Abstract
Objective:
To isolate, determine the frequency, and study the demographic trends of MBL positive Pseudomonas aeruginosa from imipenem resistant isolates collected from clinical samples in a tertiary care hospital of Pakistan.
Methods:
In this cross sectional study a total of 230 strains of Pseudomonas were isolated from various clinical specimens on the basis of culture and biochemical tests. Imipenem resistant isolates were selected by Kirby Bauer Diffusion technique, followed by screening for MBL production by Imipenem EDTA Combined Disk Test. Demographic details of each patient were recorded on a separate questionnaire. Chi-Square goodness-of-fit test was computed to review the isolation of MBL positive isolates (P-value ≤ 0.05) in different specimen.
Results:
Out of 230 strains of P. aeruginosa 49.5% were imipenem resistant; MBL production was confirmed in 64.9% of the resistant isolates. Resistance to polymyxin B (12.5%) was notable. Majority of the MBL positive strains were isolated from patients aged between 20-39 years (45.9%) and the predominant source was pus (43.24%) which was found to be statistically significant (P-value=0.04). Outpatient departments (24.3%) and burn unit (21.6%) were the major places for resistant isolates.
Conclusion:
MBL production is one of the major causes of IRPA. Increasing resistance to polymyxin B is grave. Due to acquisition of MBL strains MDR P. aeruginosa has become endemic in tertiary setups.
KEY WORDS: Imipenem-resistant Pseudomonas aeruginosa (IRPA), Metallo-beta-lactamases (MBL), combined disk test
INTRODUCTION
The notorious Metallo-beta lactamases belong to Ambler molecular class B carbapenemases. Metallo-beta-lactamases are able to hydrolyze virtually all beta-lactam antibiotics including carbapenems. They are not even inhibited by beta-lactamase inhibitors. Since the last decade MBL producing isolates have especially appeared in P. aeruginosa.1,2 Infact thirty years ago medical students did not study Pseudomonas aeruginosa as a serious pathogen, but today cosmopolitan multidrug resistant Pseudomonas aeruginosa is playing havoc with medical therapeutics.3 P. aeruginosa is amongst the commonest Gram negative nosocomial pathogen with 50% mortality rate in systemic infections. It is difficult to treat as the bacteria acquires additional modes of resistance such as acquisition of metallo-beta-lactamases (MBL) genes even during the course of therapy.4 This makes P. aeruginosa virtually invincible against our best reserve, imipenem5 employed in treatment of multi drug resistant strains.6 To complicate matters, imipenem resistant strains are often resistant to other antibiotic groups and worsen the prognosis in terms of mortality, morbidity and expenditure.2,7 Thus prompt identification of MBL strains is pertinent.
Globally, MBL strains of Pseudomonas have been detected in the subcontinent,8 Middle East9,10 and various countries worldwide.11 In Pakistan, research has been done on diagnostic techniques for prompt identification of MBL strains in Gram negative organisms.12,13 Few studies have reported 78%-100% production of MBL in carbapenem-resistant P. aeruginosa.14,15
The ubiquitous nature of the bacteria and irrational use of antibiotics via prescribers, dispensers and patients provide a fertile ground for dissemination of multidrug resistant strains in our setup.16 Therefore the current study was designed to determine the frequency and demographic trends of MBL positive strains of IRPA isolates in different clinical specimen of a tertiary care hospital of Pakistan.
METHODS
Study period and sampling
This cross sectional descriptive study was conducted from October 2013 to September 2014 at Civil Hospital Karachi. Samples were submitted to Central Lab for culture and sensitivity from indoor patients and outpatient clinics. Routine specimens were taken including pus, wound swab, blood and endotracheal tube secretions.
Culture
These samples were processed as per microbiological procedures (CLSI Guideline 2013).17 After a written informed consent detailed information of the patients and isolates was recorded on a separate questionnaire.
Biochemical identification of P. aeruginosa
All non-lactose fermenting colonies on MacConkey Agar were picked up for Gram’s stain and biochemical identification, such as motility, pigment production, citrate, catalase and oxidase test.
Antimicrobial susceptibility testing
Antimicrobial sensitivity was performed on Mueller Hinton Agar plates by Kirby-Bauer disk diffusion technique. Following antibiotic disks (Oxoid. UK) were used cefoperazone/sulbactam (SCF) 105µg, cefepime (FEP) 30 µg, ceftazidime (CAZ) 30µg, imipenem (IPM) 10µg, piperacillin/tazobactam (TZP) 100µg/10µg, aztreonam (ATM) 30µg, amikacin (AK) 30µg, tobramycin (TOB) 10µg, gentamicin (CN) 10µg, ciprofloxacin (CIP) 5µg, polymyxin B (PB) 300 units.
Detection of Metallo-beta-lactamase by imipenem EDTA (ethylenediaminetetraacetic acid) combined disk test
Only those strains of P. aeruginosa which were imipenem resistant by Kirby-Bauer disk diffusion method underwent detection for MBL production. Imipenem EDTA combined disk test was carried out according to Yong et al.18
Statistical analysis was done on SPSS Version 20. Descriptive statistics was carried out for both numerical and categorical data. Mean and standard deviation represent numerical data while frequency and percentage represent categorical data. Chi-square goodness of fit test was computed to review the isolation of MBL positive isolate considering P-value ≤ 0.05 is statistically significant. Bar charts were used to represent the resistance pattern of MBL positive isolates, while graphical presentation of frequency of MBL positive isolates in different departments was done by pie chart.
RESULTS
A total of 230 strains of Pseudomonas were isolated out of which, 114 (49.5%) were imipenem resistant by Kirby Bauer Disk Diffusion Technique. Amongst these 114 resistant isolates MBL production was confirmed in 74 (64.9%) strains by imipenem EDTA combined disk test. The least resistance was observed against polymyxin B (12.5%). The resistance pattern for the remaining therapeutic agents was between 61.1% and 98.6% (Fig.1).
Fig. 1.
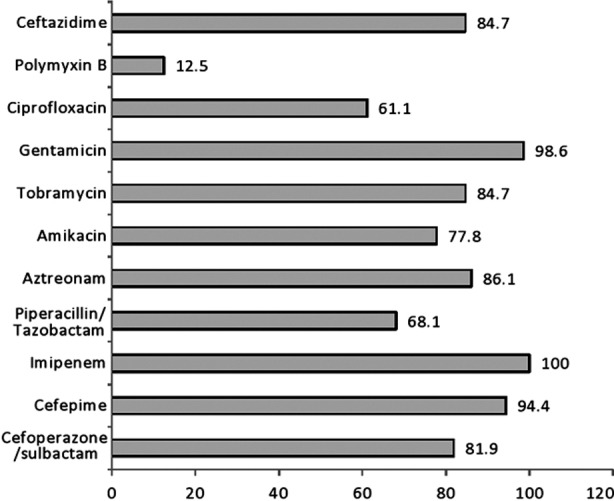
Resistance pattern of MBL positive isolates.
The mean age of patients in which MBL producing strains were isolated was 28.2 ±18years (range 1month to 85 years). Majority of the cases were isolated in age group of 20-39 years, 34(45.9%) and the least in patients sixty years or above 6 (8.1%) patients (Table-I).
Table-I.
Age distribution of MBL positive strains (n=74).
| Age in years | MBL positive strains n (%) |
|---|---|
| 1 month to19 years | 23 (31.1%) |
| 20-39 years | 34 (45.9%) |
| 40-59 years | 11(14.9%) |
| ≥ 60 years | 6 (8.1%) |
Gender wise distribution of MBL positive isolates showed that 42 (56.8%) were males and 32 (43.2%) were females. Out of 74 MBL positive isolates majority of cases were obtained from OPD, 18 (24.3%). Amongst wards the highest number of MBL positive isolates was recorded from burn ward, 16 (21.6%) and the least in paediatrics, 3 (4%). (Fig.2)
Fig.2.
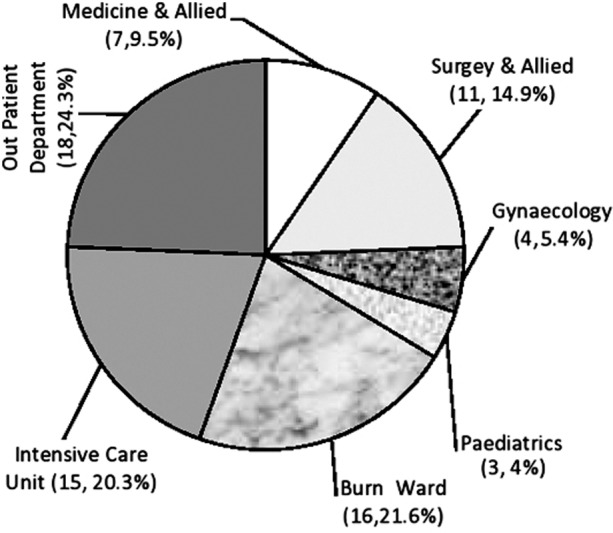
Frequency of MBL positive strains in different departments.
The predominant source of MBL positive strain was pus, 32 (43.29%) followed by wound swabs 16 (21.6%) and the least was in endotracheal tube secretions 12 (16.2%). Chi-square goodness-of-fit test was applied which showed that isolation of MBL positive isolate from pus was statistically significant (χ2 (3) = 13.58, P-value= 0.004) (Table-II).
Table-II.
Isolation of MBL positive strains from different specimen (n=74).
| Specimen | No. of isolates n (%) | P-value |
|---|---|---|
| Pus | 32 (43.24%) | 0.004 |
| Wound Swab | 16 (21.62%) | |
| Blood | 14 (18.91%) | |
| Ett secretions | 12 (16.21%) |
DISCUSSION
Imipenem is a potent cell wall synthesis inhibitor. Like all beta-lactam antibiotics it produces its therapeutic effects by crossing the cell wall through porins and binding to penicillin binding proteins (PBP) in the cell membrane.19 In P. aeruginosa porin OprD mutation in conjunction with production of AmpC6 or acquisition of MBL genes by the organism causes resistance to imipenem.20
In our study the frequency of MBL positive strains in IRPA was 64.9%; whereas in studies conducted in Pakistan, India and Brazil all IRPA strains were MBL positive.11,14,21 In this regard, Nasrin et al. has reported 43% imipenem resistant MBL strains in a tertiary care hospital of Bangladesh.22 All these figures suggest that MBL strains have the propensity to become endemic in a hospital.
In this study MBL positive isolates were predominant in males 42 (56.8%) than females 32 (43.2%). This is similar to an Indian study done by Ranjan et al.23 In comparison of different age groups, the majority of the MBL positive isolates were identified in patients in age group of 20-39 years, 34 (45.9%) and least in patients sixty years or above, 6 (8.1%) as shown in Table-I. This is in contrast to a study conducted by Bashir et al. where majority of the resistant isolates were above sixty years, 17 (51.5%).2 This contrast exists probably because in our country more patients in this age group (20-39 years) are being admitted to hospitals, from where they are acquiring resistant pathogens due to improper sterilization techniques and irrational use of antibiotics.
In our study highest number of MBL positive IRPA isolates were isolated from pus, 32 (43.29%), followed by wound swab 16 (21.6%). In contrast, Bashir et al.2 reported least in pus, 2 (6.1%). This is because in our setup majority of the patients had postoperative wound complications or burns. They remained in the ward for a longer duration. Inadequate antiseptic measures and poor hygiene in wards contributed in acquiring the resistant strains.
Among all 74 MBL positive isolates, 18 (24.32%) were detected in OPD patients. This is in contrast to Bashir et al.’s study in which all the isolates were obtained from hospitalized patients.2 This shows that in our country eventually MBL producing strains have become endemic.
Our study showed that the major contribution of MBL strains was from burn ward, 16 (21.6%) followed by ICU, 15 (20.3%) and Surgical and Allied Departments 11(14.9%) whereas Bashir et al. and Nasrin et al. has linked ICU significantly to be the major source of MBL strains.2,22
In 2005 Naqvi et al. observed that although MDR Pseudomonas was highly prevalent in burn ward at JPMC it was 77.3% sensitive to imipenem.24 Paradoxically our core finding was that imipenem resistance is increasing in burn patients.
Currently the artillery available against Pseudomonas aeruginosa include antipseudomonal penicillins (ticarcillin and piperacillin), third and fourth generation cephalosporins (ceftazidime, cefoperazone and cefepime), carbapenems (imipenem and meropenem), aminoglycosides (amikacin and tobramycin) and fluroquinolones (ciprofloxacin). However, due to acquisition of MBL strains P. aeruginosa has finally outsmarted our best treatment options. Like other studies our research has also demonstrated high resistance against all beta-lactam antibiotics. As we know by definition MBL strains are sensitive to aztreonam versus all other beta-lactams. In contrast in our study there was 86.1% resistance against aztreonam whilst an Indian study showed 100% resistance.25
Imipenem is still being used as empirical therapy in the treatment of MDR pathogens. However, due to resistance against this wonder drug, Polymyxin B a basic peptide with a narrow therapeutic index is being used as an alternate treatment.26 Though our study has revealed an emerging resistance for polymyxin B (12.5%) many countries with a high frequency of MBL positive strains have 100% sensitivity for polymyxin B.2,27
CONCLUSION
IRPA is increasing in tertiary care setups and acquistion of MBL strains is one of the major cause of resistance to this wonder drug. Emergence of polymyxin B resistant strains may push us back into a pre-antibiotic era. Since MBL strains have a propensity to disseminate globally, it is feared that MDR P. aeruginosa may soon become a pandemic pan resistant strain. The role of bacteriophage therapy in MDR strains is still in embryonic phase28; and we are far from developing molecules like MBL inhibitors there is an immediate urge to circumvent this increasing resistance to carbapenems. This can be achieved by limiting the usage of imipenem to MDR strains and avoiding irrational prescription writing.
Footnotes
Source of funding: Ziauddin University.
Conflict of interest: None.
Author’s contribution
Dr. Nadya Ameen and Dr. Ghulam Fatima conceived and designed the study beside data acquisition and analysis.
Prof. Zahida Memon and Dr. Shehla Shaheen contributed in drafting and critical review of the article. Dr. Farah Ahmed assisted in statistical analysis and interpretation.
Prof. Zahida Memon approved the final manuscript.
REFERENCES
- 1.Queenan AM, Bush K. Carbapenemases: The Versatile Beta-Lactamases. Clin Microbiol Rev. 2007;20(3):440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashir D, Thokar MA, Fomda BA, Bashir G, Zahoor D, Ahmad S, et al. Detection of Metallo-beta-lactamase (MBL) producing Pseudomonas aeruginosa at a tertiary care hospital in Kashmir. Afr J Microbiol Re. 2011;5(2):164–172. doi: 10.5897/AJMR10.694. [Google Scholar]
- 3.Levy SB. Factors impacting on the problem of antibiotic resistance. J Antimicrobial Chemotherapy. 2002;49(1):25–30. doi: 10.1093/jac/49.1.25. doi: 10.1093/jac/49.1.25. [DOI] [PubMed] [Google Scholar]
- 4.Lister PD, Wolter DJ, Hanson ND. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin Microbiol Rev. 2009;2(4):582–610. doi: 10.1128/CMR.00040-09. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam VH, Hirsch EB. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes Expert Rev Pharmacoecon Outcomes Res. 2010;10(4):441–451. doi: 10.1586/erp.10.49. doi: 10.1586/erp.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodloff A, Goldstein E, Torres A. Two decades of imipenem therapy. J Antimicrobial Chemotherapy. 2006;58(5):916–929. doi: 10.1093/jac/dkl354. doi: 10.1093/jac/dk1354. [DOI] [PubMed] [Google Scholar]
- 7.Lautenbach E, Weiner MG, Nachamkin I, Bilker WB, Sheridan A, Fishman NO. Imipenem resistance among Pseudomonas aeruginosa isolates: risk factors for infection and impact of resistance on clinical and economic outcomes. Infect Control Hosp Epidemiol. 2006;27(9):893–900. doi: 10.1086/507274. [DOI] [PubMed] [Google Scholar]
- 8.Arunagiri K, Sekar B, Sangeetha G, John J. Detection and characterization of metallo-β-lactamases in Pseudomonas aeruginosa by phenotypic and molecular methods from clinical samples in a tertiary care hospital. West Indian Med J. 2012;61(8):778–783. [PubMed] [Google Scholar]
- 9.Asghar AH. Antimicrobial susceptibility and Metallo-β-lactamase production among Pseudomonas aeruginosa isolated from Makkah Hospitals. Pak J Med Sci. 2012;28(5):781–786. [Google Scholar]
- 10.ElMasry SA, Ammar RA, Saber SM. Phenotypic and Molecular Characterization of Imipenem Resistant pseudomonas Isolates. Life Sci J. 2012;9(2s) [Google Scholar]
- 11.Wirth FW, Picoli SU, Cantarelli VV, Gonçalves A, al FRBe Metallo-β-lactamase-producing Pseudomonas aeruginosa in two hospitals from Southern Brazil. BJID. 2009;13(3):170–172. doi: 10.1590/s1413-86702009000300003. [DOI] [PubMed] [Google Scholar]
- 12.Butt T, Usman M, Ahmad RN, Saif I. Emergence of Metallo-beta-Lactamase producing Pseudomonas aeruginosa in Pakistan. J Pak Med Assoc. 2005;55(7):302–304. [PubMed] [Google Scholar]
- 13.Omair M, Usman J, Kaleem F, Hassan A, Khalid A, Fahim Q. Evaluation of combined disc method for the detection of Metallo-β-lactamase producing Gram negative bacilli. Mal J Microbiol. 2012;8(1):21–25. [Google Scholar]
- 14.Irfan S, Zafar A, Guhar D, Ahsan T, Hasan R. Metallo-β-lactamase-producing clinical isolates of Acinetobacter species and Pseudomonas aeruginosa from intensive care unit patients of a tertiary care hospital. Indian J Med Microbiol. 2008;26(3):243. doi: 10.4103/0255-0857.42035. [DOI] [PubMed] [Google Scholar]
- 15.Kaleem F, Usman J, Hassan A, Khan A. Frequency and susceptibility pattern of Metallo-beta-lactamase producers in a hospital in Pakistan. J Infect Dev Ctries. 2010;4(12):810–813. doi: 10.3855/jidc.1050. [DOI] [PubMed] [Google Scholar]
- 16.Zafar SN, Syed R, Waqar S, Irani FA, Saleem S. Prescription of medicines by medical students of Karachi, Pakistan: A cross-sectional study. BMC Public Health. 2008;8(162):1–7. doi: 10.1186/1471-2458-8-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing;Twenty-Third Informational Supplement. CLSI documnet M100-S23 Wayne Pa. 2013 [Google Scholar]
- 18.Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y. Imipenem-EDTA disk method for differentiation of Metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002;40(10):3798–3801. doi: 10.1128/JCM.40.10.3798-3801.2002. doi: 10.1128/JCM.40.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzung BG, Masters SB, Trevor AJ. 12 ed. Tata McGraw Hill Education Private Limited; 2012. Basic and Clinical Pharmacology. [Google Scholar]
- 20.Livermore DM. Multiple Mechanisms of Antimicrobial Resistance in Pseudomonas Aeruginosa: Our worst nightmare. CID. 2002;34:634–40. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhari MS, Javadekar TB, Ninama G, Pandya N, Damor J. A Study of Metallo-beta-lactatamase producing Pseudomonas Aeruginosa in clinical samples of S.S.G Hospital. Natl J Med Res. 2011;1(2):61–63. [Google Scholar]
- 22.Nasrin T, Jilani MSA, Barai L, Haq JA. Metallo-ß-Lactamase producing Pseudomonas species in a tertiary care hospital of Dhaka city. Bangladesh J Med Microbiol. 2010;4(1):43–45. [Google Scholar]
- 23.Ranjan S, Banashankari G, Babu PS. Comparison of epidemiological and antibiotic susceptibility pattern of Metallo-beta-lactamase-positive and Metallo-beta-lactamase-negative strains of pseudomonas aeruginosa. J Laboratory Physicians. 2014;6(2):109. doi: 10.4103/0974-2727.141509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naqvi ZA, Hashmi K, Rizwan QM, Kharal SA. Multidrug resistant Pseudomonas aeruginosa: a nosocomial infection threat in burn patients. Pak J Pharma. 2005;22(2):9–15. [Google Scholar]
- 25.Basak S, Attal RO, Rajurkar MN. Pseudomonas Aeruginosa and Newer β-Lactamases: An Emerging Resistance Threat. In: Christopher Sudhakar., editor. Infection Control-Update. Intech publication; 2012. Feb, pp. 181–98. [Google Scholar]
- 26.Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review J Antimicrobial Chemotherapy. 2007;60:1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- 27.Kumar R, Srivastva P, Rishi S, Dahiya SS, al KHe Detection and antimicrobial susceptibility pattern of Pseudomonas Aeruginosa isolates in various clinical samples with special reference to Metallo-beta-lactamase from a tertiary care hospital in Jaipur, India. Natl J Med Res. 2014;4(2):128–131. [Google Scholar]
- 28.Ghannad MS, Mohammadi A. Bacteriophage: Time to Re-Evaluate the Potential of Phage Therapy as a Promising Agent to Control Multidrug-Resistant Bacteria. Iran J Basic Med Sci. 2012;15(2):693–699. [PMC free article] [PubMed] [Google Scholar]


