Abstract
Controlling the assembly of basic structural building blocks in a systematic and orderly fashion is an emerging issue in various areas of science and engineering such as physics, chemistry, material science, biological engineering, and electrical engineering. The self-assembly technique, among many other kinds of ordering techniques, has several unique advantages and the M13 bacteriophage can be utilized as part of this technique. The M13 bacteriophage (Phage) can easily be modified genetically and chemically to demonstrate specific functions. This allows for its use as a template to determine the homogeneous distribution and percolated network structures of inorganic nanostructures under ambient conditions. Inexpensive and environmentally friendly synthesis can be achieved by using the M13 bacteriophage as a novel functional building block. Here, we discuss recent advances in the application of M13 bacteriophage self-assembly structures and the future of this technology.
Keywords: Biocompatibility, genetic engineering, M-13 bacteriophage, self-assembly.
1. INTRODUCTION
Controlling the assembly of basic structural building blocks in a systematic and orderly fashion is an emerging issue in various areas of science and engineering including physics, chemistry, material science, biological engineering, and electrical engineering. The self-assembly technique can be used to develop functional nanostructures associated with organic-, inorganic-nanoparticles, copolymers, and proteins, and has several unique advantages of which the most important is its easy processing [1-5]. Functional nano-structures can be achieved at low cost since the solvent-based procedure utilized in their creation can proceed without additional manipulation of the physical, chemical, and vapor pressure of the solvent of the basic structural building blocks. The regular formation of highly packed building blocks increases device performance by increasing the density of building blocks per unit volume [6, 7].
The construction of building blocks using self-assembly techniques is inspired by biological systems [8-11]. Although mimicking the biological process of assembly is still in its infancy due to the uncertainty and complexity of the structure, using natural building blocks can be a solution for overcoming this obstacle. Of the natural materials including DNA, collagen, yeast, and viruses, viruses were recently highlighted as unique natural building blocks for the self-assembly process [12-15]. Furthermore, the M13 bacteriophage has attracted significant attention in the field because of its easy growth and handling properties. The use of biological materials as templates enables nontoxic synthesis at low cost.
The M13 bacteriophage (Phage) is composed of 2, 700 copies of the helically arranged pVIII major coat protein on its body. Five to seven copies each of pIII, pVI, pIX, and pVII are located at each end of the M13 phage. It is a virus nanofiber of approximately 880 nm in length and 6.6 nm in diameter and is safe in humans. It can be easily modified genetically and chemically to reveal designated functions. It can also be used as a template to reveal homogeneous distribution and the percolated network structure of inorganic nanostructures under ambient conditions [16-19]. Inexpensive and environmentally friendly synthesis is possible through M13 bacteriophage engineering. In this review, we introduce the recent advances in the application of the M13 bacteriophage self-assembly structure and discuss the future of this technology.
2. FABRICATION OF THE M13 BACTERIOPHAGE SELF-ASSEMBLY (M13SA) BUILDING BLOCK
The traditional preparation of M13SA is based on the layer-by-layer (LBL) assembly technique. By using linear polyethyleneimine and polyacrylic acid, this controlled molecular interaction leads to regular orientation of the bacteriophage [20-22]. Recently, novel techniques for two-dimensional (2-D) and three-dimensional (3-D) assembly of the M13 bacteriophage were introduced.
2.1. 2-D Structure Fabrication
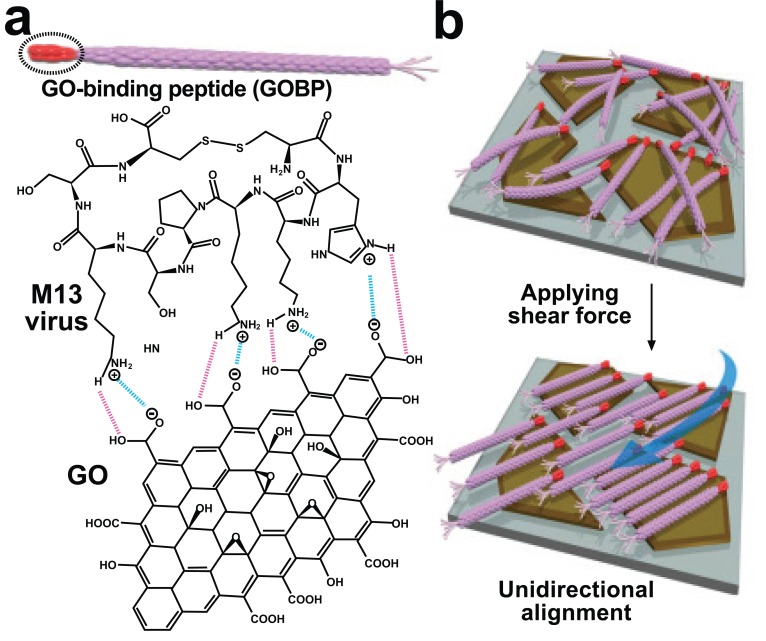
Low permeability has been a significant issue in 2-D fabrication because of the thickness of traditional membranes at the active area. Current research efforts are therefore primarily focused on making thin membranes that maintain separation efficiency with high permeability [23-26]. Interconnected basic structural building blocks can provide structural integrity and large-scale production with excellent permeability [27, 28]. However, a range in pore size distribution due to the non-uniformed integration of the building blocks limits the application of this system however using the M13 bacteriophage could be a solution to this problem. Lee et al. generated a unidirectionally aligned 2-D M13 bacteriophage structure on a graphene oxide (GO) surface using a simple fabrication method [29]. The genetically programmed pIII protein placed at the end of the M13 bacteriophage had specific binding interactions with the carboxylate-functionalized edges of the GO. The relatively neutral body of virus had a weaker connection with the GO surface, which was less chemically active than the end of the virus with the GO edge. The genetically modified pIII protein made strong salt-bridge type interactions with the GO edge via hydrogen bonding. The pIII protein, displayed with a disulfide-bond-constrained peptide made a strong cyclic structure with the edge of the GO and the aligned virus in the same direction owing to the energetic affinity of peptide geometry [30]. In contrast, the pVIII protein on the virus body demonstrated slight electrostatic repulsion due to the inherent negative charge. The fabricated M13 virus on the GO surface can be unidirectionally aligned by external shear force by water stream. This technique, involving orienting the direction of M13 by the dipping and sweeping procedure, was previously reported [31]. Fig. (1a) shows the cyclic binding interaction between the modified pIII protein and the functionalized edge of GO. Fig. (1b) shows a representative sketch of the alignment of the M13 virus by employing external shear force. Actual alignment of the M13 virus was observed using transmission electron microscopy (TEM). Through this approach, it is anticipated that highly orientated 2-D viral structures can be produced on both a large scale and at low cost, while maintaining high performance [30].
Fig. (1).
Unidirectional alignment of the M13 virus on the graphene oxide (GO) surface (2-D structure). (a) Interaction between the GObinding peptide with the functionalized edge of GO. (b) Alignment of the bacteriophage [29]. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
2.2. 3-D Structure Fabrication
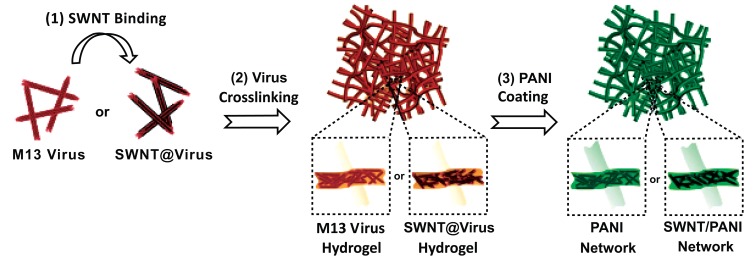
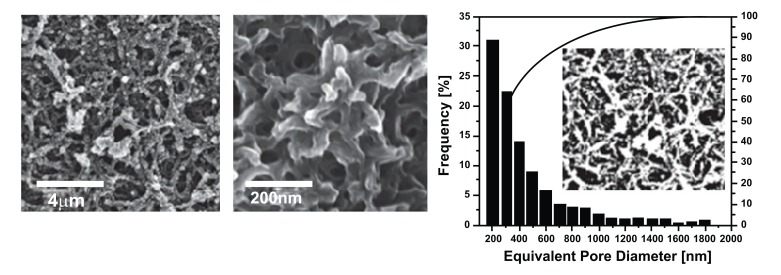
While 2-D bacteriophage formation is important for the application of thin structures such as ultrathin membranes, the 3-D structure of bacteriophage is also essential for engineering batteries [32], piezoelectric generators [33], and photovoltaics [34]. Chen et al. reported the generation of a polyaniline (PANI) and single walled carbon nanotube (SWNT) composite 3-D structure using the M13 bacteriophage as a template [35]. PANI is an excellent conductive polymer and exhibits enhanced performance when mixed with SWNT. PANI and SWNT composites are studied extensively through different assembly methods such as colloidal mixtures [36], electrostatic deposition [37], electropolymerization [38-40], radical polymerization in solution [41], and direct polymerization on SWNT supports [42-45]. However, poor dispersion and aggregation of SWNTs in composites limit the progress of these studies. Chemical modification of SWNTs [41, 43] by adding surfactants [42, 45] or binders [38, 39] was applied to overcome this hurdle. Although fabrication of composites was successful in this approach, heavy loading of SWNTs to achieve efficiency creates another obstacle for further application due to the high associated costs [38, 39]. The M13 bacteriophage was introduced as a template to generate a better PANI/SWNT composite because of its efficient dispersion ability. It was genetically engineered to bind SWNTs along the length of the bacteriophage, thereby allowing SWNTs to be clustered without aggregation. The M13/SWNTs composite was cross-linked to form a hydrogel type of scaffold. The continuous 3-D porous M13/SWNTs scaffold was successfully combined with PANI using a direct polymerization method. Fig. (2) shows a schematic illustration of the fabrication process used to create the M13/SWNTs/PANI composite. Fig. (3) shows the resulting 3-D porous film possessing 48.4% average porosity with an average pore size of 350 nm. The interconnected pore structure provides maximum interfacial contact with the electrolytes. A maximal specific capacity of 668 Fg-1 with 4.8% w/w of SWNT was achieved. This result represents one of the best cost effective solutions, since the aqueous-based synthetic process allows for reduced cost and easy large-scale production.
Fig. (2).
Fabrication of the M13/PANI composite with/without SWNTs [35]. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
Fig. (3).
Scanning electron microscope (SEM) images of PANI film with a virus-template and calculated pore size distribution [35]. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
3. VARIOUS APPLICATION FIELDS OF M13 BACTERIOPHAGE SELF-ASSEMBLY (M13SA)
3.1. Rechargeable battery
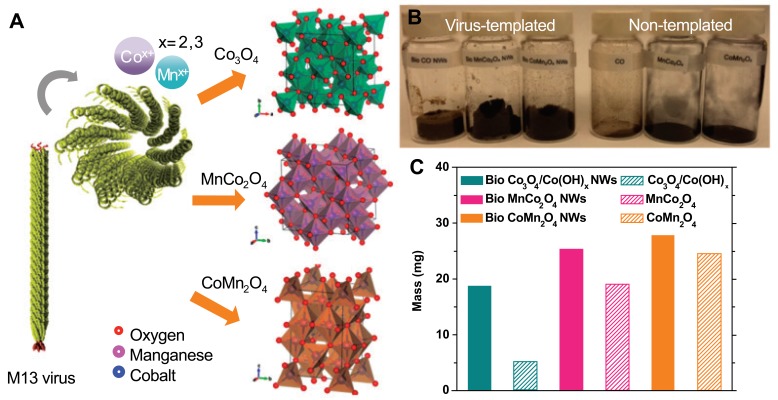
Despite the excellent energy density (~1, 000 Whkg-1) of nonaqueous Li-O2 batteries, there are currently several limitations that restrict their effectiveness, such as a large voltage hysteresis, low power, poor cycle life, and poor selectivity for oxygen molecules [46]. Single metal oxide cathodes were employed to overcome these limitations [47-50]. The single metal oxide cathode from the previous synthetic method produced random particle sizes, plates, and spheres [51-53]. Therefore, a systematic synthetic approach is required to compare different methodologies. Oh et al. reported metal oxide synthesis on a M13 bacteriophage template [54]. The viral templates provided homogeneous distribution due to the length and diameter of the M13 bacteriophage (approximately 50 nm in diameter and 880 nm in length). E3/E4 virus clones were used as templates for metal oxides due to their capacity for effective nucleation of single metal and bimetallic oxides [55-58]. The E3/E4 virus clone contains either EEAE (E3) or EEEE (E4), a sequence of triple or quadruple glutamates at pVIII. Fig. (4) shows a schematic procedure of metal oxide synthesis on the viral template and the subsequent results. Two-step synthesis produced highly porous nanowire on viral templates with a mass that was 3 times greater than nanowires without M13 bacteriophage templates. The high porosity and homogeneity of the final product led to 83, 78, and 78% Coulombic efficiency (Co3O4 (OH)x, MnCo2O4, and CoMn2O4, respectively) in the first galvanostatic cycling, tested at 400 mA g-1. However carbon electrodes demonstrated 51% efficiency. The smaller true electrode surface area of bio-metal oxides can suppress surface driven parasitic reactions. Therefore, bio-metal oxides possess higher oxygen evolution reaction and oxygen reduction reaction activity in media [59, 60]. The final products also showed superior discharge capacities, specific capacities, and longer cycle life than bare carbon electrodes.
Fig. (4).
(a) Schematic procedure of metal oxide synthesis on the viral template, (b), and (c) mass difference between samples [54]. Copyright ACS publication. Reproduced with permission.
3.2. Piezoelectric Nanogenerator
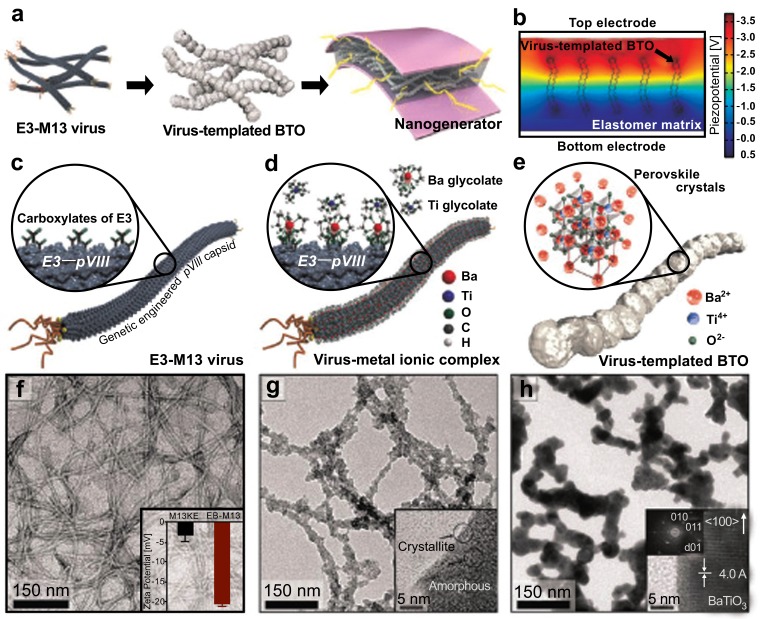
At the end of the fossil fuel-based energy era, renewable energy harvesting has become one of the most important issues [61]. The piezoelectric system is a highlighted source for energy due to the direct conversion from mechanical to electrical energy [62]. Owing to the purity issues with polymeric residues and surfactants for traditional perovskite-structured chemicals such as BaTiO3 (BTO), biological templates have attracted attention as a means of fabricating distinguished properties in ambient conditions [63, 64]. The genetically programmable M13 bacteriophage was introduced as a template in a recent study by Jeong et al. [65]. Their previous findings revealed that the homogeneous distribution of the piezoelectric nanostructure was the key point for high electrical output [66, 67]. They introduced Ba and Ti precursors into the glutamate trimer (E3)-M13 viral template to form virus template-BTO. A further calcination process at 1, 000°C resulted in high crystalline BTO. Fig. (5) shows a schematic diagram and TEM images of virus template-BTO formation. The overall procedure and simulated piezopotential are shown in Fig. (5a and 5b), respectively. The detailed scheme and TEM images of each step are shown in Fig. (5c-5h). After mixing virus template- BTO with polydimethylsioxane (PDMS), the product was coated in between a flexible indium tin oxide (ITO)-coated polyethylene terephthalate (PET) substrate. BTO is well dispersed in PDMS due to the virus template process. The final flexible device showed excellent performance of approximately 300 nA and 6 V over 21, 000 cycles at fast frequency.
Fig. (5).
Schematic diagram and TEM images of virus template-BTO formation [65]. Copyright ACS publication. Reproduced with permission.
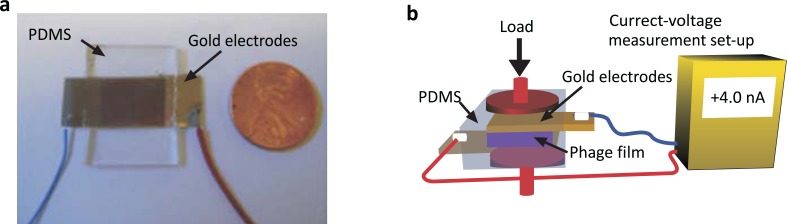
The M13 bacteriophage is however an excellent piezoelectric source. In nature, hierarchically ordered tissues such as bones, collagen fibrils, and peptide nanotubes exhibit piezoelectric properties [68-72]. The strong dipole moment of the M13 bacteriophage possesses intrinsic piezoelectric property as a result of the lack of inversion symmetry. Lee et al. reported a piezoelectric response of genetically engineered M13 bacteriophage film on substrates. Fig. (6) shows the device structure and experimental set up. The pVIII coat protein of M13 bacteriophage was engineered to have four negatively charged glutamates (4E; EEEE), resulting in a higher dipole moment than the wild type. The single phage generator exhibited a current of 6 nA and potential of 400 mV by mechanical strain which was higher in order of magnitude than the performance of natural collagen. The combination phage film demonstrated an even better performance [33]. It is anticipated that these devices could be applied in miniature-scale actuators.
Fig. (6).
Virus-based nanogenerator and experimental set up [33]. Copyright nature publishing group. Reproduced with permission.
3.3. Solar Cell
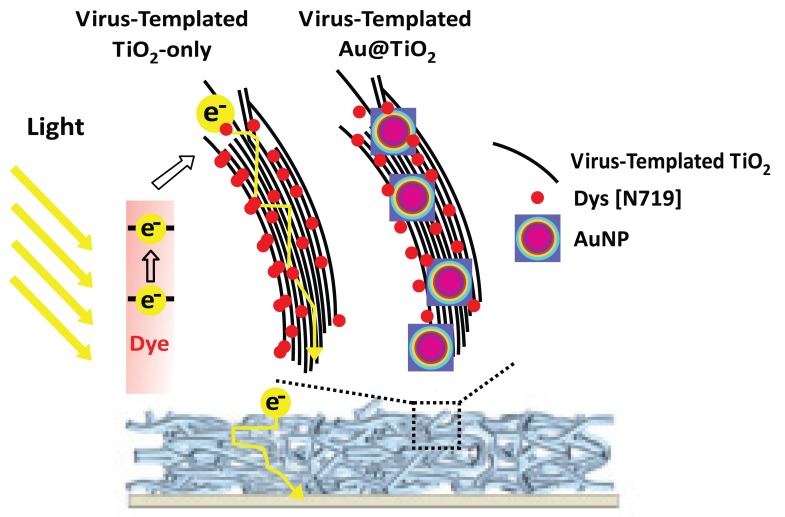
The increasingly growing requirement of green energy accelerates the development of solar energy technology. The dye-sensitized solar cell (DSSC) takes center stage on account of its relatively easy process and low cost [73]. The power conversion efficiency (PCE) of the solar cell is highly dependent on the ability of photoanodes to collect electrons [74]. TiO2 photoanodes constructed with high aspect ratio material such as nanorods, nanotubes, and nanofibers showed better performance than nanoparticles (NPs). However, a one-dimensional composite structure provides less surface area than three-dimensional structures for dye and light absorption. Therefore, a novel pathway is required to improve device performance. Belcher et al. suggested a 3-D viral network composed of TiO2 nanowire coated Au NPs to maximize localized surface plasmon resonance (LSPR) [75]. Fig. (7) shows a schematic of the mechanism of a TiO2/Au NPs structure with 3-D virus template enhancing light absorption and electron collection. Photoanodes with a 3-D plasmon-enhanced virus-template exhibited 13.72 mAcm-2 short-circuit current density and 8.46% PCE. Easy large-scale production can be anticipated with the use of the aqueous virus template process. This 3-D network morphology can also be applied to other energy-related studies.
Fig. (7).
Structures and mechanisms of TiO2 DSSC based on a virus-template with/without Au NPs [75]. Copyright ACS publication. Reproduced with permission.
3.4. Extracellular Matrix (ECM)
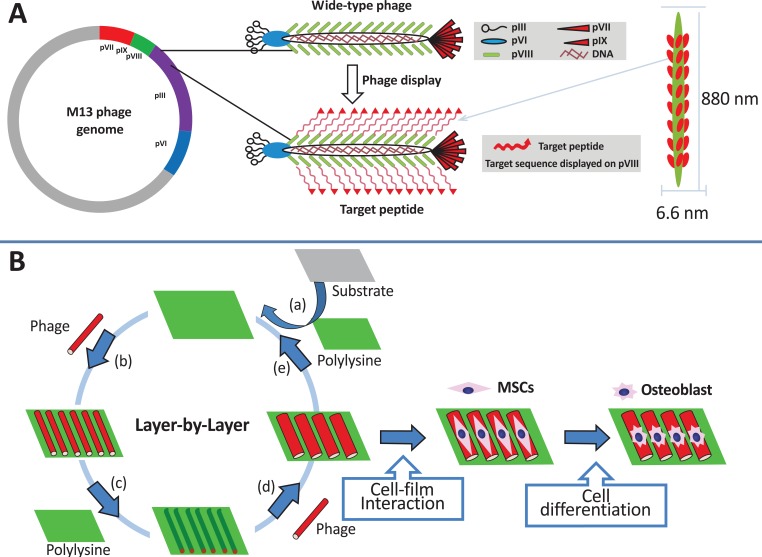
Modification of the physical and chemical properties of artificial extracellular matrix (ECM) is an important key to maintaining or controlling the proliferation or differentiation of stem cells [76-80]. Wang et al. reported that specific fibronectin peptides (RGD and PHSRN) displayed on the bacteriophage matrix with unique topology served as an ECM for differentiating mesenchymal stem cells (MSC) into osteoblasts [81]. Fig. (8) shows the process of bacteriophage fabrication with specific peptides as well as the ECM fabrication process using the LBL method. The phage-based film showed a unique rigid/groove nanostructure, and the ECM topology had a significant influence on cell behavior such as cell differentiation by cell shape elongation [82-84]. This demonstrates that the rigid/groove structure formed through self-assembly of the M13 bacteriophage significantly induced the elongation and parallel alignment of rat MSC. In addition, insertion of RGD and PHSRN peptides into the M13 bacteriophage increased cell adhesion and vitality [80]. The simultaneous functionality of ECM topology and peptides via the M13 bacteriophage could be a novel strategy in stem cell research.
Fig. (8).
M13 bacteriophage display with target peptide (a), and ECM fabrication process through LBL (b) [81]. Copyright nature publishing group. Reproduced with permission.
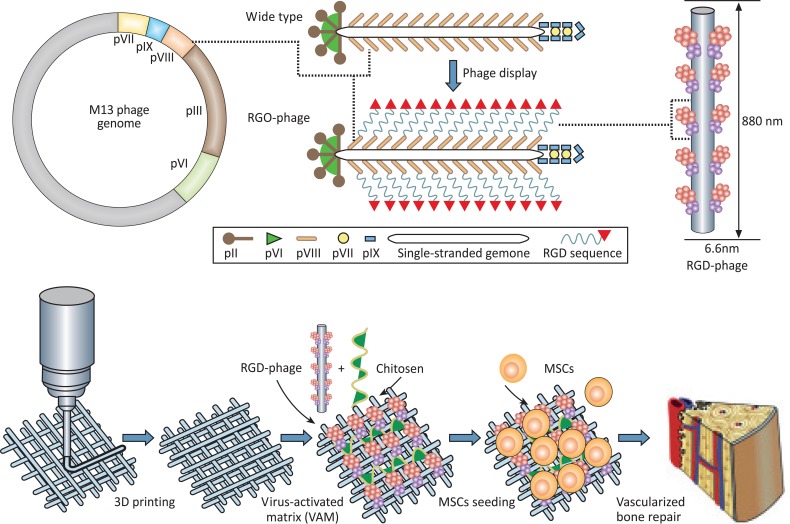
Wang et al. also suggested a 3-D structure of M13 bacteriophage-based ECM for vascularized osteogenesis of MSC [85]. It is known that new bone formation is promoted by proper angiogenesis [86]. Thus, to induce bone tissue angiogenesis in artificial ECM, RGD peptides that contain the blood vessel regeneration factor, αv group integrin, were introduced through the M13 bacteriophage. RGD peptides are highly unstable when mixed physically or chemically with ECM or media. Therefore, an RGD-displayed virus matrix could be a possible solution for maintaining RGD peptides in ECM for a long period in order to achieve successful bone regeneration in vivo. Fig. (9) demonstrates RGD display to the pVIII major coat protein on the M13 bacteriophage and illustrates scaffold fabrication. RGD-displayed virus base matrix was implanted into a rat radial bone defect. The angiogenic growth factor VEGF and wild type M13 were used as positive and negative controls, respectively, for comparison. Quantification of bone volume of the RGD-displayed virus base matrix sample showed competitive results in comparison to the normal bone tissue sample. The number of blood vessels formed was approximately 50% greater than the positive control. The RGD-displayed virus base matrix induced formation of vascularized bone without VEGF [85]. Based on these results, the application of various peptide-displayed bacteriophages in nanomedicine and regenerative medicine is highly anticipated.
Fig. (9).
RGD display to the pVIII major coat protein on M13 bacteriophage (top) and scaffold fabrication through 3-D priming with RGDphage and chitosan (bottom) [85]. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
3.5. Colorimetric Sensors
The majority of developing colorimetric sensors is inspired by nature through structurally colored biomaterials such as the butterfly wing, beetle exocuticle, cephalopod skin, mammalian skin, and avian skin and feathers [87-95].
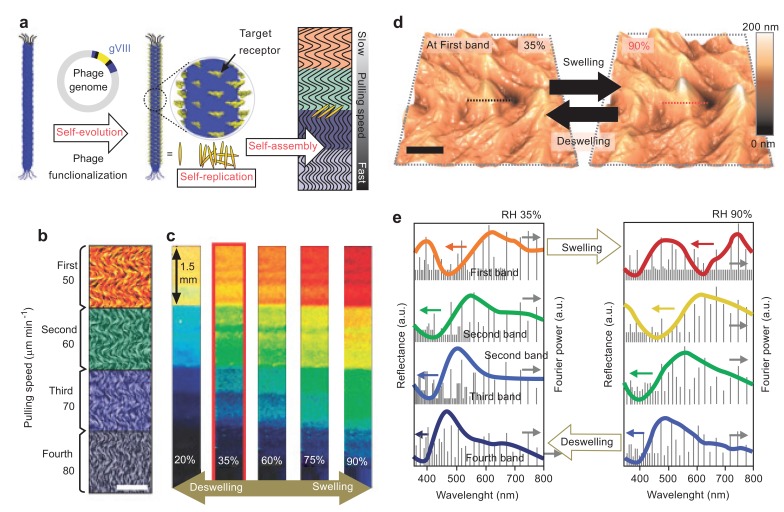
Due to the complex designs and synthetic process of incorporating analyte-responsive elements into current sensors (defined as type pattern recognition and also called ‘artificial nose’), an alternative approach is challenging but undoubtedly needed [96-99]. Oh et al. suggested a tunable M13 bacteriophage-based structure inspired by a turkey’s skin color change due to coherent scattering of light from collagen bundle structures [91, 100]. Fig. (10) demonstrates the fabrication of phage film by a simple pulling method and its associated color changes in response to changes in humidity. The hierarchically structure morphology of phage film can be generated by controlling pulling speed. The quasi-ordered phage fiber bundle exhibited angle-independent color change by polarity when exposing solvents to film.
Fig. (10).
Fabrication of phage film by the pulling method (a), changing colors by changes in humidity (b-e) [100]. Copyright nature publishing group. Reproduced with permission.
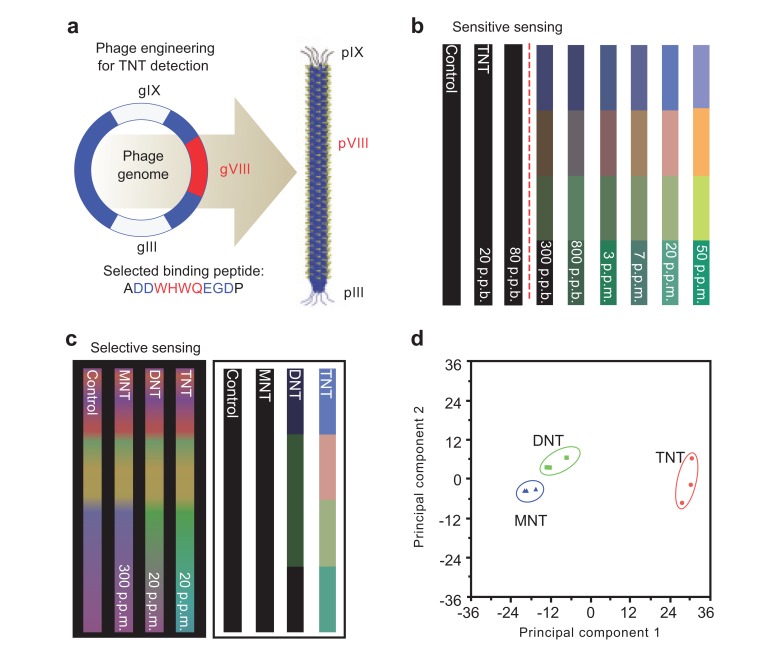
Due to the selectivity and sensitivity of the peptides displayed on phage surfaces, genetically engineered M13 bacteriophage can be applied to target-specific sensors. Oh et al. reported the creation of a TNT detective M13 bacteriophage color sensor [100]. M13 bacteriophage was genetically engineered to display a TNT-binding peptide on the major coat protein. Fig. (11) shows the phage display of identifying the peptide, gas phase TNT detection down to 300 ppb and the ability of selective sensing between TNT, DNT, and MNT by the identified peptide displaying phage. This demonstrates that the TNT sensitive virus film shows highly selective TNT sensing. Virus-based color sensors can be applied to simple solvent or humidity sensors, and they can also be used for the detection of specific chemicals and toxicants.
Fig. (11).
TNT specific-targeted phage display (a), gas phase TNT detection (b and c) and selective sensing between each TNT, DNT, and MNT (d) [100]. Copyright nature publishing group. Reproduced with permission.
4. CONCLUSION
The M13 bacteriophage has demonstrated its capacity to fabricate various noble structures with evident functionality. The characteristic self-assembly property, the aqueous mild synthesis condition, and easy functionality of the M13 bacteriophage structure allows for its significant contribution to a variety of scientific areas. Recently innovative 2-D and 3-D structures were successfully constructed using the M13 bacteriophage. Its unique properties have been exploited in several studies relating to energy storage and harvesting area such as rechargeable batteries, piezoelectric devices, and solar cells. The M13 bacteriophage was bioengineered with specific peptides and showed excellent biocompatibility and feasibility for use as a novel ECM. The structural color, one of the unique properties of the M13 bacteriophage, facilitated an innovative approach to fabricate color sensors. The M13 bacteriophage was also bioengineered with target chemical selective peptides to generate highly selective and sensitive color sensors. Furthermore, intensive studies are now actively in progress in the areas of drug delivery, gene transport, and bio imaging. A fundamental understanding of the novel assembly technique and functionality of the M13 bacteriophage is dependent on further extensive studies.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This research was supported by the Pioneer Research Center Program (2013M3C1A3065525) and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2013R1A1A3008484) and by the Korean government (NRF-2014S1A2A2027641).
REFERENCES
- 1.Whitesides GM, Mathias JP, Seto CT. Molecular self-assembly and nanochemistry a chemical strategy for the syn-thesis of nanostructures. Science. 1991;254:1312–1319. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]
- 2.Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 3.Capitol RM, Azevedo HS, Velichko YS, Mata A, Stupp SI. Self-assembly of large and small molecules into hierarchically ordered sacs and membranes. Science. 2008;319:1812–1816. doi: 10.1126/science.1154586. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 5.Ren X, Wan W, Jiang H, Hao J. Recent Progress in One-pot Synthesis of Fluorinated Building Blocks. Mini-Rev. Org. Chem. 2007;4:330–337. [Google Scholar]
- 6.Gimenez S, Lana-Villarreal T, Gomez R, Agouram S, Munoz-Sanjose V, Mora-Sero I. Determination of limiting factors of photovoltaic efficiency in quantum dot sensitized solar cells Correlation between cell performance and struc-tural properties. J. Appl. Phys. 2010;108:064310. [Google Scholar]
- 7.Karpan V, Khomyakov P.A, Starikov A A, Giovan-netti G., Zwierzycki M., Talanana M., Brocks G., van den Brink J., Kelly P.J. 10-Theoretical prediction of perfect spin filtering at interfaces between close-packed surfaces of Ni or Co and graphite or graphene. Phys. Rev. B. 2008;78:195419. [Google Scholar]
- 8.Sundar VC, Yablon AD, Grazul JL, Ilan M, and Aizen-berg J. Fibre-optical features of a glass sponge. Nature. 2003;424:899–900. doi: 10.1038/424899a. [DOI] [PubMed] [Google Scholar]
- 9.Espinosa HD, Juster AL, Latourte FJ, Loh OY, Gregoire D, Zavattieri PD. Tablet-level origin of toughening in abalone shells and translation to synthetic composite materials. Nat. Commun. 2011;2:173. doi: 10.1038/ncomms1172. [DOI] [PubMed] [Google Scholar]
- 10.Aizenberg J, Weaver JC, Thanawala MS, Sundar VC, Morse DE, Fratzl P. Skeleton of euplectella sp. Structural hierarchy from the nanoscale to the macroscale. Science. 2005;309:275. doi: 10.1126/science.1112255. [DOI] [PubMed] [Google Scholar]
- 11.Budzikiewicz H. Bacterial aromatic sulfonates - A bucherer reaction in natureκ. Mini Rev. Org. Chem. 2006;3(2):93–97. [Google Scholar]
- 12.Yan H, Park SH, Finkelstein G, Reif JH, LaBean TH. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science. 2003;301:1882–1884. doi: 10.1126/science.1089389. [DOI] [PubMed] [Google Scholar]
- 13.Mao CB, Solis DJ, Reiss BD, Kottmann ST, Sweeney RY, Hayhurst A, Georgiou G, Iverson B, Belcher AM. Virus-based toolkit for the directed synthesis of magnetic and semiconducting nanowires. Science. 2004;303:213–217. doi: 10.1126/science.1092740. [DOI] [PubMed] [Google Scholar]
- 14.Lee SW, Mao CB, Flynn CE, Belcher AM. Ordering of quantum dots using genetically engineered viruses. Science. 2002;296:892–895. doi: 10.1126/science.1068054. [DOI] [PubMed] [Google Scholar]
- 15.Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim BC, Inoue K, Mester T, Covalla SF, Johnson JP, Rotello VM, Tuomine MT, Lovley DR. Tunable metallic-like conductivity in microbial nanowire net-works. Nat. Nanotechnol. 2011;6:573–579. doi: 10.1038/nnano.2011.119. [DOI] [PubMed] [Google Scholar]
- 16.Chung WJ, Lee DY, Yoo SY. Chemical modulation of M13 bacteriophage and its functional opportunities for nano-medicine. Int. J. Nanomed. 2014;9:5825–5836. doi: 10.2147/IJN.S73883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YJ, Yi H, Kim WJ, Kang K, Yun DS, Strano MS, Ceder G, Belcher AM. Fabricating genetically engi-neered high-power lithium-ion batteries using multiple virus genes. Science. 2009;324:1051–1055. doi: 10.1126/science.1171541. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y, Kim J, Yun DS, Nam YS, Shao-Horn Y, Belch-er AM. Virus-templated Au and Au-Pt core-shell nanowires and their electrocatalytic activities for fuel cell applications. Energy Environ. Sci. 2012;5:8328–8334. doi: 10.1039/C2EE21156D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang XN, Yi HJ, Ham MH, Qi JF, Yun DS, Ladewski R, Strano MS, Hammond PT, Belcher AM. Virus-templated self-assembled single-walled carbon nano-tubes for highly efficient electron collection in photovoltaic devices. Nature. 2011;6:377–384. doi: 10.1038/nnano.2011.50. [DOI] [PubMed] [Google Scholar]
- 20.Tang Z, Wang Y, Podsiadlo P, Kotov N-A. Biomedical applications of layer-by-layer assembly From biomimetics to tissue engineering. Adv. Mater. 2006;18:3203. [Google Scholar]
- 21.Jiang C, Tsukruk V-V. Free standing nanostructures via layer-by-layer assembly. Adv. Mater. 2006;18:829. [Google Scholar]
- 22.Javey A, Woo S, Friedman RS, Yan H, Lieber CM. Layer-by-layer assembly of nanowires for three-dimensional, multifunctional electronics. Nano Lett. 2007;7:773. doi: 10.1021/nl063056l. [DOI] [PubMed] [Google Scholar]
- 23.Peng X, Jin J, Nakamura Y, Ohno T, Ichinose I. Ultrafast permeation of water through protein-based membranes. Nat. Nanotechnol. 2009;4:353. doi: 10.1038/nnano.2009.90. [DOI] [PubMed] [Google Scholar]
- 24.Karan S, Samitsu S, Peng X, Kurashima K, Ichinose I. Ultrafast viscous permeation of organic solvents through di-amond-like carbon nanosheets. Science. 2012;335:444. doi: 10.1126/science.1212101. [DOI] [PubMed] [Google Scholar]
- 25.Striemer CC, Gaborski TR, McGrath JL, Fauchet PM. Charge-and size-based separation of macromolecules using ultrathin silicon membranes. Nature. 2007;445:749. doi: 10.1038/nature05532. [DOI] [PubMed] [Google Scholar]
- 26.He J, Lin X-M, Chan H, Vukovic´ L, Kral P, Jaeger HM. Diffusion and filtration properties of self-assembled gold nanocrystal membranes. Nano Lett. 2011;11:2430. doi: 10.1021/nl200841a. [DOI] [PubMed] [Google Scholar]
- 27.Liang H-W, Wang L, Chen P-Y, Lin H-T, Chen L-F, He D, Yu S-H. Carbonaceous nanofiber membranes for se-lective filtration and separation of nanoparticles. Adv. Ma-ter. 2010;22:4691. doi: 10.1002/adma.201001863. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Zhang T, Ng J, Sun DD. High-performance multifunctional TiO2 nanowire ultrafiltration membrane with a hierarchical layer for water treatment. Adv. Funct. Ma-ter. 2009;19:3731. [Google Scholar]
- 29.Lee YM, Jung BY, Kim YH, Park AR, Han SS, Choe WS, Yoo PJ. Nanomesh-structured ultrathin membranes harnessing the unidirectional alignment of viruses on a graphene-oxide film. Adv. Mater. 2014;26:3899–3904. doi: 10.1002/adma.201305862. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Cote LJ, Kim F, Yuan W, Shull KR, Huang J. Graphene oxide sheets at interfaces. J. Am.Chem. Soc. 2010;132:8180. doi: 10.1021/ja102777p. [DOI] [PubMed] [Google Scholar]
- 31.Sawada T, Serizawa T. Immobilization of highly oriented filamentous viruses onto polymer substrates. J. Mater. Chem. B. 2013;1:149. doi: 10.1039/c2tb00066k. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Gerasopoulos K, Guo J, Brown A, Wang C, Ghodssi R, Culver JN. Virus-enabled silicon anode for lithium-ion batteries. ACS Nano. 2010;4:5366. doi: 10.1021/nn100963j. [DOI] [PubMed] [Google Scholar]
- 33.Lee BY, Zhang J, Zueger C, Chung W-J, Yoo SY, Wang E, Meyer J, Ramesh R, Lee S-W. Virus-based piezoelectric energy generation. Nat. Nanotechnol. 2012;7:351. doi: 10.1038/nnano.2012.69. [DOI] [PubMed] [Google Scholar]
- 34.Chen P-Y, Dang X.M., Klug T., Qi J., Dorval Courchesne N.-M., Burpo F.J., Fang N., Hammond P.T., Belcher A.M. Versatile three-dimensional virus-based template for dye-sensitized solar cells with improved electron transport and light harvesting. ACS Nano. 2013;7:6563. doi: 10.1021/nn4014164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen PY, Hyder MN, Mackanic D, Dorval Courchesne N-M, Qi J, Klug MT, Belcher AM, Hammond PT. Hy-drogels Assembly of viral hydrogels for three-dimensional conducting nanocomposites. Adv. Mater. 2014;26:5101–5107. doi: 10.1002/adma.201400828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Q, Nayfeh MH, Yau S-T. Brushed-on flexible supercapacitor sheets using a nanocomposite of polyaniline and carbon nanotubes. J. Power Sources. 2010;195:7480. [Google Scholar]
- 37.Hur J, Im K, Kim SW, Kim UJ, Lee J, Hwang S, Song J, Kim S, Hwang S, Park N. DNA hydrogel template carbon nanotube and polyaniline assembly and its applica-tions for electrochemical energy storage devices. J. Mater. Chem. A. 2013;1:14460. [Google Scholar]
- 38.Gupta V, Miura N. Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance superca-pacitors. Electrochim. Acta. 2006;52:1721. [Google Scholar]
- 39.Gupta V, Miura N. Influence of the microstructure on the supercapacitive behavior of polyaniline/single-wall carbon nanotube composites. J. Power Sources. 2006;157:616. [Google Scholar]
- 40.Zhou Y-K, He B-L, Zhou W-J, Li H-L. Preparation and electrochemistry of SWNT/PANI composite films for electrochemical capacitors. J. Electrochem. Soc. 2004;151:A1052. [Google Scholar]
- 41.Abdiryim T, Ubul A, Jamal R, Rahman A. Solid-State Synthesis of Polyaniline / Single-Walled Carbon Nanotubes A Comparative Study with Polyaniline/Multi-Walled Carbon Nanotubes. Materials. 2012;5:1219. [Google Scholar]
- 42.Ge J, Cheng G, Chen L. Transparent and flexible elec-trodes and supercapacitors using polyaniline / single-walled carbon nanotube composite thin films. Nanoscale. 2011;3:3084. doi: 10.1039/c1nr10424a. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Sun J, Gao L. A promising way to enhance the electrochemical behavior of flexible single-walled carbon nanotube/polyaniline composite films. J. Phys. Chem. C. 2010;114:1961,4–1962,0. [Google Scholar]
- 44.Niu Z, Luan P, Shao Q, Dong H, Li J, Chen J, Zhao D, Cai L, Zhou W, Chen X, Xie S. A “skeleton/skin” strategy for preparing ultrathin free-standing single-walled carbon nanotube/polyaniline films for high performance supercapacitor electrodes. Energy Environ. Sci. 2012;5:8726–8733. [Google Scholar]
- 45.Wang K, Zhao P, Zhou X, Wu H, Wei Z. Flexible super-capacitors based on cloth-supported clectrodes of conducting polymer nanowire array/SWCNT composites. J. Mater. Chem. 2011;21:16373–16378. [Google Scholar]
- 46.Chen Y.H., Freunberger S. A., Peng Z.Q, Fontaine O., Bruce P.G. Charging a Li-O2 battery using a redox mediator. Nat. Chem. 2013;5:489–494. doi: 10.1038/nchem.1646. [DOI] [PubMed] [Google Scholar]
- 47.Lee JH, Black R, Popov G, Pomerantseva E, Nan FH, Botton GA, Nazar LF. The role of vacancies and defects in Na0. 4MnO2 nanowire catalysts for lithium-oxygen batteries. Energy Environ. Sci. 2012;5:9558–9565. [Google Scholar]
- 48.Xu JJ, Xu D, Wang ZL, Wang HG, Zhang LL, Zhang XB. Angew. Chem. 2013;52:3887–3890. doi: 10.1002/anie.201210057. [DOI] [PubMed] [Google Scholar]
- 49.Ottakam Thotiyl MM, Freunberger SA, Peng Z, Chen Y, Liu Z, Bruce PG. A stable cathode for the aprotic Li-O2 battery. Nat. Mater. 2013;12:1050–1056. doi: 10.1038/nmat3737. [DOI] [PubMed] [Google Scholar]
- 50.Jung HG, Jeong YS, Park JB, Sun YK, Scrosati B, Lee YJ. Ruthenium-based electrocatalysts supported on re-duced graphene oxide for lithium-air batteries. ACS Nano. 2013;7:3532–3539. doi: 10.1021/nn400477d. [DOI] [PubMed] [Google Scholar]
- 51.Lavela P, Tirado JL, Vidal-Abarca C. Sol-gel preparation of cobalt manganese mixed oxides for their use as electrode materials in lithium cells. Electrochim. Acta. 2007;52:7986–7995. [Google Scholar]
- 52.Liu L, Yang Y Z. Shape-controlled synthesis of Mn-Co complex oxide nanostructures via a polyol-based precursor route and their catalytic properties. Superlattice Microst. 2013;54:26–38. [Google Scholar]
- 53.Li JF, Xiong SL, Li XW, Qian YT. A facile route to synthesize multiporous MnCo2O4 and CoMn2O4 spinel quasi-hollow spheres with improved lithium storage properties. Nanoscale. 2013;5:2045–2054. doi: 10.1039/c2nr33576j. [DOI] [PubMed] [Google Scholar]
- 54.Oh DH, Qi J, Han BH, Zhang G, Carney TJ, Ohmura J, Zhang Y, Shao-Horn Y, Belcher AM. M13 Virus-Directed Synthesis of Nanostructured Metal Oxides for Lithi-um-Oxygen Batteries. Nano Lett. 2014;14:4837–4845. doi: 10.1021/nl502078m. [DOI] [PubMed] [Google Scholar]
- 55.Rosant C, Avalle B, Larcher D, Dupont L, Friboulet A, Tarascon J M. Biosynthesis of Co3O4 electrode materials by peptide and phage engineering comprehension and future. Energy Environ. Sci. 2012;5:9936–9943. [Google Scholar]
- 56.Nam KT, Kim DW, Yoo PJ, Chiang CY, Meethong N, Hammond PT, Chiang YM, Belcher AM. Virus-Enabled Synthesis and Assembly of Nanowires for Lithium Ion Battery Electrodes. Science. 2006;312:885–888. doi: 10.1126/science.1122716. [DOI] [PubMed] [Google Scholar]
- 57.Dang XN, Yi HJ, Ham MH, Qi JF, Yun DS, Ladewski R, Strano MS, Hammond PT, Belcher AM. Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes. Nat. Nanotechnol. 2011;6:377–384. [Google Scholar]
- 58.Nuraje N, Dang XN, Qi JF, Allen MA, Lei Y, Belcher AM. Biotemplated Synthesis of Perovskite Nanomaterials for Solar Energy Conversion. Adv. Mater. 2012;24:2885–2889. doi: 10.1002/adma.201200114. [DOI] [PubMed] [Google Scholar]
- 59.Cheng FY, Shen JA, Peng B, Pan YD, Tao ZL, Chen J. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat. Chem. 2011;3:79–84. doi: 10.1038/nchem.931. [DOI] [PubMed] [Google Scholar]
- 60.Oh SH, Black R, Pomerantseva E, Lee JH, Nazar LF. Synthesis of a metallic mesoporous pyrochlore asa catalyst for lithium-O2 batteries. Nat. Chem. 2012;4:1004–1010. doi: 10.1038/nchem.1499. [DOI] [PubMed] [Google Scholar]
- 61.Aubrecht GJ. Energy Physical, Environmental and Social-Impact. Pearson Prentice Hall Upper Saddle River NJ. 2006 [Google Scholar]
- 62.Wang ZL. Nanogenerators for Self-Powered Devices andSystems. Georgia Institue of Technology Atlanta GA. 2011 [Google Scholar]
- 63.Sarikaya M, Tamerler C, Jen A.K.Y., Schulten K., Baneyx F. Molecular biomimetics nanotechnology through biology. Nat. Mater. 2003;2:577–585. doi: 10.1038/nmat964. [DOI] [PubMed] [Google Scholar]
- 64.Kim S, Park CB. Bio-Inspired Synthesis of Minerals for Energy, Environment, and Medicinal Applications. Adv. Funct. Mater. 2013;23:10–25. [Google Scholar]
- 65.Jeong CK, Kim IS, Park K-I, Oh MH, Paik HM, Hwang G-T, No KS, Nam YS, Lee KJ. ACS Nano. 2013;7(12):11016–11025. doi: 10.1021/nn404659d. [DOI] [PubMed] [Google Scholar]
- 66.Park KI, Lee M, Liu Y, Moon S, Hwang GT, Zhu G, Kim JE, Kim SO, Kim DK, Wang ZL, Lee KJ. Flexi-ble Nanocomposite Generator Made of BaTiO3 Nanoparticles and Graphitic Carbons. Adv. Mater. 2012;24:2999–3004. doi: 10.1002/adma.201200105. [DOI] [PubMed] [Google Scholar]
- 67.Park KI, Jeong CK, Ryu J, Hwang GT, Lee KJ. Flexi-ble and Large-Area Nanocomposite Generators Based on Lead Zirconate Titanate Particles and Carbon Nanotubes. Adv. Energy Mater. 2013;3:1539–1544. [Google Scholar]
- 68.Bassett C.A.L., Becker R.O. Generation of electric potentials by bone in response to mechanical stress. Science. 1962;137:1063–1064. doi: 10.1126/science.137.3535.1063. [DOI] [PubMed] [Google Scholar]
- 69.Fukada E. Piezoelectric properties of biological polymers. Q.; Rev. Biophys. 1983;16:59–87. doi: 10.1017/s0033583500004923. [DOI] [PubMed] [Google Scholar]
- 70.Minary-Jolandan M, Yu M-F. Nanomechanical Heterogeneity in the Gap and Overlap Regions of Type I Collagen Fibrils with Implications for Bone Heterogeneity. Biomacromolecules. 2009;10:2565–2570. doi: 10.1021/bm900519v. [DOI] [PubMed] [Google Scholar]
- 71.Farrar D, Ren K, Cheng D, Kim S, Moon W, Wilson WL, West JE, Yu SM. Permanent Polarity and Piezoelec-tricity of Electrospun κ-Helical Poly(κ-Amino Acid) Fibers. Adv. Mater. 2011;23:3954–3958. doi: 10.1002/adma.201101733. [DOI] [PubMed] [Google Scholar]
- 72.Kholkin A, Amdursky N, Bdikin I, Gazit E, Rosenman G. Strongpiezoelectricity in bioinspired peptide nanotubes. ACS Nano. 2010;4:610–614. doi: 10.1021/nn901327v. [DOI] [PubMed] [Google Scholar]
- 73.Yella A, Lee H-W, Tsao HN, Yi C, Chandiran AK, Nazeeruddin MK, Diau EW-G, Yeh C-Y, Zakeeruddin SM, Grätzel M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)-Based Redox Electrolyte Exceed 12 Percent Efficien-cy. Science. 2012;334:629–634. doi: 10.1126/science.1209688. [DOI] [PubMed] [Google Scholar]
- 74.Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H. Dye-Sensitized Solar Cells. Chem. Rev. 2010;110:6595–6663. doi: 10.1021/cr900356p. [DOI] [PubMed] [Google Scholar]
- 75.Chen P-Y, Dang X, Klug MT, Qi J, Dorval Courchesne N-M, Burpo F J, Fang N, Hammond PT, Belcher. AM. Versatile Three-Dimensional Virus-Based Template for Dye-Sensitized Solar Cells with Improved Electron Transport and Light Harvesting. ACS Nano. 2013;7(8):6563–6574. doi: 10.1021/nn4014164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oh S, Brammer KS, Li YS, Teng D, Engler AJ, Chien S, Jin S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chung W-J , et al. Biomimetic self-templating supramolecular structures. Nature. 2011;478:364–368. doi: 10.1038/nature10513. [DOI] [PubMed] [Google Scholar]
- 78.Merzlyak A, Indrakanti S, Lee S-W. Genetically Engineered Nanofiber-Like Viruses For Tissue Regenerating Materials. Nano Lett. 2009;9:846–852. doi: 10.1021/nl8036728. [DOI] [PubMed] [Google Scholar]
- 79.Zhu H , et al. Controlledgrowth and differentiation of MSCs on grooved films assembled from monodisperse biological nanofibers with genetically tunable surface chemistries. Bio-materials. 2011;32:4744–4752. doi: 10.1016/j.biomaterials.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kilian K.A., Bugarija B., Lahn B.T., Mrksich M. Geomet-ric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Wang Lin, Li X, Mao C. Virus activated artificial ECM induces the osteoblastic differentiation of mesenchymal stem cells without osteogenic supplements. Sci. Rep. 2013;3:1242. doi: 10.1038/srep01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Biggs M.J.P., Richards R.G., Gadegaard N., McMurray R.J., Affrossman S., Wilkinson C.D., Oreffo R.O., Dalby M.J. Interactions with nanoscale topography Adhesionquantifica-tion and signal transduction in cells of osteogenic and multi-potentlineage. J. Biomed. Mater. Res. Part A. 2009;91A:195–208. doi: 10.1002/jbm.a.32196. [DOI] [PubMed] [Google Scholar]
- 83.Biggs M.J.P., Richards R.G., McFarlane S., Wilkinson C.D., Oreffo R.O., Dalby M.J. Adhesion formation of primary human osteoblasts and thefunctional response of mesenchymal stem cells to 330 nm deep microgrooves. J. R. Soc. Interface. 2008;5:1231–1242. doi: 10.1098/rsif.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Yang M, Zhu Y, Wang L, Tomsia AP, Mao C. Phage Nanofibers Induce Vascularized Osteogenesis in 3D Printed Bone Scaffolds. Adv. Mater. 2014;26:4961–4966. doi: 10.1002/adma.201400154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Street J, Bao M, deGuzman L, Bunting S, Peale F.V., Ferrara N., Steinmetz H., Hoeffel J., Cleland J.L., Daugh-erty A., van Bruggen N., Redmond H.P., Carano R.A.D. Filvaroff,E.H. Proc. Natl. Acad. Sci. USA. 2002;99:9656. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kolle M, Salgard-Cunha PM, Scherer MR, Huang F, Vukusic P, Mahajan S, Baumberg JJ, Steiner U. Mimick-ing the colourful wing scale structure of the Papilioblumei butterκy. Nat. Nanotechnol. 2010;5:511–515. doi: 10.1038/nnano.2010.101. [DOI] [PubMed] [Google Scholar]
- 88.Vukusic P, Sambles JR. Photonic structures in biology. Nature. 2003;424:852–855. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]
- 89.Sharma V, Crne M, Park JO, Srinivasarao M. Structural origin ofcircularly polarized iridescence in jeweled beetles. Science. 2009;325:449–451. doi: 10.1126/science.1172051. [DOI] [PubMed] [Google Scholar]
- 90.Kramer RM, Crookes-Goodson WJ, Naik RR. The self-organizingproperties of squid reκectin protein. Nat. Ma-ter. 2007;6:533–538. doi: 10.1038/nmat1930. [DOI] [PubMed] [Google Scholar]
- 91.Prum RO, Torres RH. Structural colouration of mammalian skin: convergent evolution of coherently scattering dermal col-lagen arrays. J. Exp. Biol. 2004;207:2157–2172. doi: 10.1242/jeb.00989. [DOI] [PubMed] [Google Scholar]
- 92.Prum RO, Torres R. Structural colouration of avian skin convergentevolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 2003;206:2409–2429. doi: 10.1242/jeb.00431. [DOI] [PubMed] [Google Scholar]
- 93.Kinoshita S, Yoshioka S. Structural colors in nature the role ofregularity and irregularity in the structure. Chem-PhysChem. 2005;6:1442–1459. doi: 10.1002/cphc.200500007. [DOI] [PubMed] [Google Scholar]
- 94.Noh H, Liew SF, Saranathan V, Mochrie SG, Prum RO, Dufresne ER, Cao H. How noniridescent colors are generated by quasi-orderedstructures of bird feathers. Adv. Mater. 2010;22:2871–2880. doi: 10.1002/adma.200903699. [DOI] [PubMed] [Google Scholar]
- 95.Forster JD, Noh H, Liew SF, Saranathan V, Schreck CF, Yang L, Park JG, Prum RO, Mochrie SG, O'Hern CS, Cao H, Dufresne ER. Biomimetic isotropic nanostruc-tures for structuralcoloration. Adv. Mater. 2010;22:2939–2944. doi: 10.1002/adma.200903693. [DOI] [PubMed] [Google Scholar]
- 96.Holtz JH, Asher SA. Polymerized colloidal crystal hydro-gel κlms asintelligent chemical sensing materials. Nature. 1997;389:829–832. doi: 10.1038/39834. [DOI] [PubMed] [Google Scholar]
- 97.Burgess I.B., Koay N., Raymond K.P., Kolle M., Lon?ar M., Aizenberg J. Wetting in color colorimetric differentiation of organicliquids with high selectivity. ACS Nano. 2012;6:1427–1437. doi: 10.1021/nn204220c. [DOI] [PubMed] [Google Scholar]
- 98.Lim SH, Feng L, Kemling JW, Musto CJ, Suslick KS. An opto-electronic nose for the detection of toxic gases. Nat. Chem. 2009;1:562–567. doi: 10.1038/nchem.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mao S, Liu K, Lu F, Du L. Colorimetric Sensors Based on Hydrogen-bond-induced κ-delocalization and/or Anion-triggered Deprotonation. Mini-Rev. Org. Chem. 2010;7(3):221–229. [Google Scholar]
- 100.Oh J-W, Chung W-J, Heo K, Jin H-E, Lee BY, Wang E, Zueger C, Wong W, Meyer J, Kim C, Lee S-Y, Kim W-G, Zemla M, Auer M, Hexemer A, Lee S-W. Biomi-metic virus-based colorimetric sensors. Nat. Chem. 2014;5:3043. doi: 10.1038/ncomms4043. [DOI] [PubMed] [Google Scholar]