Abstract
Objectives:
To identify the STEMI patients at high risk in terms of no-reflow during percutaneous coronary intervention (PCI) with a simple risk score system that can be used before reperfusion.
Methods:
Total 173 patients who had undergone primary or rescue percutaneous coronary intervention following the diagnosis of STEMI, were classified as “no-reflow” developers and “no-reflow” non-developers, during the procedure. The pre-procedural ECGs, laboratory parameters, demographic data, time for the treatment, and the treatment methods were evaluated with univariate analysis. The independent predictors were identified by multivariate logistic regression analysis among the no-reflow risk factors. Using the independent predictors, we developed a simple risk score system proportional to area under the ROC (AUROC) curves.
Results:
The independent predictors of “no-reflow” phenomenon were identified as follows: high values of blood glucose at reference; long symptom-onset-to-balloon-time; and low lymphocyte count. The incidence rates of “no-reflow” in patients with low (0-1), moderate (2-3) and high (4-6) risk factors were 13.3%, 40.0%, and 46.7%, respectively. The risk score system demonstrated a good risk prediction between patients with various risk levels of the development of “no-reflow” with a c-statistics of 0.734 (95% CI 0.654-0.814).
Conclusion:
The development of “no-reflow” which is an adverse event in STEMI treatment can be predicted efficiently by simple clinical risk scoring method.
KEY WORDS: No-reflow, Risk score, ST-segment elevation myocardial infarction (STEMI), Percutaneous coronary intervention
INTRODUCTION
Acute coronary syndromes including the ST elevation myocardial infarction (STEMI) are the most important conditions of the ischemic heart diseases. Increase in invasive interventions in acute coronary syndromes has resulted in newer complications. In percutaneous coronary intervention; the phenomenon of no-reflow is defined as inadequate myocardial perfusion through a given segment of the coronary circulation without angiographic evidence of mechanical vessel obstruction. Dangerous arrhythmias, congestive heart failure, and cardiac death are seen more frequently in patients who develop “no-reflow” after AMI.
The choice of appropriate treatment by the identification of risk predictors in the pathophysiology of “no-reflow” phenomenon would significantly improve the prognosis of the procedure. Hence, in this study, it was aimed to identify the high risk patients before the procedure on the basis of the evaluation of demographics, laboratory parameters, ECGs, symptom-balloon time and the treatment methods of the patients that underwent primary or rescue percutaneous coronary intervention with the diagnosis of STEMI.
METHODS
Patients
One hundred seventy three patients older than 18 years who were hospitalized and had undergone primary or rescue PCI at DokuzEylül University Medical Faculty Coronary Intensive Care Unit between January 2009 and August 2011, with the diagnosis of STEMI were included in the study. STEMI was diagnosed with the presence of chest pain with electrocardiographic changes (ST-segment elevation of >1 mm in at least two extremity electrocardiographic leads or 2 mm in at least two consecutive precordial leads). Exclusion criteria were as follows: performed percutaneous interventions for stable angina pectoris or unstable angina pectoris or non-ST elevation myocardial infarction (NSTEMI), patients with malignancies, coagulation disorders. Detailed demographic, clinical, electrocardiographic, angiographic, and procedural data were collected from patient records. The study protocol was approved by the Ethical Committee of the University (Ethics Committee approval number: 2011/18-10).
Laboratory tests and revascularization procedure
The study was retrospective and all data were acquired from patient history files. Blood samples for complete blood count, glucose, renal functions, and cardiac biomarkers of STEMI patients were drawn at admission to the emergency service in DokuzEylul University. Hemoglobin A1c and lipid parameters were measured after 24 hours from admission to emergency service at the intensive care unit. The end procedural angiographic TIMI flow which is reported in patient’s catheter report was used for the diagnosis of “no-reflow”. The end procedural TIMI grades 0, 1, and 2 flows were described as “no-reflow” phenomenon. In DokuzEylul University Hospital, acetylsalicylic acid (ASA), clopidogrel therapy and intravenous heparin before PCI were administered as a standard therapy at catheterization room. GP IIb/IIIa inhibitors and/or aspiration catheter were preferred according to the coronary angiography findings. Treatment with balloon plus stent or direct stent was determined by the operator according to the characteristics of the lesion.
Statistical analysis
Statistical analysis was performed using SPSS v15.0 (Statistical Package for Social Sciences) and MedCalc 13.1.2. The continuous variables were presented as mean (±SD) and categorical variables as percentages (n(%)). Normal distributed variables were assessed with independent t-test, not normal distributed variables were assessed with Mann-Whitney U test, and all the variables were compared between both the groups. The adequacy of data for normal distribution was tested by Shapiro-Wilk test. Categorical data were compared with chi-square test. One sample z (test and confidence interval) was used for testing ratios. Univariate analysis and multivariate logistic regression models were used to identify the risk factors of no-reflow before PCI. Receiver-operating characteristics (ROC) curve analysis of categorical variables was performed to identify the optimal cutoff value for predicting no-reflow phenomenon. The p value <0.05 was considered to indicate statistical significance.
RESULTS
A total of 173 patients (33 females (19.1%) and 140 males (80.9%)) with a mean age of 58.43±11.46 years (min: 33.0, max: 85.0) were included in the study. Risk factors for the development of “no-reflow” were evaluated individually with reference to demographic characteristics, laboratory parameters, ECGs, pre-procedural medicines, initiation of symptoms, treatment, therapies given during the PCI, and STEMI treatment methods (Table-I). When all of the significant parameters were evaluated with multivariate logistic regression analysis, the independent predictors of “no-reflow” phenomenon were found as follows: high blood glucose levels at admission (OR = 1.008; 95% CI = 1.002, 1.013; p = 0.004), long symptom-onset-to-balloon-time (OR = 1.486; 95% CI = 1.248, 1.770; p <0.001) and low lymphocyte count (OR = 0.999; 95% CI = 0.999, 1.000; p = 0.026) (Table-II).
Table-I.
Study Population Basic Characteristics.
| Variable | No-reflow (n:45, %26) | Normal reflow (n:128, %74) | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (mean ± SD*) | 61.33±12.34 | 57.41±11.01 | 0.048 |
| Women (n**, %) | 16 (35.6) | 17 (13.3) | 0.002 |
| Prior Stent (n**, %) | 6 (87.5) | 19 (90.5) | 0.724 |
| Prior CABG (n**, %) | 1 (14.3) | 2 (9.5) | |
| Hypertension (n**, %) | 23 (51.1) | 52 (40.6) | 0.296 |
| Diabetes mellitus (n**, %) | 13 (28.9) | 16 (12.6) | 0.023 |
| Smoking status (n**, %) | 25 (56.8) | 93(72.7) | 0.078 |
| Current smoker (n**, %) | 18 (75) | 74 (84.1) | 0.367 |
| Previous smoker (n**, %) | 6 (25) | 14 (15.9) | |
| Smoking duration (mean ± SD*) | 40.00±12.24 | 30.875±10.49 | 0.085 |
| Medication usage before MI | |||
| ASA (n**, %) | 6 (13.3) | 24 (18.8) | 0.55 |
| Klopidogrel *** (n**, %) | 2 (4.4) | 1 (0.8) | |
| Statin (n**, %) | 6 (13.3) | 12 (9.4) | 0.57 |
| Insulin (n**, %) | 3 (25) | 4 (26.7) | 1.000 |
| OAD (n**, %) | 8 (66.7) | 10 (66.7) | |
| OAD+insulin (n**, %) | 1 (8.3) | 1 (6.7) | |
| Laboratory parameters on admission | |||
| Blood glucose level (mg/dl) (mean ± SD*) | 208.18±133.9 | 147.01±49.7 | 0.014 |
| Hb (g/dl) (mean ± SD*) | 13.19±2.2 | 14.26±1.9 | 0.005 |
| MPV (mean ± SD*) | 8.39±1.0 | 8.40±1.0 | 0.954 |
| Neutrophil count (mean ± SD*) | 9323.0±4815.8 | 8983.76±3752.7 | 0.631 |
| Lymphocyte count (mean ± SD*) | 1920.91±1595.0 | 2592.88±1922.3 | 0.023 |
| Neutrophil/lymphocyte ratio (mean ± SD*) | 8.61±9.9 | 5.88±5.0 | 0.086 |
| WBC count (mean ± SD*) | 12204.44±4997.4 | 12037.91±3969.6 | 0.822 |
| PLT count (mean ± SD*) | 231822.2±77262.6 | 239152.1±70260.7 | 0.559 |
| Sedm (mean ± SD*) | 18.40±12.8 | 18.21±15.2 | 0.956 |
| CRP (mean ± SD*) | 18.52±37.0 | 11.14±24.4 | 0.213 |
| BNP(pg/ml) (mean ± SD*) | 277.28±469.0 | 274.17±393.0 | 0.617 |
| LDL (mg/dl) (mean ± SD*) | 117.46±35.0 | 120.34±40.3 | 0.689 |
| HDL(mg/dl) (mean ± SD*) | 35.74±7.7 | 36.55±9.0 | 0.614 |
| T. cholesterol (mg/dl)(mean ± SD*) | 185.10±46.7 | 184.45±39.1 | 0.931 |
| Triglycerides (mg/dl)(mean ± SD*) | 159.28±97.8 | 149.73±98.6 | 0.286 |
| Creatinine (mean ± SD*) | 1.06±0.4 | 0.94±0.3 | 0.229 |
| HbA1c (mean ± SD*) | 6.55±1.9 | 6.59±1.7 | 0.442 |
| ECG characteristics and treatment duration | |||
| Mean ST elevation (mm) (mean ± SD*) | 10.95±5.1 | 9.48±5.9 | 0.048 |
| Mean ST depression (mm) (mean ± SD*) | 5.82±4.94 | 5.04±4.91 | 0.136 |
| QT duration (msn) (mean ± SD*) | 369.50±39.4 | 354.09±33.98 | 0.023 |
| Heart rate (beat/min) (mean ± SD*) | 83.07±20.80 | 78.34±17.69 | 0.166 |
| Anterior MI (n**, %) | 20 (%44.4) | 61 (%47.7) | 0.901 |
| Inferior MI (n**, %) | 24(%53.3) | 62 (%48.4) | |
| Lateral MI (n**, %) | 1(%2.2) | 5 (%3.9) | |
| Symptom-ballon time (h) | 5.79±4.5 | 2.43±2.2 | 0.000 |
| Treatment during procedure | |||
| Existence of thrombus | 19 (43,2) | 59 (46,1) | 0,874 |
| Glycoprotein IIb/IIIa inhibitor | 29 (64,4) | 65 (50,8) | 0,159 |
| Aspiration catheter (aspiration thrombectomy) | 16 (36,4) | 41 (32,0) | 0,733 |
| Direct stenting | 7 (15,6) | 44 (34,6) | 0.048 |
| Ballon+stenting | 30 (66,7) | 68 (53,5) | |
| Ballon therapy | 8 (17,8) | 15 (11,8) | |
SD: standard deviation,
n: number of patients,
ASA: acetylsalicylic acid, BNP: brain natriuretic peptid, CABG: coronary arterial by-pass surgery, CRP: C reactive protein, EF: ejection fraction, h: hour, Hb: hemoglobin, HDL: high density lipoprotein, LDL: low density lipoprotein, MI: myocardial infarction, MPV: Mean Platelet Volume, OAD: oral antidiabetics, PLT: platelet, RDW: red cell distribution width, Sedm: Sedimentation, T. Cholesterol: total cholesterol, WBC: white blood cell.
Statistic analysis cannot be done due to lack of patient number for clopidogrel under the heading medication usage before MI.
Table-II.
Effects of variables on the no reflow in univariate analysis and multivariate logistic regression analysis.
| Variables | Univariate Analysis | Multivariate Logistic Regression Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 1.81 (0.908 - 3.611) | 0.048 | ||
| Gander | 3.59 (1.626 - 7.983) | 0.002 | ||
| Diabetes mellitus | 2.82 (1.228 - 6.469) | 0.023 | ||
| Blood glucose level | 5.12 (2.128 - 12.347) | <0.001 | 1.008 (1.002 – 1.013) | 0.004 |
| Hemoglobin level | 3.43 (1.658 - 7.246) | 0.001 | ||
| Lymphocyte count | 2.59 (1.256 - 5.347) | 0.014 | 0.999 (0.999 – 1.000) | 0.026 |
| Mean ST elevation | 2.37 (1.310 - 4.978) | 0.033 | ||
| QT duration | 3.18 (1.467 - 6.898) | 0.005 | ||
| Rescue PCI | 4.96 (2.205 - 9.998) | <0.001 | ||
| Symptom-ballon time | 5.61 (2.678 - 11.755) | <0.001 | 1.486 (1.248 – 1.770) | <0.001 |
| Ballon+stent therapy | 2.77 (1.122 - 6.865) | 0.039 | ||
In ROC analysis, lymphocyte count of <1830 uL at admission was found to be the predictor for the development of no reflow with 54.8% sensitivity and 68.2% specificity(OR = 2.591; 95% CI = 1.256, 5.347; p = 0.014) (Fig.1). The sensitivity was 34.09%, and the specificity was 91.67% in the analysis of ROC curve for the prediction of “no-reflow” in patients with blood glucose level of >225 mg/dL at admission (OR = 5.125; 95% CI = 2.128, 12.347; p <0.001) (Fig.1). In diabetic patients, lower levels of blood glucose were found to increase the risk of “no-reflow”. This value was determined as >143 mg/dL in the analysis of ROC curve in diabetic patients (OR = 2.20; 95% CI = 1.39-3.48; p = 0.047). As the risk of development of “no-reflow” increased with lower blood glucose levels, the contribution of chronic uncontrolled high blood sugar to development of “no-reflow” was evaluated in a separate group in diabetic patients (n = 29). HbA1c level above 7 was used as the criterion to include the patients in the chronic uncontrolled blood sugar group. It was found that HbA1c level of >7 (n = 11) had no influence on the development of “no-reflow” (5 (45%) vs. 6 (54.5%); p = 1.000).
Fig.1.
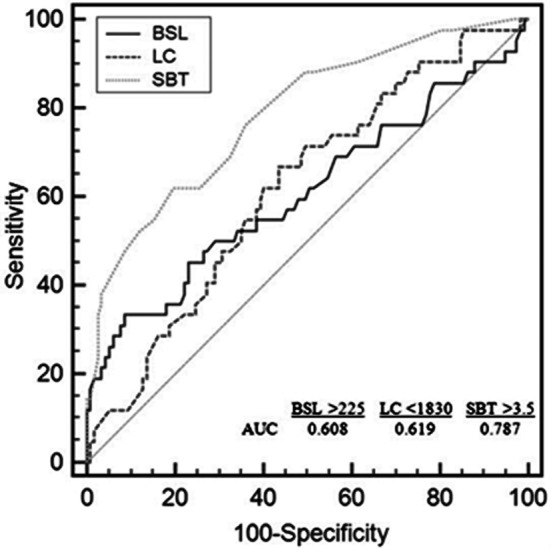
Receiver-operating characteristics curve of blood sugar level (BSL), symptom-balloon time (SBT) & lymphocyte count (LC) for predicting development of no-reflow. (AUC: Area under the Curve).
The sensitivity was 59.09%, and the specificity was 79.53% in the analysis of ROC curve for the prediction of “no-reflow” in patients with the time of symptom-onset-to-balloon-time >3.5 h (OR = 5.61; 95% CI = 2.678, 11.755; p <0.001) (Fig.2). The mean symptom-onset-to-balloon-time of primary (n = 152, 87.9%) and rescue PCIs (n = 21, 12.1%) were determined as 2.97 ±3.16 h vs. 5.61 ±3.57 h respectively. Rescue PCI was found to increase the risk of the development of no-flow in STEMI patients (p <0.001). In the analysis of ROC curve, it was found that the symptom-onset-to-balloon time of >2 h increased the risk of the development of “no-reflow” in the rescue PCI patients (OR = 4.96; 95% CI = 2.205, 9.998; p <0.001).
Fig.2.
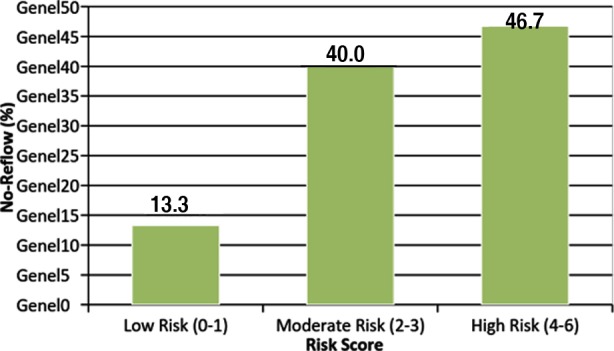
Rates of no-reflow in patients with low, moderate and high risk scores.
The independent predictors of “no-reflow” phenomenon were selected for the development of a clinical scoring. To calculate a risk score, we assigned each of the three variables a number of points that were proportional to its area under ROC (AUROC) curves. Table-III shows the variables from the score development set which were included in the final logistic regression model, alongside their associated score component values. The patients were categorized into three groups on the basis of the score as follows: low (risk score: 0-1 (n = 6)), moderate (risk score: 2-3 (n = 18)), and high (risk score: 4-6 (n = 21)). The incidence rates of “no-reflow” in patients with low, moderate, and high risk factors were 13.3%, 40.0%, and 46.7%, respectively. The AUROC was 0.734 (95% CI = 0.654, 0.814; Fig.3), which indicates the ability to efficiently discriminate between patients with various risk levels of the development of “no-reflow” in the study group.
Table-III.
Clinical risk scores for no-reflow.
| Factors | Score value |
|---|---|
| Symptom-ballon time | |
| ≥3,5h | 3 |
| <3,5h | 0 |
| Lymphocyte count | |
| ≥ 1830 uL | 2 |
| <1830 uL | 0 |
| Blood sugar level | |
| ≥225mg/dl | 1 |
| <225 mg/dl | 0 |
Fig.3.
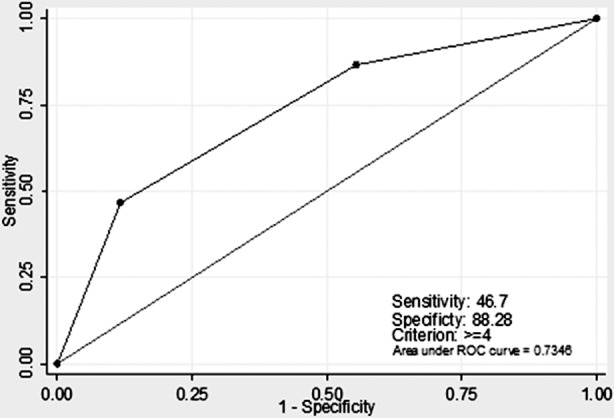
ROC analysis of the no-reflow risk model in the study group.
DISCUSSION
The parameters that are the independent predictors of “no-reflow” phenomenon are simple laboratory and clinical anamnesis informations. Clinical anamnesis information based on data at presentation, blood sugar level can be achieved by bed sided measurement and blood count is the only acquired laboratory data before the procedure especially in the first 20 minutes of admission to the hospital. With the risk scoring method performed by using the available simple clinical findings, the risk of “no-reflow” can be predicted confidentially before the initiation of PCI treatment, and the prognosis can be improved by decreasing the risk of “no-reflow” with suggested therapies. Other findings of our study were similar with the ones available in literature and validate the importance of the seemingly simple data prior to the procedure. Based on this, the results of the study were interpreted as discussed below:
Hyperglycemia can be seen in the course of acute MI irrelative to DM; and it is associated with increased mortality after MI.1 The relationship between “no-reflow” phenomenon and acute hyperglycemia can be explained by a lot of mechanisms. First, there is an increase in the obstruction of capillary bed with leucocytes by increasing the levels of ICAM-12 or P-selectin.3 The accumulation of leucocytes in coronary capillary bed after coronary perfusion is higher in diabetic animal hearts when compared to non-diabetic animals.4 Leucocyte plugs in capillary bed are among the factors that contribute to the development of “no-reflow” phenomenon. Moreover, hyperglycemia increases the occurrence of thrombus. The occurrence of microthrombus is one of the key reasons of “no-reflow” phenomenon. Finally, it is suggested that hyperglycemia is associated with reperfusion injury. In the heart of mouse, myocardial reperfusion is increased by hyperglycemia that causes the increment of the adhesion of leucocytes to capillary bed and production of free oxygen radicals.5 High blood glucose level at reference is associated with high mortality in diabetic patients that are admitted to hospital because of STEMI.6,7 The regulation of blood sugar with insulin dose during the course of MI in diabetic patients reduced the long term mortality when compared to oral anti-diabetic treatment.8,9 The normal blood sugar value is suggested to be 90-140 mg/dL.10 A cut-off value (143 mg/dL) of blood sugar in diabetic patients which was determined by ROC curve for diabetic patients, is a value to target blood sugar level when identified in diabetic patients undergoing the course of STEMI. In concordance with our current knowledge, the course of blood sugar level higher than the target value increased not only the mortality but also the risk of the development of “no-reflow” in diabetic patients of the study. It can be suggested that the effect of acute high blood sugar on the increase in mortality is related to the increased frequency of “no-reflow” in diabetic patients. The relationship between the indicator of chronic hyperglycemia (HbA1c >7) and the development of “no-reflow” was evaluated in diabetic patients. It was determined that the risk of development of “no-reflow” is higher in diabetic patients irrespective of the HbA1c level. Another data that supports the current findings is the identification of the choice of OAD or else OAD + insulin treatment has no effect on the development of “no-reflow”. According to this, high chronic blood sugar has no effect on the development of “no-reflow” but high acute blood sugar increases the risk. While chronic hyperglycemia is a risk factor for coronary artery disease, acute hyperglycemia is a risk factor for increased mortality in the course of MI.
Leukocyte count increases in the course of acute MI, and this is related with the occurrence of adverse event in the course of acute MI.11 Among the white blood cells, while the number of neutrophil and monocyte increases, the number of lymphocyte decreases. It is not clear which leukocyte sub-group is best correlated with adverse events for predicting “no-reflow” phenomenon. Lymphocytes play a prominent role in altering the inflammatory responses in atherosclerotic processes.12 Lymphopenia is a condition that occurs due to the increased corticosteroid levels in acute stress conditions13, and it is related to mortality after acute MI.14 In our study, lymphocyte count is found significantly lower in “no-reflow” developer group, and it is identified as an independent predictor for the development of “no-reflow”. This study indicated that the development of “no-reflow” can be best predicted by decreasing the number of lymphocytes among the white blood cells.
The relationship between symptom-onset-to-balloon-time and “no-reflow” development which is shown in the study is in line with available literature.15 Thrombi in early period of MI are rich in thrombocytes, and their treatment is relatively easier with pharmacotherapy. Erythrocyte load increases with prolongation of reperfusion time and thrombi become more resistant. Delayed reperfusion can result in thrombus that has an increased distal embolization risk, and more difficult to ensure TIMI-3 flow, which is more organized and older.16 Therefore, the risk of development of “no-reflow” is increased in delayed reperfusion. In concordance with this finding, development of “no-reflow” is more frequent in patients who underwent rescue PCI in the study. “No-reflow” frequency was found increased in over 2 hour’s symptom-onset-to-balloon-time in rescue PCI patients which is a shorter period for all group. Based on this data, it can be suggested that a thrombus which has a structure that cannot be dissolved with fibrinolytic treatment increases the risk of the development of “no-reflow” in a shorter period of time. This data is supported by the study performed by Zalewski et al.17 which discusses the association between the specialties of fibrin structure and “no-reflow” development for the first time. The presence of smaller pores in fibrin ball, high level of fibrinogen, and long time of lysis were found related with “no-reflow” development.
Limitations
There are some limitations in our study. First of all is the design of the study which is single centered, non-randomized, retrospective, and performed in relatively small patient group. Second, evaluated laboratory parameters are not in the same extent for individual patient and some parameters were measured in much smaller groups. Third, for evaluation of reperfusion, grading of TIMI was done instead of myocardial contrast echocardiography.
CONCLUSION
Ordinary clinic, ECG, and laboratory data offer ancillary information in decreasing long term morbidity and mortality in STEMI patients. Risk scoring method developed from simple clinical data is useful and efficient for the prediction of the risk of “no-reflow” development. The identified risk scoring method can have significant improvement in the prognosis of STEMI patients with administration of medical treatment and/or specific catheters intended to prevent the development of “no-reflow”.
Footnotes
Declaration of interest: None declared.
Source of funding: None.
Authors’ Contribution
Nazile Bilgin Dogan: Data collection and writing article. Ebru Ozpelit: Writing article. Selma Akdeniz: Data collection. Muzaffer Bilgin: Prepare statistics. Nezihi Baris: Controller.
REFERENCES
- 1.Stranders I, Diamant M, Van Gelder R, Spruijt HJ, Twisk JW, Heine RJ, et al. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004;164(9):982–988. doi: 10.1001/archinte.164.9.982. doi: 10.1001/archinte.164.9.982. [DOI] [PubMed] [Google Scholar]
- 2.Marfella R, Esposito K, Giunta R, Coppola G, De Angelis L, Farzati B, et al. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101(19):2247–2251. doi: 10.1161/01.cir.101.19.2247. doi: 10.1161/01.CIR.101.19.2247. [DOI] [PubMed] [Google Scholar]
- 3.Booth G, Stalker TJ, Lefer AM, Scalia R. Elevated ambient glucose induces acute inflammatory events in the microvasculature: effects of insulin. Am J Physiol Endocrinol Metab. 2001;280:E848–856. doi: 10.1152/ajpendo.2001.280.6.E848. [DOI] [PubMed] [Google Scholar]
- 4.Engler RL, Dahlgren MD, Morris DD, Peterson MA, Schmid-Schönbein GW. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986;251(2 Pt 2):H314–323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- 5.McDonagh PH, Hokama JY, Copeland JG, Reynolds JM. The blood contribution to early myocardial perfusion injury in amplified in diabetes. Diabetes. 1997;46:1859–1867. doi: 10.2337/diab.46.11.1859. doi: 10.2337/diab.46.11.1859. [DOI] [PubMed] [Google Scholar]
- 6.Cao JJ, Hudson M, Jankowski M, Whitehouse F, Weaver WD. Relation of chronic and acute glycemic control on mortality in acute myocardial infarction with diabetes mellitus. Am J Cardiol. 2005;96(2):183–186. doi: 10.1016/j.amjcard.2005.03.040. doi: 10.1016/j.amjcard.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626–2632. doi: 10.1161/01.cir.99.20.2626. doi: 10.1161/01.CIR.99.20.2626. [DOI] [PubMed] [Google Scholar]
- 8.Malmberg K, Ryden L, Efendic S, Herlitz J, Nicol P, Waldenström A, et al. Randomized trial of insulin–glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study):effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65. doi: 10.1016/0735-1097(95)00126-k. doi: 10.1016/0735-1097(95)00126-K. [DOI] [PubMed] [Google Scholar]
- 9.Malmberg K, Ryden L, Hamsten A, Herlitz J, Waldenström A, Wedel H. Effects of insulin treatment on cause-specific one-year mortality and morbidity in diabetic patients with acute myocardial infarction. DIGAMI Study Group. Diabetes Insulin-Glucose in Acute Myocardial Infarction. Eur Heart J. 1996;17(9):1337–1344. doi: 10.1093/oxfordjournals.eurheartj.a015067. [DOI] [PubMed] [Google Scholar]
- 10.Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, et al. Hyperglycemia and acute coronary syndrome. A Scientific Statement From the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117(12):1610–1619. doi: 10.1161/CIRCULATIONAHA.107.188629. doi: 10.1161/CIRCULATIONAHA.107.188629. [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, McCabe CH, Wilcox RG, Bentley JH, Braunwald E. Association of white blood cell count with increased mortality in acute myocardial infarction and unstable angina pectoris. OPUS-TIMI 16 Investigators. Am J Cardiol. 2001;87(5):636–639,A610. doi: 10.1016/s0002-9149(00)01444-2. doi: 10.1016/S0002-9149(00)01444-2. [DOI] [PubMed] [Google Scholar]
- 12.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12(2):178–180. doi: 10.1038/nm1343. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 13.Onsrud M, Thorsby E. Influence of in vivo hydrocortisone on some human blood lymphocyte subpopulations. I. Effect on natural killer cell activity. Scand J Immunol. 1981;13:573–579. doi: 10.1111/j.1365-3083.1981.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 14.Dragu R, Huri S, Zuckerman R, Suleiman M, Mutlak D, Agmon Y, et al. Predictive value of white blood cell subtypes for long-term outcome following myocardial infarction. Atherosclerosis. 2008;196(1):405–412. doi: 10.1016/j.atherosclerosis.2006.11.022. doi: 10.1016/j.atherosclerosis.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Ndrepepa G, Tiroch K, Keta D, Fusaro M, Seyfarth M, Pache J, et al. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv. 2010;3(1):27–33. doi: 10.1161/CIRCINTERVENTIONS.109.896225. doi: 10.1161/CIRCINTERVENTIONS.109.896225. [DOI] [PubMed] [Google Scholar]
- 16.Nagata Y, Usuda K, Uchiyama A, Uchikoshi M, Sekiguchi Y, Kato H, et al. Characteristics of the pathological images of coronary artery thrombi according to the infarct-related coronary artery in acute myocardial infarction. Circ J. 2004;68(4):308–314. doi: 10.1253/circj.68.308. doi: 10.1253/circj.68.308. [DOI] [PubMed] [Google Scholar]
- 17.Zalewski J, Undas A, Godlewski J, Stepien E, Zmudka K. No-Reflow phenomenon after acute myocardial infarction is associated with reduced clot permeability and susceptibility to lysis. Arterioscler Thromb Vasc Biol. 2007;27:2258–2265. doi: 10.1161/ATVBAHA.107.149633. doi: 10.1161/ATVBAHA.107.149633. [DOI] [PubMed] [Google Scholar]


