium red (RR), PMA, phytohemagglutinin, ATP, oxidized ATP, and the P2X7 inhibitor A-438079 were purchased from Sigma-Aldrich.
Human uPA (molecular weight, 52 kDa) was provided by Dr. Jack Henkin, Abbott Laboratories, Abbott Park, IL and Dr. Andrew P. Mazar, Chemistry of Life Processes Institute, Evanston, IL. Imipramine (Tofranil; Novartisfarma) was kindly provided by Dr. Claudia Verderio, University of Milan, Milan, Italy. Anti-human anti-P2X7R mAb was purchased from Origene Technologies. The cytotoxic detection kit for LDH was purchased from Roche Applied Science.
Isolation of Human Monocytes from Peripheral Blood Mononuclear Cells and Differentiation into MDM.
Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coats of healthy HIV-seronegative blood donors by Ficoll-Hypaque density gradient centrifugation. The monocytes were then obtained by Percoll density gradient centrifugation reaching 80–90% purity, as determined by CD14 expression and other lineage markers, as described previously (58). The cells were then washed and resuspended in DMEM containing pen/strep (1%), glutamine (1%), heat-inactivated FBS (10%), and heat-inactivated NHS (5%) (Complete Medium). Monocytes were seeded into 48-well plastic plates at a concentration of 5 × 105 cells/mL and were cultivated for 7–8 additional days at 37 °C in 5% CO2 to promote their full differentiation into MDM (≥95% CD14+), as described previously (19).
Chronically HIV-1–Infected Promonocytic U1 Cell Line and D-U1 Cells.
The promonocytic U1 cell line was obtained from a population of U937 cells surviving the cytopathic effect of acute CXCR4-dependent HIV-1LAI/IIIB infection (59). Each U1 cell contains two copies of integrated proviruses (60). No virus production is detected by conventional RT activity assay or p24 Gag antigen ELISA in cultures of unstimulated U1 cells, but virus expression at levels comparable to those achieved at peak of acute virus infection of parental U937 cells or primary MDM and T-cell blasts is rapidly induced by stimulation of U1 cells with PMA or several proinflammatory cytokines (25). U1 cells (5 × 105 cells/mL, unless indicated otherwise, in RPMI 1640 containing 10% of heat-inactivated FCS, 1% pen/strep, and 1% glutamine) were incubated with PMA + uPA to promote their differentiation to macrophage-like cells displaying abundant VCC (D-U1 cells).
CCR5-Dependent (R5) HIV-1 Infection of MDM.
Human MDM were infected with the macrophage-tropic, laboratory-adapted R5 HIV-1BaL strain at the multiplicity of infection (moi) of 0.1. Multiple aliquots of culture supernatants were collected every 3 d over a 5-wk period and stored at −20 °C to assess virus production. At the end of each infection experiment, supernatants were thawed and analyzed for their viral content by measuring the levels of virion-associated Mg2+-dependent RT activity present in the supernatant, with a radioactive assay. Considering that 99% of the RT enzyme is virion-associated, this assay is a faithful indication of the production of new progeny virions (31).
Infectivity of VCC-Release HIV-1 Virions.
The TZM-bl cell line was generated from CXCR4+ HeLa cells engineered to express CD4 and CCR5 as well (61), and contains integrated reporter genes for firefly luciferase (luc) and Escherichia coli β-galactosidase (β-Gal) under control of an HIV-1 LTR, thereby permitting sensitive and accurate measurements of infection (34).
TZM-bl cells were cultured in DMEM containing pen/strep (1%), glutamine (1%), and heat-inactivated FBS (10%). For infectivity assays, virion-containing supernatants were added on these cells, and the infectious titer was determined by measuring luc levels. In brief, the supernatants were incubated with 30,000 TZM-bl cells for 30 min, replaced with fresh medium, and cultured for additional 24 h. Then luminescent detection of luc activity was performed in the cell lysates using the Dual-Glo Luciferase Assay System (Promega).
Supernatents from infected MDM were incubated with autologous CD4+ T lymphocytes that were previously frozen at the time of PBMC isolation. T cells were prestimulated with PHA (5 mg/mL) for 3 d, then washed and resuspended in RPMI 1640, 10% FCS supplemented with IL-2 (450 U/mL).
Detection of Intracellular HIV-1 p24 Gag Antigen by Flow Cytometry.
Intracellular p24 Gag expression was analyzed by fixing and permeabilizing 2 × 105 cells using a Cytofix/Cytoperm Kit (BD Biosciences).After fixing, cells were washed with Perm/Wash buffer (BD Biosciences) and permeabilized, then stained for 20 min at room temperature with FITC-conjugated mouse anti-p24 mAb (clone KC57; Beckman Coulter) in 100 µL of Perm/Wash buffer. Stained cells were washed with Perm/Wash buffer and resuspended in 2% PFA, followed by flow cytometry analysis. The events were analyzed with FlowJo version 8.8.7 (Tree Star).
Live Imaging of HIV Infection of MDM.
HIV Gag-iGFP ΔEnv viruses were produced by transfection of the corresponding proviral cDNA in 293T cells (ATCC CRL-11268; American Type Culture Collection) with polyethylenimine. The plasmid pMD2.G (Addgene) was used for pseudotyping. Supernatants were harvested at 72 h after transfection, and then subjected to ultracentrifugation at 31,000 × g for 90 min. Pellets were resuspended in RPMI (Gibco, Life Technologies) with 20% FBS. As described previously (62), virus preparations were titrated by infecting the Ghost reporter cell line, and their infectious titer was determined at 24 h after infection in terms of percentage of GFP+ cells detected by flow cytometry using an Accuri C6 flow cytometer (BD).
For live-imaging experiments, monocytes were enriched from PBMCs of healthy donors by magnetic cell sorting by CD14-positive selection (MACS; Miltenyi), seeded on eight-well Labtek II plastic chambers (Nunc; Thermo Fisher Scientific) or a FluoroDish with a glass bottom (World Precision Instruments) and imaged after 4–5 d of infection.
Live imaging was performed on an inverted microscope Nikon TE2000-E, equipped with a piezo stage NanoScanZ mounted on a Marzhauser XYZ motorized scanning stage (Nikon Instruments France), with images recorded on a CoolSNAP HQ2 camera (Photometrics). Cells were incubated in Complete Medium enriched with Hepes (20 mM). Videos were acquired at 37 °C (LIS Cube Box; Life Imaging Services) using a 60× oil immersion objective. The microscope was driven by MetaMorph software (Molecular Devices). ImageJ software (National Institutes of Health) was used for image processing.
Ultrastructural Analysis.
At 15 d PI, coinciding with their peak of virus production, human MDM were stimulated with ATP for up to 30 min. After stimulation, the cells were washed and scraped with a rubber policeman and analyzed by transmission electron microscopy (TEM). The cells were fixed for 2 h at 4 °C with 4% paraformaldehyde and 2.5% glutaraldehyde in 125 mM cacodylate buffer, then postfixed for 1 h with 2% OsO4 in 125 mM cacodylate buffer, washed, and embedded in EPON. Conventional thin sections were collected on uncoated grids, stained with uranil and lead citrate, and examined in a Leo912-Omega transmission electron microscope (Carl Zeiss).
For RR staining, infected MDM were washed and fixed in a solution containing 1.2% glutaraldehyde and 0.5 mg/mL RR in 66 mM NaCacodylate (pH 7.1) and kept for 1 h at room temperature. Then the cells were quickly washed twice in 150 mM NaCacodylate and postfixed in 1.3% OsO4 plus 0.5 mg/mL RR in 33 mM NaCacodylate for 2 h at room temperature. The cells were dehydrated with a 70% EtOH (twice for 5 min), 95% EtOH (twice for 10 min), and 100% EtOH (twice for 30 min), then infiltrated with EPON/100% EtOH overnight. Finally the cells were embedded in fresh EPON. Conventional thin sections were collected on uncoated grids, stained with uranil and lead citrate, and examined in a Leo912-Omega transmission electron microscope.
Western Blot Analysis.
The expression of P2X7R in U1 cells and human MDM was determined by Western blot analysis. The cells were lysed in Nonidet P-40 buffer (50 mM Tris HCl pH 7.5, 150 mM NaCl, 1% Nonidet P-40, and 0.5% wt/vol deoxycholate) containing protease inhibitors. The protein concentration of cell lysates was measured by the Bio-Rad protein assay, based on the Bradford method. Proteins were separated by 10% SDS/PAGE, transferred to a nitrocellulose membrane by electroblotting, and probed with anti-P2X7R rabbit mAb (Origene Technologies), anti-caspase 3 rabbit mAb (clone 8G10; Cell Signaling Technology), or anti-caspase 1 rabbit mAb (clone C-20; Santa Cruz Biotechnology). Anti-actin goat polyclonal Ab (clone I-19; Santa Cruz Biotechnology) or rabbit anti-GAPDH mAb (Cell Signaling Technology) served as a control.
Statistical Analysis.
Statistical analyses were performed using the GraphPad Prism version 5.0 (www.graphpad.com). Results are reported as mean ± SE. Comparisons between two groups were performed using an unpaired, two-tailed t test. P values < 0.05 were considered significant. To control for interdonor variability, all assays were performed in triplicate using MDM derived from three to nine independent donors, as specified in the text.
Supplementary Material
Acknowledgments
We thank Dr. Elisa Vicenzi (San Raffaele Scientific Institute) for helpful discussions. This study was supported in part by grants from the Agence Nationale de Recherche contre le SIDA (ANRS) and Ensemble contre le SIDA (Sidaction) (to P.B.), including an ANRS fellowship to M.D., and by the EMBO short-term fellowship program (to FG.). F.G. performed this study as partial fulfillment of her PhD in Molecular Medicine at Vita-Salute San Raffaele University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500656112/-/DCSupplemental.
References
- 1.Cobos-Jiménez V, Booiman T, Hamann J, Kootstra NA. Macrophages and HIV-1. Curr Opin HIV AIDS. 2011;6(5):385–390. doi: 10.1097/COH.0b013e3283497203. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson M. HIV-1 pathogenesis. Nat Med. 2003;9(7):853–860. doi: 10.1038/nm0703-853. [DOI] [PubMed] [Google Scholar]
- 3.Coiras M, López-Huertas MR, Pérez-Olmeda M, Alcamí J. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009;7(11):798–812. doi: 10.1038/nrmicro2223. [DOI] [PubMed] [Google Scholar]
- 4.Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74(5):635–641. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- 5.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37(3):377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deeks SG, et al. International AIDS Society Scientific Working Group on HIV Cure Towards an HIV cure: A global scientific strategy. Nat Rev Immunol. 2012;12(8):607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharova N, Swingler C, Sharkey M, Stevenson M. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 2005;24(13):2481–2489. doi: 10.1038/sj.emboj.7600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambotte O, Deiva K, Tardieu M. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain Pathol. 2003;13(1):95–103. doi: 10.1111/j.1750-3639.2003.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watters SA, Mlcochova P, Gupta RK. Macrophages: The neglected barrier to eradication. Curr Opin Infect Dis. 2013;26(6):561–566. doi: 10.1097/QCO.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 10.Orenstein JM, Meltzer MS, Phipps T, Gendelman HE. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1–treated human monocytes: an ultrastructural study. J Virol. 1988;62(8):2578–2586. doi: 10.1128/jvi.62.8.2578-2586.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudin R, et al. Dynamics of HIV-containing compartments in macrophages reveal sequestration of virions and transient surface connections. PLoS ONE. 2013;8(7):e69450. doi: 10.1371/journal.pone.0069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol. 2007;177(2):329–341. doi: 10.1083/jcb.200609050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan J, Sattentau QJ. The HIV-1–containing macrophage compartment: A perfect cellular niche? Trends Microbiol. 2013;21(8):405–412. doi: 10.1016/j.tim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci USA. 2003;100(19):10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koppensteiner H, Banning C, Schneider C, Hohenberg H, Schindler M. Macrophage internal HIV-1 is protected from neutralizing antibodies. J Virol. 2012;86(5):2826–2836. doi: 10.1128/JVI.05915-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aquaro S, Perno CF. Assessing the relative efficacy of antiretroviral activity of different drugs on macrophages. Methods Mol Biol. 2005;304:445–453. doi: 10.1385/1-59259-907-9:445. [DOI] [PubMed] [Google Scholar]
- 17.Biswas P, et al. Interferon gamma induces the expression of human immunodeficiency virus in persistently infected promonocytic cells (U1) and redirects the production of virions to intracytoplasmic vacuoles in phorbol myristate acetate-differentiated U1 cells. J Exp Med. 1992;176(3):739–750. doi: 10.1084/jem.176.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfano M, Sidenius N, Blasi F, Poli G. The role of urokinase-type plasminogen activator (uPA)/uPA receptor in HIV-1 infection. J Leukoc Biol. 2003;74(5):750–756. doi: 10.1189/jlb.0403176. [DOI] [PubMed] [Google Scholar]
- 19.Graziano F, Elia C, Laudanna C, Poli G, Alfano M. Urokinase plasminogen activator inhibits HIV virion release from macrophage-differentiated chronically infected cells via activation of RhoA and PKCε. PLoS ONE. 2011;6(8):e23674. doi: 10.1371/journal.pone.0023674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantuzzi L, et al. Endogenous CCL2 (monocyte chemotactic protein-1) modulates human immunodeficiency virus type-1 replication and affects cytoskeleton organization in human monocyte-derived macrophages. Blood. 2003;102(7):2334–2337. doi: 10.1182/blood-2002-10-3275. [DOI] [PubMed] [Google Scholar]
- 21.Gaudin R, et al. Critical role for the kinesin KIF3A in the HIV life cycle in primary human macrophages. J Cell Biol. 2012;199(3):467–479. doi: 10.1083/jcb.201201144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mlcochova P, Pelchen-Matthews A, Marsh M. Organization and regulation of intracellular plasma membrane-connected HIV-1 assembly compartments in macrophages. BMC Biol. 2013;11:89. doi: 10.1186/1741-7007-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadiu I, Narayanasamy P, Dash PK, Zhang W, Gendelman HE. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J Immunol. 2012;189(2):744–754. doi: 10.4049/jimmunol.1102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berre S, et al. CD36-specific antibodies block release of HIV-1 from infected primary macrophages and its transmission to T cells. J Exp Med. 2013;210(12):2523–2538. doi: 10.1084/jem.20130566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benaroch P, Billard E, Gaudin R, Schindler M, Jouve M. HIV-1 assembly in macrophages. Retrovirology. 2010;7:29. doi: 10.1186/1742-4690-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509(7500):310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob F, Pérez Novo C, Bachert C, Van Crombruggen K. Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal. 2013;9(3):285–306. doi: 10.1007/s11302-013-9357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elia C, et al. Inhibition of HIV replication by the plasminogen activator is dependent on vitronectin-mediated cell adhesion. J Leukoc Biol. 2007;82(5):1212–1220. doi: 10.1189/jlb.0407251. [DOI] [PubMed] [Google Scholar]
- 29.Liangpunsakul S, et al. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am J Physiol Gastrointest Liver Physiol. 2012;302(5):G515–G523. doi: 10.1152/ajpgi.00455.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianco F, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28(8):1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernie BF, Poli G, Fauci AS. Alpha interferon suppresses virion but not soluble human immunodeficiency virus antigen production in chronically infected T-lymphocytic cells. J Virol. 1991;65(7):3968–3971. doi: 10.1128/jvi.65.7.3968-3971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trautmann A. Extracellular ATP in the immune system: More than just a “danger signal”. Sci Signal. 2009;2(56):pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 33.Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J Immunol. 2003;170(11):5728–5738. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- 34.Polonis VR, et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology. 2008;375(2):315–320. doi: 10.1016/j.virol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Dubyak GR. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol. 2012;14(11):1697–1706. doi: 10.1111/cmi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78(5):321–332. doi: 10.1111/j.1399-0039.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 37.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doitsh G, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505(7484):509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alfano M, Sidenius N, Panzeri B, Blasi F, Poli G. Urokinase-urokinase receptor interaction mediates an inhibitory signal for HIV-1 replication. Proc Natl Acad Sci USA. 2002;99(13):8862–8867. doi: 10.1073/pnas.142078099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11(3):213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- 41.Li J, et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat Immunol. 2013;14(8):793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- 42.Turola E, Furlan R, Bianco F, Matteoli M, Verderio C. Microglial microvesicle secretion and intercellular signaling. Front Physiol. 2012;3:149. doi: 10.3389/fphys.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee BH, et al. Activation of P2X(7) receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection. PLoS ONE. 2012;7(4):e35812. doi: 10.1371/journal.pone.0035812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulinelli S, et al. IL-18 associates to microvesicles shed from human macrophages by an LPS/TLR-4 independent mechanism in response to P2X receptor stimulation. Eur J Immunol. 2012;42(12):3334–3345. doi: 10.1002/eji.201142268. [DOI] [PubMed] [Google Scholar]
- 45.Bergamaschi A, Pancino G. Host hindrance to HIV-1 replication in monocytes and macrophages. Retrovirology. 2010;7:31. doi: 10.1186/1742-4690-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254(1):102–113. doi: 10.1111/imr.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: Lessons from human and nonhuman primate studies. J Neurovirol. 2008;14(4):318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer-Hämmerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111(2):194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS. 2014;28(15):2175–2187. doi: 10.1097/QAD.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamashita M, Emerman M. Retroviral infection of non-dividing cells: Old and new perspectives. Virology. 2006;344(1):88–93. doi: 10.1016/j.virol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Sozzani S, et al. MCP-1 and CCR2 in HIV infection: Regulation of agonist and receptor expression. J Leukoc Biol. 1997;62(1):30–33. doi: 10.1002/jlb.62.1.30. [DOI] [PubMed] [Google Scholar]
- 52.Cocucci E, Meldolesi J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Séror C, et al. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med. 2011;208(9):1823–1834. doi: 10.1084/jem.20101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paoletti A, et al. Editorial: Pannexin-1—the hidden gatekeeper for HIV-1. J Leukoc Biol. 2013;94(3):390–392. doi: 10.1189/jlb.0313148. [DOI] [PubMed] [Google Scholar]
- 55.Hazleton JE, Berman JW, Eugenin EA. Purinergic receptors are required for HIV-1 infection of primary human macrophages. J Immunol. 2012;188(9):4488–4495. doi: 10.4049/jimmunol.1102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mackenzie AB, Young MT, Adinolfi E, Surprenant A. Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J Biol Chem. 2005;280(40):33968–33976. doi: 10.1074/jbc.M502705200. [DOI] [PubMed] [Google Scholar]
- 57.Gunosewoyo H, Kassiou M. P2X purinergic receptor ligands: Recently patented compounds. Expert Opin Ther Pat. 2010;20(5):625–646. doi: 10.1517/13543771003702424. [DOI] [PubMed] [Google Scholar]
- 58.Colotta F, Peri G, Villa A, Mantovani A. Rapid killing of actinomycin D-treated tumor cells by human mononuclear cells, I: Effectors belong to the monocyte-macrophage lineage. J Immunol. 1984;132(2):936–944. [PubMed] [Google Scholar]
- 59.Folks TM, et al. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. J Immunol. 1988;140(4):1117–1122. [PubMed] [Google Scholar]
- 60.Emiliani S, et al. Mutations in the tat gene are responsible for human immunodeficiency virus type 1 postintegration latency in the U1 cell line. J Virol. 1998;72(2):1666–1670. doi: 10.1128/jvi.72.2.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72(4):2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mörner A, et al. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J Virol. 1999;73(3):2343–2349. doi: 10.1128/jvi.73.3.2343-2349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.