Abstract
Remineralization of dentin during dental caries is of considerable clinical interest. Dentin matrix protein 1 (DMP1) is a non-collagenous calcium-binding protein that plays a critical role in biomineralization. In the present study, we tested if peptides derived from DMP1 can be used for dentin remineralization. Peptide pA (pA, MW = 1.726 kDa) and peptide pB (pB, MW = 2.185), containing common collagen-binding domains and unique calcium-binding domains, were synthesized by solid-phase chemistry. An extreme caries lesion scenario was created by collagenase digestion, and the biomineral-nucleating potential of these peptides was ascertained when coated on collagenase-treated dentin matrix and control, native human dentin matrix under physiological levels of calcium and phosphate. Scanning electron microscopy analysis suggests that peptide pB was an effective nucleator when compared with pA. However, a 1:4 ratio of pA to pB was determined to be ideal for dentin remineralization, based on hydroxyapatite (HA) morphology and calcium/phosphorus ratios. Interestingly, HA was nucleated on collagenase-challenged dentin with as little as 20 min of 1:4 peptide incubation. Electron diffraction confirmed the presence of large HA crystals that produced a diffraction pattern indicative of a rod-like crystal structure. These findings suggest that DMP1-derived peptides may be useful to modulate mineral deposition and subsequent formation of HA when exposed to physiological concentrations of calcium and phosphate.
Keywords: dentin matrix protein 1, hydroxyapatite, extracellular matrix, calcification, biomimetics, dentin repair
Introduction
Dentin biomineralization is a carefully orchestrated process by which inorganic calcium phosphate impregnates the organic matrix under the direction of specialized matrix proteins. Once dentin is mineralized, appropriate levels of hydroxyapatite (HA) are regionally maintained within the collagen matrix by dynamic equilibrium to healthy levels with the aid of fluid transport through the dentinal tubules (White 1995). In the case of a cavitated caries lesion, net demineralization takes over, since there is an imbalance in the homeostatic levels of mineral. For a variety of reasons, there has been an increase in the prevalence of dental caries in recent years, despite scientific advances aiding in the early detection and prevention of disease progression to full-blown caries (Bagramian et al. 2009). One approach to preventing lesion progression is to treat the lesion with a remineralizing agent to tip the balance back to net remineralization, thus reversing the pathological process of caries formation (Liu et al. 2011).
Contemporary concepts of biomineralization describe the stabilization of calcium and phosphate ions in solution by acidic non-collagenous proteins, such as dentin matrix protein 1 (DMP1), resulting in prenucleation clusters (Dey et al. 2010; Demichelis et al. 2011; Nijhuis et al. 2014). Prenucleation clusters then aggregate to form amorphous calcium phosphate (ACP) nanoparticles and localize to intrafibrillar regions of the collagen fibrils, where crystallographic alignment takes place, eventually forming single-apatite crystallites within the gap zones of collagen molecules (Colfen 2010; Nudelman et al. 2010; Niu et al. 2014). Crystal growth from within these intrafibrillar gap regions is advantageous, because when these gap zones are occupied with mineral, the mechanical properties of the dentin extracellular matrix (ECM) are enhanced, while additionally protecting the collagen molecules from enzymatic and acidic challenges (Lees and Page 1992; Balooch et al. 2008; Bertassoni et al. 2011; Niu et al. 2014). Thus, DMP1 has been identified as a key non-collagenous protein in the dentin matrix, greatly regulating dentin biomineralization during development (MacDougall et al. 1998; He et al. 2003b; He and George 2004; Tartaix et al. 2004).
Current strategies for enamel remineralization, as summarized by Li et al., take a biomimetic approach toward tipping the balance back to a net remineralization by means of non-collagenous proteins or their analogs (Li et al. 2014). Within the dentin matrix, these strategies stabilize calcium and phosphate ions, guiding mineral deposition to the desired gap region areas within the collagen matrix (Tay and Pashley 2008; Kim et al. 2010). Reports demonstrate remineralization of dentin lesions on the order of ~200 µm by the use of biomimetic techniques (Qi et al. 2012). These successes demonstrate the need for further work in the field. The novelty of the peptides utilized in this study comes from their collagen-binding domain. Incorporation of this functional domain is a major physiological advantage in that it allows for specific binding to type I collagen, the framework of dentin, and promotes guided remineralization of the exposed dentin matrix. In the present study, we developed 2 novel DMP1-derived biomimetic peptides designed to bind collagen and stabilize calcium and phosphate in solution to nucleate HA crystals within human dentin matrix.
The hypothesis to be tested in this study was to determine if DMP1-derived biomimetic peptides would promote dentin remineralization. Therefore, the study involved testing the efficacy of the peptides to bind collagen of fully demineralized, native and collagenase-challenged, human dentin and investigation of the HA nucleation and growth process within the dentin matrix utilizing a solution containing physiological levels of calcium and phosphate.
Materials and Methods
Preparation of Dentin Wafers
Four previously extracted sound 3rd molars were selected and kept frozen following approval of the Institutional Review Board at the University of Illinois at Chicago (protocol 2009-0198). Coronal dentin cross-sections 1.5 mm thick were cut from each tooth and further sectioned to produce 250-µm-thick slices by means of a hydrated diamond blade saw (IsoMet 1000, Buehler, Lake Bluff, IL, USA). These slices represent the samples hereinafter referred to as dentin wafers.
Wafers measuring 1.5 x 3 x 0.250 mm thick were placed together in 14% ethylenediaminetetraacetic acid (EDTA) at 4°C for 10 d. Demineralization was verified by x-ray microradiography (MX-20 Faxitron, LLC, Lincolnshire, IL, USA). Demineralized dentin wafers were subjected to 1 M NaCl for 1 h at room temperature for the disruption of loosely bound non-collagenous proteins. Samples were then incubated in 0.25% trypsin-EDTA twice for 4 h at 37°C for the removal of strongly bound, endogenous non-collagenous proteins. Random dentin wafers were selected prior to trypsin digestion, after one 4-hour trypsin digestion, and after 2 consecutive 4-hour trypsin digestions. These wafers were cryosectioned to 5 µm, stained with Stains-All® (Sigma-Aldrich, St. Louis, MO, USA), and imaged for the detection of remaining acidic proteins. Half of the remaining dentin wafers having undergone 2 trypsin digestions were randomly split into treatment groups, as native dentin collagen matrix. After trypsin digestion, the other half—as collagenase-treated dentin matrix—were challenged with 100 µg/mL collagenase (Clostridium hystolyticum; Sigma-Aldrich) in 0.2 M sodium bicarbonate buffer (pH = 7.9) for 6 h at 37°C (Vidal et al. 2014). The 6-hour time frame was chosen based on the results of native wafers challenged with collagenase for different lengths of time (Appendix Fig. 2A–C).
Peptide Synthesis
Two peptides, pA (DSESSEEDR-Ahx-ESQES) and pB (DSESSEEDR-Ahx-QESQSEQDS), were produced via solid-phase peptide synthesis at the Protein Research Laboratory core facility of the University of Illinois at Chicago. A common collagen-binding domain, DSESSEEDR, found in full-length DMP1, was used in both peptides. This sequence has been previously identified to display a strong affinity for the N-telopeptide located in the gap region of type I collagen (He and George 2004). Peptide pA is linked to the ESQES sequence, while pB is linked to the QESQSEQDS sequence. Both of these sequences are acidic clusters found in native DMP1 and have been identified as HA-binding domains (He et al. 2003a, b).
Collagen-binding Analysis
Peptides pA and pB were conjugated to CdSe/ZnS quantum dots (QDs) according to the protocol of Shen et al. (2009). Solubilized type I collagen was reconstituted on copper transmission electron microscopy (TEM) grids and exposed to either QD-labeled pA or pB. Following thorough washing and uranyl acetate staining, grids were imaged with a JEOL 3010EX TEM for the investigation of peptide collagen-binding capacity.
Sample Treatment and Nucleation
Recombinant-DMP1 (rDMP1) and BSA served as positive and negative controls, respectively. Full-length rat rDMP1 was produced as previously published (Bedran-Russo et al. 2013). Five native wafers were randomly chosen for each treatment group and placed in a 4% solution of one of the following: BSA, rDMP1, pA, pB, and a mixture containing ratios (pA:pB) 1:4, 2:3, 3:2, 4:1, in phosphate-buffered saline (PBS) at 37°C overnight (15 h). Five collagenase-challenged wafers were randomly chosen per treatment group and placed in rDMP1 for 6 h and at a 1:4 ratio of peptides for 6 h, 4 h, 2 h, 1 h, and 20 min. Coated native and collagenase-challenged dentin was placed in the wells of a modified electrophoresis chamber used as a nucleation chamber (He et al. 2003a). Each well was connected to a reservoir of calcium buffer (165 mM NaCl, 10 mM HEPES, 2.5 mM CaCl2, pH = 7.4) on one side and phosphate buffer (165 mM NaCl, 10 mM HEPES, 1 m MKH2PO4, pH = 7.4) on the other side (He et al. 2003a, b; He and George 2004). A 1-mA direct current (DC) was applied to maintain constant flow of Ca2+ and PO42- ions. A pH of 7.4 was maintained by the regular changing of buffers for a period of 14 days to facilitate nucleation and growth of HA. Wafers were then removed and prepared for analysis.
Scanning Electron Microscopy (SEM)
Samples were dehydrated by ethanol gradient, fixed with hexamethyldisilazane, mounted, and carbon-coated. A Hitachi SU8030 equipped with an Oxford X-Max 80 EDS detector was used at 10 kV, 10 μA, and a working distance of 15 mm to obtain images and EDS data.
Transmission Electron Microscopy (TEM)
Native dentin samples were dehydrated and fixed as for SEM. Once fixed, each wafer was scratched with a diamond scriber and its material transferred to a copper TEM grid. Imaging and selected area electron diffraction (SAED) were performed with a JEOL JEM-3010 TEM operating at 300 kV and equipped with a Thermo Noran XEDS system.
After SEM analysis, the treated dentin wafers were also analyzed by TEM after being embedded in epoxy resin. Sections measuring 70 nm were cut, placed on 200-mesh copper grids, and viewed on a JEOL JEM 1220 electron microscope.
Reconstituted collagen solution was also coated directly on TEM grids and then adsorbed with either peptide pA or peptide pB. Mineralization was performed directly on this template for 1 h with 1 M Ca2+ and 1 M PO4 3- buffers and viewed by TEM as above.
Results
Removal of Acidic Non-collagenous Proteins from the Dentin Wafer
Stains-All® staining revealed that, following EDTA and NaCl treatment, there was still deep, heterogeneous staining (Fig. 1A) for acidic proteins. Samples that underwent trypsin digestion for 4 h had most of their acidic proteins removed (Fig. 1B), and light staining was uniform. Dentin wafers digested by 2 consecutive 4-hour trypsin digestions (Fig. 1C) displayed a greater reduction in endogenous acidic proteins within the ECM.
Figure 1.
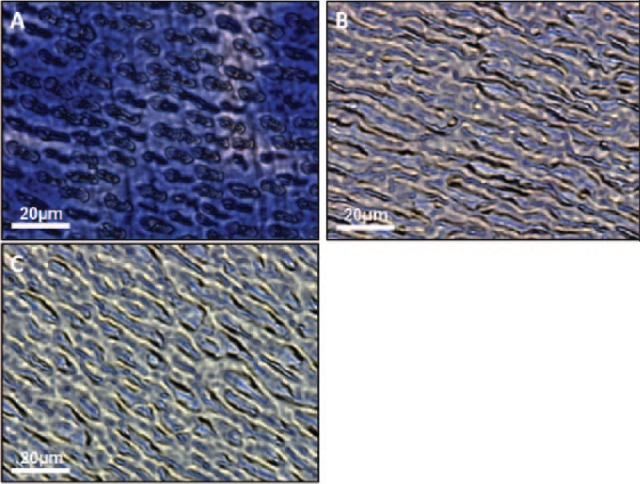
Optical microscopy images of demineralized dentin wafers treated with trypsin. (A) A 5-μm unfixed section of dentin wafer that has been decalcified by 14% ethylenediaminetetraacetic acid (EDTA) followed by 1 M NaCl treatment and stained with Stains-All. Heterogeneity is seen in staining intensity, which indicates an uneven distribution of acidic proteins still bound to the dentin extracellular matrix (ECM). (B) Dentin wafer that has been treated once with 0.25% trypsin-EDTA for 4 h. Staining is much more homogeneous, and the concentrations of acidic proteins have been greatly reduced. (C) Dentin wafer that has undergone 2 subsequent trypsin digestions. Staining is reduced to an even lesser degree, indicating further removal of acidic proteins from the demineralized dentin ECM.
Collagen Binding
Imaging of QD-conjugated pA on self-assembled collagen (Fig. 2A) showed the peptide bound to type I collagen, with preferential binding to specific regions on the helix. Analysis of pB (Fig. 2B) likewise displayed collagen-binding properties. Both QD-conjugated peptides also bound unassembled collagen molecules.
Figure 2.
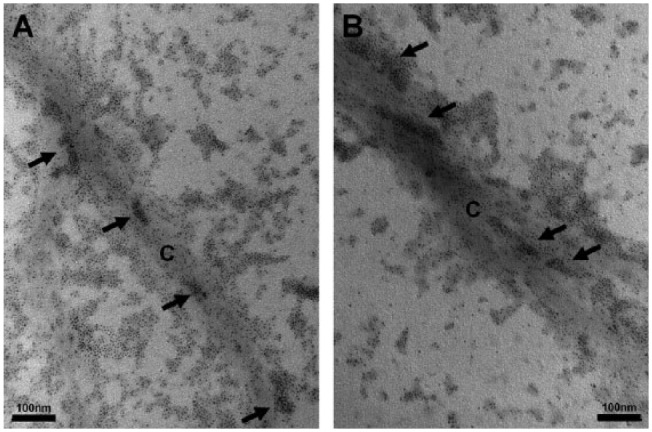
Collagen-binding properties of the peptides. (A, B) Transmission electron microscopy images of quantum dot (QD)-conjugated peptides bound to reconstituted type I collagen stained with uranyl acetate. (A) pA shows binding of the peptides labeled with the CdSe/ZnS QDs to type I collagen (labeled C) with affinity to specific regions of the helix (arrows), where clustering of the QDs can be seen. Binding to unassembled collagen molecules can also be seen. (B) The peptide pB also displays binding to the collagen (C) with preferred binding to regions of clustered radiodensities, as well as unassembled molecules.
Scanning Electron Microscopy
SEM images of in vitro nucleation samples from each treatment group on native dentin matrix (Fig. 3A–D) indicated clear differences in the crystallinity of calcium phosphate deposits for wafers treated with pA, pB, or a mixed ratio of both. BSA showed deposition of amorphous calcium phosphate (ACP) on the surface of dentin ECM (Fig. 3A), while rDMP1 produced globular HA deposits around the exposed dentinal tubules and along the collagen fibers (Fig. 3B). Groups treated with 100% pB (Fig. 3C) induced large crystalline deposits throughout the ECM and, with increasing amounts of pB, resulted in more crystalline deposits than with pA. Dentin coated with a 1:4 ratio of pA:pB (Fig. 3D) resulted in needle-like deposits of HA, and each collagen fibril was coated heavily with nanocrystallites and dense depositions were observed throughout the dentin. Treatment groups with 100% pA, 4:1, 3:2, and 2:3 of pA:pB were not as successful at producing mineral with HA-like morphology (data not shown).
Figure 3.
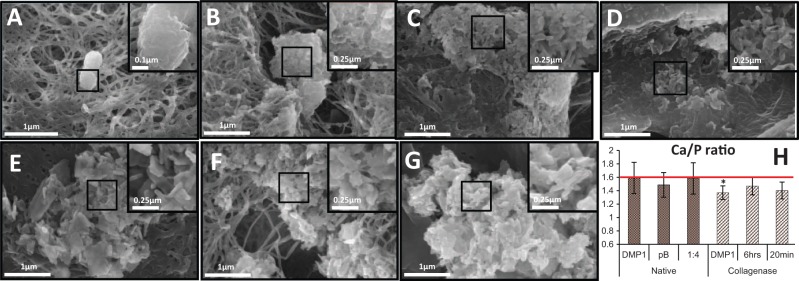
Representative field emission scanning electron microscopy (FE-SEM) images of dentin matrix after 2 wk of in vitro nucleation and energy-dispersive x-ray spectroscopy analysis (EDS). (A) BSA-coated wafer shows amorphous calcium phosphate deposits on the native collagen surface and no interfibrillar deposition; (B) recombinant dentin matrix protein 1 (rDMP1)-nucleated hydroxyapatite (HA) within dentinal tubules and along collagen fibers of the native matrix; (C) the peptide pB alone resulted in large crystalline calcium phosphate mineral throughout the native collagen matrix; (D) peptide ratio of 1:4 pA:pB-nucleated HA, which is seen as dense deposits throughout the collagen matrix with increased specificity for the peritubular region, resulting in highly mineralized native dentin; (E) 6 h of rDMP1 incubation, with the collagenase-challenged dentin matrix-nucleated plate-like HA around the dentinal tubules and visible crystal growth into the challenged collagen; (F) 6 h of 1:4 pA:pB incubation with the collagenase-challenged dentin matrix resulted in well-integrated HA crystals growing around the visibly damaged collagen fibrils of the ECM; (G) 20 min of 1:4 peptide incubation with challenged dentin matrix yielded large, needle-shaped HA crystals within the dentinal tubules; and (H) bar graph showing the atomic ratios of calcium to phosphorus within mineral nucleated within the native and collagenase-challenged dentin matrices as determined by EDS. Horizontal line represents the calcium/phosphorus atomic ratio of 1.6 for pure HA in its hexagonal close-packed unit crystal. Data are represented by mean ± SD; n = 5 unique dentin wafers; no statistical significance (P = 0.58, P = 0.72, P = 0.94) among treatments on native dentin was detected; significance between 6-hour rDMP1 and 6-hour 1:4 peptide treatments (P = 0.03) on simulated dentin matrix was detected; and no significant differences among any other treatments of challenged dentin (P = 0.29, P = 0.33) were detected by one-way analysis of variance (ANOVA) followed by Scheffé’s post hoc analysis.
Completely demineralized collagenase-treated dentin incubated for various lengths of time in a 1:4 ratio of pA:pB and rDMP1 (Fig. 3E–G) display crystalline calcium phosphate deposits within the matrix. Plate-like crystals are seen within the depression of a dentinal tubule of the collagenase-challenged matrix incubated for 6 h in rDMP1 (Fig. 3E). Dentin incubated for 6 h with a 1:4 peptide mixture (Fig. 3F) formed CaP crystals that were well-integrated into the challenged ECM. Expansion of the width of the collagen fibrils due to mineral deposition was also observed. A 20-minute incubation of collagenase-treated dentin in the 1:4 peptide mixture was also successful (Fig. 3G), yielding large, needle-shaped HA within the collagen matrix. Peptide incubation periods of 4-hour-, 2-hour, and 1-hour-nucleated mineral showed morphologies that did not deviate noticeably from the 6-hour or 20-minute lengths (data not shown).
EDS results (Fig. 3H) confirmed that the 1:4 ratio of peptides was as successful as rDMP1 at nucleating HA within the dentin scaffold, with Ca/P ratios of 1.58 ± 0.23 and 1.59 ± 0.23, respectively. Results for pB revealed a Ca/P ratio of 1.49 ± 0.18, which was not significant but was farther from the ideal atomic ratio of 1.6 seen in pure HA.
Transmission Electron Microscopy
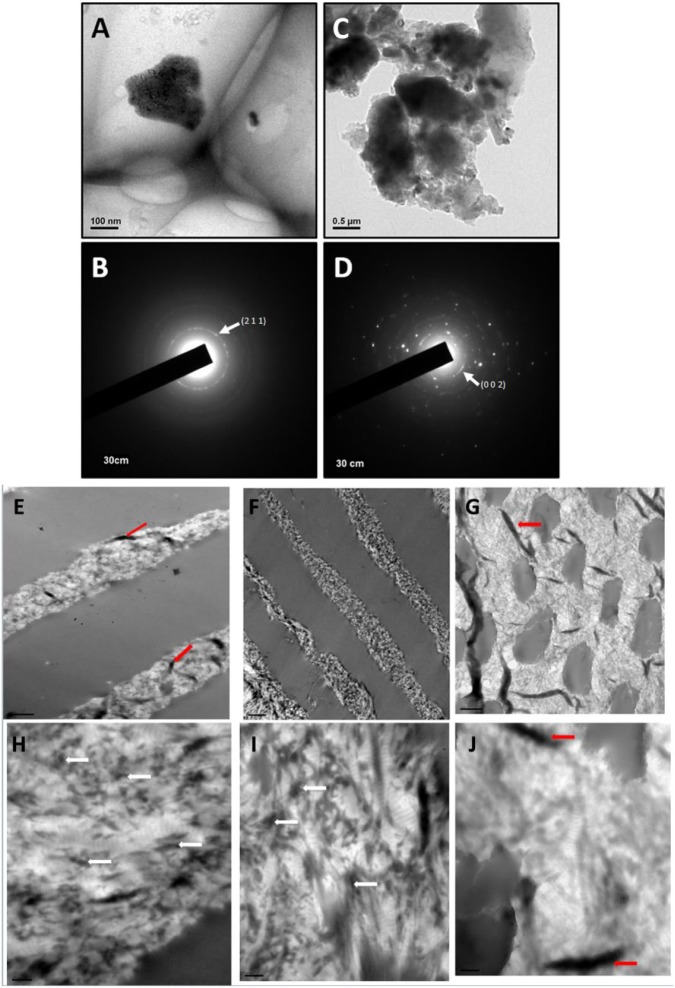
TEM and SAED of CaP mineral revealed that the crystal size was on the order of 250 nm (Fig. 4A) when nucleated with 100% pB on native dentin and resulted in a diffraction pattern (Fig. 4B) with a diffuse (2 1 1) ring, indicating impure HA and several epitaxial phases. Meanwhile, the crystal size resulting from the 1:4 ratio on native dentin (Fig. 4C) was on the order of 1-3 µm and produced a diffraction pattern with a (0 0 2) ring (Fig. 4D), indicating that crystal growth was along a uniform axis, producing rod-like mineral as seen via SEM (Fig. 3D), and individual reflections indicate the presence of a polycrystalline mineral.
Figure 4.
Transmission electron microscopy images of mineral and corresponding selected area electron diffraction (SAED) pattern. (A) Representative mineral crystal nucleated by the pB peptide; (B) corresponding SAED diffraction pattern for mineral crystal nucleated by pB that displays a diffuse (2 1 1) ring that is characteristic of impure hydroxyapatite (HA); (C) representative mineral crystal nucleated by a 1:4 ratio of pA:pB; (D) SAED diffraction pattern corresponding to mineral nucleated by a 1:4 ratio of peptides, resulting in a (0 0 2) ring as well as several concentric rings, indicating the presence of rod-like HA crystals. (E, F, G) TEM images obtained from dentin wafers coated with 1:4 peptide, DMP1, and BSA, respectively. (H, I, J) Corresponding magnified images. Intrafibrillar and interfibrillar mineral deposition is clearly observed in (H) and (I). Scale bars for E–G are 1 µm, and those for E′–G′ are 200 nm. The white arrows point to nucleated HAP, and red arrows are tissue folds. This figure is available in color online at http://jdr.sagepub.com.
Examination of the dentin wafers with either the peptide ratio of 1:4 or the rDMP1 showed nanocrystalline mineral deposits localized both intra- and interfibrillarly, while the control BSA did not show such deposits (Fig. 4E and H, F and I, G and J).
Nanocrystalline nature was confirmed by the presence of HAP deposits on reconstituted collagen fibrils coated with pA and pB at a 1:4 ratio (Fig. 5).
Figure 5.
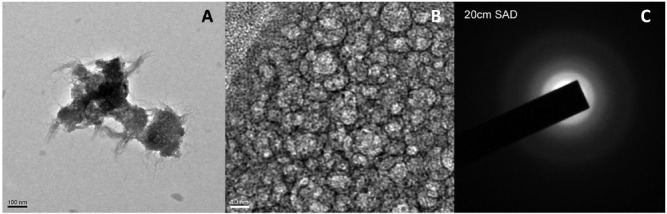
TEM image of HAP nanocrystals obtained directly from nucleation of reconstituted collagen adsorbed with the 1:4 ratio peptides and subjected to mineralization for 1 h in the presence of 1 M Ca2+ and phosphate buffer. (A, B) Nanocrystals deposited within the fibrils. (C) Selected area electron diffraction (SAED) confirms the nanocrystalline nature.
Discussion
This study provides evidence that synthetic peptides derived from DMP1 bind type I collagen and promote nucleation of HA within native and collagenase-challenged demineralized dentin substrates when exposed to physiological concentrations of calcium and phosphate ions in vitro. The biomimetic peptides used in this study are derived from C-terminal DMP1, which contains many acidic D and E residues that stabilize Ca2+ ions. When exposed to inorganic phosphate ions, PO43-, calcium phosphate minerals are nucleated within the gap regions of type I collagen. Through a series of intermediates, these ACP deposits are rearranged into stable HA through biomineralization mechanisms that are yet to be elucidated, but that are being aggressively investigated (Mahamid et al. 2008; Mahamid et al. 2010; Mahamid et al. 2011). Analysis of these data indicates that the peptides (pA 1.727 kDa; pB 2.185 kDa), when at the correct ratio, have an in vitro remineralization capacity equal to that of rDMP1 within human dentin matrix.
Recently, there has been a significant interest in casein phosphoproteins (CPPs) as remineralizing agents for the prevention and reversal of demineralized dental lesions (Rao and Malhotra 2011; Cochrane and Reynolds 2012; Nongonierma and Fitzgerald 2012). The 2 most-studied CPPs are αs1-casein F(59-79)5P and β-casein f(1-25)4P, each containing a 5aa -pSpSpSEE- acidic motif (Nongonierma and Fitzgerald 2012) that has been reported to successfully bind Ca2+ (Rose, 2000; Mekmene and Gaucheron 2011). The synthetic peptides used in this study contain acidic motifs pA -ESQES- and pB -QESQSEQDS-, which have also been shown to successfully bind Ca2+ in solution. With acidic motifs on the same size scale as CPPs, a major advantage of our peptides is the incorporation of a well-characterized collagen-binding motif -DSESSEEDR- (He and George 2004). Additionally, this motif shows specificity for the N-telopeptide region of the collagen fibril’s gap region, which is crucial for maintaining the mechanical properties of dental tissues (Kinney et al. 2003).
EDTA demineralization of the dentin substrate to expose the native collagen network was less aggressive when compared with the phosphoric acid used in our previous studies. Phosphoric acid did not solubilize collagen or GAGs and resulted in minimal detection of PGs (Bedran-Russo et al. 2013). Therefore, it is reasonable to suggest that our milder EDTA treatment should not have affected any of these organic components; however, these ECM molecules were not investigated in this study. Staining by Stains-All® indicated that at least one trypsin digestion is crucial for the removal of a great deal of heterogeneously distributed acidic proteins that remain bound to the organic matrix following decalcification. Analysis of our data suggests a total of 2 trypsin digestions to remove as many endogenous acidic proteins as possible from the type I collagen matrix, which is inherently resistant to trypsin digestion due to its triple helix structure (Seltzre and Eisen 1999).
Collagen-binding analysis of QD-conjugated peptides by TEM confirmed that the collagen-binding epitope used in the design of pA and pB is capable of binding reconstituted type I collagen. Preferential binding as seen by dense clusters of QDs is believed to be to the N-telopeptide located in the gap region of type I collagen.
SEM investigation of pA:pB ratios indicated a trend that, up to a 1:4 ratio, the more pB to which the wafer was exposed, the greater the presence of HA within the native matrix. Despite the importance of pB, the 1:4 ratio of peptides resulted in the most ideal morphology of mineral deposits, suggesting that although less effective, pA is still necessary for proper HA nucleation. Chemical analysis by EDS provided additional support that a 1:4 ratio of pA:pB is ideal and capable of nucleating HA identical to rDMP1 within demineralized, native dentin. These results are encouraging when coupled with the fact that the molecular weights of these peptides are 30 times smaller than that of rDMP1 and on the same size scale as those of the 2 CPPs discussed above. TEM further confirmed that the 1:4 ratio resulted in intra- and interfibrillarly localized nanocrystals, and with time, the HA nanocrystals grew along a single axis and had a rod-like structure.
To examine the efficacy of these synthetic peptides for clinical applications, we investigated whether a decreased incubation time of 20 min affected mineral nucleation. SEM analysis revealed that all treatments nucleated HA. Analysis of EDS data indicated that the 1:4 peptide ratio significantly outperformed rDMP1-treated, collagenase-challenged matrix incubated for 6 h. Peptide incubation for 20 min resulted in a Ca/P ratio similar to that after 6 h of rDMP1. Further investigations localizing HA nucleation within the type I collagen fiber and its ability to influence mechanical properties of the native and artificial caries-affected dentin matrices are required to understand the potential of these DMP1-derived synthetic peptides for clinically relevant remineralization applications.
In summary, these findings demonstrate that (i) synthetic polypeptides derived from DMP1 are capable of binding demineralized human dentin, (ii) these peptides can stabilize nucleation clusters from physiological levels of calcium and phosphate, (iii) an ideal ratio of these peptides exists within dentin ECM that results in HA formation utilizing both calcium-binding domains found in endogenous DMP1, and (iv) the ideal ratio of these peptides successfully promotes hydroxyapatite formation within native and collagenase-challenged dentin matrices within a short period of exposure. We envision the use of these peptides in a sequestered environment in the oral cavity for caries remineralization.
Author Contributions
J. Padovano, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; S. Ravindran, contributed to conception and design, drafted the manuscript; P.T. Snee, A. Ramachandran, contributed to data acquisition, drafted the manuscript; A. Bedran-Russo, contributed to conception and design, drafted the manuscript; A. George, contributed to conception, design, data interpretation, and analysis, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank Dr. Cristina Vidal for her assistance with this work and Ms. Linda Juarez for her assistance with the preparation of samples for TEM.
Footnotes
This work was supported by National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) grants F30DE023458, R01DE11657, T32DE018381, and K08DE017740. P.T. Snee acknowledges partial support from the Chicago Biomedical Consortium. Also, this work made use of the EPIC facility (NUANCE Center-Northwestern University), which has received support from the MRSEC program (NSF DMR-1121262) at the Materials Research Center, and the Nanoscale Science and Engineering Center (EEC-0118025/003), both programs of the National Science Foundation; the State of Illinois; and Northwestern University.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Bagramian RA, Garcia-Godoy F, Volpe AR. 2009. The global increase in dental caries. A pending public health crisis. Am J Dent. 22(1):3–8. [PubMed] [Google Scholar]
- Balooch M, Habelitz S, Kinney JH, Marshall SJ, Marshall GW. 2008. Mechanical properties of mineralized collagen fibrils as influenced by demineralization. J Struct Biol. 162(3):404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedran-Russo AK, Ravindran S, George A. 2013. Imaging analysis of early DMP1 mediated dentine remineralization. Arch Oral Biol. 58(3):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertassoni LE, Habelitz S, Marshall SJ, Marshall GW. 2011. Mechanical recovery of dentin following remineralization in vitro—an indentation study. J Biomech. 44(1):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane NJ, Reynolds EC. 2012. Calcium phosphopeptides—mechanisms of action and evidence for clinical efficacy. Adv Dent Res. 24(2):41–47. [DOI] [PubMed] [Google Scholar]
- Colfen H. 2010. Biomineralization: a crystal-clear view. Nat Mater. 9(12):960–961. [DOI] [PubMed] [Google Scholar]
- Demichelis R, Raiteri P, Gale JD, Quigley D, Gebauer D. 2011. Stable prenucleation mineral clusters are liquid-like ionic polymers. Nat Commun. 2:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Bomans PH, Müller FA, Will J, Frederik PM, de With G, Sommerdijk NA. 2010. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat Mater. 9(12):1010–1014. [DOI] [PubMed] [Google Scholar]
- He G, Dahl T, Veis A, George A. 2003a. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater. 2(8):552–558. [DOI] [PubMed] [Google Scholar]
- He G, Dahl T, Veis A, George A. 2003b. Dentin matrix protein 1 initiates hydroxyapatite formation in vitro. Connect Tissue Res. 44(Suppl 1):240–245. [PubMed] [Google Scholar]
- He G, George A. 2004. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem. 279(12):11649–11656. [DOI] [PubMed] [Google Scholar]
- Kim J, Arola DD, Gu L, Kim YK, Mai S, Liu Y, Pashley DH, Tay FR. 2010. Functional biomimetic analogs help remineralize apatite-depleted demineralized resin-infiltrated dentin via a bottom-up approach. Acta Biomater. 6(7):2740–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JH, Habelitz S, Marshall SJ, Marshall GW. 2003. The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res. 82(12):957–961. [DOI] [PubMed] [Google Scholar]
- Lees S, Page EA. 1992. A study of some properties of mineralized turkey leg tendon. Connect Tissue Res. 28(4):263–287. Published erratum in Connect Tissue Res. 1993;29(1):73. [DOI] [PubMed] [Google Scholar]
- Li QL, Ning TY, Cao Y, Zhang WB, Mei ML, Chu CH. 2014. A novel self-assembled oligopeptide amphiphile for biomimetic mineralization of enamel. BMC Biotechnol. 14(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mai S, Li N, Yiu CK, Mao J, Pashley DH, Tay FR. 2011. Differences between top-down and bottom-up approaches in mineralizing thick, partially demineralized collagen scaffolds. Acta Biomater. 7(4):1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall M, Gu TT, Luan X, Simmons D, Chen J. 1998. Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res. 13(3):422–431. [DOI] [PubMed] [Google Scholar]
- Mahamid J, Aichmayer B, Shimoni E, Ziblat R, Li C, Siegel S, Paris O, Fratzl P, Weiner S, Addadi L. 2010. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc Natl Acad Sci USA. 107(14):6316–6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamid J, Sharir A, Addadi L, Weiner S. 2008. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: indications for an amorphous precursor phase. Proc Natl Acad Sci USA. 105(35):12748–12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamid J, Sharir A, Gur D, Zelzer E, Addadi L, Weiner S. 2011. Bone mineralization proceeds through intracellular calcium phosphate loaded vesicles: a cryo-electron microscopy study. J Struct Biol. 174(3):527–535. [DOI] [PubMed] [Google Scholar]
- Mekmene O, Gaucheron F. 2011. Determination of calcium-binding constants of caseins, phosphoserine, citrate and pyrophosphate: a modelling approach using free calcium measurement. Food Chem. 127(2):676–682. [DOI] [PubMed] [Google Scholar]
- Nijhuis AW, Nejadnik MR, Nudelman F, Walboomers XF, te Riet J, Habibovic P, Tahmasebi Birgani Z, Li Y, Bomans PH, Jansen JA, et al. 2014. Enzymatic pH control for biomimetic deposition of calcium phosphate coatings. Acta Biomater. 10(2):931–939. [DOI] [PubMed] [Google Scholar]
- Niu LN, Zhang W, Pashley DH, Breschi L, Mao J, Chen JH, Tay FR. 2014. Biomimetic remineralization of dentin. Dent Mater. 30(1):77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nongonierma AB, Fitzgerald RJ. 2012. Biofunctional properties of caseinophosphopeptides in the oral cavity. Caries Res. 46(3):234–267. [DOI] [PubMed] [Google Scholar]
- Nudelman F, Pieterse K, George A, Bomans PH, Friedrich H, Brylka LJ, Hilbers PA, de With G, Sommerdijk NA. 2010. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater. 9(12):1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi YP, Li N, Niu LN, Primus CM, Ling JQ, Pashley DH, Tay FR. 2012. Remineralization of artificial dentinal caries lesions by biomimetically modified mineral trioxide aggregate. Acta Biomater. 8(2):836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Malhotra N. 2011. The role of remineralizing agents in dentistry: a review. Compend Contin Educ Dent. 32(6):26–33. [PubMed] [Google Scholar]
- Rose RK. 2000. Effects of an anticariogenic casein phosphopeptide on calcium diffusion in streptococcal model dental plaques. Arch Oral Biol. 45(7):569–575. [DOI] [PubMed] [Google Scholar]
- Seltzre JL, Eisen AZ. 1999. Native type I collagen is not a substrate for MMP2 (gelatinase A). J Invest Dermatol. 112(6):993–994. [DOI] [PubMed] [Google Scholar]
- Shen H, Jawaid AM, Snee PT. 2009. Poly(ethylene glycol) carbodiimide coupling reagents for the biological and chemical functionalization of water soluble nanoparticles. ACS Nano. 3(4):915–923. [DOI] [PubMed] [Google Scholar]
- Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, et al. 2004. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 279(18):18115–18120. [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. 2008. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 29(8):1127–1137. [DOI] [PubMed] [Google Scholar]
- Vidal CM, Aguiar TR, Phansalkar R, McAlpine JB, Napolitano JG, Chen SN, Araújo LS, Pauli GF, Bedran-Russo A. 2014. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 10(7):3288–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DJ. 1995. The application of in vitro models to research on demineralization and remineralization of the teeth. Adv Dent Res. 9(3):175–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.