Abstract
Temsirolimus is an inhibitor of the mammalian target of rapamycin (mTOR) kinase, a protein that has been shown to be particularly active in metastatic renal cell carcinoma (mRCC) with poor prognosis. Therefore, temsirolimus should be considered as the first-line treatment indicated in mRCC patients classified as poor risk. The benefits of temsirolimus are not limited to an increased survival but are also related to a better quality of life, which is certainly one of the most important aspects in the clinical management of these frail patients. Temsirolimus is a well-tolerated treatment, and the most frequent adverse events are manageable with supportive care. To this end, the identification of predictive factors of response to temsirolimus could help us to better select patients and obtain a more tailored clinical management of mRCC.
Keywords: mTOR inhibitors, renal cell carcinoma, targeted therapies, temsirolimus
Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all adult malignancies and the 5-year survival rate for mRCC is approximately 5–10% [Cohen and McGovern, 2005; Garcia and Rini, 2007]. Therefore, numerous efforts have been made to improve survival for patients with this aggressive disease. The most important result obtained in the past decade was the understanding of the molecular pathways responsible for cell growth, proliferation, cell survival and angiogenesis in this cancer; this discovery has led to a dramatic change in the treatment of metastatic RCC (mRCC).
Historically, standard therapies for mRCC were immunotherapeutic agents such as interferon α (IFN-α) and interleukin-2 (IL2), since the neoplastic cells of renal tumor present a marked resistance to chemotherapy. However, these cytokines have limited efficacy and are associated with considerable toxicity [Fyfe et al. 1995; Negrier et al. 1998; Motzer et al. 2000; McDermott et al. 2005].
The molecular targets mostly implicated in the pathogenesis and progression of RCC are platelet-derived growth factor α (PDGF-α), transforming growth factor α (TGF-α), vascular endothelial growth factor (VEGF) and the mammalian target of rapamycin (mTOR). The inhibition of these targets by monoclonal antibodies (bevacizumab), small molecules (sunitinib, pazopanib, sorafenib, axitinib) and derivatives of sirolimus (everolimus and temsirolimus) has increased the survival of patients with mRCC.
In particular, temsirolimus is an inhibitor of mTOR kinase. It has been shown to be particularly active in mRCC with poor prognosis, thus introducing the concept of predictive and prognostic factors in the management of this neoplasia.
The aim of this review is to evaluate the current role of temsirolimus in the treatment of mRCC. Articles for inclusion in this paper were selected by a review of the published literature in PubMed. Phase I, II and III trials on temsirolimus in RCC, and papers on the use of temsirolimus in the management of this disease were considered and selected for inclusion based on their relevance to the topic and according to the authors’ judgment.
mTOR pathway and temsirolimus: clinical trials
mTOR is a serine–threonine kinase, a member of the phosphatidyl inositol 3′ kinase family. It plays a central role in the regulation of cell growth, metabolism, proliferation and motility [Schmelzle and Hall, 2000].
Rapamycin (sirolimus) is an immunosuppressant macrolide that specifically inhibits mTOR action and is currently utilized to prevent organ rejection after transplantation. Temsirolimus, a rapamycin analogue, showed antitumor activities in preclinical studies that prompted the planning of subsequent clinical trials until the approval of this molecule, in 2007, for patients with advanced RCC or mRCC.
mTOR network
mTOR consists of two multiprotein complexes, namely TOR complex 1 (TORC1) and TOR complex 2 (TORC2). Each complex plays different roles: TORC2 is implicated in the regulation of cell morphology and adhesion by regulating the cytoskeleton, while TORC1 controls the induction of tumor by promoting the synthesis of proteins such as d-cyclins, c-Myc, HIF1α, HIF2α and VEGF, therefore regulating cell proliferation and angiogenesis [Inoki and Guan, 2006].
mTOR is activated in response to growth stimuli such as insulin growth factor (IGF) through PI3K/AKT signaling, and in response to depletion of nutrients and energy through the adenosine monophosphate (AMP) kinase. mTOR inhibitors, such as temsirolimus, form a complex with FKBP12, an intracellular protein, and this complex binds mTOR in the specific rapamycin domain, thus inhibiting kinase function.
It is important to stress that TORC2 has been demonstrated to be relatively resistant to rapamycin in vitro and this can be considered as a possible mechanism of resistance to mTOR inhibitors [Hudes, 2009].
Phase I
Two phase I studies evaluated temsirolimus in advanced cancer. In one study, temsirolimus was administered intravenously (IV) once daily on days 1 to 5 every 2 weeks [Hidalgo et al. 2006], while in the other, patients were treated with escalating doses of temsirolimus administered as a 30 minute IV infusion once weekly [Raymond et al. 2004]. With the first schedule, two different maximum tolerated doses (MTD) were observed: 15 mg/m2/day for patients who were heavily pretreated and 19 mg/m2/day for patients who were minimally pretreated. No MTD was observed in the second study, during which patients were treated with doses from 7.5 to 220 mg/m2. Analysis of drug exposure obtained with dosages based on body surface indicated that dose normalization did not result in improved variability in patients, compared with flat doses. In neither study were clinically relevant manifestations of an immune-suppressed state shown.
Partial responses were observed in patients affected by breast cancer, non-small cell lung cancer and also RCC, and therefore a subsequent phase II study in patients with mRCC was planned.
Phase II
After the results of the phase I studies, Atkins and colleagues evaluated the efficacy of temsirolimus at the fixed doses of 25, 75 and 250 mg administered IV once weekly in patients with advanced refractory RCC previously treated with IL2-based therapy [Atkins et al. 2004]. No differences were shown between the three doses in terms of time to tumor progression (TTP) and overall survival (OS). In fact, median TTP was 5.8 months for the total patient population, and 6.3, 6.7 and 5.2 months for patients in the 25, 75 and 250 mg dose groups, respectively. Median OS was 15.0 months in the overall population, and the results in the three dose groups were 13.8, 11.0 and 17.5 months, respectively. The toxicity profile of temsirolimus was similar in the three groups, but dose reductions and discontinuations occurred more often with the highest dose: therefore, the authors indicated 25 mg as the optimal dose in terms of biological activity and safety for the use of temsirolimus in advanced RCC.
Another key element that Atkins and his collaborators showed in this phase II trial was that temsirolimus showed activity in a subgroup of patients with a poor prognosis, according to the modified Motzer’s prognostic factors. This preliminary observation paved the way for subsequent studies and analyses.
Another phase I/II trial evaluated the association between temsirolimus and IFN-α in mRCC [Motzer et al. 2007]. This study was designed after preclinical evidence showing that temsirolimus and IFN-α have synergistic anti-angiogenesis effects [Gibbons, 2006]. After a first dose escalation phase, the established recommended dose for the association was temsirolimus 15 mg with IFN-α 6 million units (MU). In patients treated with the recommended dose, progression-free survival (PFS) of 9.1 months with the combination temsirolimus–IFN-α was observed. This result, compared with those obtained in the phase II trial by Atkins and colleagues [Atkins et al. 2004], showed the efficacy of the association of targeted and biological therapies in the treatment of mRCC.
The most frequent adverse events included asthenia, chills, stomatitis, nausea, diarrhea, anorexia, anemia, increased cough, rash and dyspnea; about 8% of patients experienced an allergic reaction. The most frequently reported grade 3–4 adverse events were leukopenia (32%), hypophosphatemia (27%), asthenia (21%), anemia (21%) and hypertriglyceridemia (15%). A dose reduction was necessary, in most cases, to control thrombocytopenia, elevated triglycerides, stomatitis, neutropenia, hyperglycemia, rash, elevated liver aminotransferases and pneumonia.
Thanks to the positive results of this study, a phase III trial was conducted to further evaluate the association of temsirolimus and IFN.
Phase III
The Global Advanced Renal Cell Carcinoma Trial (ARCC Trial) was an international, phase III, randomized trial which evaluated patients with mRCC and poor prognosis treated with IFN-α-2a alone, temsirolimus alone, or the combination of the two drugs [Hudes et al. 2007]. In this trial, a total of 626 patients with advanced or recurrent RCC were enrolled and randomly assigned to receive IFN-α-2a (207 patients) or temsirolimus (209 patients), while the combination was given to 210 patients.
In accordance to the definition of prognostic factors indicative of poor prognosis [Bukowski et al. 2004], patients had to present at least three of the following factors to be considered for inclusion in the study: a serum lactate dehydrogenase level of more than 1.5 times the upper limit of the normality; a hemoglobin level below the lower limit of the normality; a corrected serum calcium level of more than 10 mg/dl (2.5 mmol/l); a time from initial diagnosis of RCC to recurrence of less than 1 year; a Karnofsky performance score of 60 or 70; or metastases in multiple organs.
The IFN group received IFN-α-2a at the starting dose of 3 MU given subcutaneously 3 times per week for the first week, with the possibility to increase the dose up to 18 MU depending on the tolerance to treatment. The temsirolimus group received 25 mg of temsirolimus in a weekly 30-minute intravenous infusion, and the combination therapy group received 15 mg of temsirolimus in a weekly 30-minute infusion plus IFN at a starting dose of 3 MU three times per week for first week and 6 MU subcutaneously 3 times per week thereafter.
The results showed a median survival of 7.3 months in the IFN group, 10.9 months in the temsirolimus group, and 8.4 months in the combination therapy group (Figure 1). When compared with IFN alone, monotherapy with temsirolimus was associated with a reduced risk of death [hazard ratio (HR) = 0.73; 95% confidence interval (CI), 0.58–0.92; p = 0.008); no difference in the risk of death was disclosed between the combination of IFN plus temsirolimus and IFN alone (HR = 0.96; 95% CI, 0.76–1.20; p = 0.70). The clinical benefit, defined as stable disease for at least 6 months or objective response, was higher in the temsirolimus group (32.1%) and in the combination therapy group (28.1%) compared with the IFN-α group (15.5%).
Figure 1.
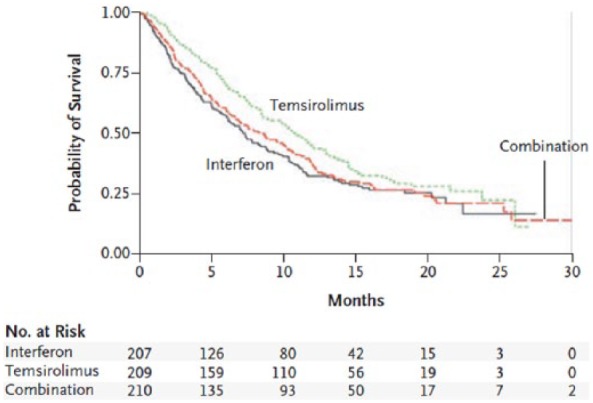
Overall survival in the pivotal Advanced Renal Cell Carcinoma trial [Hudes et al. 2007].
Patients in the temsirolimus group showed a lower incidence of grade 3–4 adverse events compared with the other groups of treatment: 67 versus 78% in the IFN group (p = 0.02) and 87% of patients in the combination therapy group (p = 0.02). In particular, grade 3 or 4 asthenia was more frequent in patients treated with IFN, either alone (26%) or in combination (28%); anemia, neutropenia and thrombocytopenia were more common in the combination therapy group than in the IFN group (p < 0.001) or in the temsirolimus group (p < 0.001 for neutropenia and thrombocytopenia, and p = 0.002 for anemia). Hyperglycemia, hypercholesterolemia and hyperlipidemia were more frequent in patients treated with temsirolimus, either alone or in combination.
This trial therefore showed that combination of temsirolimus with IFN-α does not result in an increased OS with respect to temsirolimus alone; furthermore, the combination treatment is associated with an increased incidence of adverse events.
Importantly, until the publication of the data from this study, patients with poor prognostic factors had a median OS ranging from 4 to 8 months. In this phase III randomized trial, temsirolimus allowed a median OS of 10.9 months to be obtained in this group, thus showing to be an effective drug in the management of ‘poor risk’ patients (Figure 2). On this basis, temsirolimus is currently approved in the United States and Europe as first-line treatment in patients with mRCC and poor prognosis [Escudier et al. 2014; NCCN, 2015].
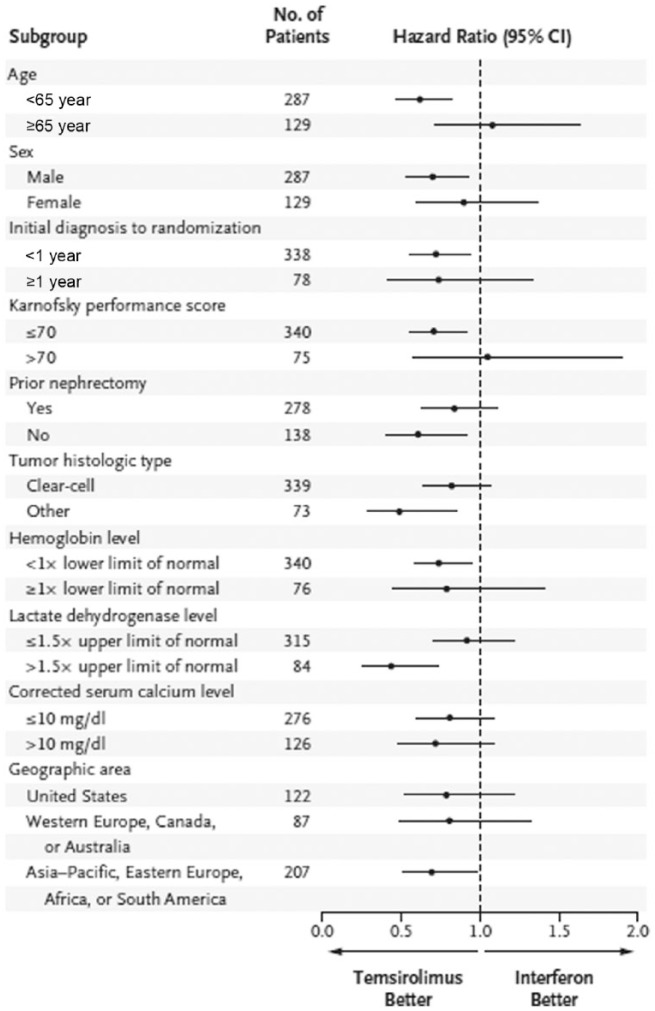
Figure 2.
Overall survival among subgroups of patients in the phase III ARCC trial [Hudes et al. 2007].
ARCC, Advanced Renal Cell Carcinoma Trial; CI, confidence interval.
Second line
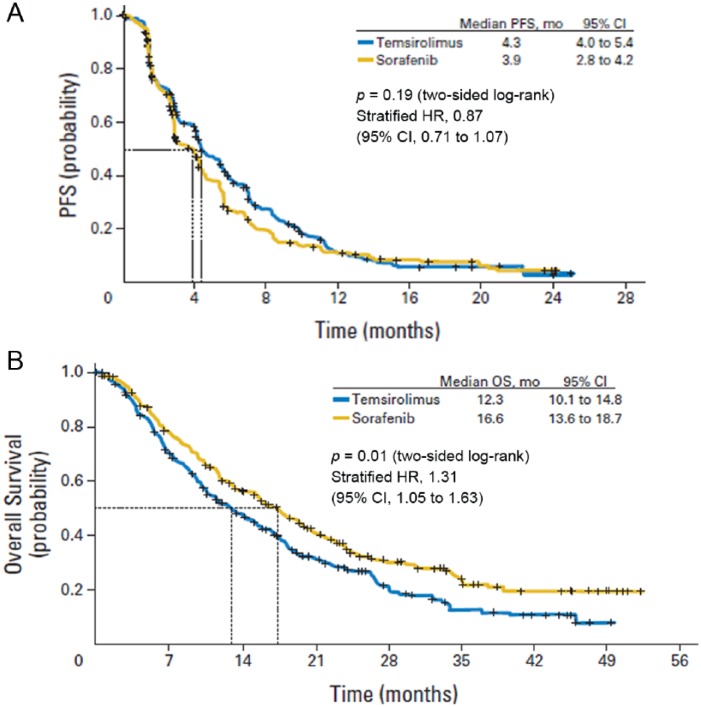
After the approval of temsirolimus as first-line treatment in poor risk patients, a phase III trial [Investigating Torisel As Second Line Therapy (INTORSECT)] evaluated treatment with temsirolimus versus sorafenib in the second-line setting [Hutson et al. 2014]. In this trial, patients who progressed after a first-line treatment with sunitinib were randomly assigned to receive intravenous temsirolimus 25 mg once weekly or oral sorafenib 400 mg twice daily.
No significant difference was seen between the two arms in PFS, the primary endpoint of the study (Figure 3a). Median OS was 12.3 months (95% CI, 10.1–14.8 months) in the temsirolimus arm and 16.6 months (95% CI, 13.6–18.7 months) in the sorafenib arm (Figure 3b). The reasons for the lack of correlation between PFS and OS remain unclear; the potential role of different therapies adopted after progression from either temsirolimus or sorafenib may be taken into account.
Figure 3.
PFS (panel A) and OS (panel B) in the second-line treatment with temsirolimus and sorafenib during the INTORSECT study [Hutson et al. 2014].
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
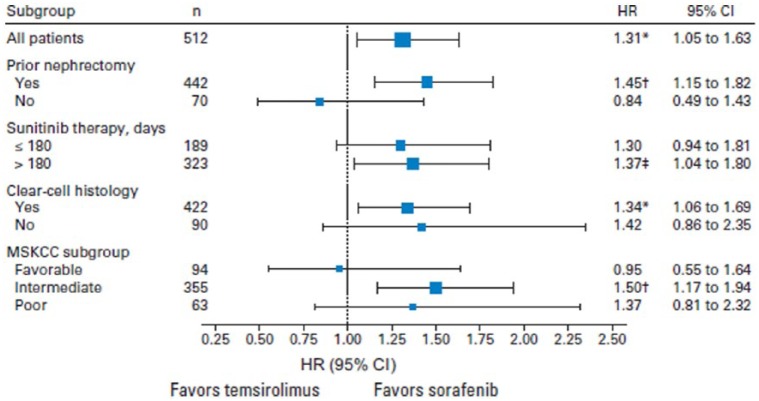
A subgroup analysis identified some characteristics that were related to improved OS in sorafenib patients compared with those assigned to temsirolimus: prior nephrectomy; longer duration of prior sunitinib; clear cell histology; and Memorial Sloan-Kettering Cancer Center (MSKCC) intermediate risk (Figure 4). Furthermore, the gain in survival with sorafenib was observed in particular subgroups of patients, such as those with intermediate risk and those who have had a longer response to a previous treatment with sunitinib. However, temsirolimus seems to be as effective as sorafenib in patients who rapidly progressed with sunitinib treatment (⩽180 days).
Figure 4.
Exploratory subgroup analysis of OS for prespecified factors in the INTORSECT study [Hutson et al. 2014].
*Indicates p = 0.01. †Indicates p = 0.002. ‡Indicates p = 0.002.
CI, confidence interval; HR, hazard ratio; MSKCC, Memorial Sloan-Kettering Cancer Center; OS, overall survival; PFS, progression-free survival.
Therefore, despite the results in OS shown in this study, as well as the retrospective analysis of Iacovelli and colleagues [Iacovelli et al. 2014], temsirolimus could be considered as a second-line treatment in patients who do not respond to a previous treatment with sunitinib, or in patients with poor tolerance to anti-VEGF drugs, temsirolimus having a completely different safety profile compared with anti-VEGF treatments.
Temsirolimus in different histologies
RCC is a heterogeneous disease, which comprises different histological subtypes with distinct responses to treatments and clinical behavior. The most common subtypes are: clear cell (70–85%); papillary (7–15%); and chromophobe (5–10%).
In the Global ARCC trial, patients with any subtype of RCC were eligible to receive treatment; a subgroup analysis was therefore carried out to evaluate the efficacy of temsirolimus in the different histological subtypes [Dutcher et al. 2009]. The histological characteristics of patients were balanced in the temsirolimus and IFN groups: 83% of patients in the IFN group and 82% in the temsirolimus group had a clear cell carcinoma, 17 and 18% had nonclear or indeterminate histology, and 15 and 12% had a papillary RCC, respectively.
The subgroup analysis showed that temsirolimus determined a similar median OS in patients with clear cell carcinoma (10.7 months, 95% CI, 8.5–13.0) and in those with other histologies (11.6 months, 95% CI, 8.9–14.5). Conversely, median OS in the IFN group was shorter in patients with other histologies compared with those with clear cell RCC (4.3 versus 8.2 months).
The HR for death with temsirolimus versus IFN in patients with clear cell histology was 0.82 (95% CI, 0.64–1.06), while in patients with other histologies, it was 0.49 (95% CI, 0.29, 0.85). In patients with papillary histology, HR for death was 0.50 (95% CI, 0.27–0.94). Similar findings were reported for PFS.
With these results taken into account, temsirolimus was shown to be active in all histological subtypes, with greater efficacy in nonclear cell tumor subgroups. Moreover, temsirolimus seems particularly active in the papillary subtype, but the number of evaluated patients was very limited (n = 5) and therefore caution is required in the interpretation of these results.
This subgroup analysis suggests that temsirolimus is indicated as first-line treatment in all renal cancer histological subtypes for patients with poor prognosis.
Clinical presentation and management of side effects related to temsirolimus
The most frequent adverse events associated with temsirolimus observed during phase II and III trials were hematologic, metabolic, pulmonary and cutaneous side effects. In the ARCC phase III trial, anemia was the most common hematologic event, reported in 33% of patients treated with temsirolimus compared with 21% in the IFN group. In particular, grade 3–4 anemia was observed in 13% of patients: in these cases, erythropoietin or blood transfusion must be considered during the patient’s treatment. Any grade hyperglycemia, hypertriglyceridemia and hypercholesterolemia were the main metabolic side effects in the temsirolimus group, and occurred in 18, 25 and 21% of patients, respectively. Notably, the occurrence of these adverse events suggests the central role of mTOR in the regulation of glucose and lipid metabolism.
The clinical manifestation of hyperglycemia may be an excessive thirst or increased urination, and this event may require initiation or increase of insulin and/or oral hypoglycemic drugs. Hypertriglyceridemia and hypercholesterolemia associated with temsirolimus are in most cases manageable with nutritional counseling and, if necessary, lipid-lowering agents. It is important to test serum cholesterol and triglyceride levels before and during treatment [Bellmunt et al. 2008].
Drug-related pneumonitis is a typical side effect associated with mTOR inhibitors. A retrospective blinded review of chest computerized tomography (CT) images in the ARCC phase III trial indicated that 29% of patients treated with temsirolimus developed temsirolimus-related pneumonitis [Maroto et al. 2011]. This incidence is similar to that reported in other retrospective radiographic analyses of patients treated with TOR inhibitors and confirms that pneumonitis is a class-effect toxicity of mTOR inhibitors.
Radiologic images are characterized by ground glass opacities and consolidation, either alone or concomitant, that often involve multiple lobes [Duran et al. 2006]. The pathophysiology of mTOR inhibitor-related pneumonitis remains unclear. Review of chest CT images in the ARCC trial indicated that the onset of pneumonitis occurred within the first 8 weeks of temsirolimus treatment in 60% of patients, between weeks 8 and 16 in 21% of patients, and after 16 weeks in 19%. Temsirolimus-related pneumonitis is not always associated with clinical symptoms, as cough or dyspnea. In fact, in the ARCC trial, investigators identified only 2% of temsirolimus-related pneumonitis, considerably fewer than pneumonitis identified by systematic radiographic retrospective analysis. In asymptomatic patients, modifications in treatment are not required, but the fact that the incidence of pneumonitis is about 30%, superior to that observed by investigators, suggests that more attention to this side effect should be paid to obtain a better management of patients. Diagnostic tests such as pulmonary function tests and bronchoscopy, as well as the initiation of empiric treatments such as corticosteroids and antibiotics, remain the cornerstones of the clinical management of these events.
Also cutaneous and mucosal toxicity are frequently observed during temsirolimus therapy. In fact, drug-related rash was reported in 34% of patients receiving temsirolimus versus 4.5% of patients treated with IFN; acne occurred in 10% of patients in the temsirolimus arm and in 0.5% of patients in the IFN arm. The nature of temsirolimus-related rash is mainly macupapular. Mucositis was observed in all the three treatment groups, but was in almost all of the cases of mild severity and was manageable with supportive measures.
With respect to gastrointestinal events, 20% of temsirolimus-treated patients reported stomatitis, 19% mucositis, 4% aphthous stomatitis and 3% mouth ulceration [Bellmunt et al. 2008].
Although all these adverse events are in most cases mild in severity, they can negatively affect quality of life (QoL). For this reason, good education of patients to allow an early identification of these adverse events and prompt use of supportive measures by the treating physician appear important.
QoL
The evaluation of QoL is an important measure to assess the global treatment benefit. Two different methods were used in the ARCC trial to test QoL in patients treated with temsirolimus: the Q-TWiST (Quality-Adjusted Time Without Symptoms and Toxicity) method and the EQ-5D™ questionnaire.
Q-TWiST is a well-recognized method which combines three different health states, Toxicity, Relapse or TWiST (neither Toxicity or Relapse), into an algorithm to provide a single measure of the quality of survival. It can be used for treatment comparisons in all oncology settings [Radice and Redaeli, 2005; Sherrill et al. 2008].
The EQ-5D questionnaire consists of two pages: the first with descriptive questions that generate the EQ-5D index score and the second with the EQ-VAS [Oppe et al. 2008]. Five dimensions are identified in the EQ-5D descriptive system: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Patients can respond to a question on each dimension indicating ‘no problems’, ‘some problems’ or ‘severe problems’. The EQ-5D questionnaire was scored using the index-based algorithm as described by Dolan (1997). In the EQ-VAS patients can rank their health state on a continuous scale from 0 to 100, in which 0 is the worst possible health status and 100 the best health condition.
Both types of evaluation of QoL showed that temsirolimus-treated patients had a better QoL with respect to those on IFN [Zbrozek et al. 2010]. In particular in the Q-TWiST analysis, the difference in Q-TWiST for patients treated with temsirolimus (7.0 months) and for those treated with IFN-α (5.6 months) was 1.4 months, resulting in a clinically significant difference of 15.7%. The mean EQ-5D index score at last measure was higher in the temsirolimus arm than in the IFN-α arm by 0.10 (p = 0.0279) and the mean EQ-VAS score at last measure was better in the temsirolimus arm than the IFN-α arm by 6.61 (p = 0.0095). These results indicate that temsirolimus improves mean EQ-5D index and EQ-VAS scores compared with IFN-α in patients with metastatic RCC and poor prognosis.
The longer survival associated with a better QoL supports the use of temsirolimus in the management of advanced RCC with poor prognosis.
Predictive and prognostic factors for temsirolimus treatment
The identification of predictive and prognostic factors is crucial in the management of patients. With respect to RCC, the International Metastatic RCC Database Consortium (IMDC) or Heng’s model [Heng et al. 2009] identified three categories of patients: good risk, intermediate risk and poor risk. To each category corresponds a different median survival.
Given the results of the Global ARCC trial, only lactate dehydrogenase (LDH) may be considered as a predictive factor of response to temsirolimus. Another important factor is the histology: chromophobe and papillary RCC have a better prognosis than clear cell RCC if disease is localized [Cheville et al. 2003; Patard et al. 2005]. In the metastatic setting, on the contrary, nonclear cell RCC is more frequently resistant to systemic therapy and is associated with shorter survival. Temsirolimus was shown, in a retrospective analysis of the pivotal phase III trial, to be effective also in nonclear cell carcinoma, and it is recommended also in patients with nonclear cell carcinoma who present poor prognosis [Dutcher et al. 2009].
Different molecular markers were analyzed in patients treated with temsirolimus to detect possible predictive factors, such as phosphatase and tensin homolog (PTEN) and HIF-1α, proteins belonging to the mTOR pathway. Temsirolimus has been shown to be effective regardless of expression of these molecules [Figlin et al. 2009].
Another post hoc analysis has evaluated the correlation between cholesterol, triglycerides and glucose in patients treated with temsirolimus versus patients treated with IFN [Lee et al. 2012]. Patients with a greater increase in cholesterol had a longer survival with temsirolimus treatment; this finding was not replicated when considering triglycerides or glucose. Further studies are necessary to fully evaluate whether the increase in cholesterol may be a predictive factor during treatment with temsirolimus.
Conclusion
Temsirolimus should be considered to be the first-line treatment indicated in mRCC patients classified as poor risk. The benefits of temsirolimus are not limited to an increased survival but are also related to a better QoL, which is definitely one of the most important aspects in clinical management of these frail patients. Temsirolimus is a well-tolerated treatment and the most frequent adverse events are manageable with supportive care.
Temsirolimus represents an option in selected cases pretreated with targeted therapies, even if it is still unclear what the optimal sequence of therapy is with anti-VEGF and mTOR inhibitors.
Duration of response to a first treatment should be considered as a prognostic and not a predictive factor to be used in defining subsequent line therapy [Procopio et al. 2014].
Patients affected by poor tolerance to anti-VEGF drugs and nonclear cell histotypes should be considered for mTOR inhibitor treatment. Moreover, the identification of predictive factors of response to temsirolimus could help us better select patients and obtain a more tailored clinical management.
Acknowledgments
Editorial assistance for the preparation of this manuscript was provided by Luca Giacomelli, PhD, and Ambra Corti; this assistance was supported by internal funds.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Contributor Information
Elisa Zanardi, Department of Medical Oncology, Istituto Nazionale dei Tumori, Milan, Italy.
Elena Verzoni, Department of Medical Oncology, Istituto Nazionale dei Tumori, Milan, Italy.
Paolo Grassi, Department of Medical Oncology, Istituto Nazionale dei Tumori, Milan, Italy.
Andrea Necchi, Department of Medical Oncology, Istituto Nazionale dei Tumori, Milan, Italy.
Patrizia Giannatempo, Department of Medical Oncology, Istituto Nazionale dei Tumori, Milan, Italy.
Daniele Raggi, Department of Medical Oncology, Istituto Nazionale dei Tumori, Milan, Italy.
Filippo De Braud, Department of Medical Oncology, Istituto Nazionale dei Tumori, Milan, Italy.
Giuseppe Procopio, Department of Medical Oncology, Unit 1 Fondazione IRCCS Istituto Nazionale dei Tumori, Via G. Venezian 1, 20133, Milan, Italy.
References
- Atkins M., Hidalgo M., Stadler W., Logan T., Dutcher J., Hudes G., et al. (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22: 909–918. [DOI] [PubMed] [Google Scholar]
- Bellmunt J., Szczylik C., Feingold J., Strahs A., Berkenblit A. (2008) Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Ann Oncol 19: 1387–1392. [DOI] [PubMed] [Google Scholar]
- Bukowski R., Negrier S., Elson P. (2004) Prognostic factors in patients with advanced renal cell carcinoma: development of an international kidney cancer working group. Clin Cancer Res 10: 6310S–6314S. [DOI] [PubMed] [Google Scholar]
- Cheville J., Lohse C., Zincke H., Weaver A., Blute M. (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27: 612–624. [DOI] [PubMed] [Google Scholar]
- Cohen H., McGovern F. (2005) Renal-cell carcinoma. N Engl J Med 353: 2477–2490. [DOI] [PubMed] [Google Scholar]
- Dolan P. (1997) Modeling valuations for EuroQol health states. Med Care 35: 1095–1108. [DOI] [PubMed] [Google Scholar]
- Duran I., Siu L., Oza A., Chung T., Sturgeon J., Townsley C., et al. (2006) Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer 42: 1875–1880. [DOI] [PubMed] [Google Scholar]
- Dutcher J., de Souza P., McDermott D., Figlin R., Berkenblit A., Thiele A., et al. (2009) Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol 26: 202–209. [DOI] [PubMed] [Google Scholar]
- Escudier B., Porta C., Schmidinger M., Algaba F., Patard J., Khoo V., et al. (2014) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25: iii49–iii56. [DOI] [PubMed] [Google Scholar]
- Figlin R., de Souza P., McDermott D., Dutcher J., Berkenblit A., Thiele A., et al. (2009) Analysis of PTEN and HIF-1alpha and correlation with efficacy in patients with advanced renal cell carcinoma treated with temsirolimus versus interferon-alpha. Cancer 115: 3651–3660. [DOI] [PubMed] [Google Scholar]
- Fyfe G., Fisher R., Rosenberg S., Sznol M., Parkinson D., Louie A. (1995) Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 13: 688–696. [DOI] [PubMed] [Google Scholar]
- Garcia J., Rini B. (2007) Recent progress in the management of advancedrenal cell carcinoma. CA Cancer J Clin 57: 112–125. [DOI] [PubMed] [Google Scholar]
- Gibbons J. (2006) CCI-779 Potentiates the inhibitory effect of the anti-angiogenesis drug interferon-alpha on the growth of a human renal cell carcinoma in nude mice: RPT-49843. Pearl River, NY: Wyeth Research. [Google Scholar]
- Heng D., Xie W., Regan M., Warren M., Golshayan A., Sahi C., et al. (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27: 5794–5799. [DOI] [PubMed] [Google Scholar]
- Hidalgo M., Buckner J., Erlichman C., Pollack M., Boni J., Dukart G., et al. (2006) A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res 12: 5755–5763. [DOI] [PubMed] [Google Scholar]
- Hudes G., Carducci M., Tomczak P., Dutcher J., Figlin R., Kapoor A., et al. (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356: 2271–2281. [DOI] [PubMed] [Google Scholar]
- Hudes G. (2009) Targeting mTOR in renal cell carcinoma. Cancer 115: 2313–2320. [DOI] [PubMed] [Google Scholar]
- Hutson T., Escudier B., Esteban E., Bjarnason G., Lim H., Pittman K., et al. (2014) Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 32: 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli R., Cartenì G., Milella M., Berardi R., Di Lorenzo G., Verzoni E., et al. (2014) Clinical outcomes in patients with metastatic renal cell carcinoma receiving everolimus or temsirolimus after sunitinib. Can Urol Assoc J 8: E121–E125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Guan K. (2006) Complexity of the TOR signaling network. Trends Cell Biol 16: 206–212. [DOI] [PubMed] [Google Scholar]
- Lee C., Marschner I., Simes R., Voysey M., Egleston B., Hudes G., et al. (2012) Increase in cholesterol predicts survival advantage in renal cell carcinoma patients treated with temsirolimus. Clin Cancer Res 18: 3188–3196. [DOI] [PubMed] [Google Scholar]
- Maroto J., Hudes G., Dutcher J., Logan T., White C., Krygowski M., et al. (2011) Drug-related pneumonitis in patients with advanced renal cell carcinoma treated with temsirolimus. J Clin Oncol 29: 1750–1756. [DOI] [PubMed] [Google Scholar]
- McDermott D., Regan M., Clark J., Flaherty L., Weiss G., Logan T., et al. (2005) Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 23: 133–141. [DOI] [PubMed] [Google Scholar]
- Motzer R., Hudes G., Curti B., McDermott D., Escudier B., Negrier S., et al. (2007) Phase I/II trial of temsirolimus combined with interferon alfa for advanced renal cell carcinoma. J Clin Oncol 25: 3958–3964. [DOI] [PubMed] [Google Scholar]
- Motzer R., Murphy B., Bacik J., Schwartz L., Nanus D., Mariani T., et al. (2000) Phase III trial of interferon alfa-2a with or without 13-cis-retinoic acid for patients with advanced renal cell carcinoma. J Clin Oncol 18: 2972–2980. [DOI] [PubMed] [Google Scholar]
- NCCN (2015) Kidney cancer. NCCN Clinical Practice Guidelines in Oncology, Version 2.2015. Fort Washington, PA: National Comprehensive Cancer Network. [Google Scholar]
- Negrier S., Escudier B., Lasset C., Douillard J., Savary J., Chevreau C., et al. (1998) Recombinanthuman interleukin-2, recombinanthuman interferon alfa-2a, or both in metastatic renal-cell carcinoma. N Engl J Med 338: 1272–1278. [DOI] [PubMed] [Google Scholar]
- Oppe M., Rabin R., de Charro F. (2008) EQ-5D user guide, version 1.0. Rotterdam: EuroQol Group. [Google Scholar]
- Patard J., Leray E., Rioux-Leclercq N., Cindolo L., Ficarra V., Zisman A., et al. (2005) Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol 23: 2763–2771. [DOI] [PubMed] [Google Scholar]
- Procopio G., Verzoni E., De Braud F. (2014) Butterfly and renal cell cancer: out of chaos comes order. J Clin Oncol 32: 3083–3084. [DOI] [PubMed] [Google Scholar]
- Radice D., Redaeli A. (2005) Q-TWiST analysis of cyclophosphamide, epirubicin, fluorouracil versus cyclophosphamide, methotrexate, fluorouracil treatment for premenopausal women with node-positive breast cancer. Pharmacoeconomics 23: 69–75. [DOI] [PubMed] [Google Scholar]
- Raymond E., Alexandre J., Faivre S., Vera K., Materman E., Boni J., et al. (2004) Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol 22: 2336–2347. [DOI] [PubMed] [Google Scholar]
- Schmelzle T., Hall M. (2000) TOR, a central controller of cell growth. Cell 103: 253–262. [DOI] [PubMed] [Google Scholar]
- Sherrill B., Amonkar M., Stein S., Walker M., Geyer C., Cameron D. (2008) Q-TWiST analysis of lapatinib combined with capecitabine for the treatment of metastatic breast cancer. Br J Cancer 99: 711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbrozek A., Hudes G., Levy D., Strahs A., Berkenblit A., DeMarinis R., et al. (2010) Q-TWiST analysis of patients receiving temsirolimus or interferon alpha for treatment of advanced renal cell carcinoma. Pharmacoeconomics 28: 577–5784. [DOI] [PubMed] [Google Scholar]