Abstract
Chytridiomycosis caused by the chytrid fungus Batrachochytrium salamandrivorans (Bsal) poses a serious threat to urodelan diversity worldwide. Antimycotic treatment of this disease using protocols developed for the related fungus Batrachochytrium dendrobatidis (Bd), results in therapeutic failure. Here, we reveal that this therapeutic failure is partly due to different minimum inhibitory concentrations (MICs) of antimycotics against Bsal and Bd. In vitro growth inhibition of Bsal occurs after exposure to voriconazole, polymyxin E, itraconazole and terbinafine but not to florfenicol. Synergistic effects between polymyxin E and voriconazole or itraconazole significantly decreased the combined MICs necessary to inhibit Bsal growth. Topical treatment of infected fire salamanders (Salamandra salamandra), with voriconazole or itraconazole alone (12.5 μg/ml and 0.6 μg/ml respectively) or in combination with polymyxin E (2000 IU/ml) at an ambient temperature of 15 °C during 10 days decreased fungal loads but did not clear Bsal infections. However, topical treatment of Bsal infected animals with a combination of polymyxin E (2000 IU/ml) and voriconazole (12.5 μg/ml) at an ambient temperature of 20 °C resulted in clearance of Bsal infections. This treatment protocol was validated in 12 fire salamanders infected with Bsal during a field outbreak and resulted in clearance of infection in all animals.
The rate at which amphibian populations have been declining the past decades is alarming1,2. One of the factors in part responsible for these declines is the infectious disease chytridiomycosis caused by the chytrid fungus Batrachochytrium dendrobatidis (Bd)3,4,5. Recently, the related chytrid fungus Batrachochytrium salamandrivorans (Bsal) has been identified as a novel threat to amphibian populations, with a potentially major impact on salamander diversity worldwide6,7. For amphibian chytridiomycosis caused by Bd, topical antimycotic treatment using voriconazole at a concentration of 1.25 μg/ml during 7 days has proven highly successful and safe8. However, applying this treatment to Bsal infected salamanders is unable to clear infections (see Case report section). Thermal treatment consisting of exposure to the critical thermal maximum for Bsal (25 °C) for 10 days was shown to be able to clear Bsal infections from infected salamanders9. However, this temperature approaches the critical thermal maximum of several urodelans10, rendering this treatment of limited use for those species. Therefore the aim of this study was to develop an antifungal treatment protocol able to eliminate Bsal in infected salamanders at temperatures below the critical thermal maximum of Bsal, tolerated by most salamanders.
Case report
Thirty-nine fire salamanders (Salamandra salamandra) from a population in the Netherlands undergoing dramatic declines from 2008 onwards due to Bsal11 were included in an ex situ conservation program. Since the susceptibility of Bsal to antimycotics was not known, the animals were treated with voriconazole (1.25 μg/ml, topical spray, twice a day for 7 days), based on the treatment protocol used to clear Bd infections from amphibians8. Skin lesions, lethargy and inappetite did not resolve in the Bsal infected animals.
Results and Discussion
In vitro susceptibility of Bsal to antimycotic compounds
The results of the experiments to determine the MICs of the tested antimicrobials for Bsal are summarized in Table 1. Florfenicol was the only compound tested that was not able to limit growth or kill Bsal at the concentrations tested. In contrast, florfenicol is capable of limiting growth of Bd at concentration of 0.5–1.0 μg/ml (Muijsers et al. 2012). Interestingly, the inhibitory concentrations of the other compounds against Bsal differed noticeably from those against Bd. The mechanism underlying this difference remains unknown. Whereas polymyxin E did not show any inhibitory potential in Bd MIC tests at the concentrations used12, Bsal was inhibited by polymyxin E at a concentration of 8000 IE/ml (Table 1). Terbinafine limited Bsal growth at a concentration of 0.2 μg/ml, which is in accordance with its activity against Bd at 0.063 μg/ml13. Itraconazole, which is frequently used to treat amphibians infected with Bd, had a MIC against Bsal 2.5–5 times lower (0.006 μg/ml) compared to its MIC against Bd (0.016–0.032 μg/ml)13. Finally, the MIC of voriconazole for inhibiting Bsal growth was 10 times higher (0.125 μg/ml) than the MIC for inhibiting Bd (0.0125 μg/ml)8. This result at least partly explains the failed initial treatment of the wild fire salamanders using the voriconazole dosage for treating chytridiomycosis in amphibians infected with Bd (1.25 μg/ml sprays, twice a day for 7 days)8. The concentrations to completely kill Bsal cultures were all close to the MIC (Table 1, one dilution higher for all compounds).
Table 1. Susceptibility of Bsal to antimicrobial compounds.
| Compound | 2-fold dilution range | MIC value | 100% killing concentration |
|---|---|---|---|
| Florfenicol | 0.016–8 μg/ml | >8 μg/ml | >8 μg/ml |
| Voriconazole | 0.016–8 μg/ml | 0.125 μg/ml | 0.25 μg/ml |
| Polymyxin E | 1250–64000 IE/ml | 8000 IE/ml | 16000 IE/ml |
| Itraconazole | 0.003–1.2 μg/ml | 0.006 μg/ml | 0.012 μg/ml |
| Terbinafine | 0.1–12.5 μg/ml | 0.2 μg/ml | 0.4 μg/ml |
An overview of the tested two-fold serial dilution range of the antimicrobial compounds, the minimum inhibitory concentrations (MICs) for Bsal and the concentrations that completely killed Bsal after an exposure period of 10 days.
Synergy between polymyxin E and voriconazole or itraconazole in inhibiting Bsal growth
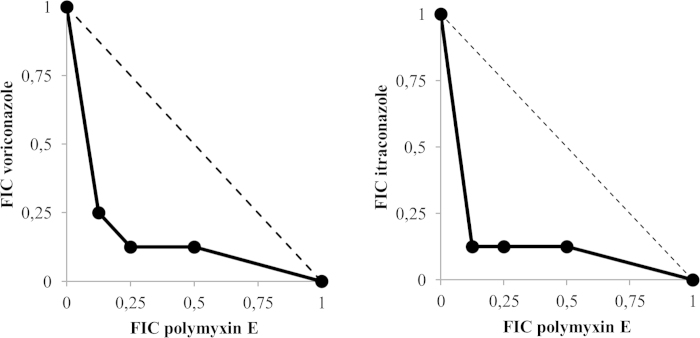
The three main techniques used for testing interactions between compounds in antifungal activity are Etest, time-kill methods and checkerboard dilution methods14,15,16,17. In synergy testing for bacterial pathogens, the biggest disadvantage is that no two methods will produce comparable results, and therefore clinical applicability of results is under debate18. These limitations also apply for antifungal synergy testing17. Furthermore, a vast amount of studies exist that describe in vitro synergy without linking (or being able to link) these results to a beneficial treatment outcome of combined treatment18. The goal of this study was to evaluate potential synergy between antimycotic compounds in inhibiting Bsal growth to allow development of an experimental treatment protocol using antifungal concentrations below toxicity levels. In this study, a checkerboard dilution method adopted from the method used to evaluate minimum inhibitory concentrations for Bd8,12 was used, which in comparison to the time-kill method, is easier to carry out and interpret17. The combinations of compounds tested both included an azole antifungal (voriconazole or itraconazole) and polymyxin E, which were already shown to be able to inhibit Bsal growth (Table 1). Apart from polymyxins exerting antifungal activity on their own, combinations of polymyxins and azole antifungals showed synergistic antifungal activity against infections with Aspergillus spp., Candida spp. and Cryptococcus spp19,20,21. The bactericidal activity of polymyxin E against Gram-negative bacteria is an added advantage for treating Bsal associated lesions, since histological preparations of skin samples of salamanders infected with Bsal often revealed severe bacterial overgrowth of the skin in concordance with Bsal infection6. Secondary bacterial infections in immunocompromised amphibians are often caused by opportunistic Gram-negative bacteria22,23. The azole antifungals voriconazole and itraconazole both have reported effectiveness in treating chytridiomycosis in amphibians caused by infections with Bd8,24,25. Negative side effects of treating amphibians with itraconazole have been reported though24,25,26, so studies evaluating the efficacy of reduced concentrations of itraconazole alone27 or in combination therapies to successfully treat chytridiomycosis could be a major advantage. The results of the experiments to determine the FICs of polymyxin E combined with voriconazole or itraconazole are graphically depicted in isobolograms (Fig. 1). Two of the tested combinations of polymyxin E with voriconazole (2000 IE/ml + 0.02 μg/ml and 1000 IE/ml + 0.03 μg/ml) and two combinations of polymyxin E with itraconazole (2000 IE/ml + 0.0016 μg/ml and 1000 IE/ml + 0.0016 μg/ml) resulted in a FICI that demonstrates synergism (FICI ≤ 0.5, Fig. 1). All combinations that inhibited Bsal growth also killed Bsal completely after 10 days of incubation.
Figure 1. FIC Isobolograms showing the interactions between two antimicrobial agents in inhibiting Bsal growth.
FIC values derived from combinations of voriconazole, itraconazole and polymyxin E were used to plot the isobolograms. A FIC value of 1 corresponds to the MIC value of the particular antimicrobial agent. The dotted line represents the theoretical additive interaction between two agents (see text for our definition of the types of interactions).
Effective treatment of Bsal infections in fire salamanders based on synergy between polymyxin E, voriconazole and temperature
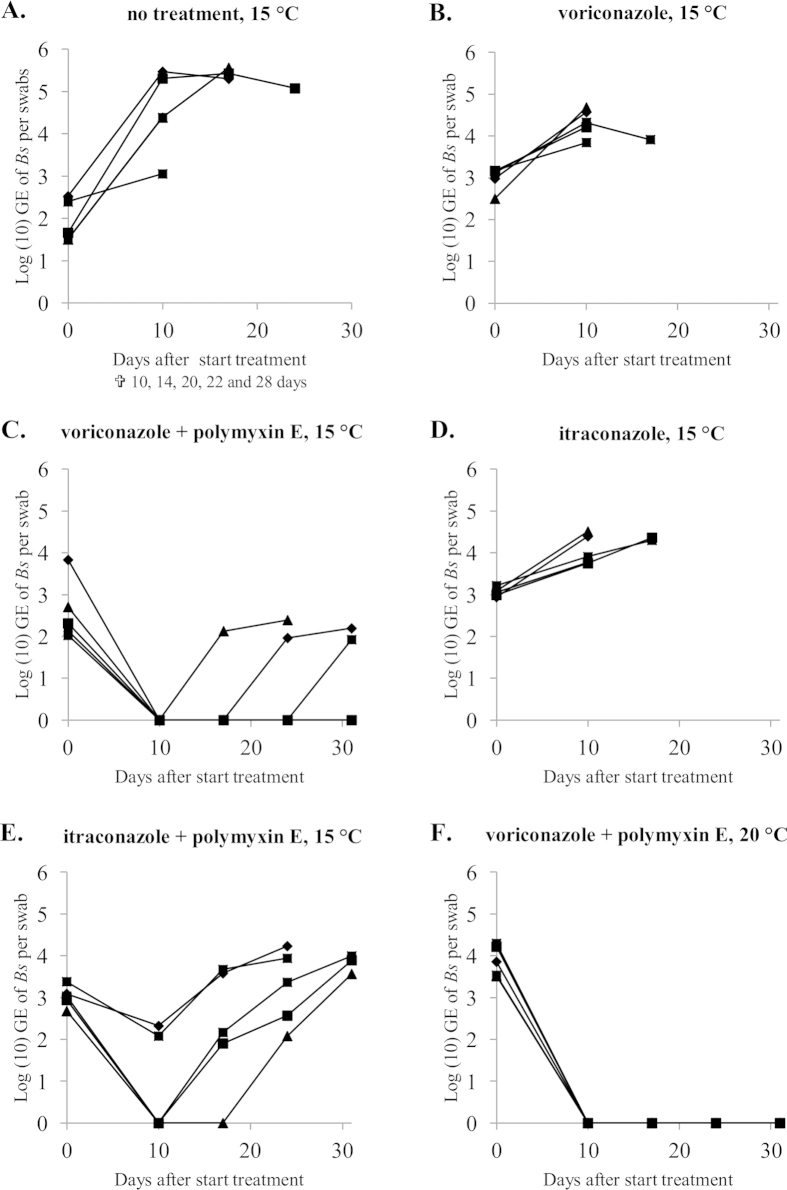
All initially tested treatment conditions, composed out of itraconazole or voriconazole alone and in combination with polymyxin E were unable to clear Bsal infections from infected amphibians (Fig. 2, panels A–E ). Although the combination therapies of itraconazole or voriconazole with polymyxin E did reduce Bsal infection loads to undetectable levels in 3 out of the 5 animals and 5 out of 5 animals respectively, recrudescence of infection did occur in all animals (Fig. 2, panels C and E). The difference between in vitro and in vivo effects of the combination therapy at 15 °C might lie in the exposure to the compounds; in the in vitro experiments, Bsal was exposed continuously to both compounds as opposed to the periodical exposure in the in vivo experiments. At least for polymyxin E (2000 IU/ml) longer exposure times are unusable due to occurrence of toxicity (personal observations). The conditions of the additional treatment (Fig. 2, panel F) instituted after failure of the initial conditions to clear Bsal infections, were based on the in vitro synergy between voriconazole and polymyxin E in inhibiting Bsal growth, the increased but suboptimal inhibition of Bsal in vivo by combined exposure to voriconazole and polymyxin E (Fig. 2, panel C) and the previously determined temperature dependent infection dynamics of Bsal9. Using the same concentrations of voriconazole and polymyxin E, but raising the temperature to 20 °C did result in successful elimination of Bsal in all infected animals (Fig. 2, panel F). The results of this study underline the key influence temperature plays in Bsal infection dynamics, which already allowed development of a Bsal temperature treatment protocol for amphibian species able to endure a continuous ambient temperature of 25 °C for 10 days9. Ethical considerations allowed only one additional treatment condition to be tested. Therefore, the positive treatment effect could theoretically be attributed to the sole influence of either one of the compounds at 20 °C, as experimental treatments with individual compounds were only tested in vivo at a temperature of 15 °C. The results of this study show that synergy between voriconazole and polymyxin E together with the temperature dependent infection dynamics of Bsal allow Bsal infections to be eliminated in amphibian species with critical thermal maxima lower than that of Bsal. The efficacy of the treatment protocol was validated by successful treatment of fire salamanders naturally infected with Bsal during a field outbreak (Fig. 3). In conclusion, in vitro synergy between antimycotic compounds in inhibiting Bsal, together with the temperature dependent infection dynamics of Bsal allowed development of a treatment protocol successful in eliminating Bsal from experimentally and naturally infected amphibians. Although exploiting synergism between temperature and chemical compounds was effective in this study, in case of therapeutic failure, acclimatisation of Bsal to higher temperatures and/or the emergence of Bsal strains with higher thermal preferences should be considered28,29.
Figure 2. Results of five treatment protocols to clear Bsal infections from experimentally infected fire salamanders.
After ascertaining presence of Bsal in all animals (day 0) they were either left untreated as control (A), treated twice a day for 10 days with voriconazole sprays (12.5 μg/ml) (B) or itraconazole sprays (0.6 μg/ml) (D) alone or with a combination of polymyxin E submersion baths (2000 IU/ml, 10 minutes) followed by spraying voriconazole (12.5 μg/ml) (C) or itraconazole (0.6 μg/ml) (E). Conditions A-E were all performed at an ambient temperature of 15 °C. An additional treatment protocol composed of polymyxin E submersion baths (2000 IU/ml, 10 minutes) followed by spraying voriconazole (12.5 μg/ml) at an ambient temperature of 20 °C was instituted after failure to clear Bsal infections in conditions A-E. Swabs for Bsal real-time PCR analysis were collected at day 0, 10, 17, 24 and 31. Animals that reached the determined experimental endpoint. Each symbol represents the course of infection of an individual animal.
Figure 3. Treatment of fire salamanders naturally infected with Bsal.
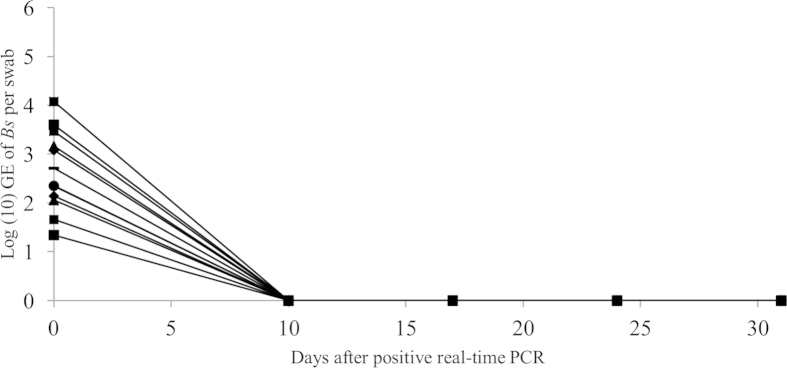
After ascertaining presence of Bsal in all animals (day 0), they were treated with polymyxin E submersion baths (2000 IE/ml, 10 minutes) followed by spraying voriconazole (12.5 μg/ml) twice a day for 10 days at an ambient temperature of 20 °C. Each symbol represents the course of infection of an individual animal.
Methods
All experiments were performed in accordance with the relevant guidelines and regulations. All experiments with experimental animals were carried out with approval of the ethical committee of the Faculty of Veterinary Medicine, Ghent University.
Chytrid strain & culture conditions
The Bsal type strain (AMFP13/1)6 was grown in TGhL broth (16 g tryptone, 4 g gelatin hydrolysate, 2 g lactose per liter of distilled water) in 25 cm3 cell culture flasks and incubated at 15 °C. To obtain a suspension containing a mixture of zoosporangia and zoospores, the walls of a cell culture flask containing 5-day-old culture were scraped with a sterile cell scraper and the suspension subsequently collected.
Determination of the minimum inhibitory concentrations of antimicrobial agents against Bsal
The minimum inhibitory concentrations (MICs) of florfenicol (20%), voriconazole (VFend IV, Pfizer, Kent, UK), itraconazole (Itrafungol, Elanco, Brussels, Belgium), terbinafine (Terbinafine hydrochloride, Sigma-Aldrich, Bornem, Belgium) and polymyxin E (Colistin sulphate, VMD, Arendonk, Belgium) for Bsal were determined using a broth macrodilution method used for MIC testing of Bd8,12. In short, two-fold dilutions series of the antimicrobial agents were prepared in TGhL broth, and 200 μl of these prepared dilutions were added to wells of 24 well cell culture plates (Table 1). Two hundred μl of a suspension containing a mixture of Bsal sporangia and zoospores (approximately 105 Bsal organisms per ml) were added to all wells. Finally, 1600 μl of TGhL broth were added to all wells resulting in a final volume of 2 ml per well. Plates were incubated at 15 °C (optimum growth temperature of Bsal6) and checked for viability and growth daily for 10 days with an inverted light microscope. Wells containing TGhL broth with viable Bsal sporangia and zoospores and wells containing heat treated (85 °C, 10 minutes) Bsal sporangia and zoospores served as positive and negative growth controls respectively. The MIC value was determined as the lowest concentration of the antimicrobial agent at which no growth could be observed after 10 days of incubation. To test which concentrations of the antimicrobial agents were lethal for Bsal after 10 days of exposure, we removed the medium and replenished all wells with fresh TGhL broth without antimicrobial agents. Plates were incubated at 15 °C and checked for viability and growth daily for an additional 14 days with an inverted light microscope. A concentration was considered to be lethal to Bsal when no signs of growth could be observed after this incubation period of 14 days (Table 1). All conditions were tested in triplicate.
Determination of the fractional inhibitory concentrations of antimicrobial agents against Bsal
To test for synergy in combinations of polymyxin E with voriconazole or with itraconazole in inhibiting Bsal growth, fractional inhibitory concentrations (FICs)30 were determined using a macrodilution broth checkerboard technique. Two-fold serial dilution series of all antimicrobial agents in TGhL broth were prepared. Polymyxin E was tested at final concentrations of 1000–64000 IE/ml, voriconazole at final concentrations of 0.016–1 μg/ml and itraconazole at final concentrations of 0.0007–0.05 μg/ml. All tested concentrations of the individual antimicrobial agents were included separately as controls for reproducibility of the earlier determined MIC values of the compounds. Twenty-four well cell culture plates were prepared with 1600 μl of TGhL broth, 200 μl of the respective compound or combination of compounds and 200 μl of a suspension containing Bsal sporangia and zoospores including Bsal positive and negative growth controls as described earlier. Plates were incubated at 15 °C and checked for signs of viability and growth daily for 10 days with an inverted light microscope. The FIC value for an individual antimicrobial agent is determined as the ratio of the MIC value of the antimicrobial agent used in combination (MICcombi) to the MIC value of the antimicrobial by itself (MICalone) after 10 days of incubation:
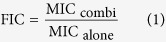 |
These FIC values are subsequently used to produce a single fractional inhibitory concentration index (FICI) as an indicator for the type of interaction between two antimicrobial agents30:
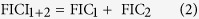 |
We determined the possible interactions as synergistic (FICI ≤ 0.5), additive (FICI > 0.5–1.0), indifferent (FICI > 1.0–4.0) or antagonistic (FICI ≥ 4.0)18,31,32. Isobolograms were used to graphically depict the FIC and FICI values of all tested combinations of antimicrobial agents (Fig. 1)33. To test which combination of concentrations of the antimicrobial agents was lethal for Bsal after 10 days of exposure, we replaced the broth containing the compound(s) with fresh TGhL broth without antimicrobial agents. Plates were incubated at 15 °C and checked for viability and growth daily for an additional 14 days with an inverted light microscope. A combination of concentrations was considered to be lethal to Bsal when no signs of growth could be observed after this incubation period of 14 days. All conditions were tested in quadruplicate.
Treatment of experimentally infected fire salamanders
Fire salamanders (Salamandra salamandra) were inoculated with Bsal in order to study in vivo efficacy of different antimicrobial treatment protocols. The animal experiment was performed with the approval of the ethical committee of the Faculty of Veterinary Medicine (Ghent University, EC2013/87 and EC2014/65). Thirty captive bred fire salamanders were housed individually in plastic containers in a climatized room with an ambient temperature of 15 °C. The animals were kept on a moist tissue, with access to a hiding place and water container. Crickets powdered with mineral and vitamin supplement were provided ad libitum as food source. All animals were clinically healthy and free of Bd and Bsal, as determined with duplex real-time PCR examination of skin swabs34. An acclimatization period of 2 weeks was admitted before the start of the experiment. The experimental animals were randomly assigned to one of the 6 experimental treatment groups (5 animals per treatment group, kept individually). All salamanders were inoculated with Bsal by topically applying one mL of inoculum containing 105 zoospores per ml on the skin. Animals were kept at 15 °C (except for the animals in group F; details below) and skin swabs for Bsal real-time PCR analysis34 were collected every 7 days. Individual treatment commenced when Bsal infection was established (determined as an increase in Bsal infection load between 2 consecutive samplings). The different groups were untreated negative control (group A), voriconazole treatment (12.5 μg/ml) alone (group B), voriconazole and polymyxin E treatment (concentrations of 12.5 μg/ml and 2000 IU/ml respectively, group C), itraconazole treatment (0.6 μg/ml) alone (group D) and itraconazole and polymyxin E treatment (0.6 μg/ml and 2000 IU/ml respectively, group E). After initial failure to clear Bsal infections in these first experimental groups, we trialed another treatment condition composed of voriconazole and polymyxin E treatment (concentrations of 12.5 μg/ml and 2000 IU/ml respectively, identical to the treatment described for group C) but with an ambient temperature of 20 °C instead of 15 °C (group F). Due to ethical considerations only one additional condition was performed. All experimental treatments were carried out twice a day for 10 days. Polymyxin E was administered through submersion baths (10 minutes) and voriconazole and itraconazole were administered through spraying the animals and tissue in their housing (after polymyxin E baths if applicable). After the treatment period, all animals were kept at 15 °C. Skin swabs for Bsal real-time PCR analysis were collected immediately after the treatment period and subsequently every 7 days for another 3 weeks. An animal was considered negative for Bsal after 3 consecutive negative real-time PCR results. Development/progression of symptoms associated with Bsal infections together with presence of Bsal as determined with real-time PCR analysis in an animal was determined as experimental endpoint and resulted in withdrawal of the animal from the experiment. If an animal tested positive for the presence of Bsal in the post-treatment follow-up phase (starting from day 10 in Fig. 2), the treatment was considered as failed. Remaining Bsal infections in animals that were removed from the experiment due to reaching the described endpoint, and animals still positive for Bsal at the last sampling time point were exposed to an ambient temperature of 25 °C during 10 days to clear the Bsal infection9.
Treatment of naturally infected fire salamanders
Thirty-five fire salamanders from the population from which Bsal (strain AMFP13/1) was originally isolated (Bunderbos, Netherlands, N50°54′51″, E5°44′59″) were transferred to our research facility for treatment. Upon arrival, 12 of the translocated animals tested positive for presence of Bsal DNA as tested with the Bsal real-time PCR34. Based on the results of the treatments of experimental infections, the animals were treated with polymyxin E submersion baths (2000 IU/ml, 10 minutes) followed by spraying voriconazole (12.5 μg/ml) twice a day for 10 days at an ambient temperature of 20 °C. Housing conditions of the animals were identical to the conditions described for the experimental animals. After the treatment period all animals were put back at 15 °C. Skin swabs for Bsal real-time PCR analysis were collected directly after the treatment period and subsequently every 7 days for another 3 weeks. An animal was considered negative for Bsal after 3 consecutive negative real-time PCR results.
Cost of treatment
A step-by-step treatment protocol can be found as supplementary file. Based on the products, volumes and estimated pricing in this protocol, the treatment cost of a single animal would be 6 €. It should be noted however, that this estimated cost applies for individual treatment of animals. Treatment of groups of amphibians with the same volumes is possible (although this has not yet been validated), resulting in a reduction of the cost of treatment per animal.
Additional Information
How to cite this article: Blooi, M. et al. Successful treatment of Batrachochytrium salamandrivorans infections in salamanders requires synergy between voriconazole, polymyxin E and temperature. Sci. Rep. 5, 11788; doi: 10.1038/srep11788 (2015).
Supplementary Material
Footnotes
Author Contributions M.B., F.P. and A.M. designed the experiments. M.B. carried out the experiments. M.B., F.P., L.R., F.H., F.V. and F.P. contributed in writing and reviewing the manuscript.
References
- Stuart S. N. et al. Status and trends of amphibian declines and extinctions worldwide. Science. 306, 1783–1786 (2004). [DOI] [PubMed] [Google Scholar]
- Houlahan J. E., Findlay C. S., Schmidt B. R., Meyer A. H. & Kuzmin S. L. Quantitative evidence for global amphibian population declines. Nature. 404, 752–755 (2000). [DOI] [PubMed] [Google Scholar]
- Berger L. et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. P. Natl. Acad. Sci. U.S.A. 95, 9031–9036 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake D. B. & Vredenburg V. T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. P. Natl. Acad. Sci. U.S.A. 105, 11466–11473 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pounds J. A. et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 439, 161–167 (2006). [DOI] [PubMed] [Google Scholar]
- Martel A. et al. Batrachochytrium salamandrivorans sp nov causes lethal chytridiomycosis in amphibians. P. Natl. Acad. Sci. U.S.A. 110, 15325–15329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel A. et al. Recent introduction of a chytrid fungus endangers western palearctic salamanders. Science. 346, 630–631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel A. et al. Developing a safe antifungal treatment protocol to eliminate Batrachochytrium dendrobatidis from amphibians. Med. Myc. 49, 143–149 (2011). [DOI] [PubMed] [Google Scholar]
- Blooi M. et al. Treatment of urodelans based on temperature dependent infection dynamics of Batrachochytrium salamandrivorans. Sci Rep. 5, 8037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury R. B. Low thermal tolerances of stream amphibians in the Pacific Northwest: Implications for riparian and forest management. Appl. Herpetol. 5, 63–74 (2008). [Google Scholar]
- Spitzen-van der Sluijs A. et al. Rapid enigmatic decline drives the fire salamander (Salamandra salamandra) to the edge of extinction in the Netherlands. Amphibia-Reptilia. 34, 233 - 239 (2013). [Google Scholar]
- Muijsers M. et al. Antibacterial therapeutics for the treatment of chytrid infection in amphibians: Columbus’s egg? Bmc Vet. Res. 8, 10.1186/1746-6148-8-175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward A., Berger L. & Skerratt L. F. In vitro sensitivity of the amphibian pathogen Batrachochytrium dendrobatidis to antifungal therapeutics. Res. Vet. Sci. 97, 364–366 (2014). [DOI] [PubMed] [Google Scholar]
- Kontoyiannis D. P. et al. Itraconazole-amphotericin B antagonism in Aspergillus fumigatus: an E-test-based strategy. Antimicrob. Agents Ch. 44, 2915–2918 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele D. J., DeLallo V. C., Lewis R. E., Ernst E. J. & Klepser M. E. Evaluation of amphotericin B and flucytosine in combination against Candida albicans and Cryptococcus neoformans using time-kill methodology. Diagn. Micr. Infec. Dis. 41, 121–126 (2001). [DOI] [PubMed] [Google Scholar]
- Perea S. et al. In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob. Agents Ch. 46, 3039–3041 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. E., Diekema D. J., Messer S. A., Pfaller M. A. & Klepser M. E. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemoth. 49, 345–351 (2002). [DOI] [PubMed] [Google Scholar]
- Doern C. D. When does 2 plus 2 aqual 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 52, 4124–4128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai B. et al. Polymyxin B, in combination with fluconazole, exerts a potent fungicidal effect. J. Antimicrob. Chemoth. 65, 931–938 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami R., Lewis R. E., Tarrand J., Leventakos K. & Kontoyiannis D. P. Antifungal activity of colistin against Mucorales species in vitro and in a murine model of Rhizopus oryzae pulmonary infection. Antimicrob. Agents Ch. 54, 484–490 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemuth H. et al. In vitro activity of colistin as single agent and in combination with antifungals against filamentous fungi occurring in patients with cystic fibrosis. Mycoses. 56, 297–303 (2013). [DOI] [PubMed] [Google Scholar]
- Mader D. R. Reptile Medicine and Surgery. second edn, (Saunders Elsevier, 2006). [Google Scholar]
- Whitaker B. & Wright K. N. Amphibian Medicine and Captive Husbandry. (Krieger Publishing Company, 2001). [Google Scholar]
- Garner T. W., Garcia G., Carroll B. & Fisher M. C. Using itraconazole to clear Batrachochytrium dendrobatidis infection, and subsequent depigmentation of Alytes muletensis tadpoles. Dis. Aquat. Organ. 83, 257–260 (2009). [DOI] [PubMed] [Google Scholar]
- Brannelly L. A., Richards-Zawacki C. L. & Pessier A. P. Clinical trials with itraconazole as a treatment for chytrid fungal infections in amphibians. Dis. Aquat. Oragn. 101, 95–104 (2012). [DOI] [PubMed] [Google Scholar]
- Pessier A. P. Management of disease as a threat to amphibian conservation. Int. Zoo Yearb. 42, 30–39 (2008). [Google Scholar]
- Jones M. E. et al. Treatment of chytridiomycosis with reduced-dose itraconazole. Dis. Aquat. Organ. 99, 243–249 (2012). [DOI] [PubMed] [Google Scholar]
- Woodhams D. C., Alford R. A., Briggs C. J., Johnson M., Rollins-Smith L. A. Life-history trade-offs influence disease in changing climates: Strategies of an amphibian pathogen. Ecology. 89, 1627–1639 (2008). [DOI] [PubMed] [Google Scholar]
- Voyles J. et al. Temperature alters reproductive life history patterns in Batrachochytrium dendrobatidis, a lethal pathogen associated with the global loss of amphibians. Ecol. Evol. 2, 2241)2249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Singer S. & Hitchings G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J. Biol. Chem. 208, 477–488 (1954). [PubMed] [Google Scholar]
- Odds F. C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemoth. 52, 1–1 (2003). [DOI] [PubMed] [Google Scholar]
- Johnson M. D., MacDougall C., Ostrosky-Zeichner L., Perfect J. R. & Rex J. H. Combination antifungal therapy. Antimicrob. Agents Ch. 48, 693–715 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung 3, 285–290 (1953). [PubMed] [Google Scholar]
- Blooi M. et al. Duplex real-time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J. Clin. Microbiol. 51, 4173–4177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.