Abstract
Blastomycosis is a fungal infection rarely seen in clinical practice. Endemic to the Midwestern United States as well as the Canadian provinces of Manitoba and Ontario, Blastomyces dermatitidis characteristically involves the skin and lungs. Central nervous system (CNS) involvement, although a rare complication of this disease, can be fatal. The current literature on CNS blastomycosis primarily centers on the spectrum of traditional imaging features of T1- and T2-weighted imaging with which this entity can present. However, here we present the direct histopathologic correlation of the imaging findings of solitary mass like CNS blastomycosis, with an emphasis on the association of diffusion restriction within the lesion with a granulomatous immune response.
Keywords: Blastomyces dermatitidis, CNS, diffusion-weighted imaging, solitary
INTRODUCTION

Costas Stavrakis
Cerebral blastomycosis is a rare entity with a highly variable clinical presentation. We report a very interesting case specifically correlating the histopathologic findings of necrotizing granulomatous response to the foci of restricted diffusion.
The patient, a 58-year-old Caucasian female, presented to her primary care physician with a 6-week history of dizziness on standing. She did not describe the dizziness as “the room was spinning”, but simply that she felt off balance whenever she stood up too quickly, particularly when getting out of her vehicle. Past surgical history included a left knee replacement done 7 months earlier. The patient did not smoke, drink, or use recreational drugs. She denied any recent travel. Aside from dizziness, she denied any other symptomatic complaints. Physical examination, including complete neurological interrogation, was normal.
Magnetic resonance imaging (MRI) of the brain demonstrated a peripherally enhancing lesion in the right temporoparietal region, suspicious of an abscess. Given the imaging findings, the decision was made to perform surgical resection. On microscopic analysis the leasion was found to be a Blastomyces dermatitidis abscess. The patient was started on Voriconazole therapy after surgical resection of the abscess.
RADIOLOGIC FEATURES
Preoperative MRI [Figure 1] demonstrated a well-circumscribed peripherally enhancing lesion within the right temporoparietal region with marked surrounding vasogenic edema. Of particular note were multiple rounded foci of reduced diffusion within the central aspect of the lesion. These foci were distinctly separate from each other and did not appear to be confluent at the time of the examination. Also, MRI of the brain as well as intraoperative computed tomography (CT) [Figure 2] demonstrated no evidence of paranasal sinus disease at the time of presentation. Given the imaging findings and peripheral location of the lesion, the decision was made for surgical resection. Differential considerations prior to resection included fungal/tuberculous/bacterial abscess, primary glial neoplasm with central necrosis, as well as metastatic disease from an unknown primary malignancy.
Figure 1.
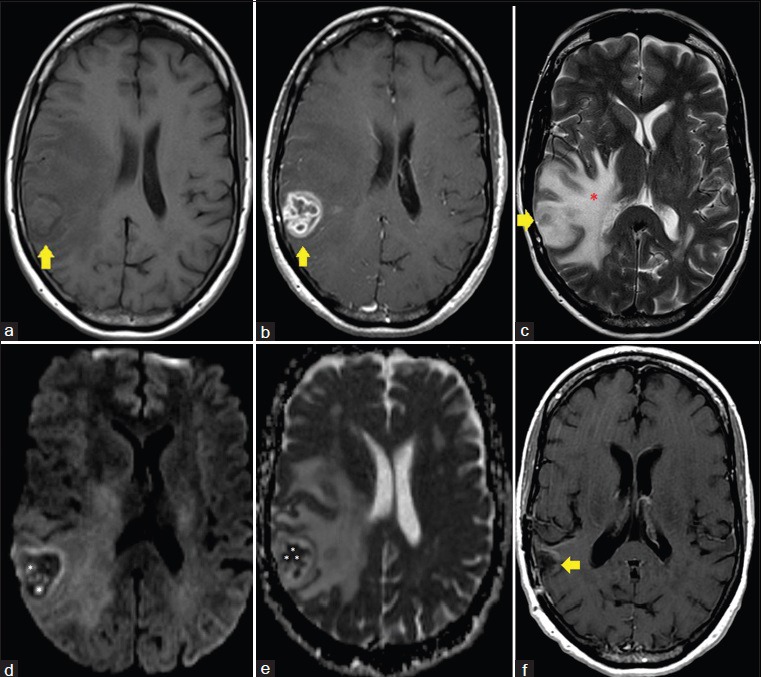
58-year-old Caucasian female presented with a 6-week history of dizziness upon standing and was diagnosed with cerebral blastomycosis. (a) Axial T1 image, pre-gadolinium administration, shows a 3.0 cm anteroposterior × 2.4 cm transverse × 3.1 cm craniocaudal lesion within the right temporoparietal region (yellow arrow). (b) Axial T1 image, post-gadolinium administration, shows a 3.0 cm anteroposterior × 2.4 cm transverse × 3.1 cm craniocaudal enhancing lesion within the right temporoparietal region (yellow arrow). (c) Axial T2 image demonstrates marked surrounding vasogenic edema throughout the right cerebral hemisphere (red *). (d) Axial diffusion-weighted image demonstrates multiple rounded foci of reduced diffusivity within the central aspect of the lesion (white *). (e) Axial apparent diffusion coefficient (ADC) map image demonstrates multiple rounded foci of reduced diffusivity within the central aspect of the lesion (white *). (f) Axial T1 image, post-gadolinium administration, following resection and antifungal treatment of the lesion demonstrates a resection cavity in the area of the previously seen enhancing lesion with no evidence of residual enhancing infection. The patient is asymptomatic 8 months post resection.
Figure 2.
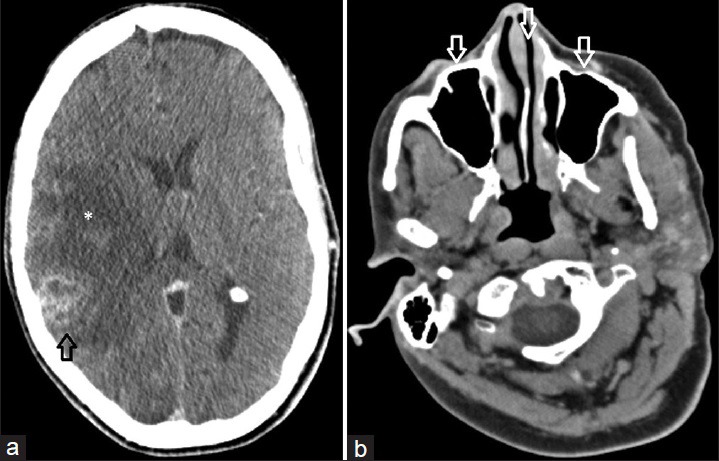
58-year-old Caucasian female presented with a 6-week history of dizziness upon standing and was diagnosed with cerebral blastomycosis. (a) Axial contrast enhanced CT image of the head obtained intraoperatively demonstrates the rim enhancing lesion within the right temporoparietal region (black arrow) resulting in significant surrounding edema (*). (b) Intraoperative axial contrast enhanced CT image at the level of the paranasal sinuses demonstrates normal aeration of the paranasal sinuses (white arrows) without evidence of mucosal thickening or other abnormality.
PATHOLOGIC FEATURES
Upon removal, the mass was found to be hard, rubbery, pedunculated, virtually avascular, and was removed as a single piece. Some purulent material was expressed from the resected mass and sent for culture. Cultures of the purulent material returned positive for B. dermatitidis. Microscopic analysis of the specimen [Figure 3] showed necrotizing granulomatous inflammation with yeast-forming fungi, consistent with B. dermatitidis.
Figure 3.
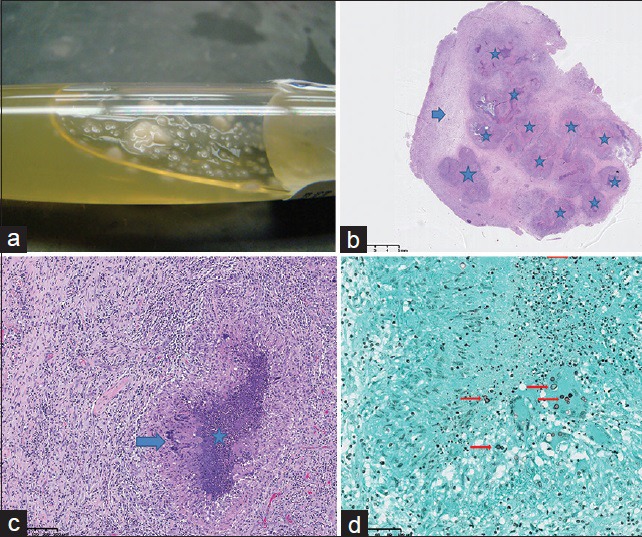
58-year-old Caucasian female presented with a 6-week history of dizziness upon standing and was diagnosed with cerebral blastomycosis. (a) Photograph of aspirated fluid from the parenchymal abscess demonstrates yeast formation (white droplets) within purulent fluid. (b) Photomicrograph of microscopic examination of the resected lesion: low-power view with 1.5 × magnification shows multiple necrotizing granulomas (stars) and a rim of reactive brain tissue (arrow). The granulomas correspond to areas of decreased signal seen on diffusion-weighted imaging. (c) Photomicrograph of microscopic examination of the resected lesion: high-power view of the granuloma with 40 × magnification shows a rim of palisading histiocytes (arrow) with central necrosis (star). d) Photomicrograph of microscopic examination of the resected lesion. stained with Grocott-methenamine silver (GMS) stain at 40× magnification, highlights the budding yeast of Blastomyces dermatitidis (arrows).
DISCUSSION
B. dermatitidis is a fungus found in certain regions of the United States and Canada. Endemic areas include the Ohio and Mississippi River valleys as well as Midwestern states and Canadian provinces that border the Great Lakes.[1] In certain cases, it can be a cause of an obscure infection in a non-endemic area.
The optimal habitat for Blastomyces colonization is wetland enriched with animal droppings and decaying vegetation.[2] Disruption of the soil containing this fungus results in the release of infectious conidia which can be subsequently inhaled by the host. Inhalation is the most common route of transmission, followed by cutaneous inoculation via direct penetration which is much less common.[2] Transmissions via dog bites have also been reported in the literature.[3] B. dermatitidis is generally not transmitted from person to person, and therefore, is not considered contagious.[2]
Patient presentation is highly variable and can range from subclinical infection to rapidly progressive dissemination and death. Constitutional symptoms such as weight loss, malaise, and fatigue can be seen.[2] Host responses that help prevent infection and dissemination include phagocytosis of the inhaled conidia via alveolar macrophages, neutrophils, and monocytes. When these responses fail, lymphohematogenous dissemination may develop.[2] Unlike other opportunistic infections, the likelihood of infection with B. dermatitidis is equal among immunocompetent and immunosuppressed individuals.[2] However, infection does tend to be more aggressive in people with compromised cellular immunity.[2] Lymphatic and hematogenous spread may ensue to potentially any organ.[4] After lungs, other sites of disease involvement include skin, bone, genitourinary system, and the CNS, in order of decreasing frequency.[2]
CNS involvement is seen in 5–10% of disseminated blastomycosis cases.[2] CNS blastomycosis without evidence of pulmonary or other organ system involvement, such as in the patient presented here, is a rare occurrence. The most common primary CNS presentation is basilar leptomeningitis. The next most common presentation is as either solitary or multiple brain parenchymal masses. Finally, CNS infection can also present as an epidural abscess, whether in the spinal canal or intra-cranially.
Many patients are initially identified through screening CT, in which if there are focal lesions, these will show low attenuation. However, the preferred imaging modality in patients with suspected CNS infection is contrast-enhanced MRI. On MRI, pyogenic bacterial parenchymal abscesses typically will have a robustly enhancing peripheral rim of tissue that correlates with the host response and capsule formation. Centrally, pyogenic abscesses will display reduced diffusion correlating with purulent material, which is typically composed of cellular debris and inflammatory cells such as polymorphonuclear cells of the immune system.[5] However, the case presented here indicates that a host inflammatory response of granuloma formation can also display similar findings of reduced diffusion. As shown in Figure 1d and e, the infectious lesion displayed small rounded foci of reduced diffusion, which ultimately correlated with multiple necrotizing granulomas on histologic section [Figure 3b]. This would also appear to correlate with the findings of reduced diffusion of intracavitary projections reported by Luthra et al.,[6] rather than in the central aspect of the cavity as is typical in pyogenic abscesses.[5] Thus, the inflammatory reaction of each individual necrotizing granuloma is reflected as a solitary focus of reduced diffusion, which has yet to be reported in the literature on CNS blastomycosis.
The imaging characteristics of solitary parenchymal CNS blastomycosis infection can be difficult to differentiate from other fungal entities and the differential should include histoplasmosis and coccidioidomycosis.
Similar to blastomycosis, Histoplasma capsulatum is endemic to the Midwestern USA and typically manifests in the setting of pulmonary infection. Intra-cranially, the fungus typically manifests as “histoplasmomas,” appearing as round lesions with peripheral ring enhancement, usually small and measuring < 2 cm in size. These are T1 hypointense and can have various signal intensities on T2-weighted imaging.[6] Degree of diffusivity can also range in appearance within the lesions. Multifocal disseminated infection is more common than singular lesions, and diffuse meningeal infection has been reported.[6] Coccidioides immitis also displays imaging features similar to both histoplasmosis and blastomycosis, although basilar leptomeningitis is the most common presentation rather than parenchymal masses. However, the main differentiating feature of coccidioidmycosis infection is not the imaging presentation, but travel history to the southwest USA, where the fungus is endemic. This is as opposed to both blastomycosis and histoplasmosis, which are both endemic to the Midwestern USA.[6]
Ultimate diagnosis of isolated fungal CNS infection can be challenging because identifying the organism in cerebrospinal fluid (CSF) culture has a low sensitivity. In one study, only 2 of 22 patients with CNS blastomycosis had a positive CSF culture.[7] CSF analysis will usually show a pleiocytosis with a neutrophilic or lymphocytic predominance.[2] If there is a diagnostic concern for blastomycosis, detection of the B. dermatitidis antigen rather than culture has been shown to be useful. However, cross reactions may occur with other fungal antigens, such as H. capsulatum.[7] Invasive procedures such as tissue sampling via biopsy and resection may have better diagnostic potential,[7] and as in the case presented here, may be nearly curative. On histopathology, tissue specimens will show the host's response to the infection as inflammatory reaction of polymorphonuclear leukocytes in a cluster of granulomas, usually of the non-caseating type.[2] Budding yeast cells with capsules will be seen, staining positive with Grocott-methenamine silver (GMS). Acid-fast stains can help distinguish B. dermatitidis from C. immitis, as it will be usually negative in the latter and weakly positive in the former.[2]
Treatment consists of antifungal formulations, with amphotericin-b being the drug of choice. Due to the possible risk of nephrotoxicity, azole antifungals can serve as a suitable substitute. Voriconazole has shown promise and continues to be used due to its ability to reach high concentrations within the CSF and brain tissue.[8] Most recently, a combination of surgical resection with antifungal therapy is considered the optimal management of solitary fungal brain abscesses.[1,2,9,10]
CONCLUSION
B. dermatitidis can result in a highly aggressive infection if left untreated. Although endemic to the Midwestern USA and Canada, it can present as an obscure infection in non-endemic areas. The case presented here highlights the imaging correlate of reduced diffusion within a solitary blastomycosis lesion of the brain with that of necrotizing granuloma formation. This is in contrast to pyogenic abscesses, in which reduced diffusion is indicative of purulent cellular debris. Also, solitary CNS involvement without pulmonary infection is a rare occurrence, not frequently reported in the literature. Although imaging characteristics can aid in the diagnosis of this entity, in the setting of isolated CNS infection, histopathology is really the only tool by which diagnosis can be confirmed with certainty. Optimal treatment of solitary CNS blastomycosis is surgical resection with antifungal therapy.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflict of interest.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/30/157854
REFERENCES
- 1.Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17:21–40. doi: 10.1016/s0891-5520(02)00038-7. vii. [DOI] [PubMed] [Google Scholar]
- 2.Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367–81. doi: 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gnann JW Jr, Bressler GS, Bodet CA, 3rd, Avent CK. Human blastomycosis after a dog bite. Ann Intern Med. 1983;98:48–9. doi: 10.7326/0003-4819-98-1-48. [DOI] [PubMed] [Google Scholar]
- 4.Fang W, Washington L, Kumar N. Imaging manifestations of blastomycosis: A pulmonary infection with potential dissemination. Radiographics. 2007;27:641–55. doi: 10.1148/rg.273065122. [DOI] [PubMed] [Google Scholar]
- 5.Luthra G, Parihar A, Nath K, Jaiswal S, Prasad KN, Husain N, et al. Comparative evaluation of fungal, tubercular, and pyogenic brain abscesses with conventional and diffusion MR imaging and proton MR spectroscopy. AJNR Am J Neuroradiol. 2007;28:1332–8. doi: 10.3174/ajnr.A0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starkey J, Moritani T, Kirby P. MRI of CNS fungal infections: Review of aspergillosis to histoplasmosis and everything in between. Clin Neuroradiol. 2014;24:217–30. doi: 10.1007/s00062-014-0305-7. [DOI] [PubMed] [Google Scholar]
- 7.Bariola RJ, Perry P, Pappas PG, Proia L, Shealey W, Wright PW, et al. Blastomycosis of the central nervous system: A multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis. 2010;50:797–804. doi: 10.1086/650579. [DOI] [PubMed] [Google Scholar]
- 8.Lutsar I, Roffey S, Troke P. Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin Infect Dis. 2003;37:728–32. doi: 10.1086/377131. [DOI] [PubMed] [Google Scholar]
- 9.Chapman SW, Dismukes WE, Proia LA, Bradsher RW, Pappas PG, Threlked MG, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Disease Society of America Clin Infect Dis. 2008;46:1801–12. doi: 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 10.Wylen EL, Nanda A. Blastomyces dermatitidis occurring as an isolated cerebellar mass. Neurosurg Rev. 1999;22:152–4. doi: 10.1007/s101430050053. [DOI] [PubMed] [Google Scholar]


