Abstract
Although the amount of progress cancer therapy has made in recent years is commendable, considerable limitations still remain. Most agents preferentially target rapidly proliferating cells, thereby destroying tumorigenic growths. Unfortunately, there are many labile cells in the patient that are also rapidly dividing, ultimately perpetuating significant side effects, including immunosuppression. Cytochalasins are microfilament-directed agents most commonly known for their use in basic research to understand cytoskeletal mechanisms. However, such agents also exhibit profound anticancer activity, as indicated by numerous in vitro and in vivo studies. Cytochalasins appear to preferentially damage malignant cells, as shown by their minimal effects on normal epithelial and immune cells. Further, cytochalasins influence the end stages of mitosis, suggesting that such agents could be combined with microtubule-directed agents to elicit a profound synergistic effect on malignant cells. Therefore, it is likely that cytochalasins could be used to supplement current chemotherapeutic measures to improve efficacy rates, as well as decrease the prevalence of drug resistance in the clinical setting.
Keywords: Actin, apoptosis, chemotherapy, cytochalasins, drug resistance, immunosuppression, microfilaments, preferential damage.
INTRODUCTION
Chemotherapy is an ever persistent quandary in the clinical setting. Although the diversity and versatility of current oncology drugs have grown substantially in recent years, clinicians and researchers face the all common issue of preferential damage. Many agents used in therapeutic measures rely on a fundamental characteristic of virtually all malignant cells; that of uncontrolled, aberrant cell proliferation. >From cisplatin to paclitaxel (taxol), such agents preferentially target rapidly proliferating cells, thereby destroying tumorigenic growths. Unfortunately, there are many labile cells in the patient that are also rapidly dividing and hence, are damaged by the cytotoxic effects of these agents. Not only do many patients begin to lose their hair and have troubles with their gastrointestinal tract as a consequence of such regimens, but patients often experience immunosuppression [1]. Most chemo-therapeutic regimens depress the immune system, often by interfering with the bone marrow’s supply of hematopoietic stem cells (HSCs). This subsequently perpetuates a substantial decrease of erythrocytes (red blood cells), leukocytes (white blood cells) and megakaryocytes (cells that give rise to platelets). As such, immunosuppression is an unintended consequence of many current chemotherapeutic options. Further, although often initially successful, many known chemotherapeutic agents run into the issue of drug resistance when a few unaffected cells end up repopulating a tumor [2]. Increasing the efficacy of these proven cytotoxic drugs would be a significant discovery in its own right and could significantly increase the rate at which successful treatments are attained.
By default, malignant cells have a perturbed cytoskeleton due to the effects of dysplasia and subsequent anaplasia [1]. With so many alterations present in malignant cells, the cytoskeleton provides an ideal opportunity for preferential damage. Cytochalasins are mycogenic toxins that bind filamentous (F)-actin, thereby blocking polymerization. Consequently, microfilament formation is considerably inhibited [4]. This in turn affects cellular morphology, inhibits cellular processes such as cell division, and even induces apoptosis [1-5]. Further, cytochalasins permeate cell membranes, prevent cellular translocation, and cause cells to enucleate [4]. In effect, cytochalasins influence other aspects of biological processes unrelated to actin polymerization, substantiating its already diverse mechanisms of action [5]. More than 60 different cytochalasins from several species of fungi have been classified into various subgroups based on the “size of the macrocyclic ring and the substituent of the perhydroisoindolyl-1-one residue at the C-3 position” [4]. Consequently, there are a substantial variety of mycogenic agents to examine for efficacy in chemotherapy.
Although cytochalasins have been predominantly used for understanding the importance of actin in various biological processes, there have been considerable in vitro and in vivo data that suggest such agents exhibit profound anticancer activity. In particular, the efficacy of cytochalasin B has been widely investigated, demonstrating promise in a variety of cell lines and mouse models [6-10]. It has also been indicated that cytochalasins have the potential to increase the efficacy of chemotherapeutic agents currently used in the clinic through a synergistic effect [2, 11]. Since cytochalasins have the ability to enlarge malignant cells, such agents have also been investigated in physicochemical treatment modalities, such as the experimental sonodynamic therapy (SDT), an ultrasound therapy that preferentially damages cells based on size [2, 12]. With their diverse mechanisms of action, cytochalasins appear to be an ideal chemotherapeutic agent to combat a diversity of neoplastic cell lines. This is particularly true for drug resistant cell lines, as the phenomenon typically occurs when the chemotherapeutic regimen has only a few methods in which to preferentially damage the malignancy. Therefore, in this review, cytochalasins will be examined in explicit detail to determine whether they indeed have clinical potential. If these congeners indeed hold promise, they may be applied in the clinical setting in combination with current chemotherapeutic approaches to increase the efficacy of applied treatments.
MOLECULAR STRUCTURE
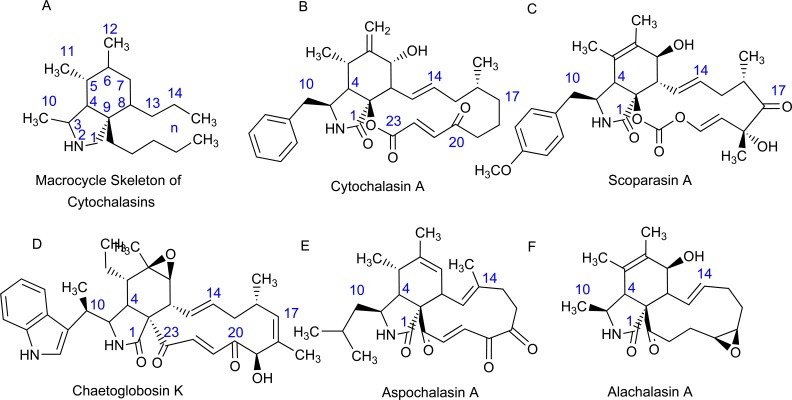
Cytochalasins comprise a tremendous diversity of fungal metabolites. As expected, the structures of these mechanistically-related molecules vary considerably, resulting in a diverse range of biological interactions. Cytochalasins are characterized by a highly substituted perhydro-isoindolone structure that is typically attached to a macrocyclic ring. This macrocycle can vary tremendously between cytochalasins as carbocycles, lactones or even cyclic carbonates have been identified [13]. Congeners often contrast in the amino acid incorporated into the polyketide carbon skeleton. Consequently, the hydrogenated isoindolone can have a variety of substituents: a benzyl group (cytochalasins), a p-methoxybenzyl group, an (indol-3-yl)methyl group, a 2-methylpropyl group (aspochalasins) or a methyl group (alachalasins) (Fig. 1) [13-15]. These variations in the size and the substitution pattern of this macrocycle further increase the chemical diversity, and subsequent biological functioning of cytochalasins.
Fig. (1).
Diversity of cytochalasins. Cytochalasins are characterized by a highly substituted perhydro-isoindolone structure that exhibits tremendous heterogeneity due to the variety of macrocyclic rings that are fused to the carbon skeleton. The hydrogenated isoindolone bears a diversity of structures: A) the numbering system of the macrocycle skeleton of cytochalasins, B) benzyl group, C) p-methoxybenzyl group, D) (indol-3-yl)methyl group, E) 2-methylpropyl group, and F) methyl group. It is the variation of the size and the substitution pattern of this macrocycle that substantially increases the chemical diversity of cytochalasins.
Although cytochalasins are typically derived from fungal metabolite extracts, there has been recent work to synthesize the fundamental carbon skeleton found in such agents [16]. Heterologous expression of the cytochalasin polyketide synthase-non-ribosomal peptide synthethase (PKS-NRPS) hybrid gene CcsA and the trans-acting enoyl-CoA reductase gene CcsC in Aspergillus oryzae (fungus known to produce cytochalasins) resulted in the production of a novel metabolite possessing the cytochalasin backbone needed for subsequent synthesis of more complex derivatives. While the synthesis was notably complex, the results established that CcsA is capable of constructing the octaketide connected with phenylalanine in collaboration with CcsC, allowing the CcsA R domain to catalyze reductive cleavage of the thio-tethered PKS-NRPS product. The reduced product can then be converted into putative intermediate analogs dihydro-1 and pyrrolinone dihydro-2 via oxidation of a primary alcohol with a subsequent Knoevenagel condensation. This ultimately suggests that an intramolecular Diels-Alder reaction (IMDA) can be used in the construction of the characteristic perhydro-isoindolone macrocyclic system needed for cytochalasin biosynthesis. The result of this study should not be understated for their importance as it suggests cytochalasins may be synthesized under controlled conditions rather than having to rely on fungal metabolite extractions for sufficient quantities of cytochalasins. If such agents do in fact exhibit profound anticancer activity, such a method opens the opportunity for industrial synthesis.
MECHANISMS OF ACTION
Cytochalasins encapsulate a tremendous diversity of mycogenic toxins that are unique in both structure and targets. As a result, the mechanisms by which these pharmacological agents damage malignant cells are immense. Cytoskeletal alterations and changes in cellular morphology, motility, and adhesiveness are characteristic features of malignant cells [17, 18]. As such, microfilaments have become promising targets for chemotherapy. In contrast to microtubules, which have been targeted successfully with antineoplastic drug classes such as epothilones (ixabepilone) taxanes (paclitaxel and docetaxel) and vinca alkaloids (vinblastine, vincristine, vindesine, vinflunine and vinorelbine), no microfilament-targeting drugs are currently being used in the clinical setting [17, 18]. Therefore, using cytochalasins to preferentially damage malignant cells through actin disruption is a novel prospect.
All cytochalasins demonstrate the propensity to bind F-actin, thereby blocking polymerization (Fig. 2). The two congeners used most extensively in anticancer research are cytochalasins B and D [4, 13]. Their mechanisms will be discussed at length, as these agents have been shown to preferentially damage a considerable variety of neoplasms. It is important to note that while cytochalasins B and D have been the most investigated, there has been a study examining the in vitro anticancer activity of eight natural cytochalasins, and three hemisynthetic derivatives of cytochalasin B on six cancer cell lines [4]. The study indicated that most cytochalasins demonstrate the capacity to inhibit tumorigenic growth, especially cancer cell lines displaying noticeable levels of resistance to pro-apoptotic stimuli when compared to cancer cell lines sensitive to these stimuli. This tentatively suggests that cytochalasins may be useful against malignancies known to circumvent apoptotic signaling, a method of drug resistance observed in the clinic. Further research will be needed to determine whether these other congeners exhibit the same propensity for preferential damage as do cytochalasins B and D [6, 9, 12, 19, 20].
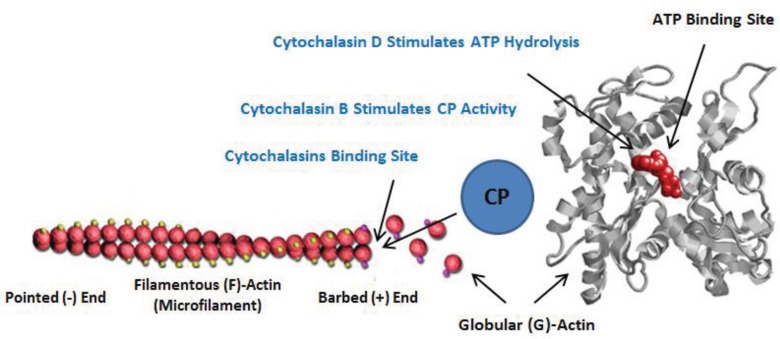
Fig. (2).
Mechanisms of cytochalasins B and D. It is known that cytochalasins bind the growing barbed (+) end of microfilaments, thereby inhibiting polymerization. In addition, cytochalasin D stimulates ATP hydrolysis in formed G-actin dimers, preventing the formation of viable microfilaments. It is also known that cytochalasin B promotes F-actin capping protein (CP) activity, considerably inhibiting the length of polymers.
Mechanisms of Cytochalasin B
Derived from the metabolic processes of Helminthosporium dematioideum and other fungi [21], cytochalasin B has been the most studied cytochalasin in terms of potential for chemotherapy. As such, its mechanism of action has been characteristically described. In terms of structure, cytochalasin B contains several highly polar keto- and hydroxyl groups and a single peripheric hydrophobic benzyl unit (Fig. 3). It has been proposed that cytochalasin B inhibits actin polymerization by binding the fast-growing (barbed) end of F-actin, as well as interacting with capping proteins (CAPZA1 and others in the F-actin capping protein α subunit family, Fig. 2) [21-24]. By doing so, cytochalasin B inhibits subsequent processes following actin polymerization, such as microfilament formation. This inhibition can disrupt all three major steps of actin polymerization (nucleation, elongation and steady state/annealing). Nucleation is essential for microfilament formation, as this oligomerization is the rate-determining step of the polymerization reaction. Once the processive reaction begins, it continues until the physiological maximum of the polymerization rate is attained [21]. As such, cytochalasin B can prevent nucleation from ever occurring, thereby destroying any potential for microfilament formation. Once the polymerization reaction has begun, elongation is favored at the barbed end of the growing filament [25]. The influence of cytochalasin B on elongation is pronounced, as the agent acts preferentially towards the barbed end of the growing microfilament. Under appropriate conditions, cytochalasin B can inhibit actin polymerization in excess of 90% [26]. Annealing is the last step in polymerization, and while cytochalasin B appears to be highly effective against nucleation and elongation, its potency in this final stage is significantly reduced [21, 23]. Therefore, cytochalasin B has its most influence on the first two steps of actin polymerization.
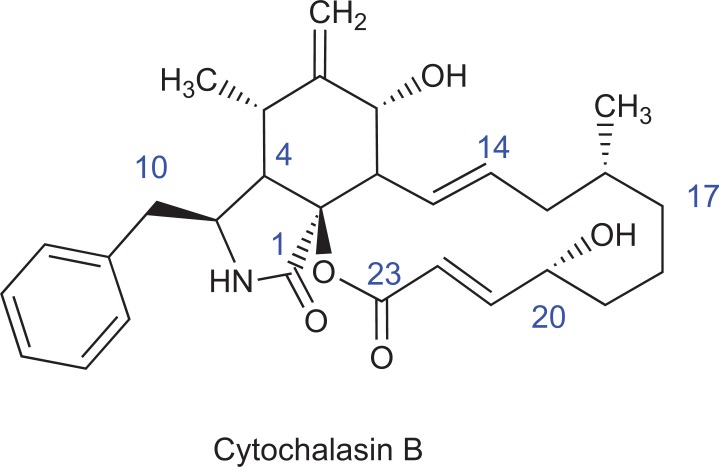
Fig. (3).
Structure of cytochalasin B. There are several highly polar ketoand hydroxyl groups, and a single peripheric hydrophobic benzyl unit. Common to virtually all cytochalasins, the characteristic rigid bicyclic isoindolone core fused to a macrocyclic appendage is present.
As indicated by its molecular structure, cytochalasin B contains a β-unsaturated ester which has a substantial propensity to undergo Michael-type conjugations with nucleophiles [27]. As such, it is likely the inhibitory potential of the pharmacological agent involves the thiol groups of several molecules within the reaction site [28, 29]. Due to this pronounced reactivity, the thiol groups are no longer available for disulfide bond formation, necessary for further actin polymerization [29, 30]. Consequently, the barbed ends of the filaments are blocked by nonreactive functional groups. An analogous principle is used by capping proteins that provide a natural limiting factor towards actin polymerization [22]. After polymer initiation, the subsequent step in actin polymerization is the deprotonation of the thiol group of monomeric globular (G)-actin. This enables the sulfur atom to become charged, making it available for actin polymerization. Sufficient concentrations of cytochalasin B compete with thiol deprotonation, as the β-unsaturated ester of cytochalasin B reacts with the actin thiol via a reaction with the charged sulfur onto the β-carbon [21]. Such action reciprocates π-bond dislocation on the left site of the β-carbon, causing resonant structures to form. This eventually perpetuates dislocation of the negative charge between the α-carbon and the oxygen, followed by protonation to mitigate the negative charge (the hydronium ion needed for reactivity is formed during sulfur activation) [21]. In summary, actin polymerization becomes aberrantly influenced by cytochalasin B reactivity, and is unable to sustain sufficient polymerization reactions to produce viable microfilaments.
Taking a broader perspective in regards to its potential efficacy in chemotherapy, cytochalasin B is a known cytokinesis inhibitor that disrupts the actin cytoskeleton and interferes with formation of the contractile ring, as well as the development of the cleavage furrow. Consequently, the cell is unable to divide, permeating a weakened cytoskeletal network. In addition, the cell continues to form nuclei and becomes enlarged and multinucleated (Fig. 4). Such cells have more DNA targets, increasing the likelihood of apoptosis when combined with a nucleic acid-directed agent; it only takes a single nucleus to undergo programmed cell death before a chain reaction is triggered, potentiating cell death [1, 3]. This is in accordance with O’Neill (1975) [10], as the study indicated substantial drug synergy between cytochalasin B and the nucleoside analog cytarabine. Further, the multinucleated cells have an increased cell volume, making them more susceptible to physical agitation, such as low frequency ultrasound [12]. Preferential damage to malignant cells is facilitated by the fact that normal cells exposed to cytochalasin B exit the cell cycle, and enter the G0 resting state until sufficient actin levels are restored [3]. Therefore, only malignant cells that have lost the ability to enter the rest phase will become enlarged and multinucleated, providing an ideal target for concomitant chemotherapy.
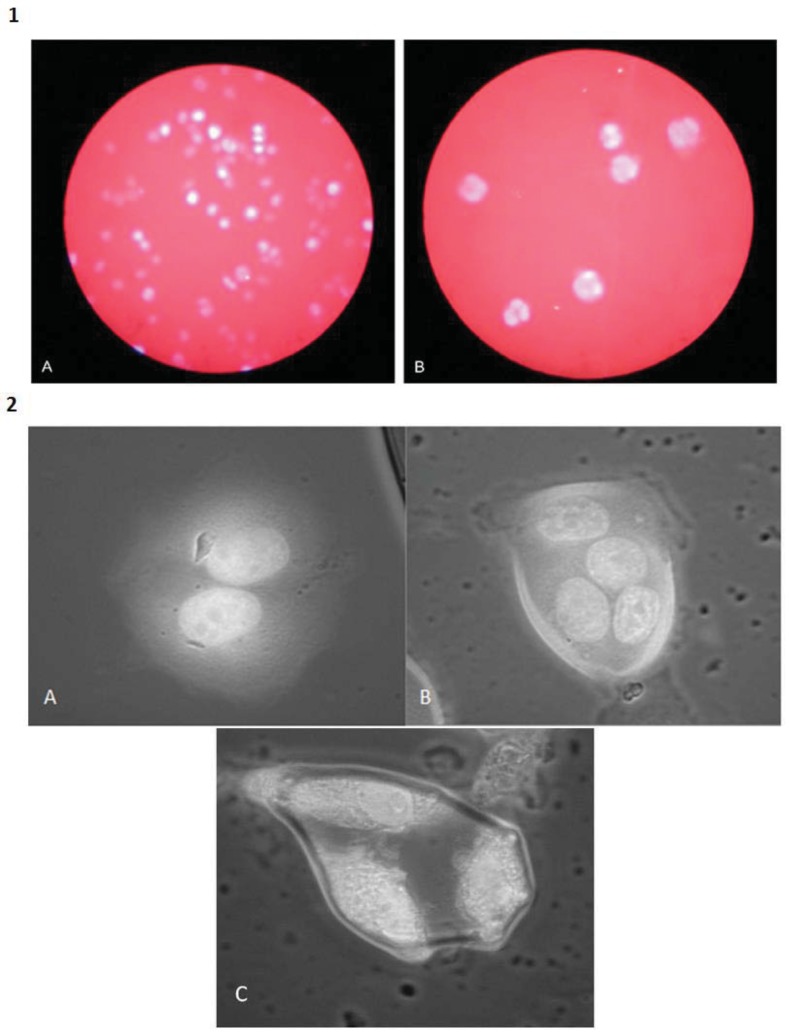
Fig. (4).
DAPI nuclear staining of U937 monocytic leukemia and MCF7 breast carcinoma cells after treatment with cytochalasin B. 1A) Untreated U937 cells are mononucleated. 1B) U937 cells 48 hours post-administration of 1.5 μM cytochalasin B are considerably enlarged and multinucleated. Photomicrographs were taken under identical conditions of magnification (100X). 2A) Binucleated MCF7 cell. 2B) Tetranucleated MCF7 cell. C) Trinucleated MCF7 cell. All MCF7 cells were exposed to 1.0 µM cytochalasin B for 7 days. Photomicrographs were taken under identical conditions of magnification (400X). Nuclear content was visualized with DAPI (4', 6-diamidino-2-phenylindole). Images are from the laboratory of the author.
Mechanisms of Cytochalasin D
Although similar in structure to the more commonly used cytochalasin B, there are noticeable differences in the functional groups of cytochalasin D. The congener is an isomeric metabolite of cytochalasin B, derived from Metarrhizium anisopliae and Zygosporium mansonii [31]. Although it retains the characteristic substituted perhydro-isoindolone attached to a macrocyclic ring, cytochalasin D has differences in the positioning of an ester group as well as the addition of a ketone group, and a contrast in the placement of double bonds (Fig. 5). While these differences may appear subtle, it has an enormous influence on efficacy, as cytochalasin D is much more efficient at disrupting actin polymerization [32]. It is so effective at disrupting microfilament formation, there are concerns as to whether cytochalasin D could act as a chemotherapeutic agent. A pharmacological agent that does not act specifically towards the intended target is not a therapeutic drug; it is a poison. Nevertheless, several studies have demonstrated the efficacy of cytochalasin D on a variety of neoplastic cell lines [20, 33-35], warranting further consideration for use as a viable chemotherapeutic agent.
Fig. (5).
Structure of cytochalasin D. Although its carbon skeleton is very similar to cytochalasin B, there are differences in positioning of an ester group as well as the addition of a ketone group, and a contrast in the placement of double bonds near the ester group. Due to these slight differences in structure, cytochalasin D is much more potent than cytochalasin B in terms of disrupting actin polymerization.
The remarkably high propensity cytochalasin D has for disrupting actin polymerization is enough by itself to warrant the understanding of its mechanism. As with cytochalasin B, cytochalasin D perturbs actin cytoskeleton dynamics by binding to the barbed end of actin filaments [36, 37]. When exposed to G-actin monomers, cytochalasin D induces G-actin dimer formation in the presence of Mg2+, thereby eliminating the polymerization lag phase due to accelerated nucleation by the dimers [38, 39]. ATP hydrolysis is also stimulated by cytochalasin D (Fig. 2), a feature that ultimately contributes to the efficacy of the agent, as perpetual ATP hydrolysis prevents the formation of viable microfilaments [40, 41]. Therefore, the mechanism can be summed up as follows: when cytochalasin D is introduced into a cellular environment, it sequesters Mg2+ which is essential for the formation of cytochalasin D-induced dimers [42]. In turn, the dimers act as nuclei to potentiate further dimer formation. Binding of Mg2+ to a low affinity site on the dimer induces a conformational change that enables enhanced ATP hydrolysis. Such action results in dissociation of the now numerous dimers, causing the release of G-actin monomers containing ADP. This considerably reduced rate of ATP exchange for bound ADP results in the aggregation of ADP-containing monomers. As these monomers have a high critical concentration, the final extent of polymerization is reduced dramatically, thereby preventing sufficient formation of microfilaments [42].
Besides its ability to substantially limit actin polymerization, cytochalasin D is cell-permeable and has been shown to activate p53-dependent pathways, causing arrest of the cell cycle at the G1-S transition [43]. Activation of p53 is of monumental clinical importance as this well-known tumor suppressor gene is critical for inducing apoptosis in aberrant cells. While many cancers lose p53 activity through a series of mutations, there are other cancers that have functioning p53 protein products, indicating that such cells could be coerced into cell death via cytochalasin D treatment. Akin to its isomeric relative cytochalasin B, cytochalsin D is also a potent cytokinesis inhibitor, transforming cells into enlarged, multinucleated cells that have a significantly perturbed cytoskeleton and multiple targets for nucleic acid-directed agents. Taken together, cytochalasin D is an effective disruptor of actin polymerization, but has yet to be sufficiently examined to determine whether it will act preferentially towards malignant cells.
CYTOCHALASINS IN CHEMOTHERAPEUTIC STUDIES
Cytochalasins are potent actin inhibitors that can have a substantial influence on cellular structure and physiology. However, as to date, cytochalasins have typically been thought of as crucial experimental agents for probing mechanisms of the cytoskeleton. Few clinicians would likely delineate cytochalasins as valid chemotherapeutic agents. In fact, there has yet to be a clinically approved microfilament-directed agent used in cancer therapy; and yet, there has been substantial evidence suggesting that cytochalasins and other actin inhibitors may be vital anticancer agents. Using chemotherapeutic agents to preferentially damage the cytoskeletons of malignant cells is a tactic long exploited in the clinical setting. Microtubule-directed agents such as taxanes and vinca alkaloids are commonly used anticancer agents that primarily act as mitotic poisons, thereby inducing cell cycle arrest, and eventual apoptosis. Since microtubules are pivotal for successful mitosis, inhibiting normal function of these polymers can be particularly devastating for rapidly proliferating cells, henceforth ideal for disrupting tumorigenic growths [44, 45]. Further, it has been shown that such agents also induce an apoptotic response in various cancer cell lines [46-48]. As has been shown and will now be further detailed, cytochalasins are also potent mitotic inhibitors, but act during cytokinesis at the end of the event. Therefore, it is likely the concomitant use of microfilament and microtubule-directed agents would have a profound synergist effect on disrupting cancer proliferation. It is hoped that the evidence provided for the efficacy of cytochalasins will inspire further research investigating the potential of such agents in the clinical setting.
Experimental Evidence of Cytochalasin B
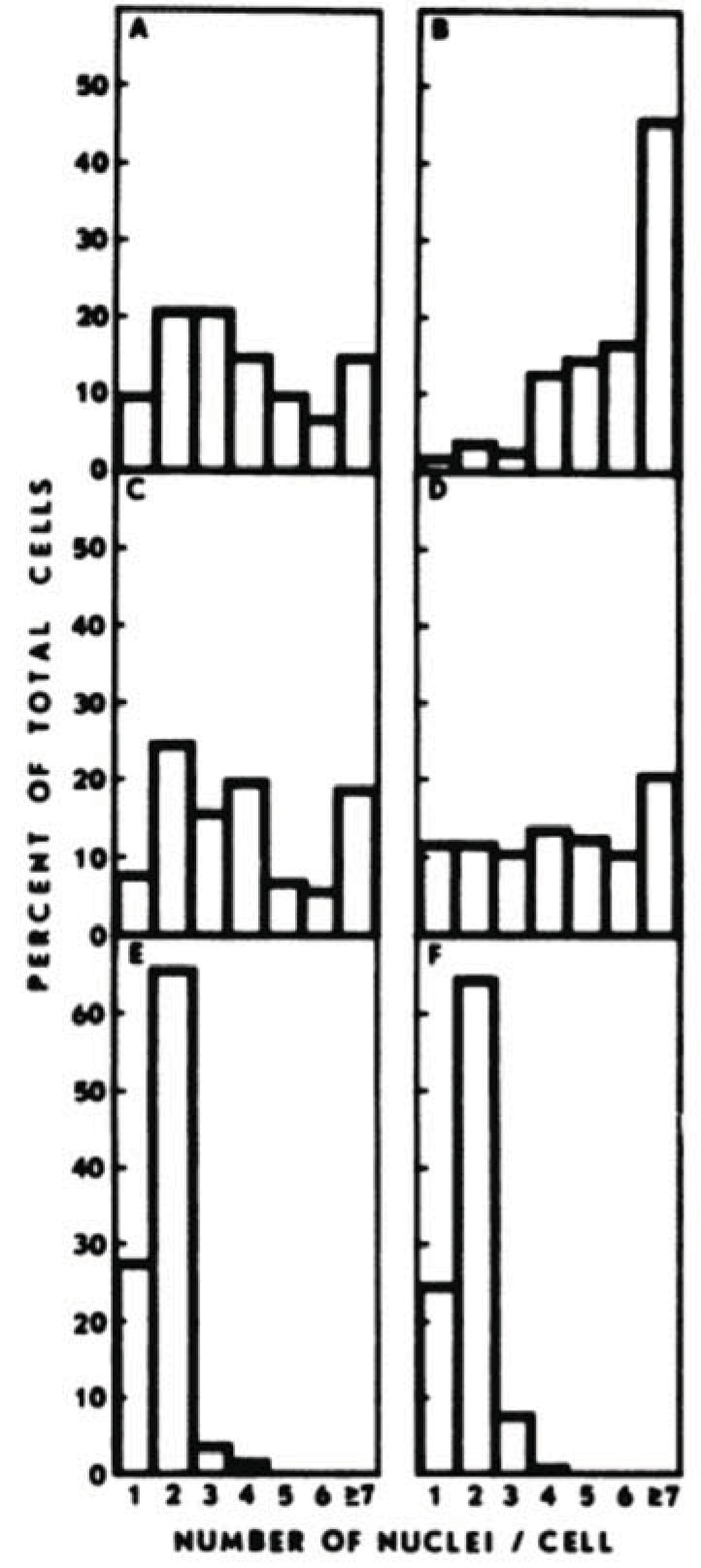
Most studies that have examined the anticancer activity of cytochalasins concentrated their efforts on cytochalasin B as it appears to be a safer alternative to the more potent cytochalasin D. As such, there is considerable experimental evidence to suggest that cytochalasin B is a viable chemotherapeutic agent. In vitro evidence of the efficacy of cytochalasin B has spanned the course of many years. Steiner et al. (1978) [7] were one of the first groups to experiment with the microfilament inhibitor on a malignant cell line when they exposed BALB/c mouse mammary cells of various tumorigenic potential to 1.0-3.0 µg/ml cytochalasin B. In response, highly tumorigenic mammary tumor cell lines with hormonal (ESD/BALB), viral (MTV-L/BALB), or chemical carcinogen (DMBA-2/BALB) etiologies were extensively multinucleated (≥ 3 nuclei/cell) after exposure (Fig. 6). By contrast, normal mammary gland epithelial cells derived from either pregnant or lactating mice were predominantly unaffected under similar conditions. Such results were later confirmed by Medina, Oborn, and Asch (1980) [8] when they exposed BALB/c mice derived breast carcinoma cells to 1 µg/ml cytochalasin B for 48 hours, as once again, neoplastic cells were much more sensitive to the microfilament-directed agent than normal epithelial cells.
Fig. (6).
Cytochalasin B preferentially multinucleates breast carcinoma cells. A) ESD/BALB-clone 3 tumorigenic mammary cells; B) MTV-L/BALB clone 2 tumorigenic mammary cells; C) DMBA 2/BALB clone 4 tumorigenic mammary cells; D) primary cells from a tumor produced by the DMBA- 2/BALB clone 4 cells; E) normal mammary gland cells of pregnant animals; F) normal mammary gland cells of lactating animals. Cells were incubated in medium supplemented with cytochalasin B (1.5 µg/ml) for 5 days. When the proportion of cells with ≥ 3 nuclei/cell is compared for A to D versus E and F, p < 0.005. While normal cells underwent slight binucleation, it was significantly less severe than the multinucleation experienced by the malignant cells. Graph courtesy of [7]
While the in vitro results are intriguing, cytochalasin B has also shown in vivo efficacy as well. When cytochalasin B was injected subcutaneously (s.c.) at 10 or 100 mg/kg single doses 24 hours after s.c. challenge of B6D2F1 mice with trocar implants of B16F10 murine melanoma cells, the appearance of measurable tumor nodules was delayed by 93 and 157% respectively and extended host survival by 26 and 65% [6]. Tumorigenic growth was also delayed when cytochalasin B treatment was given 1 day after the appearance of notable tumor nodules in the mice. To supplement these findings, the same study introduced Madison 109 murine lung carcinoma cells into CD2F1 mice challenged s.c. Cytochalasin B was then injected s.c. at 100 or 150 mg/kg 24 hours after initial tumor challenge. As a result, cytochalasin B-treated mice showed a 66% delay in the median day of tumor nodule appearance. When administered under these conditions or at the time of nodule appearance, cytochalasin B markedly inhibited the progression of tumor growth, substantially reduced tumor invasion at day 23, extended life span by 23%, and significantly inhibited spontaneous lung metastases measured 28 days after tumor challenge [6]. In a later study, the same laboratory also showed that immuno-suppression induced by cytochalasin B can be mitigated through the introduction of human recombinant interleukin-2 (rhIL-2) [9].
Since cytochalasin B is a microfilament-directed cytokinesis inhibitor, it seems possible that using the agent in tandem with a known microtubule-directed mitotic inhibitor would elicit a profound synergistic effect. In theory, this provides malignant cells very few opportunities to carry out a successful mitosis as the microtubule-directed agents would prevent proper formation of a spindle fiber, while any cells that managed to evade this mechanism and replicate their nuclei would be unable to undergo cytokinesis. Therefore, any malignant cells that manage to circumvent the microtubule-directed agents would likely be contained by the microfilament-directed agents. Multinucleated cells not only have compromised cytoskeletal integrity, but also are much more likely to experience apoptosis, as it only takes a single nucleus to trigger a chain reaction, culminating in the destruction of the cell [3].
As it turns out, there is evidence of a synergistic relationship between microfilament and microtubule-directed agents. In an effort to analyze the potential synergistic effects of cytochalasin B and vincristine, cell lines EL4 (H2b T-cell lymphoma), P815 (H2d mastacytoma), Z2B (human Epstein-Barr virus transformed), CH33 (H2k, B-cell lymphoma), and LS102.9 (H2s, B-cell lymphoma) were exposed to cytochalasin B-alone, vincristine-alone, and cytochalsin B/vincristine treatments [11]. As the results indicated, all cell lines showed the highest rate of DNA fragmentation with the cytochalasin B/vincristine treatment. It was in fact greater than the sum of DNA fragmentation caused by either agent alone, warranting the basis of a synergistic relationship. In particular, P815 cells treated with vincristine and cytochalasin B had substantial rates of DNA fragmentation. Therefore, using microfilament-directed agents in combination with microtubule-directed agents may offer a novel chemotherapeutic approach.
Experimental Evidence of Cytochalasin D
Although there is some concern over the potency of cytochalasin D, studies examining its potential for use as a chemotherapeutic agent have found promising results. In an in vivo study involving BALB/c mice successfully challenged with CT26 murine colorectal carcinoma cells, mice were injected intravenously (i.v.) with various doses of cytochalasin D (12.5, 25, 50 and 100 mg/kg in 200 µL DMSO) [33]. Subsequent results revealed the apparent efficacy of cytochalasin D. The agent readily inhibited CT26 cell proliferation in a time and dose dependent manner and induced significant CT26 cell apoptosis. The level of apoptosis was so high that it almost reached the level induced by the positive control TACS nuclease. Further, cytochalasin D effectively inhibited tumor angiogenesis, indicating that the agent can attack tumorigenic growths through multiple mechanisms.
The fact that cytochalasin D has the potential to induce apoptosis in malignant cells is very encouraging, as currently approved chemotherapeutic agents often rely on this mechanism to damage neoplasms. Apoptosis is a much more controlled event than necrosis or lysis, both of which can potentiate inflammation and other immunological complications. Inflammation is a very serious issue in chemotherapy, as the immune response will invariably elicit growth factors and cytokines that promote repair as well as growth; the same cellular signals that proliferating tumors thrive on [49, 50]. Therefore, chemotherapeutic agents that can cause widespread apoptosis in tumors and metastases are of substantial clinical utility.
However, there still remains concern regarding the safety of cytochalasin D, as it is an extremely potent actin polymerization inhibitor, and finding appropriate dosages may prove to be problematic. Fortunately, there are a variety of drug vehicles available that could potentially increase the safety and efficacy of the agent. Specifically, cytochalasin D can be encapsulated in polyethylene liposomes to prevent immunological recognition until it reaches an intended tumor. Liposomes have been extensively examined for their abilities to improve drug delivery and have been used for a substantial variety of medicines, including cytotoxic agents, antibiotics, and antifungal agents [51]. In regards to cancer therapy, liposomes have been shown to reduce side effects and promote targeted tumor damage. This is due in part to their ability to substantially aggregate at tumor sites by leaking through pores and defects in tumor capillary endothelium [52]. Perhaps even more profound is the fact that polyethylene glycol (PEG) coated liposomes can remain unnoticed by opsonins that label foreign substances for macrophage phagocytosis. This in turn delays the subsequent clearance by immune cells, allowing sustained exposure to neoplastic cells, ultimately perpetuating increased efficacy [20, 53].
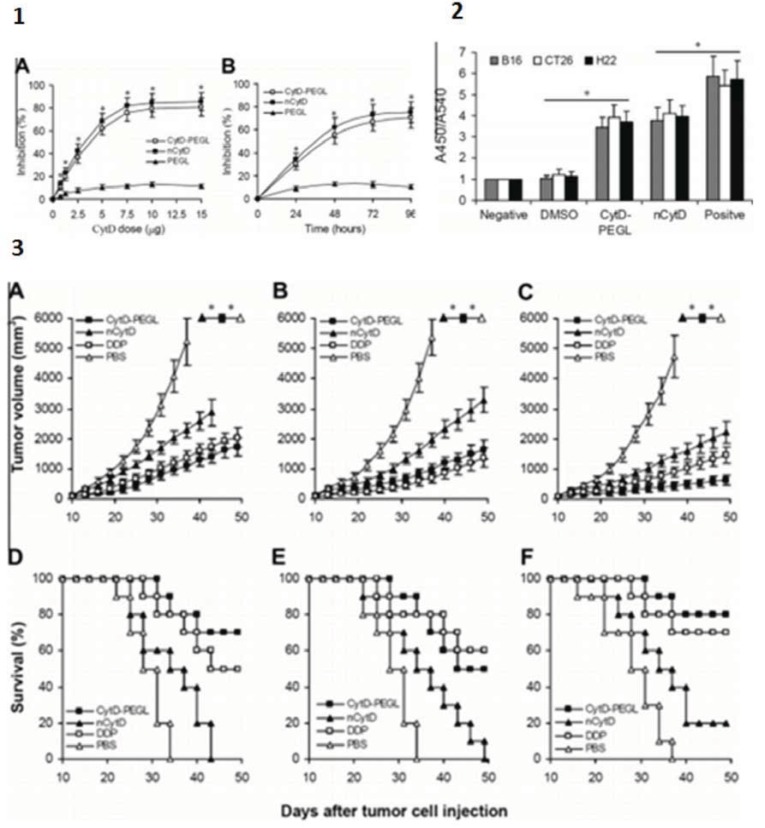
In a comparative study between pegylated cytochalasin D and its natural form, high-performance liquid chromatography (HPLC) of the biodistribution of the agents in tumor-bearing mice showed that pegylated cytochalasin D was readily dissolved in water for i.v. injection, while natural cytochalasin D proved to be problematic. Further, liposomal cytochalasin D accumulated in tumor tissues more efficiently than natural cytochalasin D. Even the half-life of liposomal cytochalasin D in the plasma (T1/2) was substantially longer than that of natural cytochalasin D (4 hours vs. 10 min). All of these benefits were observed in liposomal cytochalasin D, while still retaining the antitumor activity of the natural compound (Fig. 7). It is important to note that no significant side effects were observed in mice treated with liposomal cytochalasin D, suggesting that this enhanced form of cytochalasin D may be a viable chemotherapeutic agent.
Fig. (7).
Increased efficacy of cytochalasin D through liposome integration. 1) Inhibition of cell proliferation. A) B16 cells were treated with various doses of PEG-modified liposomal CytD (CytD-PEGL), natural cytochalasin D (nCytD) or empty pegylated liposomes (PEGL) (the same dose as the CytD-PEGL group) for 36 hours. B) B16 cells were treated with CytD PEGL (10 μg/ml, based on cytochalasin D containing PEGL about 30 μg/ml), nCytD (10 µg/ml), and PEGL (30 µg/ml). Cell viability was detected by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assays for various time intervals. All values are the mean values from three independent experiments; bars indicate SEM. *P < 0.05 or less. 2) Induction of cell apoptosis in vitro. Apoptosis induced by CytD-PEGL and nCytD almost exceeded 2/3 the level induced by the nuclease as a positive control. The graph depicts results of a representative experiment run in triplicate. Data are expressed as mean ± SEM. * P < 0.01. 3) Induction of antitumor activity in vivo. (A and D) B16, (B and E) CT26 and (C and F) H22 model mice were randomly divided into four groups (n = 10) and injected i.v. with CytD-PEGL (50 mg/kg once every 3 days for 21 days), nCytD (the same dose as CytD-PEGL), cisplatin (DDP) (5 mg/kg on days 1, 8, and 15 after initiation of CytD-PEGL treatment) and PBS, respectively. Graphs courtesy of [20].
CONCLUSION
Although there has yet to be a successfully developed microfilament-directed agent approved for use in the clinical setting, cytochalasins appear to show promise for improving current chemotherapeutic approaches. Specifically, cytochalasins B and D have demonstrated impressive in vitro and in vivo results in a diversity of cancer cell lines. These agents are predominantly known for their propensity to disrupt actin polymerization, but have a substantial variety of other mechanisms to damage tumorigenic growths. Perhaps the most provocative use of cytochalasins in chemotherapy would be to combine the agents with currently approved microtubule-directed agents such as epothilones, taxanes or vinca alkaloids. It is likely that the concomitant use of microfilament- and microtubule-directed agents would have a substantial synergist effect on disrupting cancer proliferation, as both have a unique target in the cell cycle. While microtubule-directed agents are most effective during mitosis as they disrupt the formation or degradation of spindle fibers, microfilament-directed agents exert a substantial influence on actin fibers used in cytokinesis. Malignant cells that managed to circumvent the microtubule-directed agents during mitosis would therefore also have to be resilient towards microfilament perturbation.
While the practical applications of cytochalasins have typically been limited to understanding cytoskeletal mechanisms, the microfilament-directed agents appear to have substantial promise as chemotherapeutic agents. However, the efficacy of this broad molecular family has been shown predominantly in only two congeners (cytochalasins B and D), and more research is required to determine if other cytochalasins have clinical potential. If cytochalasins and other microfilament-directed agents do prove to have clinical relevance, they could be used in combination with currently approved chemotherapeutic agents to increase the efficacy of established protocols. Before that, cytochalasins need further preclinical in vivo evaluation to properly assess their clinical potential. Only through such measures will enough data be acquired to definitively determine whether cytochalasins are capable of providing a novel avenue of chemotherapy.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The author would like to thank Syracuse University for their financial support of cytochalasin chemotherapy research as it has served a tremendous utility in advancing the therapeutic approach.
REFERENCES
- 1.Weinberg RA. 2nd ed. New York: Garland Science:; 2013. The Biology of Cancer. [Google Scholar]
- 2.Raguz S, Yagüe E. Resistance to chemotherapy: New treatments and novel insights into an old problem. Br. J. Cancer. 2008;99(3):387–391. doi: 10.1038/sj.bjc.6604510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trendowski M. The promise of sonodynamic therapy. Cancer Metastasis Rev. 2014;33(1):143–160. doi: 10.1007/s10555-013-9461-5. [DOI] [PubMed] [Google Scholar]
- 4.Van Goietsenoven G, Mathieu V, Andolfi A, Cimmino A, Lefranc F, Kiss R, Evidente A. In vitro growth inhibitory effects of cytochalasins and derivatives in cancer cells. Planta Med. 2011;77(7):711–717. doi: 10.1055/s-0030-1250523. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JA. Effects of cytochalasin and phalloidin on actin. J. Cell. Biol. 1987;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousquet PF, Paulsen LA, Fondy C, Lipski KM, Loucy KJ, Fondy TP. Effects of cytochalasin B in culture and in vivo on murine Madison 109 lung carcinoma and on B16 melanoma. Cancer Res. 1990;50(5):1431–1439. [PubMed] [Google Scholar]
- 7.Steiner MR, Altenburg B, Richards CS, Dudley JP, Medina D, Butel JS. Differential response of cultured mouse mammary cells of varying tumorigenicity to cytochalasin B. Cancer Res. 1978;38(9):2719–2721. [PubMed] [Google Scholar]
- 8.Medina D, Oborn CJ, Asch BB. Distinction between preneopastic and neoplastic mammary cell populations in vitro by cytochalasin B-induced multinucleation. Cancer Res. 1980;40(2):329–333. [PubMed] [Google Scholar]
- 9.Bogyo D, Fondy SR, Finster L, Fondy C, Patil S, Fondy TP. Cytochalasin-B-induced immunosuppression of murine allogeneic anti-tumor response and the effect of recombinant human interleukin-2. Cancer Immunol. Immunother. 1991;32(6):400–405. doi: 10.1007/BF01741335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Neill FJ. Selective destruction of cultured tumor cells with uncontrolled nuclear division by cytochalasin B and cytosine arabinoside. Cancer Res. 1975;35(11 Pt 1):3111–3115. [PubMed] [Google Scholar]
- 11.Kolber MA, Hill P. Vincristine potentiates cytochalasin B-induced DNA fragmentation in vitro. Cancer Chemother. Pharmacol. 1992;30(4):286–290. doi: 10.1007/BF00686297. [DOI] [PubMed] [Google Scholar]
- 12.Trendowski M, Yu G, Wong V, Acquafondata C, Christen T, Fondy TP. The real deal: Using cytochalasin B in sonodynamic therapy to preferentially damage leukemia cells. Anticancer Res. 2014;34(5):2195–2202. [PubMed] [Google Scholar]
- 13.Scherlach K, Boettger D, Remme N, Hertweck C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010;27(6):869–886. doi: 10.1039/b903913a. [DOI] [PubMed] [Google Scholar]
- 14.Binder M, Tamm C, Turner WB, Minato H. Nomenclature of a class of biologically active mould metabolites: The cytochalasins, phomins, and zygosporins. J. Chem. Soc. Perkin 1. 1973;11:1146–1147. [PubMed] [Google Scholar]
- 15.Binder M, Tamm C. The cytochalasans: A new class of biologically active microbial metabolites. Angew. Chem. Int. Ed. Engl. 1973;12(5):370–380. doi: 10.1002/anie.197303701. [DOI] [PubMed] [Google Scholar]
- 16.Fujii R, Minami A, Gomi K, Oikawa H. Biosynthetic assembly of cytochalasin backbone. Tetrahedron Lett. 2013;54(23):2999–3002. [Google Scholar]
- 17.Hayot C, Debeir O, Van Ham P, Van Damme M, Kiss R, Decaestecker C. Characterization of the activities of actin-affecting drugs on tumor cell migration. Toxicol. Appl. Pharmacol. 2006;211:30–40. doi: 10.1016/j.taap.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Stehn JR, Schevzov G, O'Neill GM, Gunning PW. Specialisation of the tropomyosin composition of actin filaments provides new potential targets for chemotherapy. Curr. Cancer. Drug Targets. 2006;6:245–256. doi: 10.2174/156800906776842948. [DOI] [PubMed] [Google Scholar]
- 19.Yahara I, Harada F, Sekita S, Yoshihira Y, Natori S. Correlation between effects of 24 different cytochalasins on cellular structures and cellular events and those on actin in vitro. J. Cell. Biol. 1982;92(1):69–78. doi: 10.1083/jcb.92.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang FY, Mei WL, Li YN, Tan GH, Dai HF, Guo JL, Wang H, Huang YH, Zhao HG, Zhou SL, Li L, Lin YY. The antitumour activities induced by pegylated liposomal cytochalasin D in murine models. Eur. J. Cancer. 2012;48(14):2260–2269. doi: 10.1016/j.ejca.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 21.MacLean-Fletcher S, Pollard TD. Mechanism of action of cytochalasin B on actin. Cell. 1980;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- 22.Theodoropoulos PA, Gravanis A, Tsapara A, Margioris AN, Papadogiorgaki E, Galanopoulos V, Stournaras C. Cytochalasin B may shorten actin filaments by a mechanism independent of barbed end capping. Biochem. Pharmacol. 1994;47(10):1875–1881. doi: 10.1016/0006-2952(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 23.Flanagan MD, Lin S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J. Biol. Chem. 1980;255(3):835–838. [PubMed] [Google Scholar]
- 24.Nishida E, Ohta Y, Sakai H. The regulation of actin polymerization by the 88K protein/actin complex and cytochalasin B. J. Biochem. 1983;94(5):1671–1683. [PubMed] [Google Scholar]
- 25.Kuhn JR, Pollard TD. Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys. J. 2005;88(2):1387–1402. doi: 10.1529/biophysj.104.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haidle AM, Myers AG. An enantioselective, modular, and general route to the cytochalasins: Synthesis of L-696,474 and cytochalasin B. Proc. Natl. Acad. Sci. USA. 2004;101(33):12048–12053. doi: 10.1073/pnas.0402111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little RD, Masjedizadeh MR, Wallquist O, Mcloughlin JI. The intramolecular michael reaction. Organic Reac. ions;1995, 47:315–552. [Google Scholar]
- 28.Brown SS, Spudich JA. Mechanism of action of cytochalasin: Evidence that it binds to actin filament ends. J. Cell. Biol. 1981;88(3):487–491. doi: 10.1083/jcb.88.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Painter RG, Whisenand J, McIntosh AT. Effects of cytochalasin B on actin and myosin association with particle binding sites in mouse macrophages: Implications with regard to the mechanism of action of the cytochalasins. J. Cell Biol. 1981;91(2 Pt 1):373–384. doi: 10.1083/jcb.91.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang JX, Janmey PA, Stossel TP, Ito T. Thiol oxidation of actin produces dimers that enhance the elasticity of the F-actin network. Biophys. J. 1999;76(4):2208–2215. doi: 10.1016/S0006-3495(99)77376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldridge DC, Turner WB. Structures of cytochalasins C and D. J. Chem. Soc. C. 1969:923–928. [Google Scholar]
- 32.Brown SS, Spudich JA. Cytochalasin inhibits the rate of elongation of actin filament fragments. J. Cell. Biol. 1979;83(3):657–662. doi: 10.1083/jcb.83.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang FY, Li YN, Mei WL, Dai HF, Zhou P, Tan GH. Cytochalasin D, a tropical fungal metabolite, inhibits CT26 tumor growth and angiogenesis. Asian Pac. J. Trop. Med. 2012;5(3):169–174. doi: 10.1016/S1995-7645(12)60019-4. [DOI] [PubMed] [Google Scholar]
- 34.Stracke ML, Soroush M, Liotta LA, Schiffmann E. Cytoskeletal agents inhibit motility and adherence of human tumor cells. Kidney Int. 1993;43(1):151–157. doi: 10.1038/ki.1993.25. [DOI] [PubMed] [Google Scholar]
- 35.Malecki JM, Bentke A, Ostrowska B, Laidler P, Cytochalasin D. LY294002 and olomoucine synergize in promoting death of melanoma cells through activation of caspase-3 and apoptosis. Melanoma Res. 2010;20(1):52–58. doi: 10.1097/CMR.0b013e328332f1e6. [DOI] [PubMed] [Google Scholar]
- 36.Lam WA, Rosenbluth MJ, Fletcher DA. Chemotherapy exposure increases leukemia cell stiffness. Blood. 2007;109(8):3505–3508. doi: 10.1182/blood-2006-08-043570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair UB, Joel PB, Wan Q, Lowey S, Rould MA, Trybus KM. Crystal structures of monomeric actin bound to cytochalasin D. J. Mol. Biol. 2008;384(4):848–864. doi: 10.1016/j.jmb.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schliwa M. Action of cytochalasin D on cytoskeletal networks. J. Cell. Biol. 1982;92(1):79–91. doi: 10.1083/jcb.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goddette DW, Frieden C. The binding of cytochalasin D to monomeric actin. Biochem. Biophys. Res. Commun. 1985;128:1087–1092. doi: 10.1016/0006-291x(85)91051-4. [DOI] [PubMed] [Google Scholar]
- 40.Goddette DW, Uberbacher EC, Bunick GJ, Frieden C. Formation of actin dimers as studied by small angle neutron scattering. J. Biol. Chem. 1986;261:2605–2609. [PubMed] [Google Scholar]
- 41.Sampath P, Pollard TD. Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry. 1991;30:1973–1980. doi: 10.1021/bi00221a034. [DOI] [PubMed] [Google Scholar]
- 42.Brenner SL, Korn ED. Stimulation of actin ATPase activity by cytochalasins provides evidence for a new species of monomeric actin. J. Biol. Chem. 1981;256:8663–8670. [PubMed] [Google Scholar]
- 43.Goddette DW, Frieden C. Actin polymerization: The mechanism of action of cytochalasin D. J. Biol. Chem. 1986;261(34):15974–15980. [PubMed] [Google Scholar]
- 44.Rubtsova SN, Kondratov RV, Kopnin PB, Chumakov PM, Kopnin BP, Vasiliev JM. Disruption of actin microfilaments by cytochalasin D leads to activation of p53. FEBS Lett. 1998;430(3):353–357. doi: 10.1016/s0014-5793(98)00692-9. [DOI] [PubMed] [Google Scholar]
- 45.Mani S, Macapinlac M , Jr, Goel S, Verdier-Pinard D, Fojo T, Rothenberg M, Colevas D. The clinical development of new mitotic inhibitors that stabilize the microtubule. Anticancer Drugs. 2004;15(6):553–558. doi: 10.1097/01.cad.0000131681.21637.b2. [DOI] [PubMed] [Google Scholar]
- 46.Mooberry SL. Strategies for the development of novel Taxol-like agents. Methods Mol. Med. 2007;137:289–302. doi: 10.1007/978-1-59745-442-1_20. [DOI] [PubMed] [Google Scholar]
- 47.Mollinedo F, Gajate C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis. 2003;8(5):413–450. doi: 10.1023/a:1025513106330. [DOI] [PubMed] [Google Scholar]
- 48.Bergstralh DT, Ting JP. Microtubule stabilizing agents: Their molecular signaling consequences and the potential for enhancement by drug combination. Cancer Treat. Rev. 2006;32(3):166–179. doi: 10.1016/j.ctrv.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006;4(4):221–333. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 50.Ono M. Molecular links between tumor angiogenesis and inflammation: Inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99(8):1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen TM, Cullis PR. Drug delivery systems: Entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 52.Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, Shah P, Khojasteh A, Nair MK, Hoelzer K, Tkaczuk K, Park YC, Lee LW. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J. Clin. Oncol. 2001;19(5):1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 53.Harris JM, Chess RB. Eκect of pegylation on pharmaceuticals. Nat. Rev. Drug. Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]