Abstract
Objectives:
Few studies have assessed the effect of gonadotropin-releasing hormone (GnRH) agonists, such as triptorelin, on lower urinary tract symptoms (LUTS) in patients with advanced prostate cancer. Therefore, multiple, national observational, noninterventional studies were initiated to assess the effectiveness of triptorelin in reducing moderate or severe LUTS [International Prostate Symptom Score (IPSS) >7] in men with prostate cancer starting triptorelin therapy in clinical practice.
Methods:
Prospective, noninterventional, multicentre studies of LUTS located in Algeria, Belgium, China, Hungary, Romania and South Korea, in patients who were scheduled to receive triptorelin (3-month extended release or 1-month formulation) in clinical practice. The primary effectiveness endpoint was the proportion of patients with moderate or severe LUTS after 48 weeks as assessed by IPSS. Secondary endpoints included the distribution of IPSS categories, total IPSS and prostate-specific antigen (PSA) levels at baseline, 24 and 48 weeks.
Results:
In total, 2461 patients were recruited in the studies; 1282 patients had moderate or severe LUTS at baseline (IPSS > 7), received triptorelin and had follow-up IPSS. Mean total IPSS was reduced from 18.2 [95% confidence interval (CI) 17.8–18.5] at baseline to 11.9 (95% CI 11.5–12.3; p < 0.001) and 10.6 (95% CI 10.2–11.0; p < 0.001) at weeks 24 and 48, respectively. Mean PSA levels were reduced from 117.9 ng/ml (95% CI 93.8–141.9) at baseline to 8.5 ng/ml (95% CI 5.2–11.7) and 16.6 ng/ml (95% CI 7.4–25.8) at weeks 24 and 48, respectively. There was a significant correlation between total IPSS change from baseline and PSA change from baseline at weeks 24 and 48 (ρ = 0.3 and 0.2, p < 0.001).
Conclusions:
The improvement in LUTS in men with locally advanced or metastatic prostate cancer after 24–48 weeks suggests that triptorelin is effective in improving LUTS in this subgroup of patients.
Keywords: androgen deprivation therapy, gonadotropin-releasing hormone agonists, lower urinary tract symptoms, prostate cancer, quality of life, triptorelin
Introduction
Androgen deprivation therapy (ADT) with gonadotropin-releasing hormone (GnRH) agonists is the standard treatment for many patients with prostate cancer, in particular those with advanced/metastatic disease and before, during or after radiation therapy in high-risk localized disease [Heidenreich et al. 2014]. The cytostatic and cytotoxic efficacy of ADT is well reported, as are the decreases in prostate-specific antigen (PSA) levels in nearly all patients [Labrie et al. 2005; Harris et al. 2009; Schroder et al. 2012].
In addition, when prostate tumours compress or invade proximate structures or when the prostate grows due to concomitant benign prostatic hyperplasia (BPH), patients may suffer from lower urinary tract symptoms (LUTS) [Guess, 2001; Andersson et al. 2004; Hamilton and Sharp, 2004]. One study estimated that more than 40% of patients with prostate cancer had moderate or severe LUTS [Lehrer et al. 2002]. However, there have been few reports on the effects of ADT on LUTS in patients with prostate cancer. One recent report indicated that ADT with the GnRH antagonist degarelix may result in greater reductions in LUTS [as measured by the International Prostate Symptom Score (IPSS)] than ADT with the GnRH agonist goserelin (plus the anti-androgen bicalutamide to prevent ‘flare up’) after 12 weeks, especially in those with moderate-to-severe symptoms (IPSS > 13) at baseline [Axcrona et al. 2012]. A similar finding was reported when ADT was used in the neoadjuvant setting in men with intermediate- or high-risk prostate cancer [Mason et al. 2013]. A separate 3-month study, which was stopped prematurely due to poor recruitment, compared degarelix with goserelin plus bicalutamide and suggested that degarelix treatment may be noninferior to goserelin plus bicalutamide after 12 weeks in locally advanced prostate cancer with severe LUTS [Anderson et al. 2013]. Longer-term data on the effects of ADT have not been published. Indeed, only radical prostatectomy has been shown to improve clinically significant LUTS in the long-term (up to 10 years) in men with prostate cancer [Prabhu et al. 2013].
Triptorelin is a widely used GnRH agonist with efficacy in prostate cancer [Heyns et al. 2003; Teillac et al. 2004; Lundstrom et al. 2009; Martinez-Pineiro et al. 2013; Ploussard and Mongiat-Artus, 2013]. Data on the impact of triptorelin on LUTS are limited and, therefore, multiple national, observational, open-label, noninterventional studies were initiated to assess LUTS in patients starting triptorelin therapy in routine clinical practice. The aim of these studies was to assess the effectiveness of triptorelin in reducing LUTS, after 24 and 48 weeks, in patients with locally advanced or metastatic prostate cancer and moderate or severe LUTS (IPSS > 7) at baseline.
Methods
This was a prospective grouped analysis of data from patients belonging to different noninterventional, multicentre studies with very similar protocols that were conducted in Algeria, Belgium, Hungary, Romania, South Korea and China. All six studies collected data in a similar manner (any differences are noted in the text below). The studies were noninterventional, prospective studies of LUTS in patients with locally advanced or metastatic prostate cancer who were scheduled to receive triptorelin as part of a routine ADT course. Data pooled from these six countries and interim analysis (December 2013; when follow-up data for >1000 patients were available) are reported here.
The studies in Algeria (21 centres, started October 2008 and ended August 2010 for the last patient last visit), Belgium (26 centres, started November 2006 and ended May 2010 last patient last visit) and Hungary (19 centres, started October 2009 and ended March 2012 last patient last visit) are completed, while the studies in China (26 centres, started March 2010), Romania (29 centres, started May 2009) and South Korea (21 centres, started 2009) are ongoing. All data from the completed studies and interim data from the ongoing studies are included in this analysis.
Studies were conducted in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines. Approval was obtained from all relevant institutional review boards or independent ethics committees, and local regulatory requirements. All patients gave written informed consent before entry into each study.
Participants
Men with locally advanced or metastatic prostate cancer scheduled to receive triptorelin were included in the study. Patients had to receive concomitant anti-androgen treatment to prevent ‘flare up’ when starting triptorelin according to local guidelines and practice. Inclusion of patients into the study required them to be mentally and physically capable of answering the IPSS questionnaire.
Patients were excluded if they had hypersensitivity to triptorelin or to one of the excipients of the study drug; if they had received treatment with an investigational drug in the previous 3 months; if they had received GnRH agonist treatment in the previous 6 months; or if their life expectancy was <12 months. Country-specific exclusion criteria were: in Algeria the presence of a pathology that could interfere with the results of the study; and in China the risk of a serious complication in the case of tumour flare up (e.g. vertebral metastases that could threaten spinal cord compression).
The ‘study population’ was defined as all patients with any total IPSS recorded at baseline. The ‘effectiveness population’ (EP) was defined as all patients in the study population that had at least one triptorelin injection and at least one follow-up total IPSS documented. In the ongoing studies, if patients were still in the study (i.e. it was less than 48 weeks since the first triptorelin injection and they were not recorded as withdrawn), they were excluded from the EP. The analysis reported here is focussed on the EP with moderate or severe LUTS at baseline (IPSS > 7).
Triptorelin treatment
The triptorelin treatment received in each country was partially dependent upon the availability of different formulations. In Hungary, Romania, South Korea, China and Algeria, all patients received the 3-month formulation of triptorelin pamoate (Decapeptyl®/Diphereline®) 11.25 mg every 12 weeks, and in Belgium patients received either the 3-month formulation of triptorelin pamoate 11.25 mg every 12 weeks, the 1-month formulation of triptorelin pamoate 3.75 mg every 4 weeks, or were switched between these formulations.
Assessments
Patients were assessed at baseline (when triptorelin was first prescribed), and after 24 weeks and 48 weeks of treatment. Baseline assessments included: demographic data, vital signs, previous radiation therapy, hormonal therapy or surgery for prostate cancer, eligibility criteria and the indication for prescribing triptorelin. Previous and concomitant treatments were recorded at baseline, and after 24 and 48 weeks.
The severity of LUTS and the effectiveness of triptorelin for reducing LUTS were assessed using the IPSS questionnaire at baseline, and after 24 and 48 weeks. The IPSS consists of seven symptom items (incomplete emptying, frequency, intermittency, urgency, weak stream, straining and nocturia) and one question on quality of life (QoL) due to urinary symptoms. The seven symptom questions are rated on a six-point scale, and the combined scores provide a measure of severity where: IPSS of 0 defines an absence of symptoms; IPSS 1–7 defines mild LUTS; IPSS 8–19 defines moderate LUTS; and IPSS 20–35 defines severe LUTS [Madersbacher et al. 2004]. QoL was assessed in a single question from IPSS on a seven-point scale ranging from 0 (delighted) to 6 (terrible). If the IPSS questionnaire was incomplete for any patient at any time point then the total IPSS was considered missing.
PSA levels were measured at baseline, and after 24 and 48 weeks if this schedule was the local standard of care.
Statistical analysis
Planned sample sizes in each country were based upon feasibility and were: Algeria (n = 200), Belgium (n = 300), China (n = 500), Hungary (n = 300), Romania (n = 1500) and South Korea (n = 850). In some countries if the number of screened patients exceeded these numbers, recruitment into the study was stopped.
Primary and secondary effectiveness endpoints were based upon the patients in the EP with moderate or severe LUTS. The primary effectiveness endpoint was the proportion of patients with moderate or severe LUTS after 48 weeks. Secondary effectiveness endpoints were the distribution of IPSS categories (no, mild, moderate and severe symptoms), total IPSS, QoL score and PSA level at baseline, and after 24 and 48 weeks (or last available visit within the 48 weeks); and correlation between the change from baseline in IPSS and change from baseline in PSA level. Individuals receiving 5-alpha reductase inhibitors and anticholinergic drugs were excluded from the moderate and severe LUTS analyses.
All analyses were done using SAS® version 9.2. All statistical tests were exploratory and two-sided at the 5% level of significance. Accordingly, no adjustments for multiplicity were performed for this grouped analysis. For the primary effectiveness endpoint, the proportion of patients with moderate or severe LUTS are presented using descriptive statistics including 95% confidence intervals (CI). The improvement in LUTS with time was assessed using a generalised estimating equations (GEE) model and a logit link and binomial distribution. The p-value for the time-fixed effect is presented.
Similar methods based on GEE model were used to evaluate the change in IPSS categories with time. To obtain adjusted mean of total IPSS throughout the study, a linear model with repeated measures was used, and a similar model was used for the QoL question. To assess the effect of treatment on PSA level, a repeated measures model was used. The correlation between PSA level and total IPSS was assessed using the Spearman’s rank correlation coefficient.
Results
Patients
The study population (those with a total IPSS at baseline) consisted of 2461 men with prostate cancer: 171 in Algeria, 325 in Belgium, 223 in China, 280 in Hungary, 665 in Romania, 797 in South Korea. The EP consisted of 1535 patients: of the 926 excluded from the EP, 578 patients were ongoing in the studies at the time of this analysis. In the three countries completing the study the EP was 144 (84.2% of the study population) in Algeria, 257 (79.1%) in Belgium, and 258 (92.1%) in Hungary. Reasons for withdrawal from the study are outlined in Table 1.
Table 1.
Disposition of patients in the study population (n = 2461).
| Patient status | Patients, n (%) |
|---|---|
| Effectiveness population | 1535 (62.4) |
| Excluded from the effectiveness population | 926 (37.6) |
| Reasons for exclusion* | |
| No post-baseline total International Prostate Symptom Score | 711 (76.8) |
| Failed to receive at least one triptorelin injection | 93 (10.0) |
| Ongoing in the study | 578 (62.4) |
Excluded patients may have more than one reason for exclusion.
Baseline data for the study population and EP are shown in Table 2. Of the EP, 1282 patients had moderate or severe LUTS at baseline while 253 patients had no or mild symptoms (IPSS ⩽ 7.0). Data presented here focus on these 1282 men with moderate or severe LUTS at baseline.
Table 2.
Baseline patient and disease characteristics of the study population and effectiveness population (EP).
| Characteristic | Whole study population (n = 2461 unless otherwise stated) | EP (n = 1535 unless otherwise stated) |
|---|---|---|
| Age (years), mean ± SD | 72.1 ± 8.0* | 72.2 ± 7.9 |
| Weight (kg), mean ± SD | 71.6 ± 12.4** | 73.3 ± 12.5‡ |
| Patients having metastasis (M1), n/N (%) | 513/2430 (21.1) | 293/1528 (19.2) |
| ⩾T3 stage, n/N (%) | 2318/2433 (95.3) | 1480/1532 (96.6) |
| Gleason score ⩾8, n/N (%) | 944/2327 (40.6) | 557/1486 (37.5) |
| PSA >20 ng/ml, n/N (%) | 1279/2373 (53.9) | 803/1492 (53.8) |
| Total IPSS, mean ± SD | 16.1 ± 8.3 | 15.9 ± 8.1 |
| IPSS category, n (%) | ||
| No symptoms | 10 (0.4) | 4 (0.3) |
| Mild symptoms | 394 (16.0) | 249 (16.2) |
| Moderate symptoms | 1238 (50.3) | 791 (51.5) |
| Severe symptoms | 819 (33.3) | 491 (32.0) |
| Reason for initiating triptorelin, n/N (%) | ||
| Neoadjuvant before RP | 49/2453 (2.0) | 8/1534 (0.5) |
| Neoadjuvant before RT or BT | 180/2453 (7.3) | 136/1534 (8.9) |
| Adjuvant after RP | 129/2453 (5.3) | 52/1534 (3.4) |
| Adjuvant after RT or BT | 51/2453 (2.1) | 32/1534 (2.1) |
| Rising PSA level after RP | 166/2453 (6.8) | 94/1534 (6.1) |
| Rising PSA level after RT or BT | 37/2453 (1.5) | 25/1534 (1.6) |
| Locally advanced, first line | 1176/2453 (47.9) | 776/1534 (50.6) |
| Locally advanced, after anti-androgen | 193/2453 (7.9) | 136/1534 (8.9) |
| Metastatic, first line | 489/2453 (19.9) | 292/1534 (19.0) |
SD, standard deviation; IPSS, International Prostate Symptom Score; PSA, prostate-specific antigen; RT, radiation therapy; RP, radical prostatectomy; BT, brachytherapy.
n = 2459; **n = 1850; ‡n = 1046.
Effectiveness – LUTS
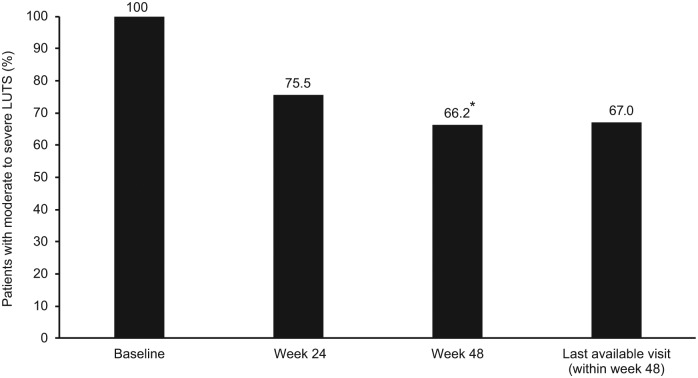
The proportion of patients with moderate or severe LUTS (according to IPSS) was reduced from baseline after 24 weeks, 48 weeks and at last available visit (Figure 1). The reduction in moderate or severe LUTS with triptorelin treatment was significantly reduced (overall time effect, p < 0.0001).
Figure 1.
Moderate or severe LUTS (according to total IPSS) at baseline, week 24, week 48 and last available visit (within week 48) in all patients in the effectiveness population with moderate or severe LUTS at baseline (n = 1282).
Data were available for 1282, 1258, 1114 and 1282 patients at baseline, week 24, week 48 and last available visit, respectively
* p < 0.0001 for overall time effect
The mean total IPSS for patients with moderate to severe LUTS was 18.2 (95% CI, 17.8–18.5) at baseline (n = 1282), and the adjusted means were 11.9 (95% CI, 11.5–12.3; p < 0.001) at week 24 and 10.6 (95% CI, 10.2–11.0; p < 0.001) at week 48.
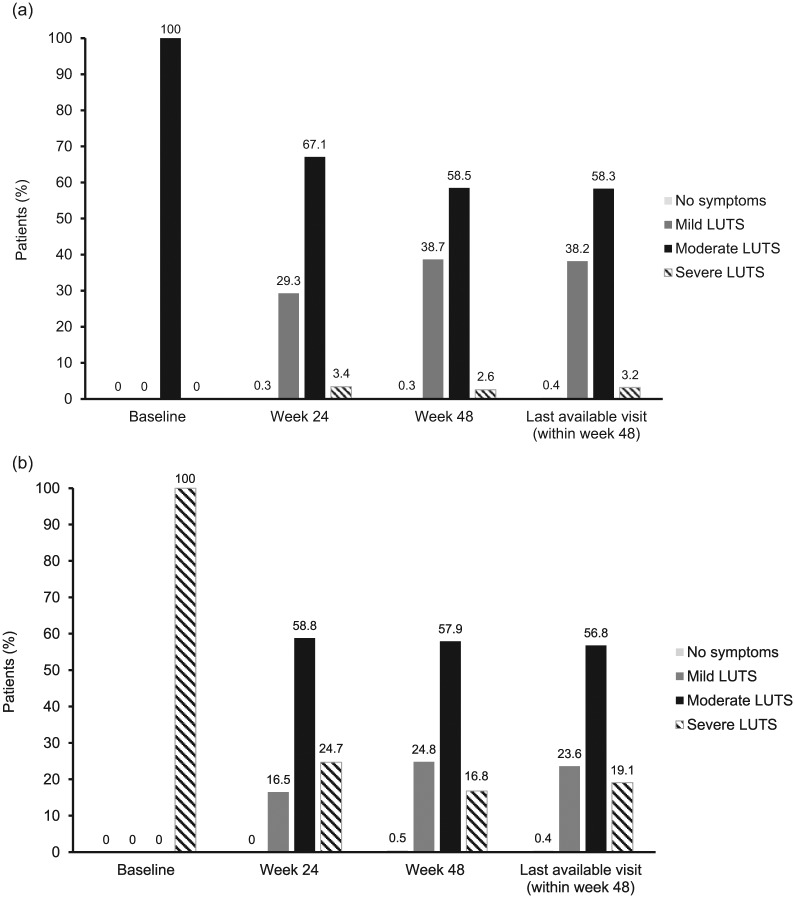
Of the 791 patients in the EP with moderate LUTS at baseline, symptoms were reduced in severity (mostly to mild LUTS) in approximately 30% of patients after 24 weeks and almost 40% of patients after 48 weeks of treatment (Figure 2a). Of the 491 patients in the EP with severe LUTS at baseline, triptorelin treatment reduced the severity of symptoms in over 75% of patients after 24 weeks and over 80% of patients after 48 weeks (Figure 2b). Almost 25% of men with severe LUTS at baseline had mild LUTS after 48 weeks treatment with triptorelin (Figure 2b).
Figure 2.
LUTS severity status (according to total IPSS) at each time point in the effectiveness population with (a) moderate LUTS at baseline (n = 791) and (b) severe LUTS at baseline (n = 491).
(a) Data were available for 791, 772, 698 and 791 patients at baseline, week 24, week 48 and last available visit, respectively
(b) Data were available for 491, 486, 416 and 491 patients at baseline, week 24, week 48 and last available visit, respectively
Mean IPSS scores in the EP were significantly reduced from baseline at week 24, week 48 and last available visit for all items (incomplete emptying, frequency, intermittency, urgency, weak stream, straining, nocturia; Table 3; p < 0.001 baseline vs. week 24 and 48).
Table 3.
Mean responses to the International Prostate Symptom Score (IPSS) symptoms questionnaire individual questions (responses on a six-point scale; 0 = not at all, 5 = almost always).
| Question | Baseline |
Week 24* |
Week 48* |
Last available visit* |
|---|---|---|---|---|
| Mean (95% confidence interval) (n = 1535)** | ||||
| Incomplete emptying | 2.2 (2.1–2.2) | 1.4 (1.4–1.5) | 1.3 (1.2–1.4) | 1.3 (1.2–1.3) |
| Frequency | 2.3 (2.3–2.4) | 1.6 (1.6–1.7) | 1.4 (1.4–1.5) | 1.5 (1.4–1.5) |
| Intermittency | 2.0 (2.0–2.1) | 1.3 (1.3–1.4) | 1.2 (1.1–1.3) | 1.2 (1.2–1.3) |
| Urgency | 2.2 (2.1–2.3) | 1.5 (1.4–1.5) | 1.3 (1.3–1.4) | 1.3 (1.3–1.4) |
| Weak stream | 2.7 (2.6–2.7) | 1.7 (1.6–1.7) | 1.5 (1.4–1.6) | 1.5 (1.5–1.6) |
| Straining | 1.9 (1.8–2.0) | 1.2 (1.2–1.3) | 1.1 (1.0–1.2) | 1.1 (1.1–1.2) |
| Nocturia | 2.6 (2.6–2.7) | 2.0 (1.9–2.0) | 1.8 (1.7–1.9) | 1.9 (1.8–1.9) |
p < 0.001 for all questions for baseline versus week 24, baseline versus week 48 and baseline versus last available visit (within week 48).
Adjusted mean values for weeks 24 and 48.
Effectiveness – PSA level
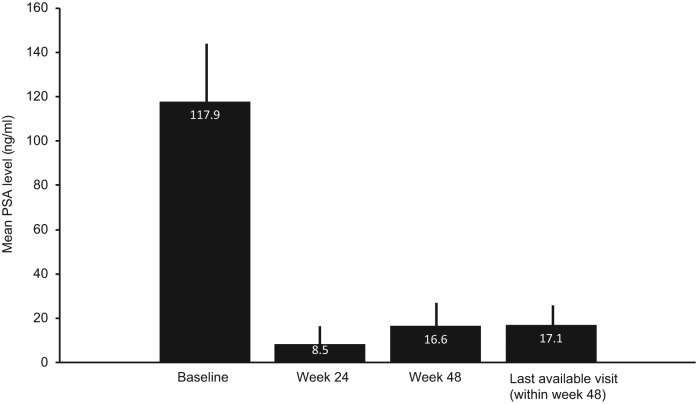
Mean PSA level in the EP was significantly reduced from baseline (Figure 3; p < 0.0001 overall time effect).
Figure 3.
Mean plus 95% confidence intervals for PSA levels for the effectiveness population with moderate or severe LUTS at baseline.
Vertical bars are 95% confidence intervals.
Data were available for 1241, 1155, 1040 and 1209 patients at baseline, week 24, week 48 and last available visit, respectively
p<0.0001 for overall time effect.
Using Spearman’s correlation coefficient there was a significant but weak correlation (p < 0.001) between total IPSS change from baseline and PSA change from baseline at week 24, week 48 and last available visit (correlation estimates were 0.2, 0.3 and 0.2, respectively).
Quality of life
The mean score for QoL assessing urinary symptoms was 4.1 (95% CI, 4.0–4.2) at baseline in the EP with moderate or severe LUTS (n = 1282). QoL was improved with triptorelin treatment, as measured by a significant reduction in the adjusted mean QoL score to 2.9 (95% CI, 2.8–3.0; p < 0.001) after 24 weeks (n = 1259) and 2.5 (95% CI, 2.5–2.6; p < 0.001) after 48 weeks (n = 1120) and 2.6 (95% CI, 2.5–2.6) at last available visit (n = 1282).
Discussion
The efficacy of triptorelin as ADT for prostate cancer is well established [Heyns et al. 2003; Teillac et al. 2004; Lundstrom et al. 2009; Martinez-Pineiro et al. 2013; Ploussard and Mongiat-Artus, 2013], but this grouped analysis of 1282 men with locally advanced or metastatic prostate cancer, derived from six countries in distinct geographical locations, is the largest to assess the effectiveness of triptorelin specifically on LUTS in this patient population. The number of patients with moderate or severe LUTS was significantly reduced after 24 weeks of treatment with triptorelin and these improvements were maintained up to 48 weeks of treatment. Similarly improvements in PSA level, which correlated with improvements in LUTS, and QoL were sustained at week 48.
Differences in patient populations and settings make comparison with other recently published papers difficult [Axcrona et al. 2012; Anderson et al. 2013; Mason et al. 2013]. However, in this grouped analysis, which consisted mostly of patients receiving first-line triptorelin therapy (approximately 70% of patients), the reductions in total IPSS among those with moderate or severe LUTS appear to be at least as large as those reported among patients receiving goserelin in previous studies (mean reduction of 7.5 points in this study compared with 0.5–3.5 in the neoadjuvant setting [Mason et al. 2013] and 4.5–9.6 in patients with more severe disease [Axcrona et al. 2012]). In previous studies the duration of follow up was 12 weeks, whereas our observations demonstrate that the benefits of GnRH agonists on reducing the severity of LUTS are maintained (and possibly improved) over the longer duration of 24 and 48 weeks.
It has been proposed that the effects of GnRH agonists on LUTS is a result of prostate volume reduction (as observed in benign prostatic hyperplasia) rather than tumour volume reduction [Oesterling, 1991; Mason et al. 2013]. Furthermore, the GnRH antagonist degarelix had a greater impact on LUTS at 12 weeks compared with the GnRH agonist goserelin, despite similar effects on PSA and testosterone suppression, which may suggest the mechanism on LUTS is independent of these markers [Axcrona et al. 2012; Anderson et al. 2013; Mason et al. 2013; Cui et al. 2014]. Although prostate volume was not measured in this observational study, there was a clear correlation between PSA suppression and LUTS improvement in this large cohort of patients.
Triptorelin treatment also had a positive impact on the QoL related to urinary symptoms of patients with a reduction in the mean score to the QoL question on the IPSS questionnaire from 4.1 (a score of ⩾4.0 mostly dissatisfied) at baseline to 2.9 and 2.5 (a score of ⩽2.0 mostly satisfied) after triptorelin treatment for 24 and 48 weeks, respectively. The change in QoL score among patients starting triptorelin therapy represents an important improvement in QoL toward the ‘normal’ range of <2.4 and from ‘dissatisfied’ to ‘satisfied’ [Viktrup et al. 2012].
One strength of the current analysis is that it includes a larger population than has previously been assessed for the impact of ADT on LUTS [Axcrona et al. 2012; Anderson et al. 2013; Mason et al. 2013; Cui et al. 2014], but observational studies have inherent limitations, such as potential selection bias and lack of control for confounding factors, which we acknowledge in this report. Furthermore, the IPSS relies on patient recall to rate symptoms, which could lead to under- or over-estimation of symptoms. However, the IPSS has been used widely to assess LUTS and is useful therefore for comparing our results with previous reports. The advantage of this study is the real-world clinical setting giving an indication of the impact of ADT on LUTS in actual practice and in a heterogeneous population.
Despite the limitations of an observational study design, this report provides evidence for the efficacy of the GnRH agonist triptorelin in reducing LUTS. The improvement in LUTS in men with locally advanced or metastatic prostate cancer after 24–48 weeks suggests that, as expected, prostate atrophy induced by triptorelin is effective in improving LUTS in this group of patients.
Acknowledgments
The authors take full responsibility for the content of the paper but thank Martin Gilmour and John Clarke of ESP Bioscience, Crowthorne, UK (supported by Ipsen) for editorial assistance in the preparation of the manuscript. The authors thank all investigators who gathered data for this analysis.
We would like to thank all participating centres in Algeria, Belgium, China, Hungary, Romania and South Korea who have enrolled patients into the local LUTS studies. We would also like to thank all of the patients who agreed to participate in the study.
Footnotes
Conflict of interest statement: Roland van Velthoven is a consultant for Ipsen. Thierry Gil and Fouad Aoun have no financial interest or financial conflict with the subject matter or materials discussed in the manuscript. Patrick Cabri and Pascal Maisonobe are employees of Ipsen Pharma, Paris, France.
Funding: These studies and this analysis were funded by Ipsen in the respective countries. Editorial assistance in the preparation of this manuscript was supported by Ipsen.
Contributor Information
Thierry Gil, Department of Urology, Institute Jules Bordet – Université Libre de Bruxelles, Brussels, Belgium.
Fouad Aoun, Department of Urology, Institute Jules Bordet – Université Libre de Bruxelles, Brussels, Belgium.
Patrick Cabri, Ipsen Pharma, Paris, France.
Pascal Maisonobe, Ipsen Pharma, Paris, France.
Roland van Velthoven, Department of Urology, Institute Jules Bordet, Université Libre de Bruxelles, Heger-Bordet Street 1, 1000 Brussels, Belgium.
References
- Anderson J., Al-Ali G., Wirth M., Gual J., Gomez Veiga F., Colli E., et al. (2013) Degarelix versus goserelin (+ antiandrogen flare protection) in the relief of lower urinary tract symptoms secondary to prostate cancer: results from a phase IIIb study (NCT00831233). Urol Int 90: 321–328. [DOI] [PubMed] [Google Scholar]
- Andersson S., Rashidkhani B., Karlberg L., Wolk A., Johansson J. (2004) Prevalence of lower urinary tract symptoms in men aged 45-79 years: a population-based study of 40 000 Swedish men. BJU Int 94: 327–331. [DOI] [PubMed] [Google Scholar]
- Axcrona K., Aaltomaa S., Da Silva C., Ozen H., Damber J., Tanko L., et al. (2012) Androgen deprivation therapy for volume reduction, lower urinary tract symptom relief and quality of life improvement in patients with prostate cancer: degarelix vs goserelin plus bicalutamide. BJU Int 110: 1721–1728. [DOI] [PubMed] [Google Scholar]
- Cui Y., Zong H., Yan H., Li N., Zhang Y. (2014) Degarelix versus goserelin plus bicalutamide therapy for lower urinary tract symptom relief, prostate volume reduction and quality of life improvement in men with prostate cancer: a systematic review and meta-analysis. Urol Int 93: 152–159. [DOI] [PubMed] [Google Scholar]
- Guess H. (2001) Benign prostatic hyperplasia and prostate cancer. Epidemiol Rev 23: 152–158. [DOI] [PubMed] [Google Scholar]
- Hamilton W., Sharp D. (2004) Symptomatic diagnosis of prostate cancer in primary care: a structured review. Br J Gen Pract 54: 617–621. [PMC free article] [PubMed] [Google Scholar]
- Harris W., Mostaghel E., Nelson P., Montgomery B. (2009) Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol 6: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich A., Bastian P., Bellmunt J., Bolla M., Joniau S., Van Der Kwast T., et al. (2014) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65: 467–479. [DOI] [PubMed] [Google Scholar]
- Heyns C., Simonin M., Grosgurin P., Schall R., Porchet H. (2003) Comparative efficacy of triptorelin pamoate and leuprolide acetate in men with advanced prostate cancer. BJU Int 92: 226–231. [DOI] [PubMed] [Google Scholar]
- Labrie F., Belanger A., Luu-The V., Labrie C., Simard J., Cusan L., et al. (2005) Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr Rev 26: 361–379. [DOI] [PubMed] [Google Scholar]
- Lehrer S., Stone N., Droller M., Stock R. (2002) Association between American Urologic Association (AUA) urinary symptom score and disease stage in men with localized prostate cancer. Urol Oncol 7: 73–76. [DOI] [PubMed] [Google Scholar]
- Lundstrom E., Rencken R., Van Wyk J., Coetzee L., Bahlmann J., Reif S., et al. (2009) Triptorelin 6-month formulation in the management of patients with locally advanced and metastatic prostate cancer: an open-label, non-comparative, multicentre, phase III study. Clin Drug Investig 29: 757–765. [DOI] [PubMed] [Google Scholar]
- Madersbacher S., Alivizatos G., Nordling J., Sanz C., Emberton M., De La Rosette J. (2004) EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol 46: 547–554. [DOI] [PubMed] [Google Scholar]
- Martinez-Pineiro L., Schalken J., Cabri P., Maisonobe P., De La Taille A. and Triptocare Study Group. (2013) Evaluation of urinary prostate cancer antigen-3 (PCA3) and TMPRSS2-ERG score changes when starting androgen-deprivation therapy with triptorelin 6-month formulation in patients with locally advanced and metastatic prostate cancer. BJU Int 114: 608–616. [DOI] [PubMed] [Google Scholar]
- Mason M., Maldonado Pijoan X., Steidle C., Guerif S., Wiegel T., Van Der Meulen E., et al. (2013) Neoadjuvant androgen deprivation therapy for prostate volume reduction, lower urinary tract symptom relief and quality of life improvement in men with intermediate- to high-risk prostate cancer: a randomised non-inferiority trial of degarelix versus goserelin plus bicalutamide. Clin Oncol (R Coll Radiol) 25: 190–196. [DOI] [PubMed] [Google Scholar]
- Oesterling J. (1991) LHRH agonists. A nonsurgical treatment for benign prostatic hyperplasia. J Androl 12: 381–388. [PubMed] [Google Scholar]
- Ploussard G., Mongiat-Artus P. (2013) Triptorelin in the management of prostate cancer. Future Oncol 9: 93–102. [DOI] [PubMed] [Google Scholar]
- Prabhu V., Taksler G., Sivarajan G., Laze J., Makarov D., Lepor H. (2013) Radical prostatectomy improves and prevents age-dependent progression of lower urinary tract symptoms. J Urol 191: 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder F., Crawford E., Axcrona K., Payne H., Keane T. (2012) Androgen deprivation therapy: past, present and future. BJU Int 109(Suppl. 6): 1–12. [DOI] [PubMed] [Google Scholar]
- Teillac P., Heyns C., Kaisary A., Bouchot O., Blumberg J. (2004) Pharmacodynamic equivalence of a decapeptyl 3-month SR formulation with the 28-day SR formulation in patients with advanced prostate cancer. Horm Res 62: 252–258. [DOI] [PubMed] [Google Scholar]
- Viktrup L., Hayes R., Wang P., Shen W. (2012) Construct validation of patient global impression of severity (PGI-S) and improvement (PGI-I) questionnaires in the treatment of men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. BMC Urol 12: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]