Abstract
Objectives:
Gonadotropin-releasing hormone agonists are widely used as androgen deprivation therapy in many men with locally advanced or metastatic prostate cancer. Gonadotropin-releasing hormone agonists are delivered by intramuscular injection every 1, 3 or 6 months, but in some patients subcutaneous injection may be more appropriate. This study assessed the efficacy and safety profile of the gonadotropin-releasing hormone agonist, triptorelin pamoate, when administered by the subcutaneous route.
Methods:
In this multicentre, open-label, single-arm study, androgen deprivation therapy-naïve men with locally advanced or metastatic prostate cancer received the gonadotropin-releasing hormone agonist triptorelin pamoate 11.25 mg (3-month formulation) by the subcutaneous route twice (at baseline and 13 weeks later). The co-primary efficacy endpoints were the proportion of patients with a castration level of serum testosterone (<50 ng/dl) after 4 weeks, and of these, those still castrated after 26 weeks.
Results:
Of the 126 treated patients, 123 [97.6%; 95% confidence interval (CI): 93.2–99.5)] were castrated 4 weeks after the first subcutaneous injection, and 115/119 patients (96.6%; 95% CI: 91.6–99.1) castrated at 4 weeks maintained castration at 26 weeks. Median prostate-specific antigen levels were reduced by 64.2 and 96.0% at 4 and 26 weeks, respectively. The probability of maintaining a testosterone level <20 ng/dl up to 26 weeks was 90.0% (95% CI: 85.0–95.0). The most frequently occurring treatment-related adverse events were typical of gonadotropin-releasing hormone agonist treatment (hot flushes, increased weight, erectile dysfunction and hyperhidrosis).
Conclusions:
This study demonstrates that triptorelin pamoate 11.25 mg administered by the subcutaneous route every 3 months is as efficacious and well tolerated as administration via the intramuscular route in men with locally advanced or metastatic prostate cancer.
Keywords: castration, prostate cancer, subcutaneous injection, testosterone, triptorelin
Trial protocol number: NCT01715129
Introduction
For the management of locally advanced and metastatic hormone-sensitive prostate cancer, the European Association of Urology (EAU) recommends initiating androgen deprivation therapy (ADT) to achieve castration levels of testosterone (<50 ng/dl) [Heidenreich et al. 2014]. Castration is most often achieved by administering a gonadotropin-releasing hormone (GnRH) agonist, which is effective for palliation of symptoms in advanced disease and improves outcomes in high-risk patients and patients with metastatic disease [Palmberg et al. 1999; Sharifi et al. 2005]. Sustained release formulations of GnRH agonists were developed to enhance patients’ quality of life and adherence [Shaheen et al. 1993]. The efficacy and tolerability of one of these sustained release agents, triptorelin pamoate (Decapeptyl®, Ipsen, Paris, France) formulated for intramuscular (IM) injection every 1 or 3 months (triptorelin is also available as a 6 month formulation), have been demonstrated in many studies [Abbou et al. 1997; Kuhn et al. 1997; Heyns et al. 2003; Teillac et al. 2004; Lundstrom et al. 2009; Mounedji et al. 2011; Martinez-Pineiro et al. 2013]. In these studies, castration serum levels of testosterone (<50 ng/dl) were achieved in >90% of patients treated with IM triptorelin 1 month after the first injection [Heyns et al. 2003; Lundstrom et al. 2009; Martinez-Pineiro et al. 2013]. These rates of castration were maintained for 6–12 months in these studies. In line with EAU guidelines [Heidenreich et al. 2014], triptorelin is indicated as first-line hormone therapy in patients with locally advanced nonmetastatic or metastatic disease as an alternative to surgical castration, and as add on to external beam radiation therapy.
In some patients, IM injections may not be recommended, for example, in patients receiving anticoagulants such as heparin and warfarin there may a possibility of developing haematomas or excessive bleeding [Lee, 1993]. In such instances, subcutaneous (SC) injections provide a recognized alternative route for the delivery of injectable medications without compromising efficacy or tolerability [Sweetser et al. 2006; Alviggi et al. 2007; Knuf et al. 2010; Madbouly et al. 2011; Zeitlinger et al. 2012; Golekoh et al. 2013]. Therefore, it would be useful to be able to inject GnRH agonists by both IM and SC routes to offer flexibility in the management of patients with prostate cancer.
We report on a study conducted to assess the efficacy and safety profile of triptorelin pamoate when administered by the SC route.
Methods
This was a multicentre, open-label, single-arm study of triptorelin pamoate 11.25 mg given by the SC route twice (at baseline and 13 weeks later), in which patients were monitored for 26 weeks. The study was conducted at 14 European centres in Bulgaria, France, Latvia, Poland and Romania between October 2012 and October 2013.
The study was conducted under the provisions of the Declaration of Helsinki and in accordance with the International Conference on Harmonisation guideline and Good Clinical Practice. The protocol was approved by all relevant Ethics Committees and Institutional Review Boards, and countries’ health authorities. All participants gave written informed consent to participate in the study.
Patients
Adult men (⩾18 and <90 years of age) were enrolled if they had histologically proven locally advanced or metastatic prostate cancer and were considered appropriate candidates for ADT. Inclusion also required: baseline testosterone levels >125 ng/dl; life expectancy >12 months; Eastern Cooperative Oncology Group (ECOG) performance status 0–1; and the ability of the patient to adhere to the study visit schedule.
Key exclusion criteria included: previous hormonal therapy for prostate cancer; previous surgery or radiation therapy for prostate cancer unless disease was verified with rising prostate-specific antigen (PSA) levels (on last two measurements at least 1 month apart); any cardiovascular condition within the previous 6 months; abnormal haematological, hepatic or renal functions; use within the last 6 months of any drug known to affect the metabolism of androgenic hormones; corticosteroid use; known brain metastases; any skin or other condition that precludes SC injection; known hypersensitivity to the study drug or any of its excipients; and any other condition that could confound the interpretation of the study findings.
Treatment and endpoints
All participants received treatment with triptorelin pamoate 11.25 mg by the SC route (injected via 20 gauge needle) at baseline and 13 weeks later.
The co-primary efficacy endpoints were the proportion of patients with a castration level of serum testosterone (<50 ng/dl) at 4 weeks (greater than a rate of 80% achieving castration), and of these, those still castrated at 26 weeks (greater than a rate of 85% maintaining castration). Secondary efficacy endpoints included: time to achieve castration; probability of testosterone levels remaining <50 ng/dl between week 4 and week 26; proportion of patients remaining castrated at week 13 (before administration of the second dose of triptorelin); proportion of patients castrated 3–4 days after administration of the second dose; plasma triptorelin levels at 13 and 26 weeks; change in PSA levels from baseline; and proportion of patients with normal PSA levels at 26 weeks (end of study visit) compared with baseline. An additional exploratory analysis was the proportion of patients with serum testosterone <20 ng/dl. Other laboratory parameters tested included follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels with limits of detection of 0.05 international units (IU)/l.
Plasma triptorelin concentrations at baseline and the minimum triptorelin plasma concentration at the end of each dosage interval immediately prior to the next dose injection (Cmin) were measured for days 92 and 183 in the intention to treat (ITT) population. The pharmacokinetic (PK) profile was assessed in a subset of 18 patients over the 24 hours following the first SC administration of triptorelin.
Safety endpoints included: injection site reactions (pain, swelling and redness) assessed daily by the participants for 2 weeks after each SC injection and at each visit by the investigators; adverse events (AEs) monitored according to the US National Cancer Institute’s Common Terminology for Adverse Events version 4.03 [National Cancer Institute, 2010]; and laboratory parameters.
Assessments
Following a screening visit up to 21 days before baseline, all patients were assessed at baseline and after 1, 2, 3, 4, 8, 13, 15, 17, 21 and 26 weeks. There was also an assessment 3–4 days after the second injection of triptorelin (after the week 13 visit).
Serum testosterone level was measured at all visits. If a testosterone level of ⩾50 ng/dl was measured in a participant after week 5, then the testosterone level was measured again within 7 days or at the next visit if scheduled within 14 days. Testosterone levels were measured using the Bayer ADVIA Centaur automated immunoassay; from 4 weeks to the end of the study, the more sensitive liquid chromatography/tandem mass spectrometry (LC-MS/MS) with a lower limit of detection of 0.104 nmol/l was used with missing data imputed by immunoassay.
PSA levels were measured using the Bayer ADVIA Centaur automated immunoassay at baseline and after 4, 8, 13, 17, 21 and 26 weeks. At baseline and week 13, blood was sampled for PSA measurement no more than 30 minutes before the triptorelin injection. FSH and LH levels were measured by a fluoroimmunometric assay with limits of detection of 0.05 IU/l.
Plasma triptorelin levels were measured in all participants immediately before the triptorelin injections to measure Cmin at baseline and after 13 and 26 weeks. In the subset of 18 patients, plasma concentrations of triptorelin were analysed at 1, 2, 3, 4, 5, 6, 7, 8 and 24 hours after the administration of the first SC dose of triptorelin, and peak plasma concentration (Cmax), time to Cmax (Tmax), and area under the concentration versus time curve between 0 and 24 hours (AUC0-24) were estimated. Triptorelin levels were measured using a radioimmunoassay technique (performed at Kymos Pharma Services, Barcelona, Spain) with a limit of quantification of 0.015 ng/ml.
AEs were assessed at all visits. Treatment-emergent AEs (TEAEs) reported by investigators were coded using MedDRA version 16.1 [MedDRA, 2013].
Statistical analyses
Due to the single-arm nature of the study, all statistical analyses were descriptive, with quantitative data summarized as mean, standard deviation (SD), median, quartiles, 95% confidence intervals (CIs) and range. Qualitative data were summarized as frequency counts and percentages.
Statistical evaluation was performed using SAS (version 9.2) on all treated participants (ITT population) and the initially castrated (IC) population (defined as all participants with testosterone levels <50 ng/dl at week 4). The safety population consisted of all patients who received at least one dose of study treatment.
The co-primary endpoint of the proportion of patients castrated at 4 weeks was based on the ITT population, and the co-primary endpoint of the proportion of patients still castrated at 26 weeks was based on the IC population. The co-primary endpoints and other endpoints based on proportions were calculated along with 95% CIs (two-sided) using exact methods (Clopper–Pearson). Time to castration and probability of testosterone level <50 ng/dl (and <20 ng/dl) was analysed using the Kaplan–Meier method. Serum PSA, LH and FSH levels were analysed throughout the study using descriptive statistics.
This single-arm study was designed to demonstrate that: (1) the proportion of patients castrated (serum level of testosterone <50 ng/dl) after 4 weeks was greater than 80% (a rate of less than 80% was considered undesirable, as this corresponds to below the lower 95% CI of the observed rate); and (2) the proportion of patients in the IC population that maintained castration levels after 26 weeks was greater than 85% (a rate of less than 85% was considered undesirable, as this corresponds to below the lower 95% CI of the observed rate). If 95% of participants maintained castration after 26 weeks, then 108 patients were required in the IC population to demonstrate that the co-primary endpoint was achieved with a 90% power and a one-sided alpha of 0.025. To achieve at least 108 patients in the IC population, and assuming 92% would achieve castration after 4 weeks (based on trial data with IM administration of triptorelin) [Heyns et al. 2003], the aim was to recruit at least 120 patients.
Results
Patient disposition and characteristics
All recruited patients (n = 126) received study treatment and were included in the ITT population, and 123 patients were included in the IC population. Patient populations and disposition are shown in Figure 1.
Figure 1.
Patient disposition.
*Patients reaching castration levels of testosterone (<50 ng/dl) at week 4 after first injection of triptorelin.
IC, initially castrated; ITT, intention-to-treat.
Baseline characteristics of the study participants are shown in Table 1. There was a wide range in the time since diagnosis of prostate cancer (1–2709 days), but the median of 25 days indicates that most patients were recently diagnosed. Most participants had locally advanced prostate cancer (109/126; 86.5%) and 14 patients (11.1%) had distant metastases. A biological relapse after curative treatment was experienced by three patients (2.4%).
Table 1.
Baseline characteristics of the intention-to-treat (ITT) population.
| Variable | All participants (n = 126) |
|---|---|
| Age, years, mean (SD) | 70.4 (7.3) |
| Weight, kg, mean (SD) | 80.6 (12.8) |
| BMI, kg/m2, mean (SD) | 27.2 (4.0) |
| Time since prostate cancer diagnosis, days, mean (SD) | 76.8 (293.9) |
| Gleason score, n (%) | |
| ⩽6 | 37 (29.4) |
| 7 | 39 (31.0) |
| ⩾8 | 50 (39.7) |
| ECOG performance status, n (%) | |
| 0 | 109 (86.5) |
| 1 | 17 (13.5) |
| Tumour staging (TNM*), n (%) | |
| T ⩾3, N0/NX, M0/MX | 105 (83.3) |
| Any T, any N and M1 | 14 (11.1) |
| Any T, N1, M0/MX | 4 (3.2) |
| T <3, N0/NX:MX | 3 (2.4) |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; SD, standard deviation.
TNM: T, tumour; N, node; M, metastasis.
Efficacy: testosterone
The primary objective of the study was met; 123/126 patients (97.6%, 95% CI: 93.2–99.5) were castrated 4 weeks after the first SC injection of triptorelin 11.25 mg (ITT population). Of the 3/126 not castrated at 4 weeks, one patient had elevated testosterone levels (202 ng/ml), one patient withdrew from the study due to protocol violation, and one patient had no testosterone measurements at 4 weeks.
At 26 weeks, 115/119 patients (96.6%, 95% CI: 91.6–99.1) who were castrated at 4 weeks maintained castration (IC population). Of the 4/119 patients who did not maintain castration at 26 weeks, one patient had elevated testosterone levels (54 ng/dl) at 26 weeks, two patients discontinued treatment before 26 weeks due to lack of efficacy (one patient withdrew at week 16 and one patient withdrew at week 17 with testosterone levels of 355 ng/dl and 58 ng/dl, respectively) and one patient discontinued due to an AE (myocardial infarction leading to death at 15 weeks).
The median time to achieve castration in the ITT population was 22 days (95% CI: 22–23). There was a short-lived flare up of testosterone levels after the first injection of triptorelin; this increase is expected with GnRH agonists (Figure 2). No such increase in testosterone levels was observed 3–4 days after the second triptorelin injection [mean (standard error of mean) testosterone level at day 95 was 12.376 (0.9481) ng/dl]; 98.3% (95% CI: 94.1–99.8) of patients were castrated at this time point.
Figure 2.
Mean serum testosterone levels* (ng/dl) in patients receiving triptorelin pamoate (11.25 mg) by the subcutaneous route (ITT population) at baseline and week 13.
Arrows denote when triptorelin injections were administered and vertical lines denote standard error of mean.
* Baseline and week 1 values measured by immunoassay, all other measurements were by the liquid chromatography/tandem mass spectrometry method with missing data imputed by immunoassay.
ITT, intention-to-treat.
The probability of achieving castration (testosterone level <50 ng/dl) after 4 weeks of treatment and remaining castrated at each measurement up to 26 weeks was 96.0% (95% CI: 92.0–99.0) in the ITT population. Furthermore, a large proportion of patients receiving triptorelin by the SC route achieved the lower cutoff value of testosterone levels <20 ng/dl from 4–26 weeks after first injection (Table 2). The probability of maintaining a testosterone level <20 ng/dl up to 26 weeks was 90.0% (95% CI: 85.0–95.0) in the ITT population.
Table 2.
Proportion of patients achieving testosterone levels of <20 ng/dl assessed using the LC-MS/MS method, with missing data imputed by immunoassay (ITT population).
| Time after first injection (weeks) | Proportion with testosterone levels <20 ng/dl, n/N % (95% CI)* |
|---|---|
| 4 | 80/103; 77.7 (68.4–85.3) |
| 8 | 110/115; 95.7 (90.1–98.6) |
| 13 | 116/121; 95.9 (90.6–98.6) |
| 17 | 113/122; 92.6 (86.3–96.5) |
| 21 | 112/121; 92.6 (86.3–96.5) |
| 26 | 109/120; 90.8 (84.2–95.3) |
Two-sided 95% confidence interval.
CI, confidence interval; ITT, intention-to-treat; LC-MS/MS liquid chromatography/tandem mass spectrometry.
Efficacy: PSA
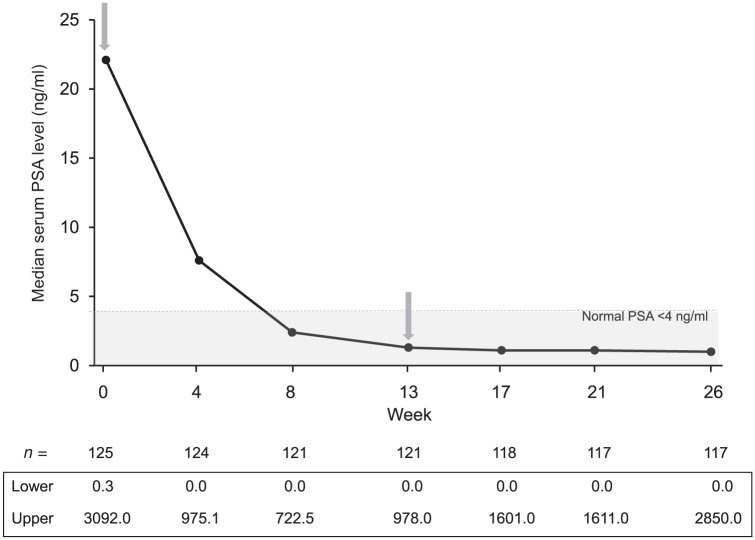
In the ITT population, median PSA levels were reduced by 64.2 and 96.0%, 4 and 26 weeks after the first SC injection of triptorelin, respectively, and median PSA levels were within the normal range (0–4 ng/ml) from 8 weeks until the end of the study (Figure 3).
Figure 3.
Median serum prostate specific antigen (PSA) levels (ng/ml) in patients receiving triptorelin pamoate (11.25 mg) by the subcutaneous route (ITT population) at baseline and week 13.
Arrows denote when triptorelin injections were administered.
ITT, intention-to-treat.
Pharmacokinetics
Following the first SC administration of triptorelin (in a subset of 18 patients), the median Cmax of triptorelin was 17.17 ng/ml (range: 7.20–32.66 ng/ml) at a median Tmax of 4.5 h (range: 1–24 h) after injection. The median AUC0-24 was 296.4 ng*h/ml (range: 120.6–575.3 ng*h/ml).
In the ITT population, pre-injection Cmin at baseline and at weeks 13 and 26 were measured. At baseline, the concentration was below the limit of quantification in 118/119 participants (one patient sample gave a detectable value <0.03 ng/ml that may have been an artefact of the assay; no samples were collected from seven patients). After 13 and 26 weeks, triptorelin concentrations were above the limit of quantification in 121/122 patients (no samples were collected from four patients) and 109/117 patients (no samples were collected from nine patients), respectively. Median Cmin at 13 and 26 weeks (excluding four outliers) was 0.062 ng/ml (range: below limit of quantification to 0.158 ng/ml) and 0.044 ng/ml (range: below limit of quantification to 0.147 ng/ml), respectively. Globally, the trough triptorelin concentrations observed at week 26 were similar to those observed at week 13. In the one patient with undetectable levels of triptorelin at week 13, the testosterone level was 8.5 ng/dl. In the eight patients with undetectable levels of triptorelin at week 26, seven had testosterone levels <50 ng/dl and one had a testosterone level of 54.2 ng/dl. No direct correlation was observed, by visual inspection, between triptorelin Cmin at weeks 13 and 26 and testosterone levels; however, no formal modelling was performed between triptorelin concentrations and testosterone levels due to the limited sampling schedule in the subset of 18 patients assessed in detail for pharmacokinetic parameters.
Efficacy: LH and FSH
There was an initial increase in LH concentrations at week 1 after the first triptorelin injection but, from week 2 until the end of the study, LH levels were reduced (Table 3). Mean FSH concentrations started to decrease at week 1 and mean decrease in FSH was maintained until the end of the study (Table 3).
Table 3.
Mean percentage change from baseline in luteinizing hormone (LH) and follicle stimulating hormone (FSH) in patients receiving triptorelin pamoate (11.25 mg) by the subcutaneous route (ITT population).
| Time after first injection (weeks) | Mean serum LH values (IU/l) | LH, % change from baseline (±SEM) | Mean serum FSH values (IU/l) | FSH, % change from baseline (±SEM) |
|---|---|---|---|---|
| 1 | 8.0 | +37.6 ± 5.1 | 6.2 | −40.8 ± 4.5 |
| 2 | 3.2 | −45.7 ± 3.5 | 3.2 | −67.1 ± 2.5 |
| 4 | 0.7 | −87.8 ± 1.6 | 3.2 | −64.0 ± 2.2 |
| 8 | 0.1 | −97.5 ± 0.2 | 4.7 | −46.8 ± 3.2 |
| 13 | 0.2 | −96.1 ± 1.4 | 5.1 | −44.6 ± 3.3 |
| 17 | 0.1 | −97.5 ± 0.2 | 5.2 | −42.7 ± 3.3 |
| 21 | 0.1 | −97.9 ± 0.1 | 5.7 | −38.3 ± 3.4 |
| 26 | 0.2 | −96.2 ± 1.1 | 5.4 | −41.1 ± 3.3 |
ITT, intention-to-treat; SEM, standard error of mean; IU, international unit.
Safety assessments
A total of 45 (35.7%) of the 126 patients treated in the study experienced 122 TEAEs (Table 4) and 27 (21.4%) patients had at least one TEAE considered related to study drug. The most frequently occurring treatment related TEAEs were hot flushes in 13 patients (10.3%) and increased weight in seven patients (5.6%). Most TEAEs were mild to moderate; one patient had a grade 3 hot flush 3–4 days after the second triptorelin injection.
Table 4.
Incidence of treatment-emergent adverse events (TEAEs) occurring in >2% of patients (safety population).
| Adverse event | Number of TEAEs (%) (n = 126) |
|---|---|
| Any | 45 (35.7) |
| Hot flush | 13 (10.3) |
| Weight increased | 12 (9.5) |
| Weight decreased | 7 (5.6) |
| Hypertension | 6 (4.8) |
| Headache | 4 (3.2) |
| Erectile dysfunction | 3 (2.4) |
| Hyperhidrosis | 3 (2.4) |
Seven SAEs were reported from six patients during the study, none of which was considered related to study drug: one patient with grade 5 myocardial infarction resulting in death; one patient with grade 3 cardiac failure still ongoing at the end of the study; one patient with grade 1 pneumonia, which resolved after treatment for pneumonia; one patient with a history of anaemia developed grade 2 worsening anaemia, which resolved with sequelae after treatment for anaemia; one patient with grade 2 chronic obstructive pulmonary disease exacerbated with pneumonia, both of which resolved after treatment; and one patient with serious grade 2 fibula fracture, from which the patient recovered with appropriate treatment.
Discussion
This study assessed the efficacy, tolerability and PK of a sustained-release GnRH agonist delivered by SC injection in patients with prostate cancer. The primary objective of the study was met, with triptorelin pamoate 11.25 mg administered by the SC route achieving castration levels of testosterone in >95% of men with prostate cancer after 4 weeks. Castration was maintained up to 26 weeks (6 months) in >95% of these men. The number of patients achieving testosterone castration levels in this study is consistent with the data of previous clinical studies performed with triptorelin and other GnRH agonists delivered by the IM route [Abbou et al. 1997; Kuhn et al. 1997; Fontana et al. 2003; Heyns et al. 2003; Teillac et al. 2004; Lundstrom et al. 2009; Mounedji et al. 2011; Spitz et al. 2012; Martinez-Pineiro et al. 2013; Wex et al. 2013].
In addition, this study showed that from 8 to 26 weeks after the first triptorelin injection, >90% of men had a testosterone level <20 ng/dl. A lower cutoff of 20 ng/dl to define castration has been proposed [Morote et al. 2007; Perachino et al. 2010] and it is therefore encouraging that triptorelin administered by the SC route suppresses testosterone to these levels. These results are also consistent with the proportion of men achieving testosterone levels <20 ng/dl following triptorelin IM injection every 3 months [Mounedji et al. 2011; Ploussard and Mongiat-Artus, 2013].
The minimal and short-lived flare up of testosterone levels after the first triptorelin injection was consistent with that widely observed with the initiation of therapy with GnRH agonists [Thompson, 2001; Damber et al. 2012; Romero et al. 2012]. The median time to achieve castration was 22 days, which suggests that the flare of testosterone levels was no more prolonged with SC injection of triptorelin than would be expected with IM injection (reported mean time to achieve castration of 22 days) [Bouchot et al. 1998], and no such flare up was observed after the second injection.
The efficacy of SC triptorelin injections was confirmed by the reduction in median PSA levels, which were reduced 4 weeks after the first triptorelin injection by 64.2% and by 96.0% at the end of the study (week 26). Median PSA values remained within normal range (0–4 ng/ml) from 8 weeks after the first injection until the end of the study. These results are also broadly consistent with PSA reductions achieved with 3-month triptorelin formulation when delivered by the IM route [Teillac et al. 2004].
Although there was variability within studies, the PK parameters of triptorelin when delivered by the SC route (as measured by median Cmax and Cmin) were within the same range as with IM administration [Bouchot et al. 1998]. For example, in the subset of 18 patients, median Cmax was 17.17 ng/ml (range: 7.20–32.66 ng/ml) with SC administration of triptorelin, and in the previous study was 32.90 ng/ml (range: 9.05–70.68 ng/ml) with IM administration [Bouchot et al. 1998]. In the whole population, Cmin was 0.044 ng/ml (range: below limit of quantification to 0.147 ng/ml) and 0.063 ng/ml (range: 0.021–0.174 ng/ml) with SC and IM administration, respectively [Bouchot et al. 1998]. Likewise, the safety profile of triptorelin administered by the SC route was similar to the known safety profile of triptorelin administered by the IM route [Abbou et al. 1997; Kuhn et al. 1997; Heyns et al. 2003; Teillac et al. 2004; Lundstrom et al. 2009; Mounedji et al. 2011; Martinez-Pineiro et al. 2013].
The findings of this study suggest that the triptorelin pamoate 11.25 mg 3-month formulation can be administered by the SC or IM route without any relevant difference in efficacy, PK and tolerability.
In men with prostate cancer, hormone therapy leading to castration is mainly used in three situations: metastatic prostate cancer; rising PSA after local treatment where micrometastases are suspected; and in adjuvant therapy in conjunction with radiotherapy. In these three situations, hormone therapy is mostly given to men over 60 years-old and in this aging population many men already use anticoagulants due to concomitant cardiac or thrombotic conditions. For this group of men, SC injections are preferable in order to avoid excessive bleeding and haematomas. Therefore, SC delivery of triptorelin pamoate provides a useful alternative to IM injection for men with prostate cancer who require chemical castration but are already receiving anticoagulant treatment.
In conclusion, this study demonstrates triptorelin pamoate 11.25 mg administered by the SC route every 3 months is as efficacious and well tolerated as administration via the IM route, and as such provides an alternative route to administer a GnRH agonist. In circumstances where IM injection is problematic or when individuals prefer SC injection, this study shows that SC injections of triptorelin can be used to reduce testosterone to current (<50 ng/dl) and proposed (<20 ng/dl) castration levels in men with prostate cancer.
Acknowledgments
We thank J.-C. Pouget (Ipsen) for statistical analyses and M.-O. Galcera and T.X.Q. Nguyen (both Ipsen) for performing PK assessments. John Clarke and Martin Gilmour (ESP Bioscience, Crowthorne, UK) provided editorial assistance funded by Ipsen.
Footnotes
Conflict of interest statement: T.L. served as the international coordinator and investigator for this Ipsen-funded study and has received fees from Novartis and Janssen. P.D. is an employee of Ipsen Innovation. The other authors have no conflicts of interest to declare in preparing this article.
Funding: This study was designed and funded by Ipsen.
Contributor Information
Thiery Lebret, Hôpital Foch - Université Versailles St Quentin en Yvelines, France.
Mathieu Rouanne, Hôpital Foch - Université Versailles St Quentin en Yvelines, France.
Oleg Hublarov, Latgalian Urology Centre, Daugavpils, Latvia.
Viorel Jinga, ‘Carol Davila’ University of Medicine and Pharmacy, Bucharest, Romania.
Lidiya Petkova, UMHAT Sveta Anna, Varna, Bulgaria.
Rumen Kotsev, UMHAT G. Stranski, Pleven, Bulgaria.
Ioanel Sinescu, Clinical Hospital Fundeni, Bucharest, Romania.
Pascale Dutailly, Ipsen Innovation, Les Ulis, France.
References
- Abbou C., Lucas C., Leblanc V. (1997) [Tolerance and clinical and biological responses during the first 6 months of treatment with 1-month sustained release LHRH agonists leuprolerin and triptolerin in patients with metastatic prostate cancer]. Prog Urol 7: 984–995. [PubMed] [Google Scholar]
- Alviggi C., Revelli A., Anserini P., Ranieri A., Fedele L., Strina I., et al. (2007) A prospective, randomised, controlled clinical study on the assessment of tolerability and of clinical efficacy of Merional (hMG-IBSA) administered subcutaneously versus Merional administered intramuscularly in women undergoing multifollicular ovarian stimulation in an ART programme (IVF). Reprod Biol Endocrinol 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchot O., Soret J., Jacqmin D., Lahlou N., Roger M., Blumberg J. (1998) Three-month sustained-release form of triptorelin in patients with advanced prostatic adenocarcinoma: results of an open pharmacodynamic and pharmacokinetic multicenter study. Horm Res 50: 89–93. [DOI] [PubMed] [Google Scholar]
- Damber J., Tammela T., Iversen P., Abrahamsson P., Boccon-Gibod L., Olesen T., et al. (2012) The effect of baseline testosterone on the efficacy of degarelix and leuprolide: further insights from a 12-month, comparative, phase III study in prostate cancer patients. Urology 80: 174–180. [DOI] [PubMed] [Google Scholar]
- Fontana D., Mari M., Martinelli A., Boccafoschi C., Magno C., Turriziani M., et al. (2003) 3-month formulation of goserelin acetate (‘Zoladex’ 10.8-mg depot) in advanced prostate cancer: results from an Italian, open, multicenter trial. Urol Int 70: 316–320. [DOI] [PubMed] [Google Scholar]
- Golekoh M., Hu S., Norman A., Horn P., Brady R., Wong B. (2013) Comparison of the immunogenicity of intramuscular versus subcutaneous administration of trivalent inactivated influenza vaccine in individuals with neuromuscular diseases. J Child Neurol 28: 596–601. [DOI] [PubMed] [Google Scholar]
- Heidenreich A., Bastian P., Bellmunt J., Bolla M., Joniau S., Van Der Kwast T., et al. (2014) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65: 467–479. [DOI] [PubMed] [Google Scholar]
- Heyns C., Simonin M., Grosgurin P., Schall R., Porchet H. (2003) Comparative efficacy of triptorelin pamoate and leuprolide acetate in men with advanced prostate cancer. BJU Int 92: 226–231. [DOI] [PubMed] [Google Scholar]
- Knuf M., Zepp F., Meyer C., Habermehl P., Maurer L., Burow H., et al. (2010) Safety, immunogenicity and immediate pain of intramuscular versus subcutaneous administration of a measles-mumps-rubella-varicella vaccine to children aged 11–21 months. Eur J Pediatr 169: 925–933. [DOI] [PubMed] [Google Scholar]
- Kuhn J., Abourachid H., Brucher P., Doutres J., Fretin J., Jaupitre A., et al. (1997) A randomized comparison of the clinical and hormonal effects of two GnRH agonists in patients with prostate cancer. Eur Urol 32: 397–403. [PubMed] [Google Scholar]
- Lee C. (1993) Thrombosis and anti-thrombotic therapy. In: Lee G., Bithell T., Foerster J., Athens J., Lukens J. (eds), Wintrobe’s Clinical Hematology, 9th edn. Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Lundstrom E., Rencken R., Van Wyk J., Coetzee L., Bahlmann J., Reif S., et al. (2009) Triptorelin 6-month formulation in the management of patients with locally advanced and metastatic prostate cancer: an open-label, non-comparative, multicentre, phase III study. Clin Drug Investig 29: 757–765. [DOI] [PubMed] [Google Scholar]
- Madbouly K., Alshahrani S., Al-Omair T., Matrafi H., Mansi M. (2011) Efficacy of local subcutaneous anesthesia versus intramuscular opioid sedation in extracorporeal shockwave lithotripsy: a randomized study. J Endourol 25: 845–849. [DOI] [PubMed] [Google Scholar]
- Martinez-Pineiro L., Schalken J., Cabri P., Maisonobe P., De La Taille A. and Triptocare Study Group. (2013) Evaluation of urinary prostate cancer antigen-3 (PCA3) and TMPRSS2-ERG score changes when starting androgen-deprivation therapy with triptorelin 6-month formulation in patients with locally advanced and metastatic prostate cancer. BJU Int 114: 608–616. [DOI] [PubMed] [Google Scholar]
- MedDRA. (2013) Medical dictionaries for regulatory activities, version 16.1. McLean, VA: MedDRA; Available at: http://www.meddra.org/how-to-use/support-documentation/english (accessed 30 October 2013). [Google Scholar]
- Morote J., Orsola A., Planas J., Trilla E., Raventos C., Cecchini L., et al. (2007) Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol 178: 1290–1295. [DOI] [PubMed] [Google Scholar]
- Mounedji N., Lundstrom E., Purcea D., Grosgurin P., Porchet H. (2011) Efficacy of triptorelin in lowering serum testosterone (sT) in patients with advanced prostate cancer. J Clin Oncol 29(Suppl. 7): A162. [Google Scholar]
- National Cancer Institute (2010) Common terminology criteria for adverse events (CTCAE), version 4.03. Rockville, MD: National Cancer Institute, National Institutes of Health, US Department of Health and Health Services. [Google Scholar]
- Palmberg C., Koivisto P., Visakorpi T., Tammela T. (1999) PSA decline is an independent prognostic marker in hormonally treated prostate cancer. Eur Urol 36: 191–196. [DOI] [PubMed] [Google Scholar]
- Perachino M., Cavalli V., Bravi F. (2010) Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int 105: 648–651. [DOI] [PubMed] [Google Scholar]
- Ploussard G., Mongiat-Artus P. (2013) Triptorelin in the management of prostate cancer. Future Oncol 9: 93–102. [DOI] [PubMed] [Google Scholar]
- Romero E., Velez De Mendizabal N., Cendros J., Peraire C., Bascompta E., Obach R., et al. (2012) Pharmacokinetic/pharmacodynamic model of the testosterone effects of triptorelin administered in sustained release formulations in patients with prostate cancer. J Pharmacol Exp Ther 342: 788–798. [DOI] [PubMed] [Google Scholar]
- Shaheen J., Amin M., Harty J. (1993) Patient compliance in treatment of prostate cancer with luteinizing hormone-releasing hormone (LHRH) agonist. Urology 42: 533–535. [DOI] [PubMed] [Google Scholar]
- Sharifi N., Gulley J., Dahut W. (2005) Androgen deprivation therapy for prostate cancer. JAMA 294: 238–244. [DOI] [PubMed] [Google Scholar]
- Spitz A., Young J., Larsen L., Mattia-Goldberg C., Donnelly J., Chwalisz K. (2012) Efficacy and safety of leuprolide acetate 6-month depot for suppression of testosterone in patients with prostate cancer. Prostate Cancer Prostatic Dis 15: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser M., Woodworth J., Swan S., Ticho B. (2006) Results of a randomized open-label crossover study of the bioequivalence of subcutaneous versus intramuscular administration of alefacept. Dermatol Online J 12: 1. [PubMed] [Google Scholar]
- Teillac P., Heyns C., Kaisary A., Bouchot O., Blumberg J. (2004) Pharmacodynamic equivalence of a decapeptyl 3-month SR formulation with the 28-day SR formulation in patients with advanced prostate cancer. Horm Res 62: 252–258. [DOI] [PubMed] [Google Scholar]
- Thompson I. (2001) Flare associated with LHRH-agonist therapy. Rev Urol 3(Suppl. 3): S10–S14. [PMC free article] [PubMed] [Google Scholar]
- Wex J., Sidhu M., Odeyemi I., Abou-Setta A., Retsa P., Tombal B. (2013) Leuprolide acetate 1-, 3- and 6-monthly depot formulations in androgen deprivation therapy for prostate cancer in nine European countries: evidence review and economic evaluation. Clinicoecon Outcomes Res 5: 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlinger M., Rusca A., Oraha A., Gugliotta B., Muller M., Ducharme M. (2012) Pharmacokinetics of a new diclofenac sodium formulation developed for subcutaneous and intramuscular administration. Int J Clin Pharmacol Ther 50: 383–390. [DOI] [PubMed] [Google Scholar]