Abstract
Dentin in permanent teeth rarely undergoes resorption in development, homeostasis, or aging, in contrast to bone that undergoes periodic resorption/remodeling. The authors hypothesized that cells in the mesenchymal compartment of dental pulp attenuate osteoclastogenesis. Mononucleated and adherent cells from donor-matched rat dental pulp (dental pulp cells [DPCs]) and alveolar bone (alveolar bone cells [ABCs]) were isolated and separately cocultured with primary rat splenocytes. Primary splenocytes readily aggregated and formed osteoclast-like cells in chemically defined osteoclastogenesis medium with 20 ng/mL of macrophage colony-stimulating factor (M-CSF) and 50 ng/mL of receptor activator of nuclear factor κB ligand (RANKL). Strikingly, DPCs attenuated osteoclastogenesis when cocultured with primary splenocytes, whereas ABCs slightly but significantly promoted osteoclastogenesis. DPCs yielded ~20-fold lower RANKL expression but >2-fold higher osteoprotegerin (OPG) expression than donor-matched ABCs, yielding a RANKL/OPG ratio of 41:1 (ABCs:DPCs). Vitamin D3 significantly promoted RANKL expression in ABCs and OPG in DPCs. In vivo, rat maxillary incisors were atraumatically extracted (without any tooth fractures), followed by retrograde pulpectomy to remove DPCs and immediate replantation into the extraction sockets to allow repopulation of the surgically treated root canal with periodontal and alveolar bone–derived cells. After 8 wk, multiple dentin/root resorption lacunae were present in root dentin with robust RANKL and OPG expression. There were areas of dentin resoprtion alternating with areas of osteodentin formation in root dentin surface in the observed 8 wk. These findings suggest that DPCs of the mesenchymal compartment have an innate ability to attenuate osteoclastogenesis and that this innate ability may be responsible for the absence of dentin resorption in homeostasis. Mesenchymal attenuation of dentin resorption may have implications in internal resorption in the root canal, pulp/dentin regeneration, and root resorption in orthodontic tooth movement.
Keywords: osteoclastogensis, dental pulp stem cells, mesenchymal stem cells, bone resorption, RANKL, OPG
Introduction
Unlike physiologically necessary bone resorption and remodeling, odontoclastogenesis—or internal dentin resorption in permanent teeth—occurs only in pathologic conditions, including trauma and inflammation. Internal dentin resorption due to trauma, infections, or unknown causes remains a severe clinical problem (Kandalgaonkar et al. 2013; Gayathri et al. 2014). Previous work has shown that dentin resorption assumes similar mechanisms to bone resorption (Wise and King 2008). Osteoclastogenesis is mediated by a cascade of cytokines through receptor activator of nuclear factor κB ligand (RANKL) and macrophage colony-stimulating factor (Lam et al. 2000; Teitelbaum 2000). Monocytes or osteoclast precursors express RANK (receptor activator of nuclear factor κB) and c-Fms, which are receptors of RANKL and macrophage colony-stimulating factor, respectively (Teitelbaum 2000). In vitro, RANK and c-Fms are sufficient to stimulate monocyte aggregation into osteoclasts (Lacey et al. 1998). In an in vitro study, dental pulp cells (DPCs) were found to express RANKL and support monocyte aggregation into osteoclasts (Uchiyama et al. 2009). However, this observation compared only the ability of DPCs to induce osteoclastogenesis with periodontal ligament (PDL) cells in vitro, showing PDL cells approximately twice more capable of inducing osteoclastogenesis than DPCs. Furthermore, DPCs show only modest RANKL expression unless they are stimulated by nucleotide-binding oligomerization domain-containing protein 2 (Lee et al. 2014). Thus, whether dental pulp of permanent teeth promotes or suppresses odontoclastogenesis remains unsettled and has tremendous significance in our understanding of not only pulp biology and regeneration but also factors that may attenuate osteoclast activity and bone resorption.
In contrast to a paucity of dentin resorption in permanent teeth, deciduous teeth undergo physiologically necessary dentin/root resorption to exfoliate. However, dentin extracts from deciduous and permanent teeth showed no significant differences in supporting osteoclastogenesis (Sriarj et al. 2009), suggesting that dental pulp in permanent teeth, in addition to or instead of dentin, may be responsible for attenuation of dentin resorption in homeostasis. Furthermore, in contrast to dentin, alveolar bone and PDL undergoes active resorption and remodeling in homeostasis (Meikle 2007). PDL cells promote osteoclastogenesis of peripheral blood monocytes by cell contact but not in conditioned medium (Kanzaki et al. 2001; Uchiyama et al. 2009). In case DPCs attenuate dentin resorption, it follows that infusion of alveolar bone cells (ABCs) and PDL cells in the root canal may enable dentin resorption (Kvinnsland and Heyeraas 1989; Bastos et al. 2014; de Paula Reis et al. 2014). Accordingly, we hypothesize that dentin resorption is inhibited by DPCs and promoted by infusion of periodontal and ABCs. Our data demonstrate that odontoclastogenesis was inhibited by mononucleated and adherent cells of dental pulp with ~20-fold lower RANKL expression but ~2-fold higher osteoprotegerin (OPG) expression than donor-matched ABCs. Replantation of surgically extracted, pulpectomized rat incisors led to remarkable dentin resorption in root canal wall. These findings suggest that DPCs may attenuate dentin resorption in homeostasis, leading to a postulate that DPCs or factors secreted by DPCs may have potential in attenuation of bone resorption or osteoporosis.
Materials and Methods
Isolation and Culture of Dental Pulp and ABCs
Mononucleated and adherent cells were separately isolated from pulp chamber and root canal of all molars and alveolar bone chips of 8-wk-old transgenic Fischer rats in which all somatic cells are tagged with green fluorescent protein (GFP; Marano et al. 2008). The advantage of this GFP rat model is to allow tracking of transplanted DPCs and ABCs. All molars in GFP rats were extracted with the attached periodontal soft tissue scraped off. Following opening of pulp chamber and root canal, all dental pulp tissue was removed, minced, and enzyme digested with 3 mg/mL of collagenase type I and 4 mg/mL of dispase (Invitrogen, Carlsbad, CA, USA), for 30 min at 37 °C, followed by passage through a 70-μm strainer (BD, Franklin Lakes, NJ, USA) per our prior methods (Yang et al. 2010; Suzuki et al. 2011). Donor-matched alveolar bone chips were cut into minute pieces per our prior methods (Yang et al. 2010; Suzuki et al. 2011). Both DPCs and ABCs were isolated and cultured in low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco BRL, OR, USA) containing 10% FBS (fetal bovine serum; Gibco BRL) per our prior methods (Yang et al. 2010; Suzuki et al. 2011). Cells were seeded into 10-cm culture plates with low-glucose DMEM supplied with 10% FBS. Passage 3 DPCs and ABCs were used for all in vitro experiments in consideration of a balance to obtain sufficient cells for study but also without extended culture that is associated with the loss of cellular phenotypes (Mao and Prockop 2012).
Osteoclastogenesis by Coculture with Primary Splenocytes
Primary splenocytes were isolated from 12-wk-old Sprague-Dawley rats per standard protocols (Lam et al. 2000). Briefly, single-cell suspensions of splenocytes were isolated from total cells of the entire spleen by a 40-mm cell strainer. Erythrocytes were removed using a Red Cell Lysis buffer. The isolated splenocytes were seeded onto 48-well plates (5 × 105) for 1 d. Subsequently, DPCs and ABCs were added to each of the wells (10,000) and cocultured with primary splenocytes in 20 ng/mL of macrophage colony-stimulating factor (M-CSF) and 50 ng/mL of RANKL (Invitrogen) for 14 d. Primary splenocytes cultured without DPCs or ABCs served as controls. The medium was changed every 2 to 3 d. Tartrate-resistant acid phosphatase (TRAP) staining was performed (Lam et al. 2000). DPCs and ABCs (<passage 3 cells) were propagated in DMEM supplied with FBS and treated with vitamin D3 (Sigma-Aldrich, St. Louis, MO, USA) at 10-8, 10-9, and 10-11 mol/L for 5 d, followed by real-time quantitative polymerase chain reaction (PCR) measurement of RANKL and OPG expression.
Osteoclast Counting and TRAP Assay
Osteoclast-like cells were counted in 10 randomly selected areas in each plate by a technician blindly in triplicate per standard protocols (Lam et al. 2000). The average osteoclast number per high-power field was subjected to statistical analysis with 1-way analysis of variance at α = 0.05 using the SPSS 11.0 statistical package (SPSS, Inc, Chicago, IL, USA).
Real-time Quantitative PCR
Total RNA of DPCs and ABCs was extracted using TRIzol (Invitrogen). RNA concentration was quantified by spectrophotometric absorbance at 260-nm wavelength. cDNA was obtained by reverse transcription of total RNA with TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA, USA). Target gene expression was quantified using TaqMan Gene specific primers (Applied Biosystems) and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Briefly, the single-stranded cDNA was subjected to PCR with specific primers: rat RANKL Rn 00589289_m1, rat OPG Rn 00563499_m1, Gapdh(rat) Rn 01775763_g1.
Dentin Resorption Model In Vivo
Twelve-wk-old Sprague-Dawley rats were placed under general anesthesia by intraperitoneal injection of 80 mg/kg of ketamine and 5 mg/kg of xylazine following Institutional Animal Care and Use Committee approval by Columbia University Medical Center. The right maxillary incisor was extracted atraumatically, followed by retrograde pulpectomy (from apical foramen) by barbed broach under saline irrigation. Due to the healthy state of dental pulp and with practice, we were able to completely extirpate the entire dental pulp as shown in the Appendix Figure. Following pulp extirpation, rat tail collagen gel (1%; BD) was infused into the root canal, followed by gelation at 37 °C. The pulpectomized incisor was immediately replanted into the extraction socket within an average of 15 min, during which retrograde pulpectomy was performed. All rats were sacrificed at 8 wk postsurgery.
Histology and Immunohistochemistry
The in vivo harvested teeth were fixed in 4% paraformaldehyde and decalcified using 0.5M ethylenediaminetetraacetic acid (EDTA) for 1 mo before histologic processing. Histologic sections (5 µm thick) were cut and stained with hematoxylin and eosin, with additional sections processed for immunohistochemistry. Tissue sections were deparaffinized in xylene, rehydrated through descending concentrations of ethanol, and rinsed in distilled water. Antigen retrieval was carried out in sodium citrate buffer (10 mmol/L, pH 6.0) for 20 min at 95 °C, followed by thorough rinse with distilled water and phosphate-buffered saline (PBS)–Tween 20 (0.1M PBS, 0.025% Tween 20, pH 7.4). Nonspecific immunoglobulin binding was blocked by incubation with fetal bovine serum in 4 mL PBS for 1 h. Treated and control sections were then incubated overnight at 4 °C with primary antibodies at dilutions determined from pilot experiments. On the following day, the samples were treated with 6 mL of 30% H2O2 in 60 mL of methanol for 15 min to suppress tissue endogenous peroxidase activity. The antibodies were goat anti-RANKL polyclonal antibody and anti-OPG a polyclonal antibody (sc-7628 and sc-8468; 1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). All the antibodies were diluted in 0.05M Tris-HCl buffer, pH 7.6, containing 154mM NaCl (TBS). After rinse in PBS containing 0.1% triton, the slides were incubated with the HRP-conjugated secondary antibody. The sections were developed using the ABC staining kit (Santa Cruz Biotechnology) following the manufacturer’s protocol. Histologic sections were deparaffinized, rehydrated, stained in acetate buffer (pH 5.2) containing 2.5mM naphthol-AS-TR-phosphate, 0.36M N,N-dimethylformamide, 0.1M sodium tartrate, and 4mM Fast Red TR Salt (Sigma-Aldrich). The stained sections were rinsed in demineralized water and counterstained with fast green.
Patient Sample of Dentin Resorption
Following approval by the Human Ethics Committee of Capital Medical University, Beijing, China, a de-identified dentin resorption sample of an extracted right maxillary lateral incisor of a 43-y-old male patient was collected as in Figure 1A.
Figure 1.
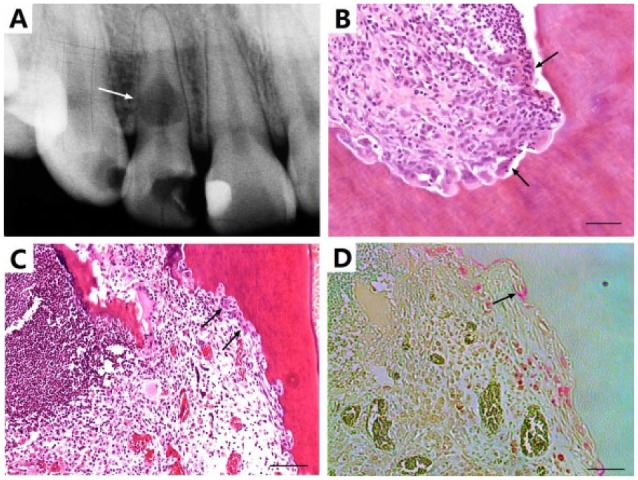
Pathologic dentin resorption. (A) Severe internal dentin resorption in a de-identified human maxillary lateral incisor with deep caries lesion in the crown (arrow), providing clinical motivation for the present study. (B) Dentin resorption in the pulp chamber showing abundant inflammatory cells and multinucleated giant cells along resorbed dentin surface (arrows). (C) Dentin resorption in a surgically extracted and retrogradely pulpectomized rat incisor following replantation into the extraction socket (arrows). (D) Tartrate-resistant acid phosphatase staining of image C (arrow). Scale = 50 µm.
Statistics Analysis
All quantitative data were analyzed by 1-way analysis of variance with Bonferroni corrections upon confirmation of normal data distribution, with significance of P ≤ 0.05.
Results
A 43-y-old male with deep carious lesion in the crown of the right maxillary lateral incisor was radiographed, showing severe internal dentin resorption (Fig. 1A). Dental pulp of the extracted tooth was processed for histology sections. Inflammatory changes consistent with chronic pulpitis and area of typical resorption lacunae were readily evident (Fig. 1B). Multinucleated cells were present on the resorption surface (Fig. 1C), with positive TRAP staining (Fig. 1D).
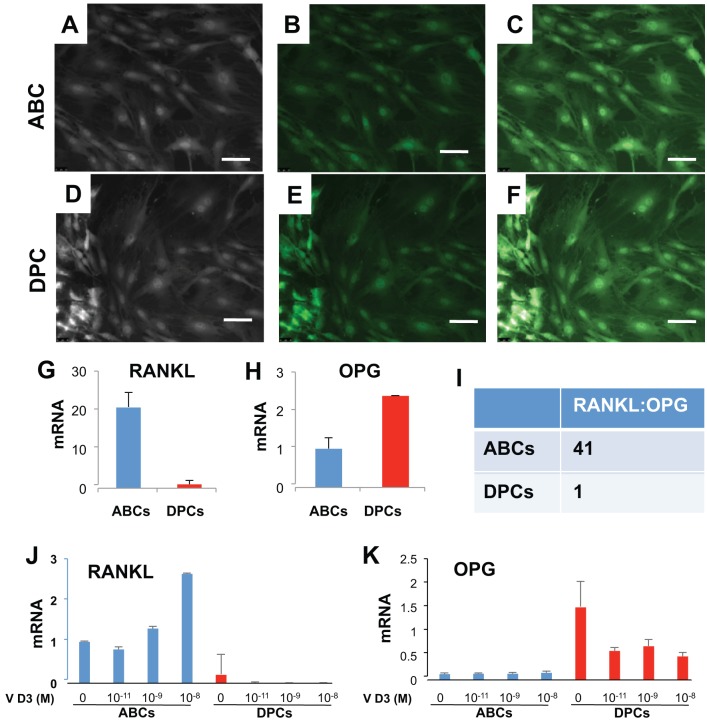
Isolated mononucleated and adherent cells from alveolar bone (ABCs; Fig. 2A) and dental pulp (DPCs; Fig. 2D) showed typical fibroblastic morphology and GFP signal under fluorescence microscope (Fig. 2B, E; overlay in Fig. 2C, F). Strikingly, RANKL expression in DPCs was significantly (~20 fold) lower than donor-matched ABCs by real-time quantitative PCR (Fig. 2G). Conversely, the expression of OPG, a RANKL decoy ligand, was significantly higher in DPCs than donor-matched ABCs (Fig. 2H). The RANKL/OPG ratio of ABCs and DPCs was 41:1 (Fig. 2I), suggesting that DPCs may have significantly greater ability to attenuate odontoclastogenesis than ABCs. Addition of vitamin D3 at various doses significantly elevated RANKL mRNA in ABCs over donor-matched DPCs (Fig. 2J). Conversely, vitamin D3 significantly attenuated OPG mRNA in ABCs but increased OPG in DPCs (Fig. 2K).
Figure 2.
Expression of receptor activator of nuclear factor κB ligand (RANKL) and osteoprotegerin (OPG) mRNA in alveolar bone cells (ABCs) and dental pulp cells (DPCs). (A) Phase-contrast image of ABCs isolated from green fluorescent protein (GFP) rats showed typical fibroblast-like morphology. (B) ABCs showed GFP fluorescence. (C) Overlay of A and B. (D) Phase-contrast image of dental pulp cells from GFP transgenic rats showed typical fibroblast-like morphology. (E) DPCs showed GFP fluorescence. (F) Overlay of D and E. Scale = 20 µm. (G, H). Expression of RANKL and OPG, by real-time quantitative polymerase chain reaction. (I) The ratio of RANKL/OPG in ABCs and DPCs. (J) Vitamin D3 at various doses significantly elevated RANKL mRNA in ABCs over donor-matched DPCs. (K) Vitamin D3 attenuated OPG mRNA in ABCs but increased OPG in DPCs.
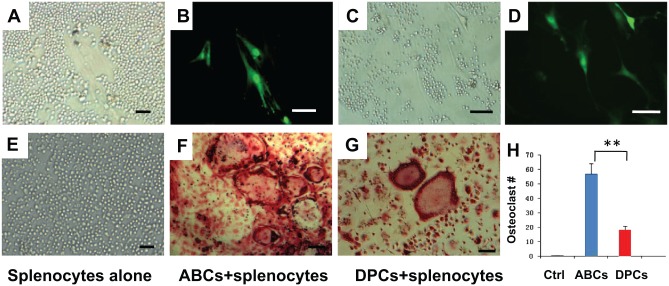
ABCs and DPCs from donor-matched GFP rats were cocultured with primary splenocytes from Sprague-Dawley rats in the presence of 20 ng/mL of M-CSF and 50 ng/mL of RANKL for 14 d. Small, round splenocytes and much larger, spindle-shaped DPCs were observed as a representative phase-contrast image (Fig. 3A). The representative fluorescent image showed GFP-positive DPCs (Fig. 3B). Similarly, small, round splenocytes and much larger, spindle-shaped ABCs were observed as a representative phase-contrast image (Fig. 3C). The representative fluorescent image showed GFP-positive ABCs (Fig. 3D). Multinucleated osteoclast-like cells (≥3 nuclei; Lam et al. 2000) were identified by TRAP staining (Fig. 3F, G) and were much larger than any of the splenocytes, DPCs, or ABCs, with multiple nuclei and a “foamy” cytosol (Fig. 3F, G). Primary splenocytes failed to form osteoclasts without induction factors (Fig. 3E). Splenocytes aggregated to form osteoclast-like cells in osteoclastogenesis permissive medium (20 ng/mL of M-CSF and 50 ng/mL of RANKL). Remarkably, primary splenocytes cocultured with ABCs generated abundant osteoclast-like cells in osteoclastogenesis permissive medium (Fig. 3F), whereas primary splenocytes cocultured with DPCs also generated osteoclast-like cells, but the numbers were few (Fig. 3G). Quantitatively, DPCs cocultured with primary splenocytes yielded significantly fewer osteoclast-like cells than primary splenocytes alone or ABCs cocultured with primary splenocytes (P < 0.01; Fig. 3H).
Figure 3.
Osteoclastogenesis by coculture of primary splenocytes with dental pulp cells (DPCs) or alveolar bone cells (ABCs). (A) Small, round splenocytes and fibroblast-like DPCs were observed in light microscope. (B) Green fluorescent protein (GFP)–positive DPCs were visible, whereas non-GFP positive splenocytes were not visible under fluorescence imaging. (C) Small, round splenocytes and fibroblast-like ABCs were observed in light microscopy. (D) GFP-positive ABCs were visible, whereas non-GFP positive splenocytes were not visible under fluorescence imaging. Scale = 20 µm. (E–G) Osteoclastogenesis via coculture of primary splenocytes with ABCs or DPCs. Multinucleated osteoclasts (≥3 nuclei; Lam et al. 2000) were identified by tartrate-resistant acid phosphatase staining. (E) Primary splenocytes. Osteoclast-like cells formed by cocultured of primary splenocytes with ABCs (F) or DPCs (G). (H) Quantification of multinucleated osteoclast-like cells (n = 6).
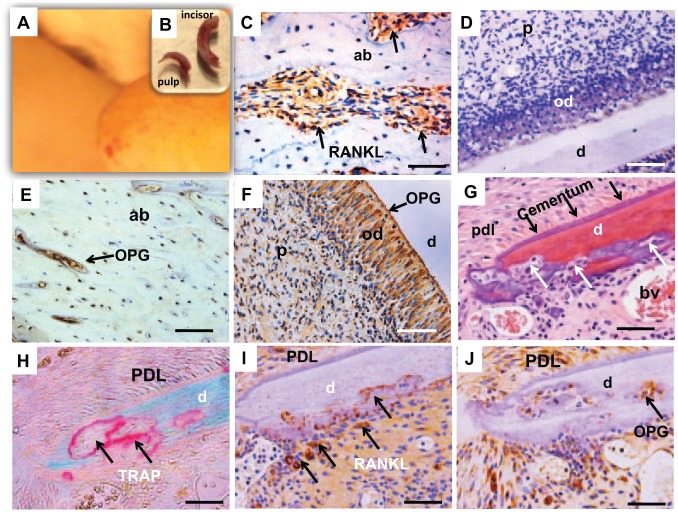
To ascertain whether osteoclastogenesis is indeed attenuated in dental pulp, we created a pulpectomized tooth replantation model. Following atraumatic extraction of the maxillary incisor in the 12-wk-old Sprague-Dawley rat (n = 7), the entire pulp tissue was extirpated retrogradely from the apical foramen by barbed broach (Fig. 4A, B). We then ascertained whether the majority of DPCs were surgically removed following dental pulp extirpation. The Appendix Figure shows that the majority of the root canal and pulp chamber space is devoid of cells. The extirpated dental pulp was virtually intact with odontoblast layers shown under microscope (Appendix Fig. B–D). High-power images showed absence of any remaining odontoblasts on the dentin wall (Appendix Fig. A–H). We found that a small number of cells—likely, pulp fibroblasts near the very coronal portion of the pulp chamber of the rat incisor—were virtually impossible to remove unless chemical ablation were used. However, chemical ablation would have defeated our purpose of in vivo cell repopulation following replantation. Following pulp extirpation, we injected 1% collagen gel into root canal and pulp chamber as a scaffold for infiltration of periapical and periodontal cells following tooth replantation. After collagen gelation, the surgically extracted maxillary incisor was immediately replanted into the extraction socket (n = 7). Following 8-wk in vivo implantation and sample retrieval, periapical and periodontal cells repopulated the root canal. RANKL-positive cells were abundant in normal alveolar bone under homeostasis (Fig. 4C). Contrastingly, no RANKL-positive cells were found in donor-matched normal dental pulp (Fig. 4D). OPG was present only in sparse areas (Fig. 4E). In contrast, abundant OPG was present in donor-matched maxillary incisor with normal dental pulp, especially in the odontoblast layer (Fig. 4F). Abundant resorption occurred in root dentin, although resorption also took place in root apex involving cementum and PDL (Fig. 4G). TRAP staining showed active resorption lacunae of various sizes in the dentin wall (Fig. 4H). Serial sections showed positive RANKL expression (Fig. 4I) and OPG expression (Fig. 4J).
Figure 4.
Internal dentin resorption in surgically pulpectomized and replanted teeth in vivo. (A) The rat maxillary incisor was extracted nontraumatically and immediately pulpectomized retrogradely. (B) A nontraumatically extracted incisor and the completely extirpated dental pulp. Following infusion of 1% collagen gel into the root canal and pulp chamber, the surgically extracted maxillary incisor was replanted in the extraction socket. (C) Receptor activator of nuclear factor κB ligand (RANKL)–positive cells (arrows) in nonoperated normal alveolar bone adjacent to the root, indicating physiologic remodeling of the alveolar bone in homeostasis. (D) Absence of RANKL staining in the pulp chamber or root canal in normal maxillary incisor. (E) Osteoprotegerin (OPG) expression in normal alveolar bone (arrow). (F) OPG obviously expressed in odontoblast layer (arrow). (G) Following pulpectomy and replantation, resorption lacunae (arrows) were present in all samples and were especially pronounced in the apical one-third with multinucleated osteoclast/odontoclast-like cells internally in the dentin wall. Cementum is demarcated by black arrows and remained intact. (H) Resorption lacunae were tartrate-resistant acid phosphatase staining positive (arrows) in the internal dentin surface. (I) RANKL-positive resorption lacunae (arrows) in consistency with G and H. (J) OPG-positive areas in serials section of J related to resorption lacunae (arrow). Scale = 20 µm. ab, alveolar bone; bv, blood vessel; d, dentin; od, odontoblast layer; p, pulp; PDL, periodontal ligament.
Discussion
The present findings indicate an innate ability of mesenchymal cells of dental pulp to attenuate dentin resorption in homeostasis and that this innate ability is lost when native DPCs are substituted by periodontal and/or ABCs. Mesenchymal cells of dental pulp express little RANKL and a great deal of OPG relative to donor-matched ABCs, yielding a striking RANKL/OPG ratio of 1:41 (DPCs:ABCs). Increased RANKL/OPG ratio is associated with dentin/root resorption (Fukushima et al. 2003). Robust RANKL and modest OPG activities in ABCs as we showed here are consistent with those in PDL cells (Uchiyama et al. 2009). Remarkably, RANKL expression is more robust in dental pulp of deciduous teeth than dental pulp of permanent teeth (Sasaki et al. 1990; Lossdorfer et al. 2002; Yildirim et al. 2008). Our data of attenuation of osteoclastogenesis by DPCs may appear to be at variance with those of Uchiyama et al. (2009) showing RANKL expression upon coculture of DPCs with peripheral blood monocytes. However, an important finding in their work is that PDL cells are approximately twice more capable of inducing osteoclastogenesis than DPCs, in consistency with our data showing that DPCs produce approximately twice as much OPG as donor-matched ABCs. Furthermore, DPCs show only modest RANKL expression unless they are stimulated by nucleotide-binding oligomerization domain-containing protein 2 (Lee et al. 2014) or vitamin D3, as we show in our present data. Thus, DPCs appear to have innate capacity to attenuate osteoclastogenesis despite exerting a low level of RANKL activity in homeostasis.
Dentin extracts from deciduous and permanent teeth showed no significant differences in supporting osteoclastogenesis (Sriarj et al. 2009). Along with the present data, it appears that dental pulp in permanent teeth, in addition to or instead of dentin, may be responsible for attenuation of dentin resorption in homeostasis. A lack of significant differences in attenuating osteoclast activity between deciduous and permanent dentin extracts further suggests that absence of osteoclast dentin resorption in permanent teeth under homeostasis (Sriarj et al. 2009) is likely attributable to dental pulp, instead of or in addition to dentin. Furthermore, in contrast to dentin, alveolar bone and PDL undergo active resorption and remodeling in homeostasis (Meikle 2007). Our finding of ~20-fold lower RANKL expression by DPCs than immediately adjacent donor-matched ABCs suggests that dental pulp indeed may harbor factors that attenuate odontoclastogenesis. An important limitation of the present work is that factors in dental pulp that attenuate osteoclastogenesis remain unidentified. Our ongoing work attempts to identify factors in dental pulp that attenuate osteoclastogenesis.
Injuries to dental pulp, such as the presently observed tooth replantation, stimulate DPCs to produce proinflammatory factors that in turn activate RANKL (Boyle et al. 2003; Chen et al. 2012). Similar insults to pulp tissue may include dental caries, pulp inflammation, trauma, pulp necrosis, and pulp revascularization. In the present replantation model, dentin resorption is present in all samples. Following pulpectomy, root canals of replanted teeth are devoid of cells. Thus, all cells present in the root canal following replantation and in vivo retrieval derive from periapical space, including PDL cells and ABCs and perhaps also systemic circulating cells. Our observation of intermittent areas of dentin resorption and areas of osteodentin formation suggests that cells migrated into pulpectomized root canal may also consist of mesenchymal origin and are consistent with previous findings by us and others (Sahara and Ozawa 2004; Kim et al. 2010; Patel et al. 2010; Yildirim et al. 2011). It is virtually impossible to remove every single cell in the most coronal portion of the pulp chamber unless chemical ablation is used, which unfortunately yields an environment that is not conducive for cell repopulation following replantation. Nonetheless, the remaining few cells at the most coronal portion of pulp chamber in the crown likely do not play significant roles in the observed dentin resorption that took place near the root apex. Cellular phenotype in dental pulp of replanted teeth warrants additional investigations. Whether cells that repopulated the root canal and pulp chamber resemble more of pulp fibroblasts and/or odontoblasts or retain their characteristics of alveolar bone and PDL cells is unknown. A panel of cell surface and other markers, such as dentin matrix protein 1 (DMP1), dentin sialophosphoprotein (DSPP), and nestin, may help to identify cell sources that populated dental pulp and root canal (Jiang et al. 2014; Zhang et al. 2015). However, genomic profiles of wild-type alveolar bone and PDL cells, in contrast to DPCs, probably need to be studied first.
In summary, native DPCs of the mesenchymal compartment appear to have an innate ability to attenuate dentin resorption in homeostasis by expressing robust OPG and diminished RANKL. Substitution of DPCs with PDL and ABCs, which have been shown to express more RANKL and less OPG than DPCs, promote dentin resorption. Our observation of 1:41 RANKL/OPG ratio by donor-matched DPCs and ABCs suggests that dental pulp may secrete factors, including RANKL and OPG, that attenuate dentin resorption in homeostasis and may have broad roles in osteoporosis.
Author Contributions
Y. Zheng, M. Chen, J.J. Mao, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; L. He, H.F. Marão, D.M. Sun, J. Zhou, S.G. Kim, S. Song, S.L. Wang, contributed to data acquisition and analysis, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank R. Burdie, F. Guo, and P. Ralph-Birkett for technical and administrative assistance.
Footnotes
This work was supported by National Institutes of Health (NIH) grants DE018248 and DE0 23112 (to J.J.M.) and National Natural Science Foundation of China grant 81171009 and 81470787 (to Y.Z.). M. Chen is supported by NIH/K12DE023583.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Bastos JV, Ilma de Souza Côrtes M, Andrade Goulart EM, Colosimo EA, Gomez RS, Dutra WO. 2014. Age and timing of pulp extirpation as major factors associated with inflammatory root resorption in replanted permanent teeth. J Endod. 40(3):366–371. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. 2003. Osteoclast differentiation and activation. Nature. 423(6937):337–342. [DOI] [PubMed] [Google Scholar]
- Chen MY, Chen KL, Chen CA, Tayebaty F, Rosenberg PA, Lin LM. 2012. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J. 45(3):294–305. [DOI] [PubMed] [Google Scholar]
- de Paula Reis MV, Moura CC, Soares PB, Leoni GB, Souza-Neto MD, Barbosa DZ, Soares CJ. 2014. Histologic and micro-computed tomographic analyses of replanted teeth stored in different kind of media. J Endod. 40(5):665–669. [DOI] [PubMed] [Google Scholar]
- Fukushima H, Kajiya H, Takada K, Okamoto F, Okabe K. 2003. Expression and role of RANKL in periodontal ligament cells during physiological root-resorption in human deciduous teeth. Eur J Oral Sci. 111(4):346–352. [DOI] [PubMed] [Google Scholar]
- Gayathri P, Pandey RK, Jain E. 2014. Management of internal resorption of central incisor using hybrid technique. BMJ Case Rep. 2014. doi: 10.1136/bcr-2013-201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Zhou J, Chen M, Schiff MD, Lee CH, Kong K, Embree MC, Zhou Y, Mao JJ. 2014. Postnatal epithelium and mesenchyme stem/progenitor cells in bioengineered amelogenesis and dentinogenesis. Biomaterials. 35(7):2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki H, Chiba M, Shimizu Y, Mitani H. 2001. Dual regulation of osteoclast differentiation by periodontal ligament cells through RANKL stimulation and OPG inhibition. J Dent Res. 80(3):887–891. [DOI] [PubMed] [Google Scholar]
- Kandalgaonkar SD, Gharat LA, Tupsakhare SD, Gabhane MH. 2013. Invasive cervical resorption: a review. J Int Oral Health. 5(6):124–130. [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, Fu SY, Koch PD, Mao JJ. 2010. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A. 16(10):3023–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvinnsland I, Heyeraas KJ. 1989. Dentin and osteodentin matrix formation in apicoectomized replanted incisors in cats. Acta Odontol Scand. 47(1):41–52. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. 1998. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 93(2):165–176. [DOI] [PubMed] [Google Scholar]
- Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. 2000. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 106(12):1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SI, Kim GT, Kim HJ, Park SH, Kim EC. 2014. NOD2 mediates odontoblast differentiation and RANKL expression. J Dent Res. 93(7):678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossdorfer S, Götz W, Jäger A. 2002. Immunohistochemical localization of receptor activator of nuclear factor kappaB (RANK) and its ligand (RANKL) in human deciduous teeth. Calcif Tissue Int. 71(1):45–52. [DOI] [PubMed] [Google Scholar]
- Mao JJ, Prockop DJ. 2012. Stem cells in the face: tooth regeneration and beyond. Cell Stem Cell. 11(3):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano JE, Sun D, Zama AM, Young W, Uzumcu M. 2008. Orthotopic transplantation of neonatal GFP rat ovary as experimental model to study ovarian development and toxicology. Reprod Toxicol. 26(3–4):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle MC. 2007. Remodeling the dentofacial skeleton: the biological basis of orthodontics and dentofacial orthopedics. J Dent Res. 86(1):12–24. [DOI] [PubMed] [Google Scholar]
- Patel S, Ricucci D, Durak C, Tay F. 2010. Internal root resorption: a review. J Endod. 36(7):1107–21. [DOI] [PubMed] [Google Scholar]
- Sahara N, Ozawa H. 2004. Cementum-like tissue deposition on the resorbed enamel surface of human deciduous teeth prior to shedding. Anat Rec A Discov Mol Cell Evol Biol. 279(2):779–791. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Shimizu T, Watanabe C, Hiyoshi Y. 1990. Cellular roles in physiological root resorption of deciduous teeth in the cat. J Dent Res. 69(1):67–74. [DOI] [PubMed] [Google Scholar]
- Sriarj W, Aoki K, Ohya K, Takagi Y, Shimokawa H. 2009. Bovine dentine organic matrix down-regulates osteoclast activity. J Bone Miner Metab. 27(3):315–323. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Lee CH, Chen M, Zhao W, Fu SY, Qi JJ, Chotkowski G, Eisig SB, Wong A, Mao JJ. 2011. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J Dent Res. 90(8):1013–1018. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. 2000. Bone resorption by osteoclasts. Science. 289(5484):1504–1508. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Nakamichi Y, Nakamura M, Kinugawa S, Yamada H, Udagawa N, Miyazawa H. 2009. Dental pulp and periodontal ligament cells support osteoclastic differentiation. J Dent Res. 88(7):609–614. [DOI] [PubMed] [Google Scholar]
- Wise GE, King GJ. 2008. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res 87(5):414–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Chen M, Lee CH, Yoon R, Lal S, Mao JJ. 2010. Clones of ectopic stem cells in the regeneration of muscle defects in vivo. PLoS One. 5(10):e13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim S, Fu SY, Kim K, Zhou H, Lee CH, Li A, Kim SG, Wang S, Mao JJ. 2011. Tooth regeneration: a revolution in stomatology and evolution in regenerative medicine. Int J Oral Sci. 3(3):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim S, Yapar M, Sermet U, Sener K, Kubar A. 2008. The role of dental pulp cells in resorption of deciduous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 105(1):113–120. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jiang Y, Qin C, Liu Y, Ho SP, Feng JQ. 2015. Essential role of Osterix for tooth root but not crown dentin formation. J Bone Miner Res. 30(4):742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.