Abstract
Purpose
To prospectively determine the prognostic significance of the TEL-AML1 fusion in children with acute lymphoblastic leukemia (ALL).
Patients and Methods
TEL gene status was determined for 926 patients with B-precursor ALL enrolled on the Pediatric Oncology Group ALinC 16 trials and patients were followed for a median time of eight years.
Results
Rearrangements of the TEL gene were detected in 244 (26%) patients. The estimated 5-year event-free survival rate (± SE) for patients with TEL rearrangements was 86% ± 2%, compared with 72% ± 2% for those with germline TEL (p<0.0001). TEL rearrangements were associated with a superior outcome among patients with standard-risk ALL, high-risk ALL, and rapid early responses to therapy. In a multivariate analysis that included risk group, sex, and day 15 marrow status, TEL status was an independent predictor of outcome (p=0.0002).
Conclusion
We conclude that TEL gene status should be incorporated into risk classification schemes and suggest that patients who have the TEL-AML1 fusion and rapid early responses to therapy should be treated with antimetabolite-based therapy designed to maintain their high cure rates and avoid late effects.
Introduction
The TEL-AML1 gene fusion, created by the t(12;21)(p12;q22), is the most common translocation in childhood acute lymphoblastic leukemia (ALL), occurring in about 25% of B-precursor cases.1-3 Soon after the fusion was cloned in 1995, several retrospective studies suggested that it was associated with an excellent outcome.4-6 We reported that among patients with ALL treated at St. Jude Children's Research Hospital, TEL gene rearrangements, representing the TEL-AML1 fusion, were associated with a 5-year event-free survival (EFS) rate >90%.4 In that study, the favorable impact of TEL rearrangements was independent of age and leukocyte count. Similarly, we demonstrated that TEL rearrangements were associated with an improved survival rate of patients treated on a Pediatric Oncology Group (POG) trial.5 The TEL-AML1 fusion was also associated with an outstanding outcome among patients treated on a Dana-Farber Cancer Institute (DFCI) trial.6 Investigators from St. Jude and from the DFCI also demonstrated a very low frequency of TEL-AML1 in patients with relapsed ALL, consistent with the excellent outcome of patients with this translocation.7,8
Despite the plethora of studies that suggest that TEL-AML1 is an independent favorable predictor of outcome and should be used in risk classification, questions remain regarding its true impact.9 For example, some reports demonstrated a high incidence (20% to 24%) of the TEL-AML1 fusion in relapsed cases of ALL, thereby casting doubt as to the prognostic significance of this genetic alteration.10,11 The retrospective nature of many of the studies and the short follow-up of others further suggested that a large prospective study should be performed. Hence, in 1996, investigators from the DFCI and from the POG simultaneously undertook prospective studies to determine the prognostic significance of the TEL-AML1 fusion in childhood ALL. The DFCI recently reported that TEL-AML1 is associated with an excellent outcome (5-yr EFS=89%), but that TEL-AML1 status is not an independent predictor of outcome.12 We report here the results of the POG study.
Patients, materials, and methods
Patients and samples
Bone marrow samples were received for TEL testing from 1150 consecutive patients who were eligible for the POG ALinC 16 (9201, 9605, and 9406) treatment protocols for B-precursor ALL from December 29, 1995 to June 16, 1998.13,14 These patients constituted a subset of all patients who had enrolled on these trials from November 15, 1994 to November 15, 1999. Definitive results regarding TEL status were obtained in 926 patients, who are the subject of this report. At the time of diagnosis, patients were assigned to induction treatment based on National Cancer Institute (NCI) risk status:15 patients with standard-risk ALL received a 3-drug induction regimen (prednisone, vincristine, l-asparaginase), and patients with high-risk ALL received the same 3 drugs plus daunorubicin. At the completion of induction therapy, patients were further stratified into 3 risk groups for postinduction therapy. Patients with low-risk disease (POG 9201) were those with NCI standard-risk features and either simultaneous trisomy of chromosomes 4 and 10, or, in the absence of informative cytogenetics, a DNA index >1.16. Patients with standard-risk disease (POG 9605) included those with NCI standard-risk features who lacked the low-risk characteristics and those with NCI high-risk features and both trisomies 4 and 10 or a DNA index >1.16. Patients with poor-risk ALL (POG 9406) included any patients with CNS3 status, a t(1;19), t(9;22), or t(4;11) and patients with NCI high-risk features who did not have trisomies 4 and 10 or a DNA index >1.16.
Post induction therapy on POG 9201 consisted of consolidation therapy with intermediate dose methotrexate (1 gm/m2) given every 3 weeks for 6 doses, and daily mercaptopurine. This was followed by continuation therapy with weekly standard dose methotrexate and daily mercaptopurine. Vincristine/prednisone pulses were given throughout continuation therapy and triple intrathecal therapy was used for central nervous system prophylaxis.13 Patients with standard-risk ALL (POG 9605) received the same consolidation therapy as the low risk patients, but were randomly assigned, in a 2 × 2 factorial design, to receive divided dose oral methotrexate every other week versus standard weekly dose methotrexate during the first 6 months of continuation therapy and oral mercaptopurine in a single-dose versus divided dose fashion throughout continuation. Patients on 9201 and 9605 received no anthracyclines, epipodophyllotoxins, alkylating agents or cranial irradiation.
Patients with poor risk ALL (POG 9406) received multi-agent intensified consolidation and continuation using rotating agents. The standard arm of 9406 used rotating courses of methotrexate (1 gm/m2) with intravenous mercaptopurine (1 gm/m2), followed by teniposide with standard dose cytarabine, followed by daunorubicin, standard dose cytarabine, vincristine, prednisone, and l-asparaginase. On POG 9406, patients were randomly assigned in a 2 × 2 factorial design to determine whether 2.5 gm/m2 methotrexate was more effective than 1.0 gm/m2 and to determine whether substitution of high dose cytarabine with PEG asparaginase for the teniposide with standard dose cytarabine would give equally good or better EFS, with less chance of causing second malignancy.
All three studies (POG 9201, 9605, and 9406) used triple intrathecal therapy until July 29, 1999, when the protocols were amended to give only intrathecal methotrexate.
Genomic DNA was extracted, and the TEL gene status was analyzed as previously described.2 Written informed consent was obtained from patients or their legal guardians, and all studies were approved by the institutional review board at each collaborating site.
Study design
At the onset of the ALinC 16 studies, the POG accrued approximately 600 patients with B-precursor ALL each year. The expected proportion of cases with rearranged TEL in each group was as follows: low risk, 0% with rearranged TEL; standard risk, 40% with rearranged TEL; and poor risk, 17% with rearranged TEL. None or very few cases with rearranged TEL were expected among patients with low-risk ALL because of the lack of overlap between cases with hyperdiploidy (DNA index > 1.16) and cases with TEL rearrangements.4 This prospective study required the submission of diagnostic marrow samples from each patient who agreed to participate. Samples were received for TEL testing from 1150 patients enrolled on these studies.
Statistical Methods
The Fisher exact test was used to compare the characteristics between patients who were studied and those who were not on each of the 3 clinical trials. EFS estimates were obtained by using the Kaplan-Meier method,16 and standard errors of the estimates were calculated by the method of Peto and Peto.17 Time to event was calculated as the time from study entry to first event (relapse, secondary malignancy, or death) or date of last contact. The log-rank test was used for comparison of survival curves between various groups. Multivariate analysis was conducted by using Cox proportional hazards regression.18 Categorical data were compared between groups by using the chi-square test. All tests were conducted at a significance level of 5%.
Results
Patient characteristics
A total of 2676 patients were enrolled on the POG ALinC 16 therapeutic trials for B-precursor ALL: 692 patients with low-risk ALL were enrolled on POG 9201; 1077 patients with standard-risk ALL were enrolled on POG 9605; and 907 patients with poor-risk ALL were enrolled on POG 9406. Of these, 926 (152 on POG 9201, 470 on POG 9605, and 304 on POG 9406) had adequate diagnostic bone marrow samples submitted for TEL gene analysis. The Fisher exact test revealed only minor differences in characteristics between patients who were and were not studied for TEL status on each trial (data not shown). TEL gene rearrangements were present in 244 (26%) of 926 cases analyzed, including 7 of 152 (5%) treated on POG 9201, 173 of 470 (37%) treated on POG 9605, and 64 of 304 (21%) treated on POG 9406 (Table 1).
Table 1.
Patient Characteristics in Relation to TEL Status
| Characteristic | Rearranged TEL | Germline TEL |
|---|---|---|
| Median age (years) | 4.4 (1.4-19.7) | 5.1 (1.1-21.1) |
| Age group | ||
| < 10 years | 222 (29%) | 531 (71%) |
| ≥ 10 years | 22 (13%) | 151 (87%) |
| Median WBC (× 109/L) | 12 (1-219) | 12 (1-848) |
| WBC group | ||
| < 50 × 109/L | 198 (27%) | 549 (73%) |
| ≥ 50 × 109/L | 46 (26%) | 133 (74%) |
| NCI risk group | ||
| Standard | 178 (29%) | 435 (71%) |
| High | 66 (21%) | 247 (79%) |
| Sex | ||
| Male | 123 (24%) | 382 (76%) |
| Female | 121 (29%) | 300 (71%) |
| Race | ||
| White | 184 (29%) | 447 (71%) |
| Black | 12 (16%) | 62 (84%) |
| Hispanic | 32 (21%) | 124 (79%) |
| Other | 16 (25%) | 49 (75%) |
| CNS Status | ||
| Negative | 232 (95.1%) | 586 (86.0%) |
| Positive | 12 (4.9%) | 96 (14.0%) |
| Day-15 marrow status | ||
| M1 | 214 (27%) | 566 (73%) |
| M2/M3 | 14 (18%) | 63 (82%) |
| Treatment study | ||
| 9201 | 7 (5%) | 145 (95%) |
| 9605 | 173 (37%) | 297 (63%) |
| 9406 | 64 (21%) | 240 (79%) |
| Median follow-up (yrs) | 8 (0.0-10.0) | 7.7 (0.0-10.0) |
| Total | 244 (26%) | 682 (74%) |
TEL-rearranged cases had a median age of 4.4 years (range, 1.4 to 19.7 years) and a median presenting leukocyte count of 12 × 109/L (range, 1 to 219 × 109/L), whereas those with germline TEL had a median age of 5.0 years (range, 1.1 to 21.1 years) and a median presenting leukocyte count of 12 × 109/L (range, 1 to 848 × 109/L). TEL rearrangements were detected in 29% of patients in the standard-risk group and in 21% of patients in the high-risk group (Table 1).15
Only 27% of patients with TEL rearrangements and 36% of patients with germline TEL (p=0.0094) had high-risk ALL. Of the 22 patients who had TEL rearrangements and were at least 10 years old, 20 (91%) had presenting leukocyte counts < 50 × 109/L.
Impact of TEL rearrangements
At a median follow-up of 7.8 years, the estimated 5-year EFS rate (± SE) for patients with TEL rearrangements was 86% ± 2%, compared with 72% ± 2% for those with germline TEL (Figure 1A, p<0.0001). TEL rearrangements were associated with a favorable outcome for patients with standard-risk ALL (5-yr EFS, 88% ± 3% vs. 78% ± 2%; p=0.0011; Figure 1B) and for patients with high-risk ALL (5-yr EFS, 81% ± 5% vs. 62% ± 3%; p=0.0032; Figure 1C). The small group of patients 10 years of age or older with TEL rearrangements also appeared to have a good outcome: their 5-year EFS estimate was 85% ± 8%, and 21 of 22 patients are alive. Finally, late events did not appear to constitute a significant problem in patients with rearrangements of TEL. In fact, only 4 (2%) of 198 patients with rearranged TEL genes and sufficient follow-up suffered events more than 5 years after the time of diagnosis; in comparison, 19 (4%) of 467 of patients with germline TEL genes experienced late events.
Figure 1. Event-free survival (EFS) estimates of patients treated on ALinC 16 and their relation to TEL gene status.
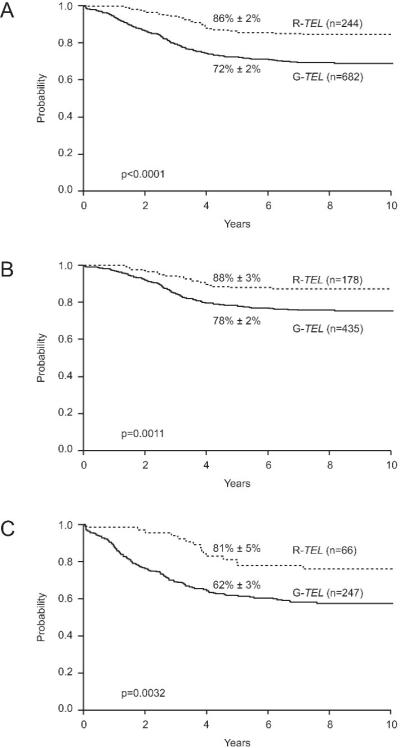
(A) EFS estimates of patients with TEL rearrangements (R-TEL) compared with those of patients with germline TEL (G-TEL). (B) EFS estimates of patients with NCI standard-risk ALL shown in relation to TEL status. (C) EFS estimates of patients with NCI high-risk ALL shown in relation to TEL status.
Because we previously demonstrated that patients with trisomies 4 and 10 have an excellent outcome,19 we compared the outcome of cases with rearranged TEL with that of cases with trisomies 4 and 10 and cases with neither feature (Figure 2). Patients with TEL rearrangements had an outcome similar to that of patients with trisomies 4 and 10 (5-yr EFS, 86% ± 2% vs. 82% ± 3%; p=0.18), and both groups fared significantly better than did the group of patients with neither feature (5-yr EFS, 69% ± 2%; overall p<0.0001).
Figure 2.
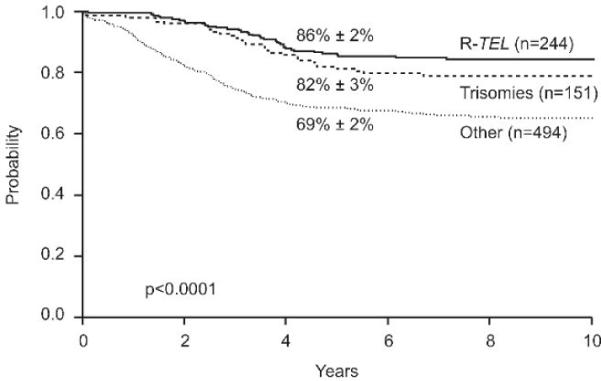
Event-free survival (EFS) estimates of patients with TEL rearrangements compared with those of patients with trisomies 4 and 10 and with those of patients with neither feature (Other).
Impact of early response to therapy
Early response to therapy is one of the best predictors of outcome in childhood ALL.20,21 We therefore studied the association between day 15 bone marrow status and TEL gene rearrangements. Day 15 marrow status was available for 857 of the patients with known TEL status. Of the 228 patients with a TEL rearrangement, 214 (94%) experienced rapid early responses, defined as M1 marrow at day 15, and 566 (90%) of 629 patients with germline TEL genes experienced such responses (p= 0.104). As expected, rapid early responses, compared with slow early responses (M2/M3 marrow at day 15), were associated with significantly improved outcomes for patients with rearranged TEL genes (5-yr EFS, 87% ± 2% vs. 71% ± 12%; p=0.043) and for those with germline TEL genes (5-yr EFS, 75% ± 2% vs. 56% ± 6%, p<0.0001). In addition, TEL rearrangements were associated with a superior outcome in patients with rapid early responses (5-yr EFS, 87% ± 2% vs. 75% ± 2%; p=0.0001; Figure 3A), but the difference in outcome did not attain statistical significance among those with slow early responses (5-yr EFS, 71% ± 12% vs. 56% ± 6%; p=0.16; Figure 3B).
Figure 3.
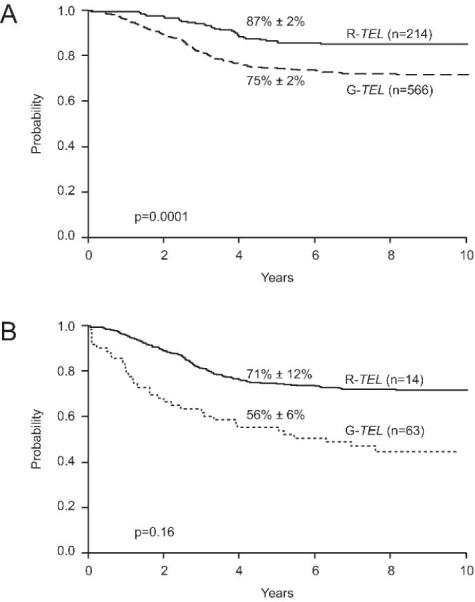
Event-free survival (EFS) estimates of patients with TEL rearrangements compared with those of patients with germline TEL and either rapid early responses to therapy (A) or slow early responses (B).
Cox proportional hazards regression was used to determine the effect of TEL status on EFS. After adjustment for NCI risk group (standard risk versus high risk), sex, and day 15 marrow status (M1 versus M2/M3), TEL status was an independent predictor of outcome (HR=0.51; p=0.0002; Table 2).
Table 2.
Results of the Multivariate Cox Regression Analysis
| Variable | Hazard Ratio | 95% CI for Hazard Ratio | P value |
|---|---|---|---|
| M2/M3 marrow on day 15 | 2.432 | (1.722, 3.437) | <0.0001 |
| Male Sex | 2.105 | (1.582, 2.798) | <0.0001 |
| NCI high-risk ALL | 1.954 | (1.504, 2.540) | <0.0001 |
| No TEL rearrangement | 1.972 | (1.379, 2.815) | 0.0002 |
Discussion
In this prospective study of more than 900 patients with ALL treated on the POG ALinC 16 study with approximately 8 years of follow-up, we demonstrated that TEL status is a highly significant and independent predictor of outcome of patients overall and of patients with standard-risk and high-risk ALL. This finding is in contrast to that in a recent report from the DFCI, which found that NCI risk group, but not TEL status, was an independent predictor of outcome. In the DFCI study, TEL status was not significantly associated with better EFS within the standard-risk or the high-risk group; however, the power to detect a statistically significant difference was limited by relatively small numbers of patients in each group. In addition, the fact that the outcome of patients with germline TEL genes in the DFCI study (5-yr EFS, 80%) was superior to that of patients with germline TEL genes in the present study (5-yr EFS, 72%) might have decreased the impact of TEL gene rearrangements. These results also suggest that the ALinC 16 therapy may have been inadequate for patients with high-risk ALL and no TEL rearrangements.
In the present study, patients with TEL rearrangements who had standard-risk ALL by NCI/Rome criteria had an excellent outcome in response to treatment with antimetabolite-based therapy (POG 9201 or 9605) that did not include anthracyclines, epipodophyllotoxins, alkylating agents, and cranial irradiation. This result suggests that current treatment protocols for this large group of patients should continue to be based on antimetabolites and to avoid agents that are associated with significant long-term sequelae. Patients with TEL rearrangements and high-risk ALL as defined by NCI/Rome criteria also had an excellent outcome, which may be attributed to the more intensive therapy that they received on POG 9406. The only subgroup of patients with TEL rearrangements and a relatively poor outcome (5-year EFS, 71%) was the approximately 5% of patients with slow early responses to therapy, who probably needed more aggressive or alternative therapies.
What is the optimal therapy for children with ALL and the TEL-AML1 fusion? The strikingly similar outcomes of patients with TEL-AML1–positive ALL treated on the present study, on the DFCI 95-01 trial,12 and at St. Jude22 (EFS estimates of 86%, 89%, and 88%, respectively) indicate that different treatment strategies may result in equally good outcomes. Leukemic blasts from patients with TEL-AML1–positive ALL are sensitive to steroids, vincristine, and asparaginase in vitro,23 and it has been suggested that the outstanding outcome of such patients on DFCI trials is related to the intensive use of asparaginase.12 However, the lack of intensive asparaginase administration in ALinC 16 or in the St. Jude trials suggests that the use of antimetabolite-based therapy or multiagent chemotherapy is also effective. Because of the excellent outcome of patients with TEL-AML1–positive ALL, very large randomized clinical trials will be required to determine the best possible therapy for these children.
In summary, our results demonstrate that early response to therapy, sex, NCI risk group, trisomies 4 and 10, and TEL status are all independent, significant predictors of outcome and should be used concurrently to determine risk classification in childhood ALL. We suggest that patients who have the TEL-AML1 fusion and are negative for minimal residual disease as indicated by flow cytometry or polymerase chain reaction at the end of remission induction therapy should be treated with antimetabolite-based therapy designed to maintain their high cure rates and avoid late effects. In contrast, we believe that patients who have the TEL-AML1 fusion and are positive for minimal residual disease are candidates for more intensive treatment.
Acknowledgments
This work was supported in part by the St. Jude Children's Research Hospital Cancer Center Support (CORE) grant P30 CA-21765; the Pediatric Oncology Group grant CA 30969, Pediatric Oncology Group Statistical Office grant U10 CA-29139, and grant R21 CA-73983 from the National Institutes of Health; by the American Lebanese Syrian Associated Charities (ALSAC) and CA 98543 COG grant . We thank Julia Cay Jones for expert editorial review. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm
References
- 1.Romana SP, Mauchauffe M, Le Coniat M, et al. The t(12;21) of acute lymphoblastic leukemia results in a tel-AML1 gene fusion. Blood. 1995;85:3662–3670. [PubMed] [Google Scholar]
- 2.Shurtleff SA, Buijs A, Behm FG, et al. TEL/AML1 fusion resulting from a cryptic t(12;21) is the most common genetic lesion in pediatric ALL and defines a subgroup of patients with an excellent prognosis. Leukemia. 1995;9:1985–1989. [PubMed] [Google Scholar]
- 3.Romana SP, Poirel H, Leconiat M, et al. High frequency of t(12;21) in childhood B-lineage acute lymphoblastic leukemia. Blood. 1995;86:4263–4269. [PubMed] [Google Scholar]
- 4.Rubnitz JE, Downing JR, Pui CH, et al. TEL gene rearrangement in acute lymphoblastic leukemia: a new genetic marker with prognostic significance. J Clin Oncol. 1997;15:1150–1157. doi: 10.1200/JCO.1997.15.3.1150. [DOI] [PubMed] [Google Scholar]
- 5.Rubnitz JE, Shuster JJ, Land VJ, et al. Case-control study suggests a favorable impact of TEL rearrangement in patients with B-lineage acute lymphoblastic leukemia treated with antimetabolite-based therapy: A Pediatric Oncology Group study. Blood. 1997;89:1143–1146. [PubMed] [Google Scholar]
- 6.McLean TW, Ringold S, Neuberg D, et al. TEL/AML1 dimerizes and is associated with a favorable outcome in childhood acute lymphoblastic leukemia. Blood. 1996;88:4252–4258. [PubMed] [Google Scholar]
- 7.Rubnitz JE, Behm FG, Wichlan D, et al. Low frequency of TEL-AML1 in relapsed acute lymphoblastic leukemia supports a favorable prognosis for this genetic subgroup. Leukemia. 1999;13:19–21. doi: 10.1038/sj.leu.2401257. [DOI] [PubMed] [Google Scholar]
- 8.Loh ML, Silverman LB, Young ML, et al. Incidence of TEL/AML1 fusion in children with relapsed acute lymphoblastic leukemia. Blood. 1998;92:4792–4797. [PubMed] [Google Scholar]
- 9.Loh ML, Rubnitz JE. TEL/AML1-positive pediatric leukemia: prognostic significance and therapeutic approaches. Curr Opin Hematol. 2002;9:345–352. doi: 10.1097/00062752-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Seeger K, Adams HP, Buchwald D, et al. TEL-AML1 fusion transcript in relapsed childhood acute lymphoblastic leukemia. The Berlin-Frankfurt-Munster Study Group. Blood. 1998;91:1716–1722. [PubMed] [Google Scholar]
- 11.Harbott J, Viehmann S, Borkhardt A, et al. Incidence of TEL/AML1 fusion gene analyzed consecutively in children with acute lymphoblastic leukemia in relapse. Blood. 1997;90:4933–4937. [PubMed] [Google Scholar]
- 12.Loh ML, Goldwasser MA, Silverman LB, et al. Prospective analysis of TEL/AML1-positive patients treated on Dana-Farber Cancer Institute Consortium Protocol 95-01. Blood. 2006;107:4508–4513. doi: 10.1182/blood-2005-08-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chauvenet AR, Martin PL, Devidas M, et al. Anti-metabolite therapy for lesser risk B-lineage acute lymphoblastic leukemia of childhood: a report from Children's Oncology Group Study P9201. Blood. 2007;110:1105–1111. doi: 10.1182/blood-2006-12-061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz KR, Pullen DJ, Sather HN, et al. Risk-and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG). Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Non-parametric estimation for incomplete observations. Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 19.Harris MB, Shuster JJ, Carroll A, et al. Trisomy of leukemic cell chromosomes, 4 and, 10 identifies children with B-progenitor cell acute lymphoblastic leukemia with a very low risk of treatment failure: a Pediatric Oncology Group study. Blood. 1992;79:3316–3324. [PubMed] [Google Scholar]
- 20.Pui CH, Campana D, Evans WE. Childhood acute lymphoblastic leukaemia--current status and future perspectives. Lancet Oncol. 2001;2:597–607. doi: 10.1016/S1470-2045(01)00516-2. [DOI] [PubMed] [Google Scholar]
- 21.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 22.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 23.Ramakers-Van Woerden NL, Pieters R, Loonen AH, et al. TEL/AML1 gene fusion is related to in vitro drug sensitivity for L-asparaginase in childhood acute lymphoblastic leukemia. Blood. 2000;96:1094–1099. [PubMed] [Google Scholar]


