Abstract
After cord blood (CB) transplantation, early platelet recovery in immune-deficient mice is obtained by expansion of CB CD34+ cells with thrombopoietin (TPO) as single growth factor. Moreover, improvement of hematopoietic engraftment has been shown by cotransplantation of mesenchymal stem cells (MSC). We investigated whether a combination of both approaches would further enhance the outcome of CB transplantation in NOD SCID mice. NOD SCID mice were transplanted with either CB CD34+ cells, CD34+ cells with MSC, TPO-expanded CD34+ cells or TPO-expanded CD34+ cells with MSC. We analyzed human platelet recovery in the peripheral blood (PB) from day 4 after transplantation onward and human bone marrow (BM) engraftment at week 6. The different transplants were assessed in vitro for their migration capacity and expression of CXCR4. TPO expansion improved the early platelet recovery in the PB of the mice. Cotransplantation of MSC with CD34+ cells improved BM engraftment and platelet levels in the PB 6 weeks after transplantation. Combining TPO expansion and MSC cotransplantation, however, neither resulted in a more efficient early platelet recovery, nor in a better BM engraftment, nor even very low or absent BM engraftment occurred. In vitro, MSC boosted the migration of CD34+ cells, suggesting a possible mechanism for the increase in engraftment. Our results show that cotransplantation of MSC with TPO-expanded CD34+ cells at most combines, but does not increase the separate advantages of these different strategies. A combination of both strategies even adds a risk of non engraftment.
Introduction
Cord blood (CB) is an alternative hematopoietic graft source for almost 20% of the patients for whom no HLA-matched donor can be found [1–4]. However, CB contains relatively low numbers of hematopoietic stem cells (HSCs), which translates into delayed neutrophil recovery and slow and impaired platelet engraftment when compared with transplantation with bone marrow (BM) or G-CSF-mobilized peripheral blood stem cell (PBSC) grafts [5–7]. Several strategies to overcome this are under investigation such as the selection of CB units containing large cell numbers, double CB transplantation, the ex vivo manipulation of CB cells and cotransplantation of accessory cells, such as MSC [8]. Ex vivo culture of CB cells, depending on culture conditions and growth factors, often alters the functionality of the CB cells and/or the composition of the CB graft [9,10]. In this respect, ex vivo culture with thrombopoietin (TPO) as single growth factor accelerates platelet recovery in the PB of mice, without impairment of engraftment in the BM [11–13]. These platelets are derived from TPO-induced lineage-negative (CD34-CD61-Lin-) cells preceding megakaryocyte formation [12].
Mesenchymal stem cells (MSC) are also investigated to boost engraftment. These multipotent stromal cells are characterized by three characteristics, (1) plastic adhesion in culture, (2) the expression of a set of distinct markers, and (3) the ability to differentiate into three mesodermal lineages [14]. MSC can be isolated from both adult [15,16] and fetal tissues [17,18]. MSC have antiproliferative, immunosuppressive, and anti-inflammatory effects [19] and are currently evaluated in clinical studies for the treatment of immune-mediated disorders such as Crohn's disease [20], systemic lupus [21], and systemic sclerosis [22]. In hematopoietic stem cell transplantation (HST), posttransplant infusions of MSC [23–28] as well as cotransplantation of MSC [29,30] are explored for the treatment and prophylaxis of graft versus host disease and/or graft rejection. In animal models, cotransplantation of MSC improves engraftment after HST [18,31–35]. Clinical studies have so far shown variable results when MSC cotransplantation was compared with neutrophil and/or platelet recovery of historical controls [36,37]. While improved neutrophil recovery is reported more consistently [38,39], the median time to platelet engraftment remained delayed, compared with unrelated BM or PBSC transplants [40].
Combining TPO expansion of CB CD34+ cells and MSC cotransplantation could enhance post CB transplant PB platelet recovery as well as BM engraftment. In this study, we, therefore, compared the engraftment potential of both approaches in an NOD SCID mouse model.
Materials and Methods
CD34+ cell purification
Umbilical CB was collected with written consent from the mother according to Netcord–FACT standards and with ethical permission from the medical ethics board of the Leiden University Medical Center (LUMC). Mononuclear cells were isolated from CB using a Ficoll density gradient. The CD34+ cell fraction was isolated using magnetic CD34+ isolation beads (Miltenyi Biotec, Bergisch Gladbach). The purity of the isolated CD34+ cell fraction was verified by flow cytometry (Beckman Coulter) with CD45-FITC and CD34-PE (Beckman Coulter). The percentage of CD34+/CD45+ cells in the isolated fraction was 91%±3%.
Expansion of the CD34+ cells
CD34+ cells were cultured at 37°C and 5% CO2 in a humidified atmosphere in IMDM medium (Gibco) supplemented with 20% (v/v) AB heparin plasma (Sanquin Blood Supply Foundation), 0.5 mg/mL human transferrin saturated with FeCl3.H2O (Sigma), 0.34% (v/v) human serum albumin 20% (Cealb® CLB), 1% (v/v) penicillin/streptomycin (Bio-Whittaker), 0.05 mM β-mercaptoethanol (Sigma), and 50 ng/mL mpl-ligand (TPO; kind gift from KIRIN Brewery Ltd.). The cells were plated in 24-well sterile TC plates, at a concentration of 5×104 cells/mL. At day 7, the medium was refreshed by semi-dilution, with a medium containing 50 ng/mL TPO. At day 9 the cells were split into two new wells and 1:1 diluted with a medium without TPO. At day 10, the cells were harvested and the total cell expansion was calculated and subsequently the composition of the cultured cells was analyzed by flow cytometry using mouse anti-human CD45, CD61, CD34, CD14, and CD15 antibodies (all Beckman Coulter).
Culture of MSCs
MSCs were obtained from fetal lung tissue as previously described [18]. The cells were cultured in M199 supplemented with 10% FCS, 1% pen/strep, 20 μg/mL EGF, and 8 U/mL heparin, in gelatin-coated tissue culture flasks at 37°C and 5% CO2 in a humidified atmosphere.
Transplantation in NOD/SCID mice
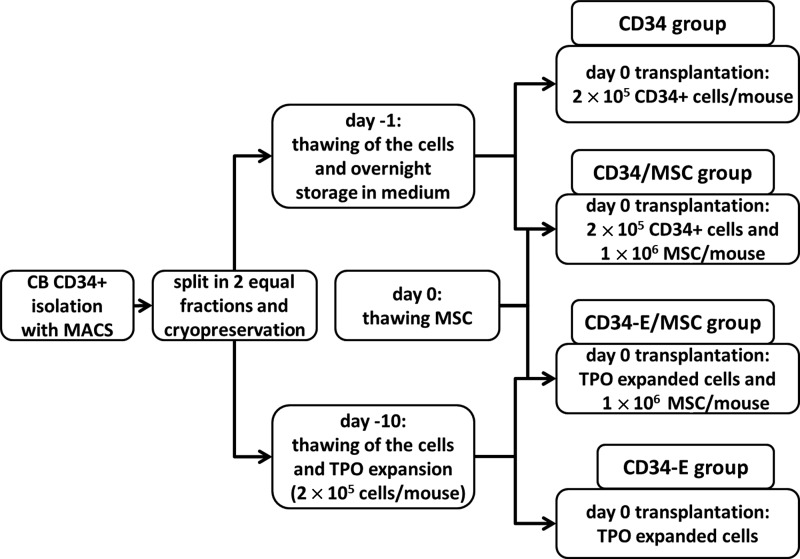
Female, 5–6 weeks old, NOD/SCID mice (Charles River) were kept at the animal facilities of the LUMC. The animal ethics committee of the LUMC approved all animal experiments. The mice were irradiated sublethally (3.5 Gy) 24 h before i.v. transplantation with the different transplants: (1) 2×105 CD34+ cells (hereafter referred to as CD34), (2) 2×105 CD34+ cells 10 days expanded with TPO (hereafter referred to as CD34-E), (3) 2×105 CD34+ cells +1×106 FL MSC (hereafter referred to as CD34/MSC), and (4) 2×105 CD34+ cells 10 days expanded with TPO+1×106 FL MSC (hereafter referred to as CD34-E/MSC). A schematic representation of the experiment is shown in Fig. 1. Blood collection through a tail vein incision was performed twice weekly during the first 3 weeks after transplantation and once weekly thereafter. Blood collection and human platelets measurements were performed as described previously [41]. Briefly, human platelets were stained with a noncross-reactive mouse anti-human CD41-PE (Beckman Coulter) and erythrocytes were lysed with the IO Test3 Lysing solution (Beckman Coulter). Flow-Count™ fluorospheres (Beckman Coulter) were added to enable the measurement of the absolute number of circulating human platelets. The detection limit was 1×103 platelets/mL of PB. Analysis was performed with flow cytometry (EPICS® XL-MCL; Beckman Coulter) running system II software.
FIG. 1.
Flow chart of the transplantation experiment. Four different types of transplants were prepared (cell numbers/mouse): CD34: 2×105 unmanipulated CD34+ cells (control group) CD34/MSC: 2×105 unmanipulated CD34+ cells with 1×106 fetal lung mesenchymal stem cell (MSC) CD34-E/MSC: the total expansion product of 2×105 CD34+ cells expanded with thrombopoietin (TPO) with 1×106 fetal lung MSC CD34-E: the total expansion product of 2×105 CD34+ cells expanded with TPO.
Six weeks after transplantation, mice were sacrificed and the BM was obtained from the femur. Cells were resuspended in IMDM and cells were labeled with goat anti-mouse-CD45-PE (LCA, Ly-5, 30-F11; Pharmingen), mouse anti-human CD45-FITC (Beckman Coulter), and the appropriate isotype controls. Subsequently erythrocytes were lysed with IO Test 3 Lysing solution according to the manufacturer's procedures (Beckman Coulter). Analysis was performed with flow cytometry (EPICS® XL-MCL; Beckman Coulter) running system II software.
Migration experiments
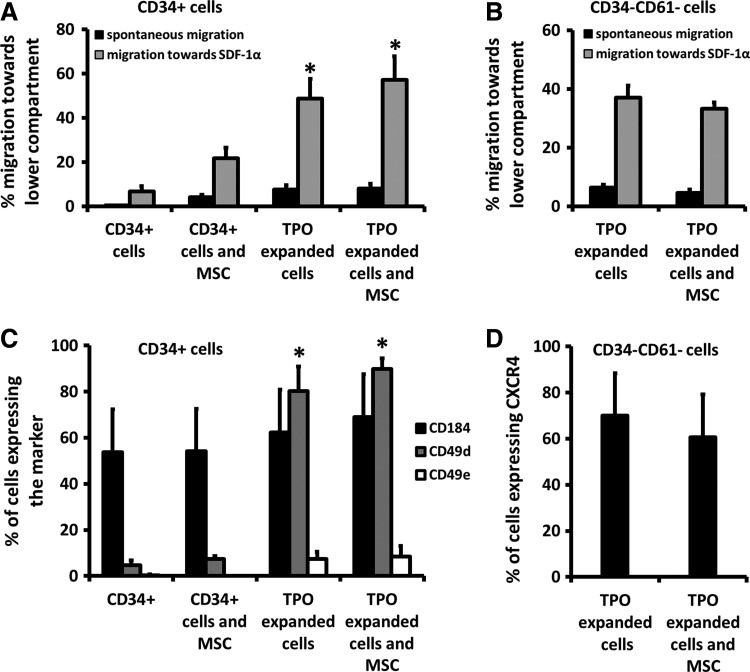
Four different cell suspensions were prepared identical to the in vivo experiment. After 30 minutes of incubation, cells were analyzed for the expression of CD45, CD34, CD61, CXCR4, CD49d, and CD49e with flow cytometry and placed in the upper compartment of a transwell plate (Costar) with a 100 ng/mL SDF gradient in the lower compartment, both containing IMDM. (Gibco). Plates were incubated for 5 h at 37°C and 5% CO2 in a humidified incubator. After incubation, cells were harvested from both compartments. After incubation all cells were analyzed for the expression of CD45, CD34, and CD61 (all Beckman Coulter) to calculate the number of cells that have migrated.
Statistical analysis
All statistics were done with the SPSS, version 20. All results are presented as mean±SEM. To compare groups, a Student's t-test (normally distributed) or Mann–Whitney test (not normally distributed) was used. Differences were considered significant when P<0.05. If multiple groups were compared, an ANOVA or a Holm's sequential Bonferroni adjustment was applied.
Results
Platelet recovery in the PB of NOD/SCID mice
To study the different strategies to overcome delayed and reduced engraftment of CB cells, mice were transplanted with either unmanipulated CD34+ cells (CD34 group), TPO-expanded CD34+ cells (CD34-E group), unmanipulated CD34+ cells with MSC (CD34/MSC group), or TPO-expanded CD34+ cells with MSC (CD34-E/MSC group) whereas platelet recovery as well as BM engraftment were studied.
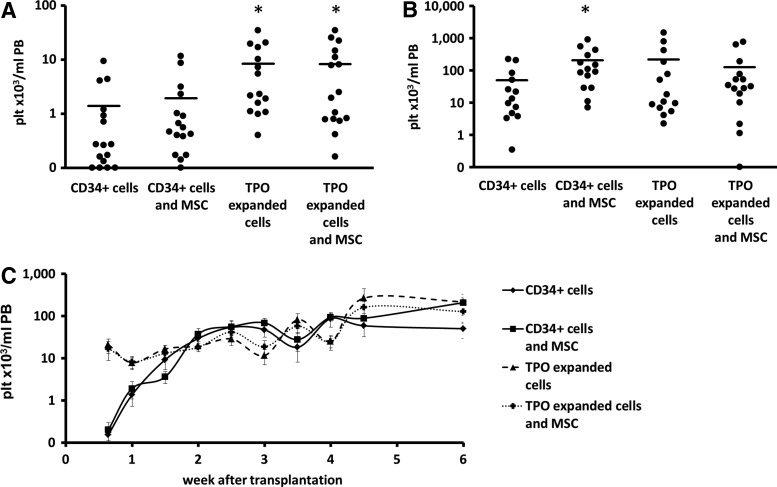
Similar to earlier studies [11–13,42], in the first week after transplantation, mice that received TPO-expanded cells (CD34-E and CD34-E/MSC groups) showed accelerated early platelet (plt) recovery in the PB when compared with mice transplanted with only CD34+ cells (CD34 group) (Fig. 2A, C, Supplementary Table S1), as shown by significantly higher platelet concentrations in the PB of the mice 8 days after transplantation (CD34-E group:7.8±2.5×103 plt/mL PB, CD34-E/MSC group: 8.3±2.7×103 plt/mL, compared with the control group, CD34: 1.4±0.6×103 plt/mL, P<0.005 for both groups). The TPO-induced increased PB platelet concentration was present for 2 weeks after transplantation whereas after week 2, the mean PB platelet levels in all groups slowly increased.
FIG. 2.
(A) Platelet concentration in the peripheral blood (PB) of each of the mice 8 days after transplantation. Bars represent the mean platelet concentration of each group. On an average, both groups that were transplanted with TPO-expanded cells had significantly higher concentrations of platelets in the PB (*P<0.02). (B) Platelet concentration of each of the mice 40 days after transplantation. Bars represent the mean platelet concentration of each group. On an average, all groups had higher concentrations of platelets in the PB than the control group, but this difference was only significant for the group that was transplanted with CD34+ cells and MSC (*P<0.05). (C) Kinetics of platelet recovery in the mice throughout the experiment. Shown are the mean±SEM values of the four different transplants.
Cotransplantation of MSC in contrast, did not significantly increase early platelet repopulation as compared with CD34+ cells or TPO-expanded CD34+ cells, respectively (CD34/MSC: 1.9±0.8×103 plt/mL compared with CD34: 1.4±0.6×103 plt/mL and CD34-E/MSC: 8.3±2.7×103 plt/mL compared with CD34-E: 7.8±2.5×103 plt/mL). Compared with unmanipulated CD34+ grafts, all mice that received a manipulated graft and/or additional MSCs showed higher platelet concentrations 6 weeks after transplantation (Fig. 2B, C, Supplementary Table S1, mean platelet concentration±SEM in PB: CD34/MSC: 207.4±68.2×103, CD34-E: 216.2±112.2×103, and CD34-E/MSC: 127.0±60×103 plt/mL) as compared with the mice that received unmanipulated CD34+ cells (CD34: 49.8±21.0×103 plt/mL PB). However, only for the CD34/MSC group this difference was significant (P<0.01). The mean increase of long-term platelet engraftment in the CD34-E group was due to a wide SD not significant because a number of mice engrafted exceptionally well, thereby increasing the mean value of the group. The median values of these groups, 18.0×103 plt/mL PB for CD34-E versus 17.4×103 plt/mL PB for CD34, were similar. Vice versa, the (nonsignificant) lower mean platelet concentration of the group that was transplanted with CD34-E/MSC was the result of a number of mice with very low or nonengraftment.
Human engraftment in the BM
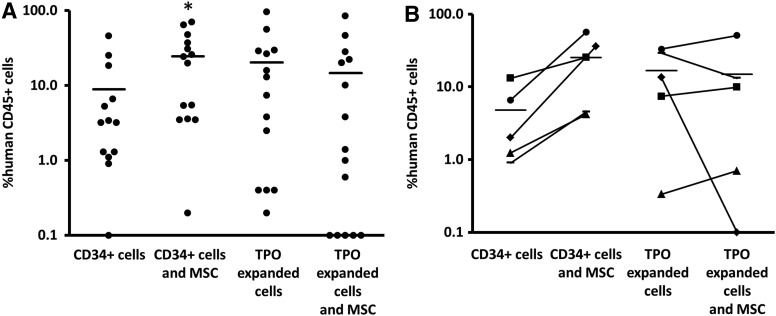
The percentage of human CD45 cells in the BM 6 weeks after transplantation of the mice is shown in Fig. 3A. Cotransplantation of MSC increased the engraftment when compared with transplantation of CD34+ cells alone with higher percentages of human cells in the BM (CD34/MSC: 24.5%±6.3% vs. CD34: 8.9%±3.7%; P<0.05). In line with the 6 weeks PB platelet counts, TPO-expanded cells also induced engraftment, but again this was not significant (CD34-E: 20.2%±7.4%; P=0.402 compared with the CD34 group).
FIG. 3.
(A) Percentage of human CD45 cells as a percentage of the total CD45 cells in the BM of the mice 6 weeks after transplantation. Bars represent mean values of each group. All groups had higher mean percentages of human CD45 in their BM than the control group, but this was only significant for the group that received CD34+ cells and MSC (P<0.05). (B) The mean percentage of human CD45+ cells of the mice that were transplanted with cells from the same cord blood unit were calculated and compared for each cell type (CD34+ cells or TPO-expanded cells) when transplanted with or without MSC, showing an increase or a decrease in engraftment when MSC were added to the CD34+ cells or the TPO-expanded cells of this cord blood. Cotransplantation of MSC with CD34+ cells increased the engraftment of the cells of all of the cord blood units. Cotransplantation of MSC with TPO-expanded only increased the engraftment of the cells of three cord blood units. The average fold increase in engraftment by the cotransplantation of MSC with CD34+ cells was 7.1±2.1, The average fold increase in engraftment by the cotransplantation of MSC with TPO-expanded cells was 1.1±0.4.
Interestingly, whereas cotransplantation of MSC with CD34+ cells significantly improved the BM engraftment of the mice at week 6, cotransplantation of MSC with TPO-expanded CD34+ cells did not enhance BM engraftment and seemed even less favorable when compared with transplantation of TPO-expanded cells alone (%human CD45 cells in the BM for CD34-E/MSC: 14.7%±6.2% vs. 20.2%±7.4% for CD34-E).
Again, as seen with the low platelet counts at week 6, low or nonengraftment in a subset of mice did account for this difference. To study if this finding was related to the quality of a particular CB unit, we compared the mean BM engraftment of mice that received cells from the same CB unit (Fig. 3B). Again, cotransplantation of MSC with CD34+ cells increased the engraftment (on average 7.1±−2.1-fold) for all of the five different CB units that were used in this study. Cotransplantation of MSC with TPO-expanded cells, however, again showed very low engraftment for one of the CB units and no engraftment for a second unit (0%–0.1% of human CD45+ cells in the BM). Whereas cotransplantation of MSC with TPO-expanded cells of these two specific units impaired engraftment, cotransplantation of MSC with the unmanipulated CD34+ cells of these two units increased the engraftment 5 and 17-fold, respectively. The latter was in line with the average increase in engraftment (7.1±2.1-fold) when MSC are cotransplanted with unmanipulated CD34+ cells, while they decreased the engraftment (1.1±0.4-fold) when cotransplanted with TPO-expanded cells.
Cotransplantation of MSC with TPO-expanded cells on different time points
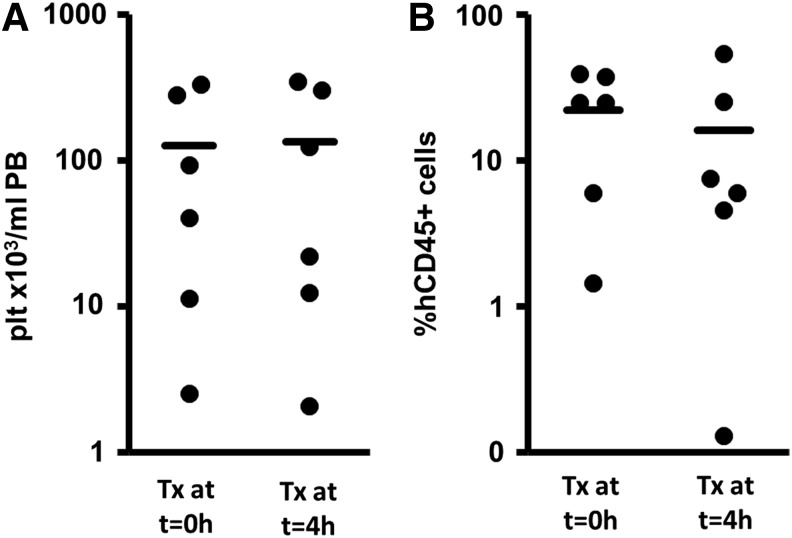
TPO-expanded grafts contain a higher cell number as compared with unmanipulated CD34+ cells. Thus, mice transplanted with CD34-E grafts receive more cells. Cotransplantation of MSC with these high cell numbers might result in, for example, obstruction of the lung circulation and impairment of the expected positive influence of MSC on marrow engraftment contributing to the nonengraftments we observed in some cases. To prevent this possible blocking of circulatory beds by MSC, we performed a pilot experiment in which we infused MSC 4 h after TPO-expanded cells. As shown in Fig. 4, this later timing of MSC cotransplantation did not significantly alter the level of platelets in the PB 6 weeks after transplantation (Fig. 4A, 125.8±58.2 plt/mL PB for MSC transplantation concomitantly with the hematopoietic cell transplantation versus 134.4±62.6×103 plt/mL PB for MSC transplantation 4 h after hematopoietic cell transplantation, P=0.873). Moreover, the percentage of human CD45+ cells in the BM was not significantly changed when MSC were directly infused with CD34+ cells or after 4 h (Fig. 4B, 22.2%±6.4% vs. 16.1%±8.3%, P=0.522).
FIG. 4.
Engraftment results of mice transplanted with TPO-expanded cells and cotransplanted with MSC at the time of hematopoietic cell transplantation (Tx at t=0 h) or 4 h after transplantation (Tx at t=4 h). Bars represent mean values of each group. No differences in the platelet engraftment (A) in the peripheral blood or the human CD45+ cell engraftment (B) in the BM were detected when the MSC were transplanted on different time points.
Migration properties and expression of migration-related molecules of the transplanted cells
SDF-1α is the main chemoattractant for hematopoietic cells to home to the BM and migration capacity of cells toward SDF-1α is, therefore, a crucial step in engraftment [43–46]. To investigate if TPO expansion and/or MSC affect the migratory capacity of the cells, we analyzed the migration capacity of the grafts toward a 100 ng/mL SDF-1α gradient in Transwell plates (Fig. 5A). Addition of MSC improved the migration of the CD34+ cells by three-fold (21.6%±4.8% with MSC vs. 6.7%±2.4% for CD34+ cells alone, P<0.05). Also TPO expansion improved the migration of the remaining CD34+ cells in the transplant eight to nine-fold (48.7%±8.9% P<0.005). Addition of MSC did not further enhance the already increased migration of residual CD34+ cells in the TPO-expanded CB (57.2%±10.5%).
FIG. 5.
(A) Percentage of CD34+ cells that have migrated through a transwell system toward the lower compartment of the plate containing medium with 100 ng/mL SDF-1α gradient (black bars) or medium alone (gray bars, spontaneous migration). CD34+ cells are either unmanipulated cells (control group and group that was transplanted with CD34+ cells and MSC) or the CD34+ subpopulation of the cells after expansion with TPO. Both addition of MSC and TPO expansion improved the migration of the CD34+ cells significantly. Addition of MSC to TPO-expanded cells did not improve the migration of the subpopulation (*P<0.05). (B) Percentage of cells of the CD34-CD61-Lin subpopulation found after TPO expansion of CD34+ cells that have migrated through a transwell system toward the lower compartment of the plate containing medium with 100 ng/mL SDF-1α gradient (black bars) or medium alone (gray bars, spontaneous migration). Addition of MSC to TPO-expanded cells did not improve the migration of the subpopulation. (C) Percentage of CD34+ cells expressing CXCR4, CD49d, or CD49e. There was no difference in the expression of CXCR4 between the different transplants suggesting that the differences found in the transwell migration are not due to a difference in the expression of the receptor for SDF-1α. TPO-expanded cells did express higher percentages of CD49d and to a lesser extend CD49e (*P<0.0001). (D) Percentage of cells of the CD34-CD61 subpopulation found after TPO expansion of CD34+ cells expressing CXCR4. No differences in the expression of the receptor of SDF-1α were found.
Because TPO expansion generated CD34-CD61-Lin cells that establish PB platelet recovery, we also investigated the effect of MSC on migration of this subpopulation [12]. Figure 5B in this respect shows that, similarly as with residual CD34+ cells, cotransplantation of MSC does not alter the migration of CD34-CD61 cells (37.0%±4.1% for TPO-expanded transplants, 33.2%±2.1% for TPO-expanded cells with MSC, P=0.818).
The expression of CXCR4, the receptor for SDF1α, on (residual) CD34+ (Fig. 5C) or CD34-CD61-Lin cells (Fig. 5D) was analyzed with flow cytometry (Fig. 5C and 5D), but was not significantly altered by their incubation with MSC nor by TPO expansion alone or in combination with MSC. However, the expression of CD49d and CD49e was upregulated in TPO-expanded CD34+ cells as opposed to nonexpanded CD34+ cells and CD34+ cells that were incubated with MSC.
Discussion
Several studies have shown that the cotransplantation of MSC improves the BM engraftment in animal models [18,31–35], but the effect on the speed of platelet recovery has not been investigated. In this study, we observed that MSC cotransplantation with either unmanipulated CD34+ cells or with TPO-expanded CD34+ cells had no effect on the recovery of platelets in the PB within 2 weeks after transplantation. The early platelet recovery seen after transplantation of CB cells expanded with TPO originates from CD34-CD61-Lin cells. This population that is (partly) committed to the megakaryocyte lineage is unique for CB and not observed among TPO-expanded adult stem cell sources and this maturation pattern may contribute to the delayed and slow platelet recovery observed after CB transplantation. [12,47]. The absence of this Lin-neg population in unmanipulated CD34+ CB explains the lack of improvement of early platelet recovery when MSC are cotransplanted with uncultured CB CD34+ cells.
Also, in line with earlier studies in NOD/SCID mice showing that MSC cotransplantation improved BM engraftment and repopulation for CD34+ CB cells [12,13,18,31–34], we observed that MSC boosted both PB platelet levels after 6 weeks and BM engraftment.
The mechanism behind the improved engraftment by MSC is still unknown. We found enhanced SDF-1α migration capacity of CD34+ cells by MSC in vitro, which was not related to a change in the expression of CXCR4. This suggests that MSC-induced improvement of engraftment might be partly attributed to this increased migration capacity. However, the interaction between MSC and stem cells on homing is complex [48,49] and a conclusive proof for this or other mechanisms is still lacking. Homing of MSC to the marrow was studied as determinant for the observed engraftment potentiating effect and these studies have shown conflicting results. Both Noort et al. and Kim et al. did not find MSC in the BM of the mice after transplantation [18,34]. Noort et al analyzed the presence of MSC in multiple organs with RT-PCR and did not find any MSC in the BM, spleen, liver, or thymus, but only sequestration of the MSC in the lung. This lung barrier was corroborated by Schrepfer et al. [50], who IV injected labeled MSC and found high bioluminescence in the lungs with in vivo imaging and ex vivo analysis in contrast to only trace signals from other organs such as the spleen, the tibia, and the liver. Moreover, efforts from Noort et al. to bypass the lung barrier by intracardiac injection did not result in the detection of MSC in the BM either. Only Hiwase et al. suggested that MSC and HSC can migrate in conjunction to the marrow. [32]. Other mechanisms induced by MSC-secreted cytokines and growth factors might also play a role. MSC are known to support and maintain blood vessels [51] and might contribute to marrow regeneration by inducing vascularization. Even MSC-mediated immunomodulation enhancing allogeneic tolerance has to be considered [36].
The aim of our study was to investigate whether cotransplantation with MSC had a synergistic effect on accelerated platelet recovery and/or improved BM engraftment of TPO-expanded CB. We observed that MSC did not potentiate short-term platelet engraftment of TPO-expanded CD34+ cells. This could be associated with an unchanged migration pattern of CD34-CD61-Lin cells toward an SDF-1α gradient in vitro since MSC do not influence the homing capacity of the TPO-generated CD34-CD61-Lin cells responsible for early platelet repopulation. However, a causal role on the lack of synergy in platelet recovery between the two populations is elusive. Furthermore, the increase in BM engraftment seen with the cotransplantation of MSC with unmanipulated CD34+ cells is not seen when MSC are cotransplanted with TPO-expanded cells. Strikingly, in some situations the combination seemed to decrease the 6 week PB platelet numbers as well as human CD45+ BM engraftment. This could largely be attributed to the fact that some mice treated with the combination of TPO expansion and MSC virtually showed nonengraftment (only 0%–0.1% of human CD45 cells in the BM). If these non or very low engrafters were not taken into account when calculating the mean engraftment percentage of this group, the level of human CD45+ cells in the BM rose from 14.7% to 22.0%, which is similar to mice transplanted with only TPO-expanded cells (20.2%). A synergy of the two approaches which each separately have shown to improve platelet recovery or BM engraftment in NOD/SCID mice was clearly absent. Most importantly, adding MSC to TPO-expanded cells may result in engraftment failure.
These non or very low engraftment cases in the combined approach could hypothetically be caused by a phenomenon called the lung barrier. Because of their larger size, MSC might become trapped in the lung. In addition, MSC can adhere to HSC, further impairing their homing to the marrow [50]. The high cell numbers in the TPO-expanded transplants in combination with the MSC may be critical to develop this complication. Although we did observe acute deaths in both groups that were cotransplanted with MSC, possibly caused by pulmonary embolism, nonengrafters were only found in the group with TPO-expanded cells combined with MSC. To see whether the simultaneous presence of high numbers of cells transplanted after TPO expansion together with MSC influenced the engraftment, we performed additional experiments in which we transplanted the MSC 4 h after the transplantation of the TPO-expanded cells. Although no differences in the engraftment of both platelets in the PB and human CD45 cells in the BM after 6 weeks were discerned, one mouse again showed hardly any BM engraftment after 6 weeks. Interestingly, this mouse was given MSC 4 h after infusion of TPO-expanded cells. Thus, entrapment of MSC adhered to TPO-expanded progenitor cells in the lung does not seem to be an explanation for nonengraftment.
TPO-expanded CD34+ cells migrate better in vitro than fresh CD34+ cells or fresh CD34+ cells with MSC. Whether this improved in vitro migration also translates into better in vivo homing is not definite. A previously conducted homing study with 99mTc-tropolone-labeled cells [13], which showed a similar proportion (approximately 0.5%) of the fresh CD34+ or TPO-expanded CD34+ cells that were transplanted, homed toward the femur. In these experiments fresh CD34+ cells or their expanded equivalent were transplanted, that is, higher numbers of cells were transplanted in the TPO-expanded group. The absolute number of homing cells is, therefore, higher in the group that was transplanted with TPO-expanded cells. However, the experiment did not discern the proportion of each of the three major subpopulations found after TPO expansion that homed to the BM. Although more cells homed to the BM in the mice that received TPO-expanded cells and the largest population of these cells (CD61+) migrated less in vitro than the two other populations (CD34+ and CD34-CD61 cells (48.7%±8.9% and 37.0%±4.1%, respectively vs. 15.4%±2.1% for CD61+ cells), we cannot with certainty conclude that TPO-expanded CD34+ cells home better to the BM than fresh CD34+ cells. Despite the higher migration rate of TPO-expanded cells in vitro, the lack of improvement in engraftment suggests that other characteristics such as TPO-induced changes to their immature characteristics, their stemness, and thus long-term engraftment potential, are more important in this respect.
In this article we additionally focused on the added engraftment effect by MSC cotransplantation with TPO-expanded cells. It is likely that MSC do not improve the homing capacity of TPO-expanded CD34+ cells since they do not affect migration in vitro and transplantation of MSC 4 h after the transplantation of CD34+ cells does not alter the engraftment of the cells.
Superior migration of TPO-expanded cells over the fibronectin-coated transwell plates, despite a lack of difference in CXCR4 expression, might be explained by their higher expression of adhesion molecules for which fibronectin is a ligand, such as CD49d and CD49e [52]. Blocking of CD49d and CD49e with antibodies reduces the migration of CD34+ cells in fibronectin-coated plates [53]. In the migration experiments, short-term incubation with MSC did not lead to a change in the expression of adhesion markers on CD34+ cells. The increased migration and enhanced engraftment of nonexpanded CD34+ cells in the presence of MSC can, therefore, not be explained by a change in adhesion molecule expression. Strikingly, expansion with CD34+ cells, either with MSC or TPO might change the longer term of the engraftment capacity of the cells. This was suggested by studies showing that after transplantation of one CB unit expanded by culture ex vivo with MSC or TPO and one unmanipulated unit, the unmanipulated CB graft establishes long-term engraftment [42,54].
In conclusion, cotransplantation of MSC can improve engraftment after 6 weeks whereas TPO expansion improves early platelet recovery. MSC cotransplantation combined with TPO expansion at best combines, but gives no synergy on either of these effects. However, the combination of high cell numbers introduces the risk of nonengraftment. More precise characterization of these nonengrafting events will be essential to combine these approaches.
Supplementary Material
Acknowledgments
The authors thank the staff of the experimental animal facility of the Leiden University Medical Center for technical assistance. This work was funded by grant PPOC 06-030 of the Sanquin Blood Supply Foundation, the Netherlands.
Author Disclosure Statement
All authors state that no competing financial interests exist.
References
- 1.Ballen KK, Gluckman E. and Broxmeyer HE. (2013). Umbilical cord blood transplantation: the first 25 years and beyond. Blood 122:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballen KK, Koreth J, Chen YB, Dey BR. and Spitzer TR. (2012). Selection of optimal alternative graft source: mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood 119:1972–1980 [DOI] [PubMed] [Google Scholar]
- 3.Fuchs E, O'Donnell PV. and Brunstein CG. (2013). Alternative transplant donor sources: is there any consensus? Curr Opin Oncol 25:173–179 [DOI] [PubMed] [Google Scholar]
- 4.Watt SM. (2014). Article 04238. In: Umbilical Cord Blood Banking. Reference Module in Biomedical Sciences. Caplan M, ed. Elsevier Press, Kidlington, United Kingom [Google Scholar]
- 5.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, DeFor TE, Gooley TA, Verneris MR, Appelbaum FR, Wagner JE. and Delaney C. (2010). Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 116:4693–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eapen M, Rubinstein P, Zhang M-J, Stevens C, Kurtzberg J, Scaradavou A, Loberiza FR, Champlin RE, Klein JP. and Horowitz MM. (2007). Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet 369:1947–1954 [DOI] [PubMed] [Google Scholar]
- 7.Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, Jacobsen N, Ruutu T, de Lima M. and Finke J. (2004). Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 351:2276–2285 [DOI] [PubMed] [Google Scholar]
- 8.Danby R. and Rocha V. (2014). Current Strategies to Improve Engraftment in Cord Blood Transplantation. J Stem Cell Res Ther 4:2 [Google Scholar]
- 9.Kelly S, Sola C, De Lima M. and Shpall E. (2009). Ex vivo expansion of cord blood. Bone Marrow Transplantat 44:673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tung S, Parmar S, Robinson S, De Lima M. and Shpall E. (2010). Ex vivo expansion of umbilical cord blood for transplantation. Best Pract Res Clin Haematol 23:245–257 [DOI] [PubMed] [Google Scholar]
- 11.Mattia G, Milazzo L, Vulcano F, Pascuccio M, Macioce G, Hassan HJ. and Giampaolo A. (2008). Long–term platelet production assessed in NOD/SCID mice injected with cord blood CD34+ cells, thrombopoietin–amplified in clinical grade serum–free culture. Exp Hematol 36:244–252 [DOI] [PubMed] [Google Scholar]
- 12.Schipper LF, Brand A, Fibbe WE. and Van Hensbergen Y. (2012). Functional characterization of TPO‐expanded CD34+ cord blood cells identifies CD34–CD61– cells as platelet‐producing cells early after transplantation in NOD/SCID mice and rCD34+ cells as CAFC colony‐forming cells. Stem Cells 30:988–996 [DOI] [PubMed] [Google Scholar]
- 13.van Hensbergen Y, Schipper LF, Brand A, Slot MC, Welling M, Nauta AJ. and Fibbe WE. (2006). Ex vivo culture of human CD34+ cord blood cells with thrombopoietin (TPO) accelerates platelet engraftment in a NOD/SCID mouse model. Exp Hematol 34:943–950 [DOI] [PubMed] [Google Scholar]
- 14.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 15.Caplan AI. (1991). Mesenchymal stem cells. J Orthop Res 9:641–650 [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain G, Fox J, Ashton B. and Middleton J. (2007). Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739–2749 [DOI] [PubMed] [Google Scholar]
- 17.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I. and Fisk NM. (2001). Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98:2396–2402 [DOI] [PubMed] [Google Scholar]
- 18.Noort WA, Kruisselbrink AB, Kruger M, van Bezooijen RL, de Paus RA, Heemskerk MH, Löwik CW, Falkenburg J, Willemze R. and Fibbe WE. (2002). Mesenchymal stem cells promote engraftment of human umbilical cord blood–derived CD34+ cells in NOD/SCID mice. Exp Hematol 30:870–878 [DOI] [PubMed] [Google Scholar]
- 19.Nauta AJ. and Fibbe WE. (2007). Immunomodulatory properties of mesenchymal stromal cells. Blood 110:3499–3506 [DOI] [PubMed] [Google Scholar]
- 20.Duijvestein M, Vos ACW, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ. and Fidder HH. (2010). Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut 59:1662–1669 [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S. and Hu X. (2010). Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum 62:2467–2475 [DOI] [PubMed] [Google Scholar]
- 22.Christopeit M, Schendel M, Föll J, Müller L, Keysser G. and Behre G. (2007). Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia 22:1062–1064 [DOI] [PubMed] [Google Scholar]
- 23.Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R. and Schuster M. (2009). Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant 15:804–811 [DOI] [PubMed] [Google Scholar]
- 24.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME. and Remberger M. (2008). Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371:1579–1586 [DOI] [PubMed] [Google Scholar]
- 25.Lucchini G, Introna M, Dander E, Rovelli A, Balduzzi A, Bonanomi S, Salvadè A, Capelli C, Belotti D. and Gaipa G. (2010). Platelet-lysate-expanded mesenchymal stromal cells as a salvage therapy for severe resistant graft-versus-host disease in a pediatric population. Biol Blood Marrow Transplant 16:1293–1301 [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Simon JA, Lopez-Villar O, Andreu EJ, Rifon J, Muntion S, Campelo MD, Sánchez-Guijo FM, Martinez C, Valcarcel D. and del Cañizo C. (2011). Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial. Haematologica 96:1072–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasad VK, Lucas KG, Kleiner GI, Talano JAM, Jacobsohn D, Broadwater G, Monroy R. and Kurtzberg J. (2011). Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal™) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant 17:534–541 [DOI] [PubMed] [Google Scholar]
- 28.Ringden O. and Le Blanc K. (2011). Mesenchymal stem cells for treatment of acute and chronic graft-versus-host disease, tissue toxicity and hemorrhages. Best Pract Res Clin Haematol 24:65–72 [DOI] [PubMed] [Google Scholar]
- 29.Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L, Vanbellinghen J-F, Hafraoui K, Lejeune M. and Gothot A. (2010). Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant 16:838–847 [DOI] [PubMed] [Google Scholar]
- 30.Kuzmina LA, Petinati NA, Parovichnikova EN, Lubimova LS, Gribanova EO, Gaponova TV, Shipounova IN, Zhironkina OA, Bigildeev AE. and Svinareva DA. (2011). Multipotent mesenchymal stromal cells for the prophylaxis of acute graft-versus-host disease—a phase II study. Stem Cells Int 2012: Article ID 968213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C. and Klingemann H. (2007). Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant 13:1477–1486 [DOI] [PubMed] [Google Scholar]
- 32.Hiwase SD, Dyson PG, To LB. and Lewis ID. (2009). Cotransplantation of placental mesenchymal stromal cells enhances single and double cord blood engraftment in nonobese diabetic/severe combined immune deficient mice. Stem Cells 27:2293–2300 [DOI] [PubMed] [Google Scholar]
- 33.in 't Anker PS, Noort WA, Kruisselbrink AB, Scherjon SA, Beekhuizen W, Willemze R, Kanhai HH. and Fibbe WE. (2003). Nonexpanded primary lung and bone marrow–derived mesenchymal cells promote the engraftment of umbilical cord blood–derived CD34+ cells in NOD/SCID mice. Exp Hematol 31:881–889 [DOI] [PubMed] [Google Scholar]
- 34.Kim D-W, Chung Y-J, Kim T-G, Kim Y-L. and Oh I-H. (2004). Cotransplantation of third-party mesenchymal stromal cells can alleviate single-donor predominance and increase engraftment from double cord transplantation. Blood 103:1941–1948 [DOI] [PubMed] [Google Scholar]
- 35.Masuda S, Ageyama N, Shibata H, Obara Y, Ikeda T, Takeuchi K, Ueda Y, Ozawa K. and Hanazono Y. (2009). Cotransplantation with MSCs improves engraftment of HSCs after autologous intra-bone marrow transplantation in nonhuman primates. Exp Hematol 37:1250–1257 [DOI] [PubMed] [Google Scholar]
- 36.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F. and Fibbe WE. (2007). Cotransplantation of ex vivo–expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 110:2764–2767 [DOI] [PubMed] [Google Scholar]
- 37.Macmillan M, Blazar B, DeFor T. and Wagner J. (2008). Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I–II clinical trial. Bone Marrow Transplant 43:447–454 [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Lee M, Yoo K, Kim D, Son M, Sung K, Cheuh H, Choi S, Oh W. and Yang Y. (2013). Co-transplantation of third-party umbilical cord blood-derived MSCs promotes engraftment in children undergoing unrelated umbilical cord blood transplantation. Bone Marrow Transplant 48:1040–1045 [DOI] [PubMed] [Google Scholar]
- 39.Wu K-H, Sheu J-N, Wu H-P, Tsai C, Sieber M, Peng C-T. and Chao Y-H. (2013). Cotransplantation of umbilical cord–derived mesenchymal stem cells promote hematopoietic engraftment in cord blood transplantation: a pilot study. Transplantation 95:773–777 [DOI] [PubMed] [Google Scholar]
- 40.Chen Y-B, Aldridge J, Kim HT, Ballen KK, Cutler C, Kao G, Liney D, Bourdeau G, Alyea EP. and Armand P. (2012). Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biol Blood Marrow Transplant 18:805–812 [DOI] [PubMed] [Google Scholar]
- 41.Schipper LF, Van Hensbergen Y, Fibbe WE. and Brand A. (2007). A sensitive quantitative single‐platform flow cytometry protocol to measure human platelets in mouse peripheral blood. Transfusion 47:2305–2314 [DOI] [PubMed] [Google Scholar]
- 42.van der Garde M, van Hensbergen Y, Brand A, Slot MC, de Graaf-Dijkstra A, Mulder A, Watt SM. and Zwaginga JJ. (2015). Thrombopoietin treatment of one graft in a double cord blood transplant provides early platelet recovery while contributing to long term engraftment in NSG mice. Stem Cells Dev 24:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer G N, Lerrer B, Cohen HY, Nagler A. and Fibach E. (2012). Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol 40:342–355 [DOI] [PubMed] [Google Scholar]
- 44.Quesenberry PJ. and Becker PS. (1998). Stem cell homing: rolling, crawling, and nesting. Proc Natl Acad Sci USA 95:15155–15157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vermeulen M, Le Pesteur F, Gagnerault M-C, Mary J-Y, Sainteny F. and Lepault F. (1998). Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood 92:894–900 [PubMed] [Google Scholar]
- 46.Voermans C, Rood P, Hordijk P, Gerritsen W. and Van Der Schoot C. (2000). Adhesion molecules involved in transendothelial migration of human hematopoietic progenitor cells. Stem Cells 18:435–443 [DOI] [PubMed] [Google Scholar]
- 47.Schipper LF, Brand A, Reniers N, Melief CJ, Willemze R. and Fibbe WE. (2003). Differential maturation of megakaryocyte progenitor cells from cord blood and mobilized peripheral blood. Exp Hematol 31:324–330 [DOI] [PubMed] [Google Scholar]
- 48.Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L. and Krause DS. (2003). Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol 31:413–420 [DOI] [PubMed] [Google Scholar]
- 49.Kang SK, Shin IS, Ko MS, Jo JY. and Ra JC. (2012). Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int 2012: Article ID 342968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schrepfer S, Deuse T, Reichenspurner H, Fischbein M, Robbins R. and Pelletier M. (2007). Stem cell transplantation: the lung barrier. Transplant Proc 39:573–576 [DOI] [PubMed] [Google Scholar]
- 51.Watt SM, Gullo F, van der Garde M, Markeson D, Camicia R, Khoo CP. and Zwaginga JJ. (2013). The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull 2013:1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turner ML, Masek LC, Hardy CL, Parker AC. and Sweetenham JW. (1998). Comparative adhesion of human haemopoietic cell lines to extracellular matrix components, bone marrow stromal and endothelial cultures. Br J Haematol 100:112–122 [DOI] [PubMed] [Google Scholar]
- 53.Voermans C, Gerritsen WR, von dem Borne AE. and van der Schoot CE. (1999). Increased migration of cord blood-derived CD34+ cells, as compared to bone marrow and mobilized peripheral blood CD34+ cells across uncoated or fibronectin-coated filters. Exp Hematol 27:1806–1814 [DOI] [PubMed] [Google Scholar]
- 54.de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, Alousi A, Saliba R, McMannis JD. and Kaur I. (2012). Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 367:2305–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.