Abstract
The aims of this study were to assess the effectiveness of calcium silicate cement (Biodentine) versus glass ionomer cement (GIC; control group) as indirect pulp capping materials in patients with reversible pulpitis and to compare the effectiveness of cone beam computed tomography (CBCT) versus periapical (PA) radiographs in detecting PA changes at baseline (T0) and at 12 mo (T12) postoperatively. Seventy-two restorations (36 Biodentine, 36 Fuji IX) were placed randomly in 53 patients. CBCT/PA radiographs were taken at T0 and T12. Two calibrated examiners assessed the presence/absence and increase/decrease in the size of existing PA radiolucencies under standardized conditions. The Kappa coefficient evaluated statistically the effectiveness of CBCT versus PA radiographs in detecting PA changes. Chi-square/Mann-Whitney tests were used to evaluate the association between PA changes in CBCT with various clinical measures. Significance was predetermined at α = 0.05. Clinical success rates for Biodentine and Fuji IX GIC were 83.3%. CBCT was significantly more effective in detecting PA radiolucencies compared with radiographs (P = 0.0069). Of the teeth, 65.4% and 90.4% were deemed healthy using CBCT and PA radiographs, respectively, at T12. Healing/healed rates were 17.3%/0%, while new/progressed radiolucency were 30.8%/9.6% with CBCT/PA radiographs, respectively. Seventy-one percent of healed lesions had received Biodentine; 88% of new/progressed lesions received Fuji IX GIC. Teeth presenting with an initial CBCT PA lesion had a failure rate of 63%, whereas teeth with no initial lesion had a failure rate of 16%. Although no statistically significant difference was detected in the clinical efficacy of Biodentine/Fuji IX when used as indirect pulp capping materials in patients with reversible pulpitis, CBCT showed a significant difference in that most healed CBCT lesions had received Biodentine while most that did not heal received Fuji IX. Longer-term follow-up is needed to establish their effect on the healing dynamics of PA tissues (ClinicalTrials.gov NCT02201641).
Keywords: pulpitis, periapical disease, dental radiography, cone beam computed tomography, glass ionomer cements, dental caries
Introduction
There is no reliable objective method of evaluating clinically the extent of pulp inflammation and/or its pathological condition. Identifying reversible/irreversible pulpitis relies on patients’ subjective descriptions of symptoms, pulp sensibility testing, and radiographic examination (Bjørndal 2002; Pitt Ford and Patel 2004). In addition, treating deep carious lesions can prove to be challenging especially when approaching the pulp as an increased risk of pulp exposure reduces the predictability of the treatment outcome (Barthel et al. 2000; Bjørndal et al. 2010; Dammaschke et al. 2010). Indirect pulp capping (IPC) is one treatment modality that maintains pulp vitality by facilitating healing/repair (Tziafas et al. 2000). Calcium silicate cements (Biodentine; Septodont, Saint Maur des Fosses, France) can be used both for pulp capping and provisional restoration. Biodentine encourages dentine bridge formation with no inflammatory pulp response through secretion of transforming growth factor (TGF)–b1 (Laurent et al. 2012; Zanini et al. 2012; Nowicka et al. 2013). Glass ionomer cements (GICs) are used as liners/sealers and dentine replacement materials in the sandwich technique with no pulp exposure (Sidhu 2011). They exhibit biocompatibility with minimal cytotoxic effect when used indirectly over the pulp (Hume and Mount 1988; Six et al. 2000). Fuji IX (GC Corporation, Tokyo, Japan) contributes to carious dentine remineralization by releasing fluoride and strontium ions (Ngo et al. 2006).
Cone beam computed tomography (CBCT) is more sensitive than intraoral periapical (PA) radiography in detecting PA radiolucencies in teeth subsequently requiring root canal treatment (Estrela et al. 2008; Patel et al. 2009; Paula-Silva et al. 2009; Patel et al. 2012). To date, the ability of CBCT to detect PA changes longitudinally in teeth diagnosed clinically with reversible pulpitis, after minimally invasive (MI) treatment, has not been determined.
This randomized controlled clinical trial following CONSORT guidelines investigated the dentine-pulp response to calcium silicate cement in teeth with reversible pulpitis symptoms compared with GIC clinically. It also assessed the effectiveness of CBCT in detecting early PA changes associated with reversible pulpitis, which may not be detected using PA radiographs, and to monitor any changes over a 1-y period posttreatment. The null hypotheses were the following: there is no clinical difference in the dentine-pulp response between Biodentine and Fuji IX, and there is no difference in the effectiveness of CBCT versus PA radiographs in detecting PA lesions following IPC in patients with symptoms of reversible pulpitis.
Materials and Methods
Study Design and Sample Size
This single-blinded, 2-arm, randomized controlled clinical trial compared calcium silicate cement (Biodentine, Septodont) as the test material and GIC (Fuji IX GP; GC Corporation, Alsip, IL, USA) as the control. The study was not operator blinded because of the different clinical consistency and appearance of the 2 materials.
The study was conducted in compliance with the principles of the Declaration of Helsinki and Good Clinical Practice after approval from the London Westminster research ethics committee (11/LO/1893). Patient information sheets were distributed and informed written consent obtained prior to study commencement. Based on the work of Falster et al. (2002), this investigation was designed to have 80% power to detect a difference between the 2 materials, whose proportion of failures were assumed to be 1% and 22% over a period of 1 y. A sample size of 72 restorations to detect differences at the 5% level of significance using the z test for testing 2 independent proportions was calculated with an anticipated loss to follow-up of 10% included.
On recruiting patients from King’s College Dental Institute at Guy’s Hospital, London, randomization was performed centrally by the Biostatistics Unit using tabular randomization, with the unit being the tooth. Cavity size (1 wall, 2 walls, or more) was considered a prognostic factor and taken into consideration during randomization. Inclusion and exclusion criteria are presented in Table 1. The intensity of the pulp symptoms was recorded. Patients’ descriptions of sensitivity to hot, cold, and sweet lasting up to 15 to 20 s and settling spontaneously were considered mild, while increased pain for more than several minutes and needing pain killers were considered severe. Teeth with symptoms of irreversible pulpitis including persistent dull throbbing pain, sharp spontaneous pain, or pain exacerbated by lying down were excluded. In addition, patients were withdrawn from the study if pulp exposure occurred during the baseline operative intervention.
Table 1.
Inclusion and Exclusion Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Patients male or female older than 18 y in good general health | 1. Clinical symptoms of irreversible pulpitis requiring endodontic treatment |
| 2. A minimum of 1 deep carious lesion penetrating three-quarters or more into the dentine as identified with the periapical (PA) radiograph; Clinically mICDAS score 4 (Banerjee and Watson 2011) | 2. Presence of fistulas or swelling |
| 3. Clinical symptoms of reversible pulpitis | 3. Mobile teeth or tenderness to percussion |
| 4. Positive pulp response to electric pulp test or thermal stimulation | 4. Anterior teeth with aesthetic concerns |
| 5. No PA changes viewed on PA radiographs | 5. Pregnant women, in view of requirements for radiographs |
| 6. Patients younger than 18 y | |
| 7. Patients unable to give consent |
Interventions
The single operator was trained to standardize the MI operative procedures. Methods of clinical assessment included electric pulp test (Kerr Vitality Scanner 2006; SybronEndo, Orange, CA, USA) and thermal test (Roeko Endo-Frost, Coltène/Whaledent, Germany), palpation and percussion, and the presence of signs of inflammation (pain, abscess, sinus tract, and abnormal mobility). PA radiographs were taken at baseline (T0) and assessed to exclude any signs of irreversible pulpitis (widening of periodontal ligament [PDL] or PA lesions). A CBCT was taken at T0 but not assessed at this stage to avoid bias in the diagnosis.
Under local anesthesia and rubber dam isolation, using a standardized MI operative protocol, superficial, soft, infected dentine was excavated using carbon-steel rose-head burs (Ash Instruments, Dentsply, Gloucester, UK) in a slow-speed WA56A handpiece (W&H Dentalwerk Bürmoos GmbH, Bürmoos, Austria) and hand excavators after gaining suitable access through the cavitated enamel using a high-speed TA-98 handpiece (W&H Dentalwerk GmbH) under copious irrigation. Deeper caries-infected/affected dentine present more than three-quarters into dentine (Bjørndal 2008) was removed using chemo-mechanical gel and hand instrumentation (Carisolv; Rubicon Lifesciences, Gothenburg, Sweden) to help maintain consistency in the quantity of caries removed between the different teeth. Residual caries-affected dentine was retained on the pulp aspect of the cavity, as any additional excavation would lead to pulp exposure (Kerkhove et al. 1967).
Material randomization was accomplished following caries removal and identifying the cavity size. Each tooth was restored according to the relevant manufacturer’s instructions. The definitive resin composite veneer restoration (N’Durance; Septodont, Louisville, KY, USA) was placed 1 mo after baseline in a “closed sandwich” technique where achievable. A standardized bonding procedure was followed using a total-etch adhesive for both groups, following the manufacturer’s instructions (Scotchbond Universal, 3M ESPE, St. Paul, MN, USA). Follow-up was longitudinal at T1, T6, and T12 months (±2 wk) at which time PA radiographs/CBCT were repeated.
Radiographic Assessment
Digital PA radiographs using Vistascan phosphor plates were taken with a dental X-ray unit (Heliodent; Sirona, Bensheim, Germany) using a paralleling technique with Rinn film holders permitting standardization, in addition to small-volume (40 mm3) CBCT scans at 0.125-mm resolution and no dose-reduction programming (Accuitomo; J Morita Corporation, Osaka, Japan). Exposure parameters at T0/T12 were standardized for each patient. For each tooth, the CBCT scan that best confirmed the presence/absence of PA radiolucency in the sagittal, coronal, and/or axial planes was selected, following manipulation of the data set to optimize slice position by an experienced clinician. The PA/CBCT images were viewed as a keynote presentation (Apple, Cupertino, CA, USA) on a computer (MacBook Pro; Apple), with a 15.5-inch backlit LED screen (1680 × 1050 pixel resolution) in a quiet, dimly lit room.
A consensus panel of 2 trained, calibrated experienced endodontists assessed the CBCT/PA radiographs jointly. The reliability of the consensus panel was evaluated by jointly repeating the assessment of radiographic images after 4 wk. The interexaminer agreement was evaluated by individual randomized assessment of 50% of the PA/CBCT images and repeated after 4 wk.
The paired images of the roots of each tooth were viewed together by examiners blinded as to which image was taken at T0/T12. Each root was examined for the presence, absence, and change (increase/decrease) in size of any PA radiolucency. A PA radiolucency referred to widening of the PDL space or a PA lesion. Widening of the PDL space was defined as less than double that of the equivalent healthy PDL space of the adjacent healthy tooth. A PA lesion was defined as radiolucency associated with the radiographic apex of the root, ≥2 times the width of the PDL space (Low et al. 2008; Bornstein et al. 2011).
Statistical Analysis
The first outcome of the study was a binary variable indicating whether the restored tooth failed to maintain its vitality at T12. Clinical success was evaluated by a positive response to cold/electric pulp testing, absence of spontaneous pain, negative sensitivity to percussion, absence of sinus/fistula/swelling and abnormal mobility, and absence of PA radiolucencies as determined by PA radiographs. The second outcome was that CBCT scans could either detect or not the presence of early PA lesions in patients with symptoms of reversible pulpitis.
Descriptive statistics summarized various study characteristics and outcome variables. Using IBM SPSS Statistics version 22 (IBM, Armonk, NY, USA) for the clinical results, a χ2 test/Fisher’s exact test assessed the association between pulp vitality and material, extent of cavity, intensity of symptoms, and gender. Logistic regression analyzed the effect of these variables on vitality. Radiographic analysis included kappa values to assess intraconsensus panel and interexaminer agreement. The χ2 tests assessed the association between the presence of PA lesions and variables including material, extent of cavity, intensity of symptoms, and gender. Sensitivity, specificity, positive and negative predictive values, and overall diagnostic accuracy were calculated using CBCT results as a reference standard. The significance level was set to P < 0.05.
Results
Clinical Assessment
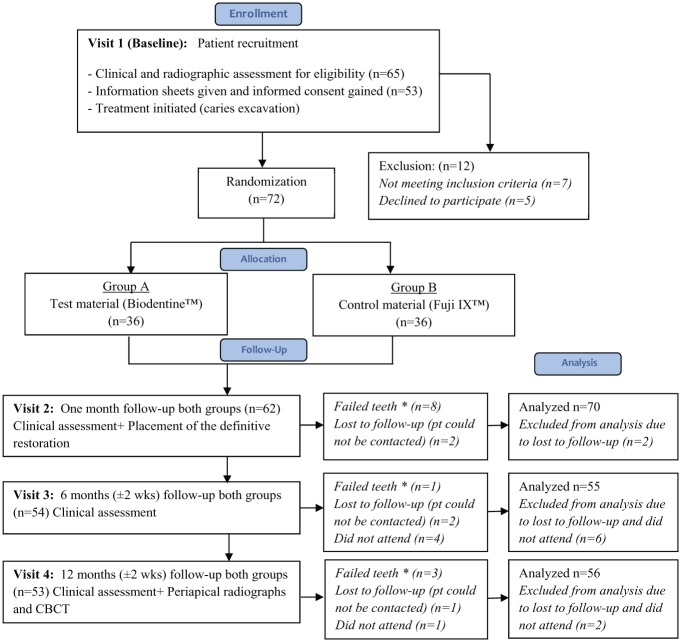
Seventy-two restorations (36 Biodentine and 36 Fuji IX) were placed in 53 patients (21 [39.6%] women and 32 [60.4%] men) with a limit of 2 restorations per patient. The patients’ age ranged from 18 to 76 y (median, 28 y). Eighty-five percent of restorations were placed in molars. The clinical success rates for both Biodentine and Fuji IX were equal (83.3%). Twelve teeth lost vitality by T12 (6 Biodentine/6 Fuji IX). Among 53 patients, 5 patients with 6 restorations dropped out of the trial by failing to attend subsequent appointments (Fig. 1). The total number of teeth analyzed excluded the dropouts (n = 66).
Figure 1.
Flow diagram indicating patient recruitment and follow-up. Adapted from the CONSORT flow diagram. *Failed teeth are ones that developed irreversible pulpitis and underwent root canal treatment.
The χ2 test/Fisher’s exact test showed no significant statistical differences between pulp vitality and type of restorative material used (P = 0.91), cavity extent (P = 0.41), gender (P = 0.33), or age (P = 0.99). The distribution of symptom intensity between each material at T0 was not equal (see Table 2A). A significant correlation was found between pulp vitality and symptom intensity (P = 0.007). In patients suffering from mild reversible pulpitis at T0, 4 teeth (9.75%) became nonvital at T12, whereas in patients suffering from severe symptoms of reversible pulpitis at T0, 8 teeth (32%) became nonvital.
Table 2.
(A) Clinical Assessment of Tooth Vitality and Symptoms Distribution for Biodentine and Fuji IX; (B) Radiographic Assessment Including Number of Teeth and Percentage Identified in Both Cone Beam Computed Tomography (CBCT) and Periapical Radiographs at T0 and T12.a
| (A) | Material |
||||
|---|---|---|---|---|---|
| Biodentine (n/Total) |
Fuji IX (n/Total) |
||||
| Symptoms at T0 | Mild | Severe | Mild | Severe | Total |
| Vital (at T0) | 21/66 | 10/66 | 18/66 | 17/66 | 66/66 |
| Nonvital (at T12) | 3/66 | 3/66 | 1/66 | 5/66 | 12/66 |
| Lesions at T0 | 4/60 | 4/60 | 3/60 | 5/60 | 16/60 |
| Lesions at T12 | 1/52 | 1/52 | 4/52 | 3/52 | 9/52 |
| Healed lesions | 4/52 | 1/52 | 0/52 | 1/52 | 6/52 |
| New/progressed lesions | 1/52 | 1/52 | 4/52 | 3/52 | 9/52 |
| (B) | CBCT |
PA |
|||
| T0 (n = 60), n (%) | T12 (n = 52), n (%) | T0 (n = 60), n (%) | T12 (n = 52), n (%) | ||
| Healthy | 29 (48.3) | 34 (65.4) | 60 (100) | 47 (90.4) | |
| Radiolucencyb | 31 (51.6) | 18 (34.6) | 0 (0) | 5 (9.6) | |
| Lesions | 16 (26.6) | 9 (17.3) | 0 (0) | 2 (3.8) | |
| Healing/healed radiolucency | — | 9 (17.3) | — | 0 (0) | |
| New/larger radiolucency | — | 16 (30.8) | — | 5 (9.6) | |
| Healing/healed lesion | — | 6 (11.5) | — | 0 (0) | |
| New/larger lesion | — | 9 (17.3) | — | 2 (3.8) | |
Dropouts excluded from analysis.
Radiolucency includes both widening and lesions.
Radiographic Assessment
Fifty-two paired (T0+T12) CBCT and PA radiographs were analyzed. Six teeth had no T0 or T12 CBCT scans taken, whereas 8 teeth had no T12 CBCT scan. CBCT was statistically significantly more effective at detecting PA changes compared with radiographs (P < 0.05). Of the teeth, 65.4% and 90.4% were deemed healthy using CBCT and PA radiographs, respectively, at T12. Healing and healed rates were 17.3% and 0% (Fig. 2), respectively, whereas new/progressing radiolucencies were 30.8% and 9.6% with CBCT and PA radiographs, respectively (Fig. 3). A significant difference (P = 0.02) was observed between teeth with healing/healed lesions identified using CBCT receiving Biodentine (71%) and those with new/progressed lesions receiving Fuji IX (88%). No significant difference was found in the development of T12 lesions between teeth with mild and severe symptoms within each material (P > 0.05; Table 2A).
Figure 2.
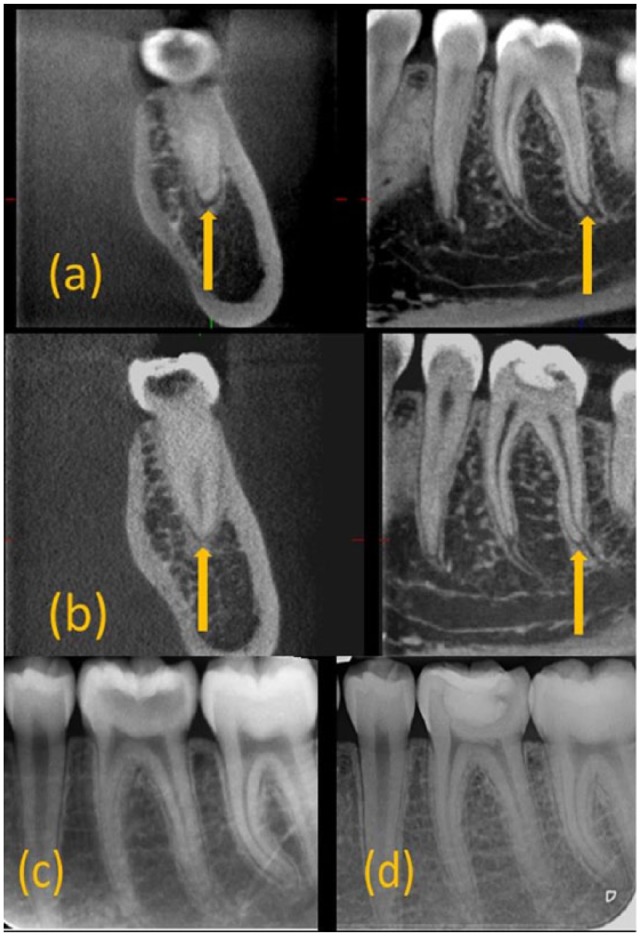
Example of healed lesion (a) cone beam computed tomography (CBCT) at T0 revealing lesion in the distal root of 36. (b) CBCT at T12 revealing resolved lesion around the distal root of 36. (c) Periapical (PA) radiograph at T0 revealing healthy periapical tissues around 36. (d) PA radiograph at T12 revealing healthy periapical tissues around 36.
Figure 3.
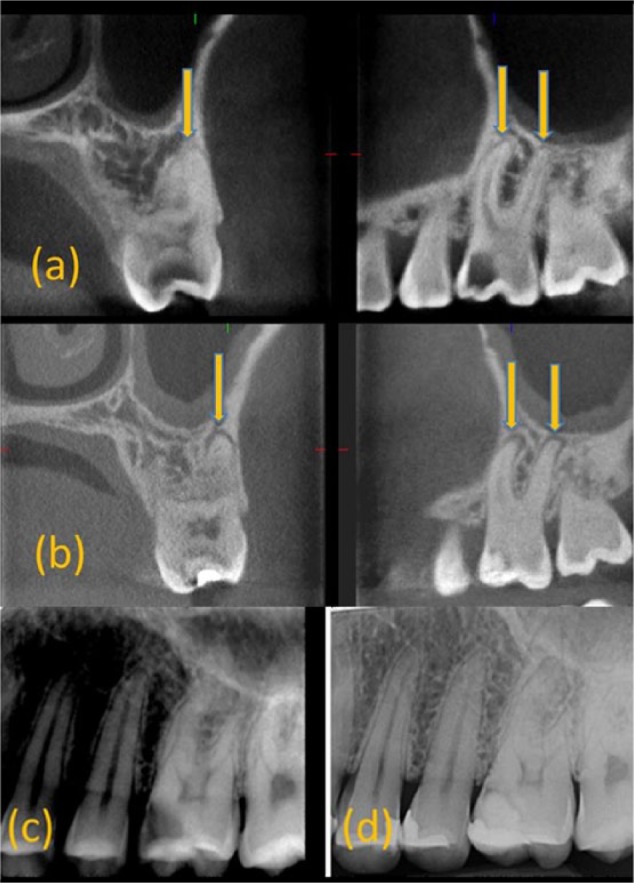
Example of new lesion (a) cone beam computed tomography (CBCT) at T0 revealing healthy periapical (PA) tissues surrounding the roots of 26. (b) CBCT at T12 revealing lesions around the roots of 26. (c) PA radiograph at T0 revealing healthy periapical tissues around 26. (d) PA radiograph at T12 revealing slight widening of the periodontal ligament around 26.
Of all teeth, 51.6% had signs of CBCT PA radiolucency at T0 and 26.6% had a PA lesion (Table 2B). Teeth presenting with a CBCT PA lesion at T0 had a failure rate of 63%, whereas teeth with no lesion at T0 had a failure rate of 16%. This was statistically significant (P = 0.02).
Correlations between CBCT PA changes and symptom intensity, cavity size, material, and patient age were not significant (P > 0.05).
Kappa values for intraconsensus agreement were 0.68 and 0.66 for CBCT and PA radiographs, respectively, and the interexaminer agreement was 0.53 and 0.26. Sensitivity, specificity, positive and negative predictive values, and overall diagnostic accuracy of PA radiographs were calculated, using CBCT as the gold standard (Table 3).
Table 3.
Sensitivity, Specificity, Positive and Negative Predictive Values, and Overall Diagnostic Accuracy of Periapical Radiographs (PA) Compared with Cone Beam Computed Tomography Findings as a Reference.
| Scan | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Diagnostic Accuracy |
|---|---|---|---|---|---|
| PA | 0.24 | 1 | 1 | 0.63 | 0.66 |
Discussion
In this study, the efficacy of Biodentine was compared with that of Fuji IX when used as an IPC agent in patients diagnosed clinically and radiographically with reversible pulpitis. The materials were selected as they have similar clinical applications in terms of being used as restorative dentine replacements and as provisional bulk restorative materials in deep cavities. It was found that both materials shared similar clinical T12 success rates of 83.3%. GICs are acidic in nature, releasing fluoride and calcium/strontium ions (Watson et al. 2014). In Biodentine, the alkaline caustic effect causes degradation of the collagenous component of the underlying dentine, leading to formation of porosities that enable diffusion of high concentrations of calcium, hydroxyl, and carbonate ions, leading to increased mineral deposition (Atmeh et al. 2012; Watson et al. 2014).
There are no randomized clinical trials comparing these 2 materials. The only clinical study in which Biodentine was used to restore deep cavities with no pulp exposure reported a high success rate for the material at 3 y (Koubi et al. 2013). Although pulp vitality was monitored in this study, its use as an IPC agent was not a primary outcome and was not controlled.
The boundary between severe symptoms of reversible pulpitis and irreversible pulpitis is blurred as the degree of pain does not necessarily reflect pulp histopathology. Studies have reported that the more severe the pain, the worse the histopathosis (Bender 2000; Aguilar and Linsuwanont 2011); however, that is not always the case (Mejare et al. 2012). Nevertheless, reports of symptom intensity are subjective, and patients seeking treatment can exaggerate its intensity. In this study, patients were interviewed exactingly about their symptoms by explaining how their descriptions might affect the treatment they received. Results showed a significant positive correlation between symptom intensity and treatment outcome. Teeth that became nonvital following treatment tended to express more severe baseline symptoms compared with others at T0.
A diagnosis of reversible pulpitis depends on the absence of apical periodontitis determined using PA radiographs. However, early histological changes in the PA hard tissues are not visible easily using PA radiographs (Patel et al. 2009).
CBCT overcomes this issue, and indeed, 51.6% of the teeth had signs of T0 PA change when viewed using CBCT and 26.6% had PA lesions. This is consistent with histological studies demonstrating the presence of chronic pulp inflammation without symptoms or clinical/conventional radiographic signs of the true state of pulp pathosis (Hilton 2009).
Of 26.6% teeth with T0 CBCT PA lesions, 38% healed following MI IPC as they were vital with a blood supply fundamental for generating repair and periradicular healing (Torabzadeh and Asgary 2013). In 2 previous studies, teeth diagnosed clinically with irreversible pulpitis and PA lesions observed in PA radiographs showed healing of the PA tissues following IPC (Jordan et al. 1978; Torabzadeh and Asgary 2013). In another study, vital teeth with PA radiolucencies observed in PA radiographs and planned for endodontic therapy or extraction were treated with either direct or IPC (Moore 1967). The results showed the pulp capable of repair with absence of PA radiolucencies and normal vitality after a period varying from 6 to 36 mo. General consensus states that PA involvement demonstrated radiographically indicates total pulp necrosis or irreversible pulpitis. However, early PA pathosis may not necessarily indicate total pulp necrosis, and the disease may be limited to the pulp chamber with inflammatory cells and vasodilation in the apical pulpal tissue (Çaliskan 1995). Therefore, to avoid overtreatment, minor changes such as widening of the PDL space should not necessarily contraindicate MI pulp vitality preservation procedures including IPC.
In this study, the majority of teeth with healed PA lesions received Biodentine, whereas teeth with new/progressing lesions received GIC. Teeth with baseline severe symptoms of reversible pulpitis had received more GIC restorations compared with Biodentine as symptom intensity was not considered during the material randomization. However, the distribution of symptom severity in lesions in the GIC group at T12 was almost equal. This association may be explained by the release of silica ions from Biodentine into the underlying dentine, a recognized promoter of remineralization in addition to its high alkalinity, which enhances apatite formation and remineralization (Watson et al. 2014). Furthermore, its ability to modulate pulp cell TGF-β1 secretion helps induce reparative dentine synthesis (Laurent et al. 2012; Zanini et al. 2012; Nowicka et al. 2013). However, this result must be interpreted with caution, as the experimental numbers were low, although statistically significant (P = 0.02).
The CBCT interexaminer agreement was better than for PA radiographs, conforming to studies assessing PA radiolucencies (Sogur et al. 2009; Lennon et al. 2011; Patel et al. 2012), confirming the superior reliability of CBCT in detecting PA radiolucency compared with PA radiographs. Both examiners were specialists in the use of CBCT and detection of radiographic signs of apical periodontitis. The radiographic techniques and viewing conditions were standardized. Viewing sessions were kept as short as possible to reduce examiner fatigue (Patel et al. 2012). Unlike previous studies, this study assessed vital teeth that underwent MI IPC as opposed to root canal treatment. The PDL space can demonstrate significant natural variation, and widening viewed in CBCT images may be considered an initial sign of disease or a variation of health. Therefore “moderate”/“fair” variability in agreement can be attributed to the practical difficulty in discerning between a healthy (slightly wide) PDL space and pathological widening. This concurs with another study comparing CBCT and PA (Pope et al. 2014). Moreover, interexaminer agreement is subject to wide variation and can be as low as 25% even between the most experienced examiners (Sogur et al. 2009; Tewary et al. 2011). This reflects the complexity of the decision-making process and the diagnostic difficulty when encountering subtle periodontal changes (Lennon et al. 2011).
The sensitivity of PA radiographs in detecting PA changes was lower than CBCT, concurring with previous studies comparing the 2 imaging techniques (Stavropoulos and Wenzel 2007; Estrela et al. 2008; Patel et al. 2009; Paula-Silva et al. 2009). A histological reference standard to compare the 2 radiologic techniques was not possible because of the nature of this study. A longer-term follow-up in addition to more extensive clinical trials are currently ongoing. At present, routine use of CBCT to support the decision to undertake vital pulp therapy or root canal treatment is not recommended; however, CBCT is indicated to aid diagnosis of radiographic signs of PA pathosis when there are contradictory (nonspecific) signs and/or symptoms (Patel et al. 2014).
Conclusions
The null hypothesis stating there is no difference in the dentine-pulp response between Biodentine and Fuji IX clinically was accepted but not radiographically. The null hypothesis stating there is no difference in the effectiveness of CBCT and PA radiographs in detecting periradicular lesions following IPC in patients with symptoms of reversible pulpitis was rejected.
CBCT detected lesions in teeth diagnosed clinically and using PA radiographs with reversible pulpitis. Although both Biodentine and Fuji IX are clinically effective when used as IPC materials in teeth with reversible pulpitis, CBCT demonstrated a statistically significant difference between the 2 materials. The majority of teeth with healing/healed lesions identified using CBCT had received Biodentine, whereas the majority of teeth with new/progressing lesions had received Fuji IX. However, further studies are needed to establish their effect on the healing dynamics of the PA tissues.
Author Contributions
D. Hashem, A. Banerjee, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; F. Mannocci, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; S. Patel, contributed to data analysis and interpretation, critically revised the manuscript; A. Manoharan, contributed to data analysis, critically revised the manuscript; J.E. Brown, contributed to data acquisition, critically revised the manuscript; T.F. Watson, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors also acknowledge the Saudi Cultural Office in London for sponsoring and supporting the work.
Footnotes
This study was supported by the Centre of Excellence in Medical Engineering funded by the Wellcome Trust and by the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy’s & St. Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aguilar P, Linsuwanont P. 2011. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: a systematic review. J Endod. 37(5):581–587. [DOI] [PubMed] [Google Scholar]
- Atmeh AR, Chong EZ, Richard G, Festy F, Watson TF. 2012. Dentin-cement interfacial interaction: calcium silicates and polyalkenoates. J Dent Res. 91(5):454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Watson TF. 2011. Pickard’s manual of operative dentistry. Oxford (UK): Oxford University Press. [Google Scholar]
- Barthel CR, Rosenkranz B, Leuenberg A, Roulet JF. 2000. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: a retrospective study. J Endod. 26(9):525–528. [DOI] [PubMed] [Google Scholar]
- Bender IB. 2000. Pulpal pain diagnosis—a review. J Endod. 26(3):175–179. [DOI] [PubMed] [Google Scholar]
- Bjørndal L. 2002. Dentin and pulp reactions to caries and operative treatment: biological variables affecting treatment outcome. Endod Top. 2(1):10–23. [Google Scholar]
- Bjørndal L. 2008. Indirect pulp therapy and stepwise excavation. J Endod. 34(7 Suppl):S29–S33. [DOI] [PubMed] [Google Scholar]
- Bjørndal L, Reit C, Bruun G, Markvart M, Kjaeldgaard M, Näsman P, Thordrup M, Dige I, Nyvad B, Fransson H, et al. 2010. Treatment of deep caries lesions in adults: randomized clinical trials comparing stepwise vs. direct complete excavation, and direct pulp capping vs. partial pulpotomy. Eur J Oral Sci. 118(3):290–297. [DOI] [PubMed] [Google Scholar]
- Bornstein MM, Lauber R, Sendi P, von Arx T. 2011. Comparison of periapical radiography and limited cone-beam computed tomography in mandibular molars for analysis of anatomical landmarks before apical surgery. J Endod. 37(2):151–157. [DOI] [PubMed] [Google Scholar]
- Çaliskan MK. 1995. Pulpotomy of carious vital teeth with periapical involvement. Int Endod J. 28(3):172–176. [DOI] [PubMed] [Google Scholar]
- Dammaschke T, Leidinger J, Schäfer E. 2010. Long-term evaluation of direct pulp capping—treatment outcomes over an average period of 6.1 years. Clin Oral Investig. 14(5):559–567. [DOI] [PubMed] [Google Scholar]
- Estrela C, Bueno MR, Leles CR, Azevedo B, Azevedo JR. 2008. Accuracy of cone beam computed tomography and panoramic and periapical radiography for detection of apical periodontitis. J Endod. 34(3):273–279. [DOI] [PubMed] [Google Scholar]
- Falster C, Araujo F, Straffon L, Nör J. 2002. Indirect pulp treatment: in vivo outcomes of an adhesive resin system vs calcium hydroxide for protection of the dentin-pulp complex. Pediatr Dent. 24(3):241–248. [PubMed] [Google Scholar]
- Hilton TJ. 2009. Keys to clinical success with pulp capping: a review of the literature. Oper Dent. 34(5):615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume W, Mount G. 1988. In vitro studies on the potential for pulpal cytotoxicity of glass-ionomer cements. J Dent Res. 67(6):915–918. [DOI] [PubMed] [Google Scholar]
- Jordan R, Suzuki M, Skinner D. 1978. Indirect pulp-capping of carious teeth with periapical lesions. J Am Dent Assoc. 97(1):37–43. [DOI] [PubMed] [Google Scholar]
- Kerkhove BC, Jr, Herman SC, Klein AI, McDonald RE. 1967. A clinical and television densitometric evaluation of the indirect pulp capping technique. J Dent Child. 34(3):192–201. [PubMed] [Google Scholar]
- Koubi G, Colon P, Franquin JC, Hartmann A, Richard G, Faure MO, Lambert G. 2013. Clinical evaluation of the performance and safety of a new dentine substitute, Biodentine, in the restoration of posterior teeth—a prospective study. Clin Oral Investig. 17(1):243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent P, Camps J, About I. 2012. Biodentine™ induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 45(5):439–448. [DOI] [PubMed] [Google Scholar]
- Lennon S, Patel S, Foschi F, Wilson R, Davies J, Mannocci F. 2011. Diagnostic accuracy of limited-volume cone-beam computed tomography in the detection of periapical bone loss: 360° scans versus 180° scans. Int Endod J. 44(12):1118–1127. [DOI] [PubMed] [Google Scholar]
- Low KM, Dula K, Bürgin W, von Arx T. 2008. Comparison of periapical radiography and limited cone-beam tomography in posterior maxillary teeth referred for apical surgery. J Endod. 34(5):557–562. [DOI] [PubMed] [Google Scholar]
- Mejare I, Axelsson S, Davidson T, Frisk F, Hakeberg M, Kvist T, Norlund A, Petersson A, Portenier I, Sandberg H. 2012. Diagnosis of the condition of the dental pulp: a systematic review. Int Endod J. 45(7):597–613. [DOI] [PubMed] [Google Scholar]
- Moore DL. 1967. Conservative treatment of teeth with vital pulps and periapical lesions: a preliminary report. J Prosthet Dent. 18(5):476–481. [DOI] [PubMed] [Google Scholar]
- Ngo HC, Mount G, Mc Intyre J, Tuisuva J, Von Doussa R. 2006. Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: an in vivo study. J Dent. 34(8):608–613. [DOI] [PubMed] [Google Scholar]
- Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, Kaczmarek W, Buczkowska-Radlińska J. 2013. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 39(6):743–747. [DOI] [PubMed] [Google Scholar]
- Patel S, Dawood A, Mannocci F, Wilson R, Pitt Ford T. 2009. Detection of periapical bone defects in human jaws using cone beam computed tomography and intraoral radiography. Int Endod J. 42(6):507–515. [DOI] [PubMed] [Google Scholar]
- Patel S, Durack C, Abella F, Roig M, Shemesh H, Lambrechts P, Lemberg K; European Society of Endodontology. 2014. European Society of Endodontology position statement: the use of CBCT in endodontics. Int Endod J. 47(6):502–504. [DOI] [PubMed] [Google Scholar]
- Patel S, Wilson R, Dawood A, Foschi F, Mannocci F. 2012. The detection of periapical pathosis using digital periapical radiography and cone beam computed tomography—part 2: a 1-year post-treatment follow-up. Int Endod J. 45(8):711–723. [DOI] [PubMed] [Google Scholar]
- Paula-Silva FW, Wu MK, Leonardo MR, Da Silva LA, Wesselink PR. 2009. Accuracy of periapical radiography and cone-beam computed tomography scans in diagnosing apical periodontitis using histopathological findings as a gold standard. J Endod. 35(7):1009–1012. [DOI] [PubMed] [Google Scholar]
- Pitt Ford TR, Patel S. 2004. Technical equipment for assessment of dental pulp status. Endod Top. 7(1):2–13. [Google Scholar]
- Pope O, Sathorn C, Parashos P. 2014. A comparative investigation of cone-beam computed tomography and periapical radiography in the diagnosis of a healthy periapex. J Endod. 40(3):360–365. [DOI] [PubMed] [Google Scholar]
- Sidhu S. 2011. Glass-ionomer cement restorative materials: a sticky subject? Aust Dent J. 56(Suppl 1):23–30. [DOI] [PubMed] [Google Scholar]
- Six N, Lasfargues JJ, Goldberg M. 2000. In vivo study of the pulp reaction to Fuji IX, a glass ionomer cement. J Dent. 28(6):413–422. [DOI] [PubMed] [Google Scholar]
- Sogur E, Baksi B, Gröndahl HG, Lomcali G, Sen B. 2009. Detectability of chemically induced periapical lesions by limited cone beam computed tomography, intra-oral digital and conventional film radiography. Dentomaxillofac Radiol. 38(7):458–464. [DOI] [PubMed] [Google Scholar]
- Stavropoulos A, Wenzel A. 2007. Accuracy of cone beam dental CT, intraoral digital and conventional film radiography for the detection of periapical lesions: an ex vivo study in pig jaws. Clin Oral Investig. 11(1):101–106. [DOI] [PubMed] [Google Scholar]
- Tewary S, Luzzo J, Hartwell G. 2011. Endodontic radiography: who is reading the digital radiograph? J Endod. 37(7):919–921. [DOI] [PubMed] [Google Scholar]
- Torabzadeh H, Asgary S. 2013. Indirect pulp therapy in a symptomatic mature molar using calcium enriched mixture cement. J Conserv Dent. 16(1):83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziafas D, Smith A, Lesot H. 2000. Designing new treatment strategies in vital pulp therapy. J Dent. 28(2):77–92. [DOI] [PubMed] [Google Scholar]
- Watson TF, Atmeh AR, Sajini S, Cook RJ, Festy F. 2014. Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry: biophotonics-based interfacial analyses in health and disease. Dent Mater. 30(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini M, Sautier JM, Berdal A, Simon S. 2012. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod. 38(9):1220–1226. [DOI] [PubMed] [Google Scholar]