Abstract
In Brief
This article describes available insulin products and published guidelines to aid clinicians in making treatment decisions for insulin-dependent patients with type 2 diabetes. It establishes the need for a thorough evaluation of the literature regarding ambulatory insulin dosing to further inform providers who manage insulin therapy for patients with type 2 diabetes.
The World Health Organization estimates that 347 million people worldwide have diabetes, making it a chronic disease of epidemic proportions.1 In 2010, the Centers for Disease Control and Prevention (CDC) estimated that 26.9% of the U.S. population > 65 years of age had diabetes, including 11.3% of adults > 20 years of age (25.6 million).2 Based on population estimates from the 2005–2008 National Health and Nutrition Examination Survey, the CDC also estimated that ∼ 79 million Americans had prediabetes in 2010.2 Diabetes was the cause of death for 3.4 million people worldwide in 2004 and is currently the seventh leading cause of death in the United States, with a projected increase in deaths from diabetes of two-thirds between 2008 and 2030.1,2
Treatment of type 2 diabetes incorporates lifestyle modifications related to diet and exercise, usually in addition to oral or injectable pharmacotherapy, depending on disease control and progression. Medications for diabetes represent the fourth largest category of medications in both sales and prescribing in the United States, with > $19 billion in spending and 173 million prescriptions dispensed.3,4 Insulin glargine is among the top 25 individual products in terms of spending, accounting for $2 billion, or 10.5% of total prescription costs for the treatment of diabetes.5 Despite these staggering numbers, ∼ 16% of patients with diabetes do not take any medication, and only 26% of patients with diabetes (type 1 and type 2 diabetes combined) are prescribed some form of insulin.2
Insulin is an endogenous hormone produced and secreted by the β-cells of the pancreas. The loss of pancreatic function is a hallmark for the diagnosis of type 1 diabetes and is the reason insulin is a necessary treatment modality for patients with that form of the disease. Type 2 diabetes is also partially defined by a loss of pancreatic function. At the time of diagnosis of type 2 diabetes, ∼ 50% of pancreatic function is already lost. This is a progressive loss that continues throughout treatment, leading to poor glycemic control and its associated complications. The continued decline of β-cell function results in the need for medication(s) and eventually exogenous administration of insulin for type 2 diabetes.6
Once medications are indicated, package inserts for pharmaceutical products, prepared by their manufacturers, are common resources to aid clinicians in dosing. The manufacturers of the two available long-acting insulin products—glargine and detemir—provide guidance about the initiation of therapy with a fixed or weight-based dose, depending on how the product was studied in clinical trials. Although the package insert for glargine notes that it can be administered at any time of the day and that the average initial dose for patients in clinical trials was 10 units daily, the statement regarding dosage adjustment is vague, stating that after initiation, the dose was “. . . subsequently adjusted according to the patient’s need to a total daily dose ranging from 2 to 100 IU.” Similarly, dose determination statements by the manufacturer of detemir focus on conversion from other types of basal insulin and report the mean dose of detemir required by patients in comparison to NPH insulin at the end of a clinical trial.7,8 The prescribing information for NPH insulin (sold under the trade names Novolin N and Humulin N) is available in “patient package inserts,” which provide a brief description of how the insulin is manufactured, along with patient education regarding diabetes and administration of the product. Dosing recommendations for health care professionals are not available in these package inserts.9,10
The package inserts for available mealtime insulin products—human regular insulin (sold as Novolin R or Humulin R) and the rapid-acting analogs aspart, lispro, and glulisine—make general recommendations for use based on the estimated total daily insulin requirement for a patient and the expected percentage of mealtime insulin as part of a basal-bolus regimen.11–15
Although insulin therapy is an integral part of treatment for patients with type 2 diabetes, a lack of consensus exists regarding insulin dosing in the ambulatory setting. With the availability of four distinct types of synthetic and analog insulin products, initial dosing and titration may vary depending on the regimen selected and patient-specific characteristics. Clinical practice guidelines provide direction for clinicians on initiating insulin in appropriate patients, with limited statements regarding titration and adjustment.16–20
Prospective, randomized trials have consistently shown the benefit of glycemic control in the treatment of type 2 diabetes,21–24 but do not go into detail regarding optimal insulin dosing. The Treating to Target in Type 2 Diabetes Study Group (4-T trial)25 compared three insulin regimens, further supporting the practice of insulin initiation and intensification as an addition to or replacement of oral therapy in patients who are not reaching their glycemic control goals. However, a lack of detailed methodology on the titration of insulin in the 4-T trial and other primary literature limits guidance for providers.25
Although dosing information is available for some insulin preparations based on clinical trial data, this guidance is not universally implemented in practice. A patient-centered approach to insulin therapy is essential to ensure optimal outcomes and safety given the varying levels of evidence regarding the use of insulin.
This article is the first of a two-part review of the use of insulin in the ambulatory care setting. The second article will be published in a subsequent issue of this journal. The remainder of this article will focus on available insulin products and guidelines to facilitate treatment decisions. The overall aim of this two-part review is to evaluate the available literature on ambulatory insulin dosing and create an evidence-based treatment algorithm to aid clinical practitioners in managing insulin therapy in patients with type 2 diabetes.
Review of Available Insulin Products
Insulin pharmacotherapy can be divided into four types based on pharmacodynamic and pharmacokinetic parameters. Insulin products available on the U.S. market include rapid-, short-, intermediate-, and long-acting products, as well as premixed formulations7–15,26–32 (J.A.G., personal communication). Furthermore, insulin therapy can be categorized as either basal or bolus therapy. Basal therapy includes long- and intermediate-acting insulin products used to mimic physiological insulin secretion in the absence of food. Rapid- and short-acting insulin products constitute bolus therapy, which is used to mimic the secretion of insulin from the pancreas in response to food. The pharmacokinetic and pharmacodynamic parameters are vaguely mentioned in the package inserts of the various insulins, but these parameters vary between individuals and even within a single individual. A summary of the available insulin products and their properties can be found in Tables 1 and 27–15,26–33 (J.G., personal communication).
Table 1.
Summary of Currently Available Insulin Products*
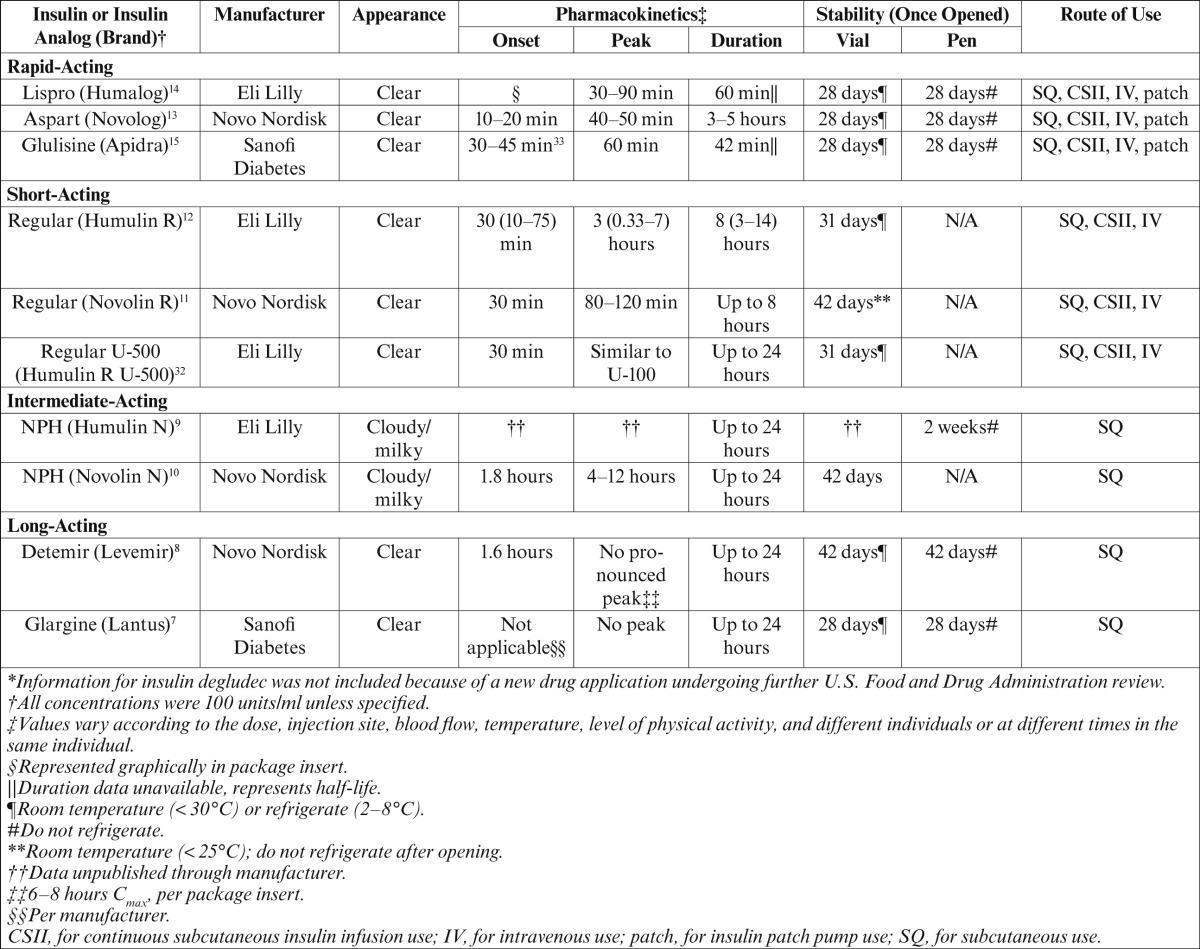
Table 2.
Currently Available Premixed Insulin Products*
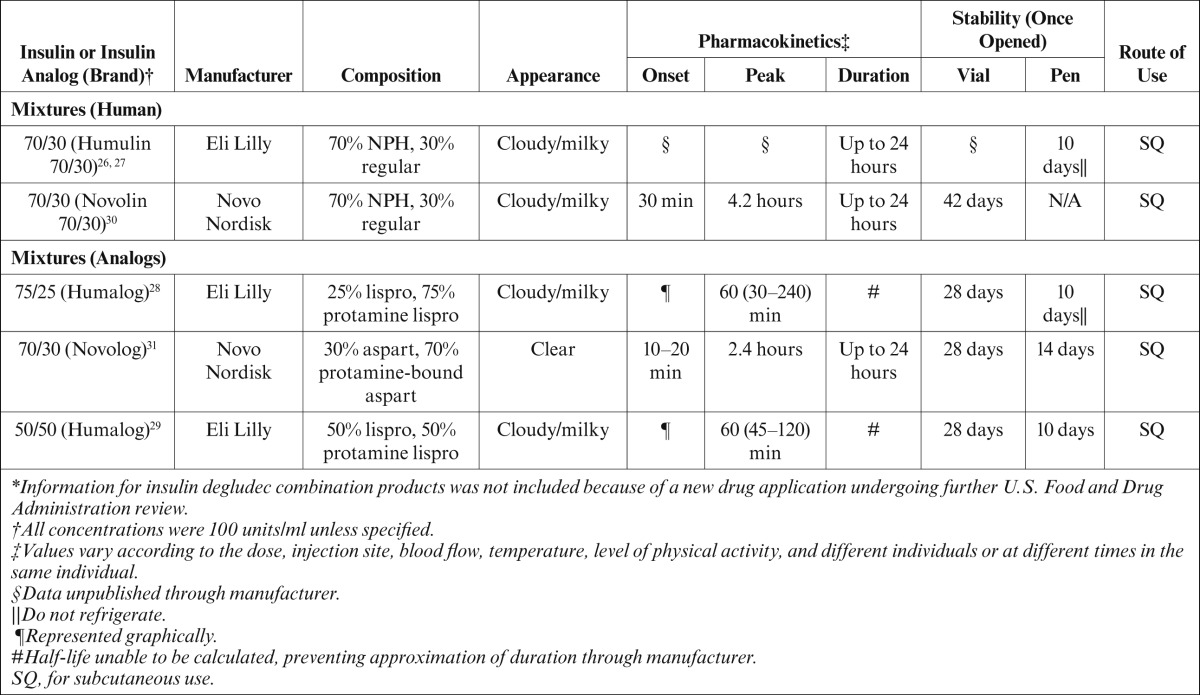
Each unit of insulin, regardless of type, has equal effects in lowering blood glucose within its duration of action; however, the variability in the products is derived from the chemical modifications that alter the pharmacokinetic and pharmacodynamic parameters. The human insulin analogs aspart, lispro, glulisine, glargine, and detemir have been chemically altered via recombinant DNA technology.34 These chemical alterations change the amino acid sequence of the insulin protein, thus adjusting the pharmacokinetic and pharmacodynamic parameters.34 Regular and NPH insulin are both physiological insulin, but NPH has altered parameters resulting from the addition of zinc and protamine moieties, thus giving it a cloudy appearance, delaying its onset, and extending its duration of action.9
There are currently four delivery options available for insulin administration, including subcutaneous injections, continuous subcutaneous insulin infusion (CSII), insulin patch pumps, and intravenous infusion. The most common route of administration is by subcutaneous injection. Patients administering insulin through subcutaneous injection have the option of using insulin pens or vials with syringes to inject. CSII, also known as insulin pump therapy, provides a constant infusion of insulin, allowing patients more control over their therapy.35 Insulin patch pumps are mechanical, standardized 24-hour insulin delivery devices.36 Intravenous infusions of insulin are required for the inpatient setting to treat diabetic ketoacidosis and other life-threatening conditions but are typically not used in the ambulatory care setting.
Guidelines for the Treatment of Type 2 Diabetes
Because there are various types of insulin, methods of administration, and dosage forms available, it is essential that providers use guidelines and primary literature to ensure appropriate initiation and titration of insulin therapy. Three sets of guidelines relating to the therapeutic management of type 2 diabetes are used in clinical practice. These include the Standards of Medical Care in Diabetes, published annually by the American Diabetes Association (ADA),17 a position statement of the ADA and the European Association for the Study of Diabetes (EASD) released in 2012,16 and an update from the American Association of Clinical Endocrinologists (AACE) released in 2011.18 In addition, AACE released an updated series of algorithms in March/April 2013 with a corresponding consensus statement published in May/June to supplement the existing guidelines.19 These guidelines all take an evidence-based approach to the management of type 2 diabetes and offer guidance as it relates to pharmacotherapy. Although the majority of the guideline statements do not focus specifically on providing prescriptive recommendations for insulin dosing during titration and intensification of therapy, the newest AACE algorithms and consensus statement do provide improved direction. Recommendations from these guidelines serve as the foundation for creating an individualized, patient-centered treatment regimen for patients with type 2 diabetes.
ADA Standards of Medical Care in Diabetes—2013
The 2013 version of the ADA standards of care guidelines supported a team-based approach to diabetes management and recommended that treatment plans be formulated in collaboration with a multidisciplinary team of health care professionals. Although oral therapy is generally the preferred option for most patients with type 2 diabetes at diagnosis, the progressive loss of β-cell function means that insulin replacement therapy will eventually become necessary for many patients. Despite this continued loss of β-cell function, most patients maintain at least some endogenous insulin secretion even in late states of type 2 diabetes.6 Therefore, multiple factors should be taken into account when assessing diabetes control and, subsequently, considering the initiation and impact of insulin therapy. These factors include the patients’ A1C level and self-monitoring of blood glucose (SMBG) records, in combination with patient interviews.
SMBG is recommended for all patients with diabetes regardless of their medication regimen. The self-monitoring plan should be individualized for each patient’s specific insulin regimen. For example, patients taking multiple daily doses of insulin should check their glucose levels at various times throughout the day, ranging from before and after meals, to occasionally at bedtime, before exercise, and when hypoglycemia is suspected. With appropriate patient education and instruction, these results can be useful to help guide treatment decisions and also as part of the patient’s self-management regimen. When patients are provided with an algorithm for self-titration of insulin doses, improved glycemic control has been noted in patients newly initiated on insulin therapy.37 However, although the literature pertaining to these algorithms is referenced, it is not discussed explicitly in the standards of care guidelines.
SMBG is also imperative to help detect and appropriately manage hypoglycemia, a major risk and limitation of insulin therapy. For patients who suffer from severe hypoglycemia, short-term relaxation of glycemic targets with tapering of insulin therapy may be appropriate, especially in light of recent data suggesting potential associations with cognitive impairment and mortality.17,38–40
Despite the evidence for SMBG and the reference to potential algorithms for insulin pharmacotherapy, the ADA standards of care guidelines provide only limited information about pharmacotherapy for patients with type 2 diabetes. The document does not focus on specific recommendations relating to dosing of insulin therapy, but rather references a 2012 position statement released by the ADA and EASD for details on insulin pharmacotherapy.16,17
ADA/EASD 2012 Position Statement
In 2012, the ADA and EASD partnered to release a position statement offering guidance on the individualization of medication regimens for nonpregnant adults with type 2 diabetes. The authors noted that the guidelines modified recommendations from previous years regarding pharmacotherapy; they are less prescriptive and offer fewer algorithms to allow for more individualization of therapy because of the lack of comparative effectiveness research relating to diabetes pharmacotherapy. The ADA/EASD guidelines support the use of patient-specific insulin therapy to achieve a glycemic profile as close to normal as possible while minimizing adverse effects such as weight gain and hypoglycemia. Proper patient education, including SMBG training, is cited as a crucial component to minimize adverse effects.16
Through patient-specific self-monitoring plans, individuals with type 2 diabetes should be aware of both their fasting and postprandial glucose levels and the respective targets for each, which may vary depending on which guidelines their provider is following. The ADA/EASD glycemic goals are in agreement with the recommendations found in the ADA standards of care document, targeting fasting blood glucose at 70–130 mg/dl and postprandial glucose at <180 mg/dl for most patients. The guidelines also recommend individualized SMBG frequency, as well as individualized A1C goals, similar to the ADA standards of care.16
In accordance with the ADA standards of care, the ADA/EASD generally recommends oral therapy as first-line treatment for patients newly diagnosed with type 2 diabetes, typically initiating with one agent. After ∼ 3 months of monotherapy, providers may consider a second oral agent, the addition of a glucagon-like peptide-1 (GLP-1) receptor agonist, or the addition of basal insulin if glycemic goals are not attained. Often, insulin therapy is an adjunct, when mono- or dual therapies do not achieve or maintain desired glucose targets (generally if a patient’s A1C is ≥ 8.5% on dual oral therapy). The guidelines note that higher A1C levels often increase the likelihood of requiring the addition of basal insulin to adequately achieve the necessary A1C reduction.16
However, the ADA/EASD guidelines reference certain situations in which immediate initiation of insulin therapy is likely indicated, specifically in patients who exhibit significant symptoms of hyperglycemia or in those who present with drastically elevated plasma glucose levels (i.e., > 300–350 mg/dl) or A1C (i.e., ≥ 10–12%). In such cases, some patients may require immediate multiple daily insulin doses rather than a more gradual progression into insulin therapy. When catabolic features, including weight loss or ketonuria, are present, implementation of insulin therapy is considered mandatory.16
Basal insulin alone is generally the initial insulin of choice when added to a pharmacotherapy regimen, thus allowing for uniform insulin coverage over the course of a day. As previously mentioned, basal insulin encompasses two types: intermediate-acting (NPH) or long-acting (glargine or detemir). In most patients, a single injection of basal insulin is initiated at a low dose of 0.1–0.2 units/kg/day. However, in patients with more severe hyperglycemia (undefined by the guidelines), therapy can begin with larger doses of 0.3–0.4 units/kg/day. An advantage of both glargine and detemir is that they have been shown to cause less nocturnal hypoglycemia than NPH. Comparatively, detemir is associated with slightly less weight gain and a higher average unit requirement when dosing. The main drawback to the long-acting insulins is increased cost.16
After initiation, titration is described in the guidelines as the addition of 1–2 units of basal insulin to the daily dose made once or twice weekly for elevated fasting glucose readings.41 For patients who have been titrated gradually and are now prescribed more robust doses of basal insulin, clinicians may consider further titrations in the range of 5–10% of the daily dose. The guidelines go on to recommend that, as patients near their glycemic goals, dose modifications should occur less often and generally consist of fewer additional units of insulin. Alternatively, clinicians must be aware of situations in which tapering of the dose may be needed, such as for recurrent episodes of hypoglycemia, especially when there is no identifiable cause.16
The ADA and EASD suggest that elevations in postprandial glucose may be a contributing factor to elevated A1C when fasting glucose levels are at goal. Postprandial blood glucose excursions contribute to the majority of elevation in A1C levels that are close to goal. For A1C levels in the range of 7.3–8.4%, fasting and postprandial glucose levels contribute equally to overall glycemia.42 This concept provides support for the clinical practice of intensifying the insulin regimen to achieve glycemic control.
When basal insulin is not sufficient to maintain glycemic control, bolus insulin therapy with short-acting (human regular) or rapid-acting (aspart, lispro, and glulisine) insulin just before meals is recommended. Rapid-acting insulins offer better postprandial glucose control than regular human insulin, likely because of their pharmacokinetic parameters. Nevertheless, cost considerations still make regular human insulin a viable option in cases in which cost containment is an issue and prandial insulin therapy is required.16
Available guidelines provide vague recommendations for the dosing range for bolus insulin. Providers should be aware that when a patient’s daily dose of basal insulin becomes > 0.5 units/kg/day, the need for intensification with bolus insulin increases. When the total daily dose of basal insulin nears 1 unit/kg/day, the addition of bolus insulin is generally required to achieve glycemic control. The guidelines suggest initiating prandial insulin with a single dose just before the meal that contains the largest carbohydrate content of the day. For most patients, this is the evening meal. From there, a second and third injection may be added before the other two meals if they require additional coverage to limit glucose excursions. This combination of long-acting basal insulin and rapid-acting mealtime insulin is described as “basal-bolus therapy” in most guidelines.
Authors of the ADA/EASD position statement advocate for the use of the basal-bolus approach to insulin therapy. This technique allows both precision and flexibility with dosing, providing a patient-centered medication regimen. However, some patients, such as those with a history of nonadherence to their diabetes treatment regimen, may not be appropriate candidates for basal-bolus therapy. In such cases, premixed insulin products are available to increase convenience but come with the drawback of reduced flexibility in dosing.26–31 Generally, such products are dosed twice daily, before the morning and evening meals. A final option supported by the ADA/EASD statement is the “split-mixed” technique, which uses a fixed amount of intermediate-acting insulin mixed by the patient with a variable amount of prandial insulin.16
Overall, the guidelines advocate for the use of insulin in the treatment of type 2 diabetes and provide multiple dosing methodologies; however, they explicitly state that “extensive dosing instructions for insulin are beyond the scope of this statement,” and little guidance regarding insulin titration is provided. Nevertheless, they support teaching patients to self-titrate their insulin doses based on published algorithms and frequent contact and communication with their provider.23,43,44
AACE 2011 and 2013 Guidelines
Current AACE guidelines recommend initiation of insulin therapy for patients whose A1C level is > 9% and those who have not achieved their glycemic targets with combination oral therapy. The guidelines were last updated in 2011, and, in 2013, AACE published its Comprehensive Diabetes Management Algorithm.19,20 There is much overlap between the 2012 ADA/EASD position statement and the 2011 AACE guidelines. However, the 2013 AACE algorithm and consensus statement offer expanded recommendations, including a discussion on the initiation of insulin in patients with an A1C as low as 7.5%, as an adjunct to other therapies.
The AACE guidelines acknowledge the importance of SMBG in all levels of diabetes control and care but, similar to other guidelines, they especially emphasize the need for close glucose monitoring by all patients during periods of regimen intensification and adjustment. However, glycemic goals represent a major difference between the previously described guidelines and the AACE recommendations; AACE advocates for tighter glycemic control, with a fasting blood glucose target of 70–110 mg/dl and a postprandial target of < 140 mg/dl.18
AACE also favors long-acting basal insulin to target fasting glucose as the initial insulin therapy in most situations, with glargine or detemir preferred for the same reasons discussed previously. The AACE 2013 algorithm and consensus statement place basal insulin as the fifth pharmacotherapy choice for dual therapy if a patient’s A1C is ≥ 7.5% but as the fourth option for triple therapy. When patients have an A1C > 9% with no symptoms, the same recommendations apply. However, if symptoms are present with an A1C > 9%, insulin therapy should be recommended because patients with this profile are likely to derive greater benefit.19,20
The AACE guidelines are in agreement with ADA/EASD in recommending initiation with 0.1–0.2 units/kg/day of a basal insulin analog, generally equating to ∼ 10 units once daily. The 2013 algorithm and consensus statement divide dosing recommendations for the initiation of basal insulin based on whether patients have an A1C > 8% or < 8%. For those with an A1C > 8%, a higher weight-based dose of 0.2–0.3 units/kg/day is recommended, as opposed to the standard 0.1–0.2 units/kg/day.45,46
As with the ADA/EASD guidelines, the AACE 2011 guidelines provide very little information regarding insulin titration. These guidelines do, however, cite published titration algorithms to provide guidance on insulin dose escalation and also support patient self-titration when patients are given the appropriate education and instruction.43,45 Fortunately, the 2013 AACE publications provide specific guidance on insulin titration based on either a fixed or variable methodology. The fixed methodology option suggests titrating basal insulin by 2 units every 2–3 days. If the variable methodology is preferred, titration is based on fasting blood glucose levels, with 4 units added for fasting readings > 180 mg/dl, 2 units added for fasting readings of 140–180 mg/dl, and 1 unit added for fasting readings of 110–139 mg/dl. In either methodology, dose recommendations for patients who experience hypoglycemia are based on a percentage reduction of the total daily dose.19,20
The 2011 AACE guidelines support the use of bolus insulin for postprandial hyperglycemia, with rapid-acting analogs preferred. Similar to the ADA/EASD guidelines, these guidelines recommend that bolus insulin therapy should augment an established basal insulin regimen. Unlike the ADA/EASD guidelines, the AACE guidelines recommend specific dosing instructions for prandial insulin, initiating at 5 units before a meal, representing ∼ 7% of the basal insulin dose, although the guidelines do not identify a specific meal. The 2013 algorithm and consensus statement also support a basal-bolus regimen for patients with symptomatic hyperglycemia and an A1C level > 10%. Ideally, a full basal-bolus regimen is preferred. If simplification of the regimen is necessary, a single prandial injection initiated with the largest meal can still aid in glycemic control. Furthermore, the 2013 algorithm and consensus statement recommend that, for patients with a total daily dose of 0.3–0.5 units/kg/day, 50% of that dose should constitute the prandial insulin analog when initiated.19,20
The 2011 AACE guidelines recommend titration of prandial insulin via small, weekly changes based on patients’ 2-hour postprandial glucose levels, whereas the updated algorithm discusses dose increases every 2–3 days based on percentages. When a rapid-acting analog is used, the prandial dose should be increased by 10% for postprandial glucose levels > 180 mg/dl.19 If postprandial glucose readings are not available, providers may rely on premeal glucose readings for the next meal to make adjustments.18 Finally, the 2013 algorithm discusses the initiation of a GLP-1 receptor agonist or a dipeptidyl-peptidase-4 inhibitor as alternatives to bolus insulin for prandial control.
Both the ADA/EASD and the AACE guidelines suggest that the basal-bolus approach is the preferred technique for patients requiring intensive insulin therapy. Although premixed preparations are cited as an option for patients with adherence issues, inflexibility in dosing may increase the risk of hypoglycemia in patients who use these formulations. When adjusting the dose of premixed insulin, AACE recommends that providers consider predinner glucose levels for doses administered before breakfast and fasting glucose levels for adjustment of the predinner dose. The updated algorithm discusses a specific increase of 10% of the total daily dose based on fasting or premeal readings > 180 mg/dl.19,20
The AACE guidelines provide more in-depth insulin pharmacotherapy recommendations than other guideline statements, but they come with a more stringent set of glycemic goals. Specific initial doses are provided for basal and prandial bolus therapy in two of three sets of guidelines. However, all three guideline statements offer limited information regarding insulin titration and adjustment. Furthermore, there is little delineation between specific products with regard to dosing information. Fortunately, the most recently published algorithm and consensus statement from AACE provide more specific initiation and titration recommendations and may prove useful in clinical practice.
Conclusion
Awareness on the part of health care providers of the characteristics of the type of insulin a patient is prescribed and its impact on blood glucose is crucial when devising an insulin regimen and self-monitoring plan that may aid in insulin adjustment. Available guidelines cite several published algorithms and support patient self-management. Previously, guidelines for the management of type 2 diabetes lacked prescriptive recommendations for titration after insulin initiation. The 2013 AACE publications provide the most guidance regarding insulin initiation and titration available today.
This article is the first in a two-part series reviewing ambulatory insulin therapy. Part 2, to be published in a future issue of this journal, will focus on the characteristics of the individual agents and evidence-based dosing recommendations for each. Primary literature exists for the initiation of basal insulin analogs, and various titration methods have been used and will be described in greater detail in Part 2. However, clinical trial data for NPH, regular insulin, and rapid-acting analogs are limited and, in several cases, exist only as unpublished data available through the manufacturers. A thorough evaluation of the primary literature is needed to fully understand and identify appropriate insulin titration techniques that may be applied to patient-specific insulin regimens for type 2 diabetes.
REFERENCES
- 1.World Health Organization : Diabetes: Fact Sheet No. 312 [article online]. Available from http://www.who.int/mediacentre/factsheets/fs312/en/index.html. Accessed 9 March 2013
- 2.Centers for Disease Control and Prevention : National diabetes fact sheet, 2011 [article online]. Available from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed 9 March 2013
- 3.IMS Health : Top therapeutic classes by U.S. spending: data updated 23 February 2012. Available from http://www.imshealth.com/deployedfiles/ims/Global/Content/Corporate/Press%20Room/Top-Line%20Market%20Data%20&%20Trends/2011%20Top-line%20Market%20Data/Top_Therapy_Classes_by_Sales.pdf. Accessed 9 March 2013
- 4.IMS Health : Top therapeutic classes by U.S. dispensed prescriptions: data updated 23 February 2012. Available from http://www.imshealth.com/deployedfiles/ims/Global/Content/Corporate/Press%20Room/Top-Line%20Market%20Data%20&%20Trends/2011%20Top-line%20Market%20Data/Top_Products_by_RX.pdf. Accessed 9 March 2013 [Google Scholar]
- 5.IMS Health : Top U.S. pharmaceutical products by spending: data updated 23 February 2012. Available from http://www.imshealth.com/deployedfiles/ims/Global/Content/Corporate/Press%20Room/Top-Line%20Market%20Data%20&%20Trends/2011%20Top-line%20Market%20Data/Top_Products_by_Sales.pdf. Accessed 9 March 2013
- 6.Fonseca VA: Defining and characterizing the progression of type 2 diabetes. Diabetes Care 32 (Suppl. 2):S151–S156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanofi Diabetes : Lantus® (insulin glargine injection) product information. Bridgewater, N.J., Sanofi-Aventis U.S., March 2007
- 8.Novo Nordisk : Levemir® (insulin detemir injection) product information. Princeton, N.J., Novo Nordisk, July 2009
- 9.Eli Lilly and Company : Humulin® N (NPH, human insulin, rDNA origin) isophane suspension patient information. Indianapolis, Ind., Eli Lilly and Company, 2009 [Google Scholar]
- 10.Novo Nordisk : Novolin® N (NPH, human insulin isophane suspension injection) patient information. Princeton, N.J., Novo Nordisk, May 2010
- 11.Novo Nordisk : Novolin® R (regular, human insulin injection) product information. Princeton, N.J., Novo Nordisk, May 2012
- 12.Eli Lilly and Company : Humulin® R (regular, human insulin, rDNA origin) product information. Indianapolis, Ind., Eli Lilly and Company, 2011 [Google Scholar]
- 13.Novo Nordisk : NovoLog® (insulin aspart injection) product information. Princeton, N.J., Novo Nordisk, June 2011
- 14.Eli Lilly and Company : Humalog® (insulin lispro injection) product information. Indianapolis, Ind., Eli Lilly and Company, May 2011 [Google Scholar]
- 15.Sanofi Diabetes : Apidra® (insulin glulisine injection) product information. Bridgewater, N.J., Sanofi-Aventis U.S., February 2009
- 16.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR: Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 35:1364–1379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association : Standards of medical care in diabetes—2013. Diabetes Care 36 (Suppl. 1):S11–S66, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handelsman Y, Mechanick JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez G, Davidson MH: American Association of Clinical Endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract 17 (Suppl. 2):327–335, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez G, Davidson MH: AACE comprehensive diabetes management algorithm 2013. Endocr Pract 19:327–336, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez G, Davidson MH: AACE comprehensive diabetes management algorithm 2013 consensus statement. Endocr Pract 19:1–38, 2013 [DOI] [PubMed] [Google Scholar]
- 21.DCCT Research Group : The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 22.U.K. Prospective Diabetes Study Group : Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853, 1998 [PubMed] [Google Scholar]
- 23.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M: Intensive insulin therapy prevents the progression of diabetic macrovascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 28:103–117, 1995 [DOI] [PubMed] [Google Scholar]
- 24.ACCORD Study Group : Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, Paul SK: Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 361:1736–1747, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Eli Lilly and Company : Humulin® 70/30 (70% human insulin isophane suspension and 30% human insulin injection, rDNA origin) patient information. Indianapolis, Ind., Eli Lilly and Company, 2011 [Google Scholar]
- 27.Eli Lilly and Company : Humulin® 70/30 pen (70% human insulin isophane suspension and 30% human insulin injection, rDNA origin) patient information. Indianapolis, Ind., Eli Lilly and Company, 2009 [Google Scholar]
- 28.Eli Lilly and Company : Humalog® Mix 75/25 (75% insulin lispro protamine suspension and 25% insulin lispro injection, rDNA origin) product information. Indianapolis, Ind., Eli Lilly and Company, 2009 [Google Scholar]
- 29.Eli Lilly and Company : Humalog® Mix 50/50 (50% insulin lispro protamine suspension and 50% insulin lispro injection, rDNA origin) product information. Indianapolis, Ind., Eli Lilly and Company, 2009 [Google Scholar]
- 30.Novo Nordisk : Novolin® 70/30 (70% NPH, human insulin isophane suspension, 30% regular, human insulin injection, rDNA origin) patient information. Princeton, N.J., Novo Nordisk, 2010 [Google Scholar]
- 31.Novo Nordisk : NovoLog® Mix 70/30 (70% insulin aspart protamine suspension, 30% insulin aspart injection, rDNA origin) product information. Princeton, N.J., Novo Nordisk, September 2011
- 32.Eli Lilly and Company : Humulin® R U-500 (concentrated) insulin human injection, USP, rDNA origin product information. Indianapolis, Ind., Eli Lilly and Company, 2011 [Google Scholar]
- 33.Becker RHA, Frick AD: Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin Pharmacokinet 47:7–20, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Mooradian AD, Bernbaum M, Albert SG: Narrative review: a rational approach to starting insulin therapy. Ann Intern Med 145:125–134, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Corp Insulet. OmniPod® Insulet CPT Portal user guide. Insulet Corp., Bedford, Mass., October 2010 [Google Scholar]
- 36.Valeritas : The V-Go®: helps control blood glucose with simple basal-bolus insulin delivery [article online]. Available from https://www.valeritas.com/vgo. Accessed 9 March 2013
- 37.Blonde L, Merilainen M, Karwe V, Raskin P, TITRATE Study Group : Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analog: an assessment of two different fasting plasma glucose targets: the TITRATE study. Diabetes Obes Metab 11:623–631, 2009 [DOI] [PubMed] [Google Scholar]
- 38.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA: Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 35:1897–1901, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cryer PE: Diverse causes of hypoglycemia associated autonomic failure in diabetes. N Engl J Med 350:2272–2279, 2004 [DOI] [PubMed] [Google Scholar]
- 40.ADVANCE Collaborative Group : Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R, for the ATLANTUS Study Group : Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care 28:1282–1288, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Monnier L, Lapinski H, Colette C: Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients. Diabetes Care 26:881–885, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Riddle MC, Rosenstock J, Gerich J, on behalf of the Insulin Glargine 4002 Study Investigators: The Treat-to-Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 26:3080–3086, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Yki-Jarvenin H, Juurinen L, Alvarsson M, Bystedt T, Caldwell I, Davies M, Lahdenpera S, Nijpels G, Vahatalo M: Initiate Insulin by Aggressive Titration and Education (INITIATE): a randomized study to compare initiation of insulin combination therapy in type 2 diabetic patients individually and in groups. Diabetes Care 30:1364–1369, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Menenghini L, Koenen C, Weng W, Selam J-L: The usage of a simplified self-titration dosing guideline (303 algorithm) for insulin detemir in patients with type 2 diabetes: results of the randomized, controlled PREDICTIVE 303 study. Diabetes Obes Metab 9:902–913, 2007 [DOI] [PubMed] [Google Scholar]


