Abstract
Purpose
The aim of this systematic literature review was to identify research-based evidence for an effect of antimicrobial therapeutic approaches on the cariogenic microbiota and early childhood caries (ECC) outcomes. Additionally, we reviewed methods used to perform microbial assessments in clinical studies of ECC.
Methods
Multiple database searches were conducted; only clinical cohort studies and randomized controlled trials published from 1998 to 2014 were selected for the review. A total of 471 titles and abstracts were identified; 114 studies met the inclusion criteria for a full review, and finally 41 studies were selected for the meta-analyses.
Results
Moderate reductions in cariogenic bacterial levels, mainly in mutans streptococci (MS), were demonstrated following the use of antimicrobial agents. The results varied depending on the different approaches used. In most of the reviewed studies MS levels were reduced after treatment, but the bacterial regrowth occurred once the treatment had ceased, and new caries lesions developed, particularly in high-risk children. Relatively consistent findings suggested that anti-cariogenic-microbial interventions in mothers significantly reduced MS acquisition by children. However, studies of the long-term benefits of ECC prevention are lacking.
Conclusion
Based on the meta-analyses, antimicrobial interventions and treatments show temporary reductions in MS colonization levels. However, insufficient evidence suggest that the approaches used produced sustainable effects on cariogenic microbial colonization, caries reduction, and ECC prevention.
Keywords: dental caries, oral microbiota, treatment effectiveness
INTRODUCTION
Despite a continuous decline in caries in the permanent dentition for many children, the prevalence of early childhood caries (ECC) in the United States remains overwhelmingly high among certain low-income or immigrant families, minority populations, and indigenous communities.1–5 The overall percentage of children with ECC was 17% from 1971–1975;6 16% from 1988–1994, and 28% from 1999–2004.7 Currently, ECC affects more than 25% of American preschool-aged children of all races8 with rates as high as 46% in Hispanic9, 66% to 70% of American Indian/American Native children,1,10. Although ECC is considered preventable, it remains the most frequently experienced and critically important chronic disease of young children because of its tenaciously high prevalence, high treatment costs, and negative effect on the oral health-related quality of life in children.11
The pathophysiological etiology of ECC is associated with early colonization and high levels of the cariogenic microorganism, e.g. Streptococcus mutans, an abundance of dental plaque, enamel defects in primary teeth, and childhood diets high in sugar and carbohydrates. Interactions among these primary risk factors produce an acidic environment in dental plaque, resulting in enamel and dentin decalcification. Other bacteria associated with ECC development and severity include S. sobrinus and Lactobacillus (LB) species. Dr. Horowitz’s 1998 report on “Research issues in early childhood caries”12 noted that “only limited research has been done on chemotherapeutic approaches to prevent or reduce the incidence of ECC” and that research on chemotherapeutic interventions should therefore focus on “Determining the effectiveness of individual and logical combinations of chemotherapeutic agents for preventing ECC”.12
Numerous antimicrobial clinical trials or intervention programs have been conducted worldwide since 1998 with the goal of suppressing cariogenic bacteria and reducing children’s caries experiences. Several antimicrobial agents (e.g., fluoride, chlorhexidine, iodine, xylitol, silver compounds) combined with a range of application methods (e.g., mouth rinse, gel, varnish, cleaning wipe, restorative materials) have been used, with remarkable reductions in S. mutans and S. sobrinus levels. Almost all of the “successful” results, however, lasted for only weeks to a few months post intervention, and reductions in S. mutans and S. sobrinus colonization were diminished when treatment was suspended. Few chemotherapeutic interventions have targeted the critical link between the pathogenic mechanisms of bacteria in ECC development. A recent search of the Cochrane library revealed 17 systematic reviews related to fluoride and ECC, 4 reviews on chlorhexidine plus fluoride and dental caries, 3 reviews on xylitol, and 5 reviews on other interventions or treatments of ECC. None of these reviews addressed the microbiological effects of antimicrobial agents on ECC outcomes. High post-treatment caries relapse rates were reported, suggesting that most of the interventions had limited long-term beneficial effects on ECC. Thus, there is a lack of understanding as to the sustainability of bacterial reductions and how antimicrobial interventions can alter the ECC-associated microbial community. As such, the research mission set up a decade ago has not yet been accomplished.
Most microbiology in clinical studies of ECC focus on mutans streptococci (MS) and lactobacilli (LB), which are routinely detected using selective-culture-based methods. However, the microbiota of caries-associated biofilms have long been recognized to contain a wide diversity of bacteria, including species of Actinomyces, Fusobacterium, Scardovia, Bifidobacterium, Atopobium, Prevotella, Veillonella, and Candida.13–17 Advanced clinical study designs and the selection of acid-tolerant bacteria have been explored to distinguish the key contributors to caries progression. The caries-free and ECC microbiotas differ, suggesting that a disturbance of the whole polymicrobial community, and not just the levels of MS and LB, plays a role in caries etiology.13,18,19 The review identified several reports of microbial diversity in ECC, some of which linked treatment outcomes with changes in S. mutans subtypes or in the microbiota as a whole.
METHODS
The systematic review and meta-analysis were conducted according to the methods of the Cochrane Handbook.20 Multiple searches were performed based on PubMed (NLM), Ovid Medline, the Library of Congress, the Web of Science Core Collection, and the Cochrane Database of Systematic Reviews. Our strategy first limited searches to clinical trials, randomized controlled trials, systemic reviews, and meta-analysis; then the 1998 to “Current” database published in English; and finally limited the keywords to three groups based on the methods and antimicrobial agents used for interventions. These groups were as follows: (1) ECC, dental caries, tooth, deciduous, child, infant, preschool, risk factors; (2) clinical trial, fluoride, chlorhexidine, iodine, xylitol, topical therapeutic use, silver compounds, silver, silver proteins, silver nitrate, silver diamine fluoride; and (3) bacterial Infections, anti-bacterial agents, antimicrobial therapy, Streptococcus, saliva, sequence analysis, mouth, bacteria, anaerobic, metagenome, oral microbiome, DNA, bacterial proteins, RNA, ribosomal.
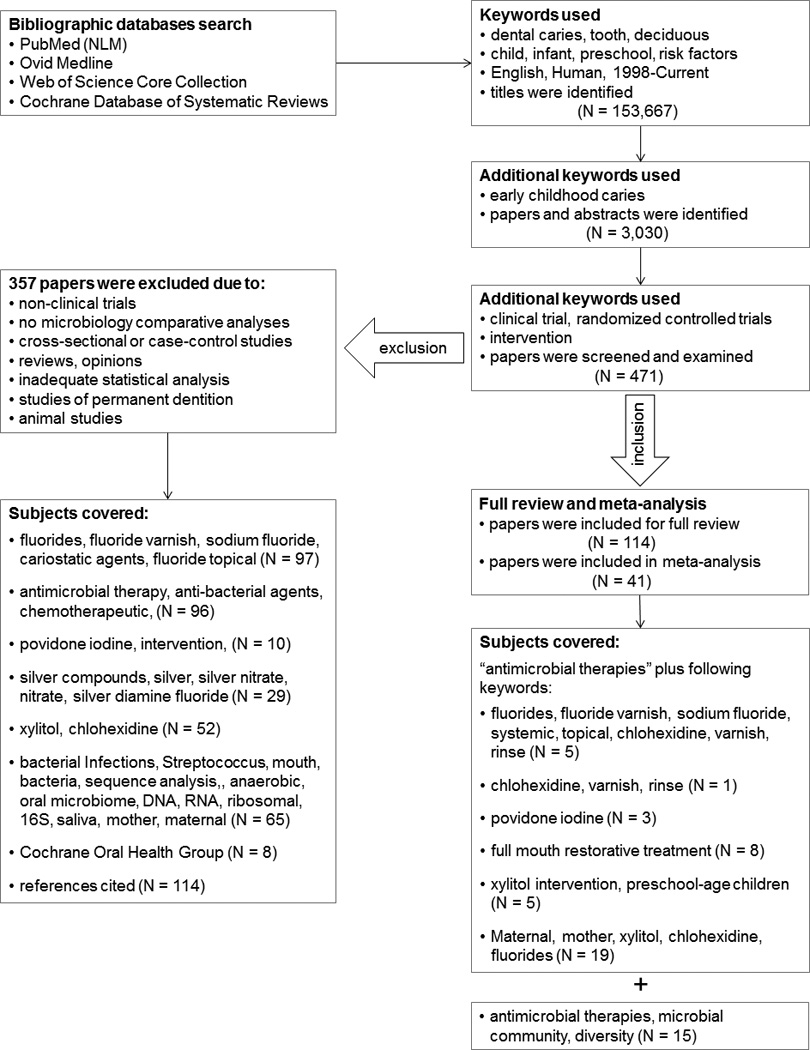
The search strategies, as well as the inclusion and exclusion criteria, are illustrated in Figure 1. Among those excluded were non-clinical trials, cross-sectional studies, case-control studies, studies without microbiological analysis, studies of permanent dentitions, and animal studies. Randomized controlled trials selected for analysis had to consist of at least 4 weeks of observation, and prospective cohort studies that were selected had to include at least 3 months of observation. The main outcome evaluations for all of the clinical trials were the reduction of cariogenic microbiota and the incidence of new ECC lesions after the antimicrobial treatment. Data were extracted according to study design, number of participants, intervention approach, duration of trials, microbiological assessment methods, outcome measurements, and valid statistical methods used.
Figure 1.
The effect size of each antimicrobial intervention on the cariogenic microbiota in preschool-aged children was further examined by a meta-analysis using the Comprehensive Meta-Analysis Program (Version 2 Biostate, Englewood, NJ). The variables used for the statistical analysis included estimates of means, variances, proportions, and rates of changes of bacterial measurements, and caries scores, as well as ECC incidence in each experimental, treatment, or control group for a given sample size. For all of the clinical studies, only data at the baseline and at the end of the treatment/intervention period were used for comparisons in the meta-analysis. Statistics for each study and summary effects included odds ratios and 95% confidence intervals, which were displayed as forest plots. Cochran’s Q test and the Hinging Index (I2) were used to determine the significance of the heterogeneity among studies.21 A fixed-effect model was used to determine the summary results. Heterogeneity tests were employed to validate the fixed-effect model assumption that all studies in the meta-analysis shared a common effect size. A two-sided P < 0.05 was considered significant for all analyses.
MAIN FINDINGS
According to the search criteria, we initially identified 471 titles and abstracts. Examination of these abstracts resulted in 114 publications for detailed review under seven categories: (1) studies using fluoride varnish (FV) topical therapeutic applications; (2) studies using chlorhexidine (CHX) varnish and all other antimicrobial therapies; (3) studies using Povidone iodine (PVP-I) applications; (4) studies of full-mouth restorative treatment with or without antimicrobial treatment; (5) studies of xylitol intervention in MS levels in children; (6) studies of the effect of maternal antimicrobial intervention on MS colonization of children and ECC outcome; and (7) studies using silver and other heavy metal compounds as antimicrobial agents. Finally, only 41 studies met all inclusion criteria (Fig 1.) and were selected for meta-analyses under the different review categories. Taking into account the diversity of the ECC-microbiome, we extended the search to include studies that described some measure of microbial diversity related to the different treatment regimens.
Most clinical studies of ECC that included microbial monitoring limited their bacterial detections to MS with or without testing for Lactobacillus species. The microbiological methods consisted of either selective culture or commercial tests based on selective culture principals. The most frequently used tests were mitis salivarius bacitracin (MSB)22,23 agar for S. mutans, the Dentocult SM Strip mutans ® test (Orion Diagnostica, Espoo, Finland) and the Caries Risk Tests (CRT®) (Ivoclar Vivadent) for MS or Lactobacillus species (Tables 1–4). Most selective media formulations for S. mutans were based on a mitis-salivarius agar (MSA) described by Chapman in 1946 for the detection of enteric streptococci.24 For S. mutans detection, MSA was modified by the addition of sucrose to facilitate species detection from colony morphology and antibiotics to suppress the non-MS microbiota, e.g. mitis-salivarius-sucrose-bacitracin medium (MSB)22 and mitis-salivarius-kanamycin-bacitracin medium (MSKB).25 Those selective media were formulated for the specific identification of S. mutans without “contamination” from other bacteria. Another selective medium for S. mutans is trypticase-yeast-cysteine-sucrose-bacitracin agar (TYCSB), which contains fewer inhibitors than MSA and offers a 10-fold higher recovery rate for S. mutans.26,27 For the optimal identification of S. mutans in clinical studies without microbiology laboratory assistance, MSB, MSKB and commercial tests (e.g., Dentocult SM at www.oriondiagnostica.fi, CarioCheck at www.hainlifescience.com/products/dentaldiag-nostics.html, the CRT test28) would be appropriate. For the sensitive detection of S. mutans and S. sobrinus, TYCSB medium which has fewer inhibitory agents but still distinctive S. mutans and S. sobrinus colonies, can be used. Additional selective media and derived commercial tests include low-pH SL agar29 and LBS agar30 for Lactobacillus species, Veillonell agar for Veillonella species31, and Sabaouraud dextrose agar32 for yeast or Candida species.
Table 1.
Effects of antimicrobial intervention on the oral microbiota of ECC children
| Author, Year | Study Design, Country |
Sample Size Age |
Treatment & Interventions |
Duration | Microbiological Method |
Evidence |
|---|---|---|---|---|---|---|
|
Fluoride application combined with chlorhexidine and other treatments | ||||||
| Lobo, et al., 200845 |
Randomized clinical trial Brazil |
N=35, ECC 4–8 years |
Grp1, 1.23% NaF gel, Grp2, 1% CHX gel Applied for 10 min, every 24 h for 6 consecutive days |
1 month | Selective culture: MSB for MS |
-A 6-day treatment with a 1% CHX gel was effective in reducing salivary MS. There was a significant MS increase once treatment was suspended. -The use of 1.23% NaF under the same regimen was not able to reduce salivary MS levels. |
| Plonka, et.al, 2013111 |
Randomized clinical trial Australia |
N=622 0.5–2 years |
Twice daily tooth- brushing with fluoride toothpaste with: Grp1, 10% casein phosphopeptide- amorphous calcium phosphate (CPP- ACP) paste Grp2, 0.12% CHX Grp3, Control (SC, no additive) |
24 months | Chairside test: CRT Bacteria (Ivoclar Vivadent) for MS and LB |
-At the 12-month and 18-month of age, MS detection rates were 0% and 5% in CPP-ACP group; 22% and 72% in CHX group, and 16% and 50% in SC groups. -At the 24-month recall, the caries incidence rates were 1% in the CPP-ACP group, 2% in the CHX group, and 2% in the SC group. -In addition to daily use of fluoride toothpaste, there is insufficient evidence to justify the daily use of CPP-ACP paste or CHX gel to control early childhood caries. |
| Plotzitza, et al ., 2005112 |
Prospective follow-up study Germany |
N=172 1 year Low, high risk, control |
Fluoride tables + fluoride salt + fluoride toothpaste Grp1, 1% CHX varnish used 3-month intervals Grp2, No CHX treatment controls |
24 months | Chairside test: CRT Bacteria (Ivoclar Vivadent) for MS and LB |
-The mean dmft value increased from 0.05 ±0.4 to 0.8 ±2.9, and the mean dmfs value rose from 0.08 ± 0.8 to 1.8 ± 5.9. -At 24 months of age, 26.2% of the two-year-olds had salivary scores of MS ≥105 CFU/ml in saliva. There were no significant differences in MS scores between the CHX and control groups. |
| Pukallus, et al .,2013113 |
Randomized clinical trial Australia |
N=234 0.5–2 years |
Twice-daily tooth- brushing using 0.304% w/w fluoride toothpaste alone with: Grp1, 0.12% CHX gel Grp2, Control, low dose fluoride toothpaste |
24 months | Chairside test: CRT Bacteria (Ivoclar Vivadent) for MS and LB |
-At 24 months, the caries prevalence rates were 5% in the CHX group and 7% in the control group. -There were no differences in percentages of MS-positive children between the CHX (54%) and control groups (53%). -Tooth brushing using low-dose fluoride toothpaste with or without the application of CHX 0.12% reduced ECC from 23% found in the general community to 5–7%. |
| Stecksen- Blicks, et al., 2009114 |
Randomized clinical trial Sweden |
N=248 1–5 years |
Grp1, fluoride and probiotic bacteria in skim milk Grp2, skim milk only |
21 months | Selective culture: MSKB (mitis salivarius, kanamycin, bacitracin) for MS |
-The proportion of MS compared with the total cultivable microflora was lower in the intervention group compared with the control group after 21 months. The mean MS levels remained unchanged throughout the study period. -There was a significant difference in the caries increment after 21 months between the groups with a prevented fraction of 75%. |
| CHX application as the main treatment | ||||||
| Twetman, et al ., 199944 |
Prospective follow-up study Sweden |
N=37 1.5 years |
1% CHX gel twice daily brush for 14 days |
3 months | Chairside test: Dentocult SM Strip for MS |
-A significant reduction of MS detection after 1 month compared with baseline. After 3 months, the difference from baseline was diminished. |
| Topical application of PVP-I | ||||||
| Berkowitz, et al., 2009115 |
Clinical exploratory study United States |
N=77 2 – 5 years |
Caries restorative treatment followed by Grp1, 10% PVP-I solution Grp2, 1.23% APF foam |
3 months | Selective culture: MSB for MS |
-Approximately 50% of subjects had a >95% reduction in MS in the saliva at the follow-up visit compared to the MS level at baseline. -PVP-I with dental surgery significantly suppressed salivary MS levels for S-ECC for at least 90 days. -Treatment with PVP-I may be an important adjunct to dental surgery for S-ECC. |
| El-Housseiny, et al. 2005116 |
Randomized clinical trial Saudi Arabia |
N=54 4–6 years |
Grp1, 1.23% APF weekly for 4 weeks, then every 3 months for one year Grp2, 1.23% APF + 10% PVP-I for 4 weeks. |
12 months | Chairside test: CRT bacteria for both MS and LB |
-There were no significant differences in MS and LB counts between the two groups in all of the evaluation periods, excluding LB at the 3-month evaluation. -The number of carious lesions was significantly reduced at the follow-up evaluation compared to baseline, but there were no significant differences between the two groups in the intervening evaluation periods. |
| Lopez, et al, 200253 |
Randomized clinical trial Puerto Rico |
N=83 1–1.5 years |
-10% PVP-I -Placebo solution |
12 months | Selective culture: MSB for MS |
-Kaplan-Meier survival estimates showed that among disease- free children, 91% received treatment compared to 54% in the control group. -Topical antimicrobial therapy increases disease-free survival in children at a high risk for ECC. |
Table 4.
Effects of maternal antimicrobial intervention on cariogenic microbial reductions and ECC outcomes in children
| Author, Year |
Study Design Country |
Sample Size Children Age |
Treatment & Interventions |
Duration | Microbiological Evaluation Method |
Evidence |
|---|---|---|---|---|---|---|
| Alamoudi, et al., 2014121 |
Randomized clinical trial Saudi Arabia |
N=60 Mother-child dyads 10–36 months |
Grp1, chewing xylitol gum after three meals for 3 months Grp2, fluoride varnish (5% NaF) every 6 months |
24 months | Chairside test: Dentocult SM Strip methods for MS |
-Children with high MS counts: no significant difference was found between the two groups. There was a significant increase in caries in the control group compared to baseline. -Caries (dmft) scores: more than a 60% increase in the control group, less than 20% increase in the experimental group, but the difference was significant only at the 24-month recall. -Compared with fluoride varnish, maternal xylitol consumption seems to provide preventive outcomes in salivary MS and caries levels in children. |
| Brambilla, et al., 1998122 |
Prospective observational study Italy |
N=60 Mother-child dyads 0–24 months |
Grp1, F tablet daily + rinsed daily with 0.05% NaF and 0.12% CHX, for 6 months Grp2, F tablet daily for 6 months only |
30 months (started at 6 months pregnancy) |
Selective culture: MSB agar for MS level |
-Over the 30-month study period, the NaF and CHX treatment regimen significantly reduced the salivary MS level in the mothers. -Fewer children in the experimental group were colonized by MS in saliva compared to those in the control group. -The treatment significantly reduced salivary MS levels in mothers and delayed bacterial colonization in their children for approximately 4 months. |
| Dasanayake, et al., 200243 |
Randomized clinical trial United States |
N=75 Mother-child dyads 6–48 months |
Grp1, 10% CHX varnish (Chlorzoin®) Grp2, varnish contained 1% hydroxypropyl cellulose, 0.2% quinine hydrochloride |
24 months | Selective culture: MSB agar for MS level |
-Mothers in the CHX group exhibited a significant reduction in S. mutans levels in the saliva compared to the control group for up to 12 months. -There were no significant differences in the percentage of children with detectable levels of S. mutans in plaque during the study period. -There were no significant differences in caries increment either among mothers or among children. |
| Fontana, et al., 200992 |
Randomized clinical trial United States |
N=97 Mother-child dyads 9–14 months |
Grp1, Xylitol gum (3x/day for 9 months) Grp2, Xylitol gum (3x/day for 3 months) Grp3, Sorbitol gum 3x/day for 9 months Grp4, No gum |
9–10 months |
Selective culture: MSB for MS counts MSA for total streptococci counts |
-MS could be recovered from one third of the predentate infants. -There were no statistically significant differences in the effects of maternal use of xylitol-containing chewing gum for 3 or 9 months on MS colonization and total bacterial counts in 9- to l4-month- old infants. |
| Gripp, et al., 2002123 |
Randomized clinical trial Germany |
N=44 Mother-child dyads 6–24 months |
Grp1, high MS score, received 40% CHX varnish (EC-40), 3- month intervals Grp2, high MS score, no CHX Grp3, low MS score, received CHX varnish at 6-month intervals |
24 months | Mothers: Chairside test: Dentocult SM Strip methods for MS counts Children: Selective culture: MSB for MS counts |
-For mothers: a significant decrease in high MS values in the CHX group compared to baseline. -For children at 24 months, 19% were MS positive in the CHX group: 40% in Grp2 and 20% in Grp3. The difference was significant. |
| Gunay, et al., 1998124 |
Prospective observational study Germany |
N=86 Mother-child dyads 0–6 years |
Grp1, recalled every 6 months and intervention: - oral hygiene instructions - professional tooth cleaning - topical fluoride varnish application - CHX mouth rinsing - dietary counselling Grp2, no intervention |
4 years (started in the 3rd trimester of pregnancy) |
Chairside test: Dentocult SM Strip methods for MS counts |
-There were significant reductions in MS score and percentage of MS positivity in saliva for both mothers and children. -Pre- and postnatal preventive programs may significantly improve the oral health of mothers and their children. -The study prophylaxis concept is recommended for incorporation into the routine (dental) care of mothers and their young children. |
| Hanno, et al., 2011125 |
Randomized clinical trial Saudi Arabia |
N=60 Mother-child dyads 2–5 years |
Grp1, - mother-xylitol chewing gums; children-xylitol chewable tablets. Grp2, NaF varnish |
3 months | Chairside test: CRT kit (Vivadent- Ivoclar, Lichenstein) for MS counts |
-At 3 month examination, the number of mother-child pairs with high MS levels in experimental group significantly decreased, but not in control group. -No difference in caries scores of the children. |
| Isokangas, et al., 2000126 Soderling, et al., 2000 & 2001127,128 Laitala, et al., 2012 & 201369,70 |
Randomized clinical Trial Finland |
N=169 Mother-child dyads 0–10 years |
Grp1, Xyl, xylitol gum 2–3 times per day Grp2, CHX, received CHX varnish at 6, 12, 18, mo. Grp3, FV, received FV at 6, 12, 18, mo. |
10 years | Selective culture: MSB agar for MS counts |
At 2 years of age: -The differences in MS levels were not significant between the FV and CHX groups. At the evaluation at 3 years of age: - Compared with the Xyl group, the risk of MS colonization was 2.3-fold higher in the F group. The differences between the FV and CHX groups were significant. At the evaluation at 5 years of age: - Dentinal caries (dmf) in the Xyl group were reduced by 71% compared to the FV group and 74% compared to the CHX group. The difference between the CHX and FV group was not statistically significant. At the evaluation at 6 years of age: - 51.6% of the children in the Xyl, 83.9% in the CHX, and 86.4% in the FV group were colonized by MS. The difference was significant between the Xyl and FV groups. At the evaluation at 10 years of age: -The children who were not colonized by MS at the age of 2 years had a longer caries-free survival time and fewer caries experience compared with MS-colonized children. Conclusions: Maternal use of xylitol chewing gum can prevent dental caries in their children by suppressing transmission of MS from mother to child. |
| Nakai, et al., 2010129 |
Randomized clinical trial Japan |
N=107 Mother-child dyads 0–2 years |
Grp1, Xylitol gum, chew 5 min, 4 times/day Grp2, no-xylitol control |
24 months (started at 3–5 months of pregnancy) |
Chairside test: Dentocult SM Strip methods for MS counts |
-Children in the xylitol group were significantly less likely to be MS-positive than those in the control group. -Children in the control group acquired MS 8.8 months earlier than those in the Xylitol group. -Maternal xylitol gum chewing in Japan shows beneficial effects. |
| Olak, et al., 2012130 |
Randomized clinical trial Estonia |
N=90 Mother-child dyads 2–3 years |
Grp1 & 2, Xylitol gum chew 4 times daily for 33 months Grp3, no-xylitol control |
36 months | Chairside test: Dentocult SM Strip methods for MS counts |
-The numbers and proportions of caries-free children were 80% at 2 years of age and 64% at 3 years of age. -The number of caries-free children was significantly higher in the intervention group than in the control group at both 2 and at 3 years of age. |
| Plonka, et al., 2013111 |
Randomized clinical trial Australia |
N=622 Mother-child dyads 6–18 months |
Grp1, 0.12% CHX gel Grp2, 10% CPP- ACP) cream Grp3, Control |
24 months | Chairside test: CRT kit (Vivadent- Ivoclar, Lichenstein) for MS and LB counts |
- MS-positive at 24 months: 72% in the CHX group; 5% in the CPP-ACP group; 50% in the control group. - LB-positive at 24 months: 63% in the CHX group; 63% in the CPP-ACP group; 65% in the control group. - Caries incidence at 24 months: 2% in the CHX group; 1% in the CPP-ACP group; 2% in the control group. - There is insufficient evidence to justify the daily use of APP-ACP or CHX gel to control early childhood caries. |
| Ramos-Gomes, et al., 2012131 |
Randomized clinical trial United States (Mexican- American, CA) |
N=361 Mother–child dyads 12–36 months |
Intervention: -mother received CHX (0.12% mouthrinse) twice daily for 3 months -children received FV (5% NaF) every 6 months from age12 to 36 months Control: -children received FV only if precavitated lesions developed. |
36 months (started at 4 months postpartum for all mothers) |
Selective culture: BHI agar for MS counts |
- Maternal MS levels declined during CHX use but increased following discontinuation. - At 36 months of age, 34% of the children in each group developed caries. There were no significant differences in the incidence of caries in children between the two groups. - Approximately half of the control group developed precavitated lesions and received therapeutic FV. - Maternal postpartum CHX regimen, oral health counselling and preventive child FV applications were not more efficacious than maternal counselling with child therapeutic FV for precavitated lesions for ECC prevention. |
| Thorid, et al., 2004, 2006, & 201271–73 |
Randomized clinical Trial Sweden |
N=173 Mother–child dyads 3–10 years |
Mothers with high counts of salivary MS were randomly assigned into 3 groups: Grp1, xylitol (n = 61) Grp2, chlorhexidine/xylitol/ sorbitol (n = 55) Grp3, sodium fluoride/xylitol/ sorbitol (n = 57) |
10 years | Chairside Test Dentocult SM Strip methods for MS counts |
At the evaluation at 3 years of age: -Lower but non-significant levels of salivary MS and dental decay were observed in 3-year-old children of mothers who used high- content xylitol gums. At the evaluation at 4 years of age: -The difference between the Xyl and F/Xyl/Sor groups was significant. Thus, fewer caries were observed in children of Xyl- gum mothers compared to non-Xyl-gum groups. At the evaluation at 10 years of age: -The overall caries prevalence in the combined groups at 10 years of age was 31%. There were no significant differences between the three experimental groups. Conclusions: -No long-term beneficial effects of maternal xylitol gum exposure on their children’s dental health were demonstrated when compared with gums containing CHX and fluoride. The study demonstrated a significant positive effect on the reduction of salivary MS colonization at 18 months of age and lower caries experience at the age of 10 years in children as a result of xylitol usage in a Swedish population. |
1. Effect of fluoride applications on the reduction of the oral microbiota
There is considerable evidence supporting a correlation between professionally applied fluoride and caries reduction in children and adolescents.33–35 The role of fluoride as an anti-caries agent is supported by many epidemiological investigations.36 The mechanism by which fluoride inhibits carbohydrate metabolism by acidogenic microorganisms has been demonstrated based in vitro studies.37 Currently, the most frequently used agents are 5% sodium fluoride varnish (NaFV; 22,500 ppm F), 1.23% acidulated phosphate fluoride gel (APF; 12,300 ppm F), 0.2% sodium fluoride (NaF) mouthrinse (900 ppm F), and 1.1% NaF (5,000 ppm F) brush-on paste/gels. Fluoride varnish (FV) has been shown to be a safe and effective chemo-preventive agent and is increasingly incorporated into dental and medical clinical practices and in community-based interventions for ECC.38 Although administering FV treatment at least twice a year is highly recommended by the American Dental Association (ADA) and the American Academy of Pediatric Dentistry (AAPD) for children with an increased caries risk,36,38 very few studies have described FV antimicrobial efficacy in children with ECC.
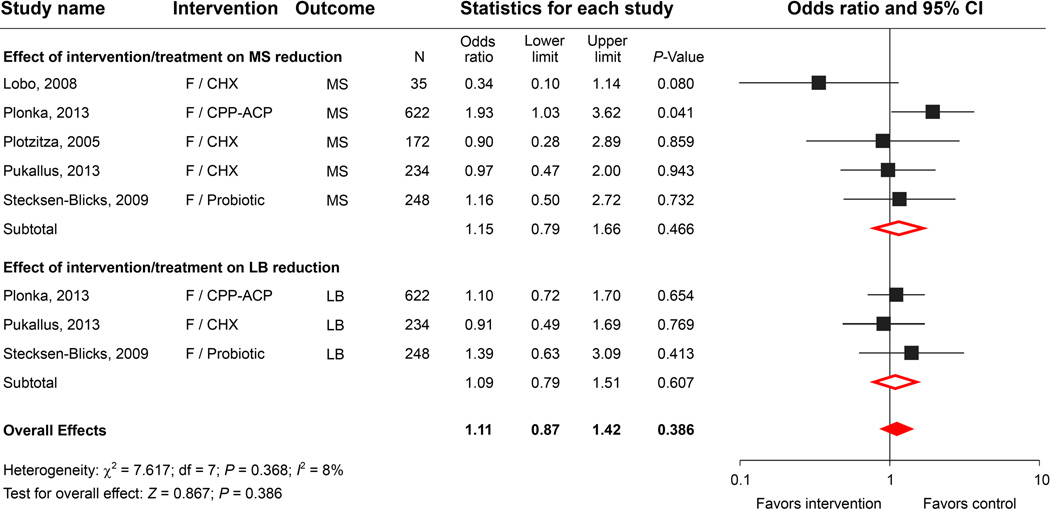
Our initial literature search revealed 338 articles on topical fluoride application in children, among which 178 were clinical trials with differing designs. None of the 178 studies incorporated microbiological evaluations of fluoride as a single agent for intervention. We found only 5 studies used different fluoride applications combined with other interventions that met the selection criteria and were included in the meta-analysis (Table 1). The meta-analysis indicated that combining NaF application with other antimicrobials showed some degree of MS and LB reduction. The odds ratio for the summary effect was 1.11, with a 95% confidence interval of 0.87 to 1.42 and a P-value of 0.386, indicating that the overall reduction was not statistically significant (Fig. 2A).
Figure 2.
2. Effect of chlorhexidine varnish intervention on the reduction of the oral microbiota
Chlorhexidine has a long history of use in caries prevention trials.39,40 A previous meta-analysis of eight studies published between 1975 and 1994 reported that the caries-inhibiting effect of CHX treatment was approximately 46%.41 More recent findings, however, has been inconclusive regarding the use of CHX varnishes for caries prevention, mostly for permanent dentitions, in high-risk groups.42 It has been suggested that the observed inconsistencies might not be simply due to the agent itself but to a combination of factors, such as the concentration used, the nature of delivery, the frequency and the duration of the application.43
Although there are a number of clinical trials using CHX varnish or CHX gel for young children, very few of these studies included microbial assessments after CHX application. Using the search strategy, we identified 50 studies of CHX and dental caries. As listed in Table 1, 4 studies reported combined treatment with various CHX agents and fluoride or other antimicrobial applications. We found only one prospective observational study that evaluated the effect of 1% CHX varnish as an ECC intervention agent on MS colonization.44 In a comparison study, Lobo, et al. observed that CHX treatment demonstrated a significantly higher efficacy in MS reduction when compared to NaF.45 A study performed by Klinke’s group demonstrated that daily brushing with a 0.2% CHX gel for two weeks was effective in reducing salivary MS, LB and additionally Candida species.46 However, because all of the children in the study received a comprehensive restorative treatment after the CHX regimen, either the CHX or the restorative treatment could have contributed to the microbial reductions. Using only CHX as a preventive agent, Twetman, et al. reported a significant reduction in MS at an early stage of the intervention, but after 3 months, the significance of the reduction was diminished.44 Results from the meta-analysis indicated that there is insufficient evidence to conclude that the daily use of CHX alone or in combination with fluoride application for an extensive period would reduce MS or LB levels in young children (Fig. 2B).
3. Effect of povidone iodine treatment on the reduction of the oral microbiota
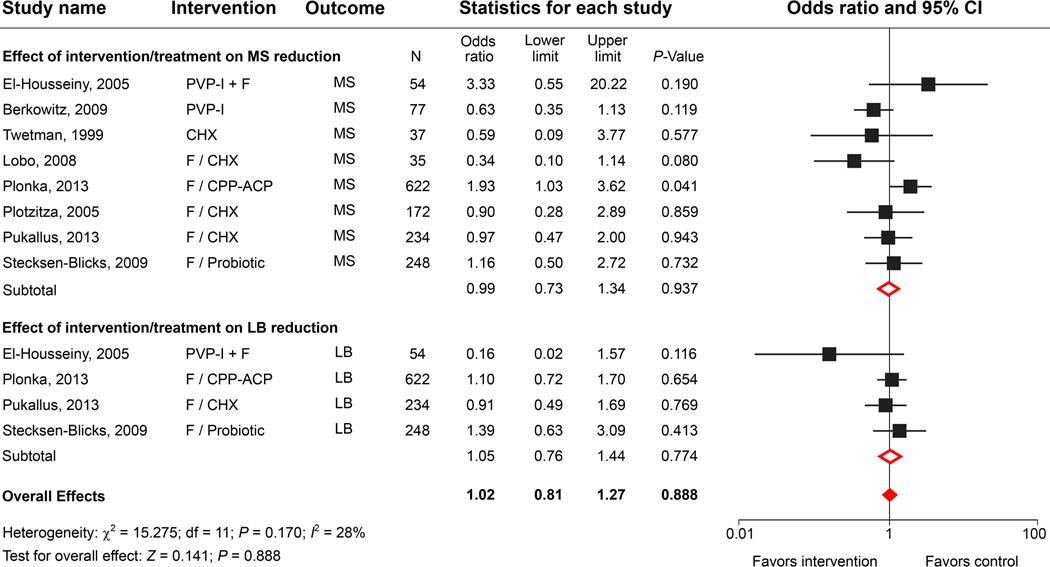
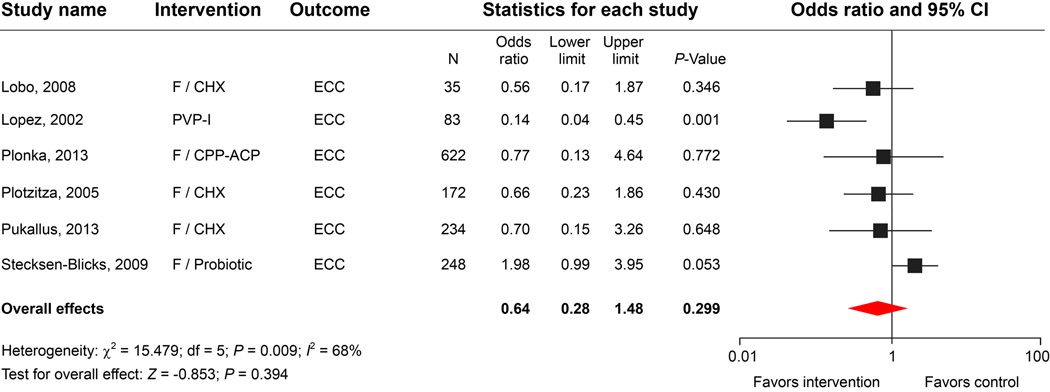
Povidone-iodine solutions are stable chemical complexes that are used as effective broad-spectrum topical antimicrobial agents with less toxicity towards mammalian cells than other commonly used agents.47 PVP-I has been used for many decades as a topical antimicrobial therapy in the treatment and prevention of dental caries in clinical studies.48 Several studies found that PVP-I temporarily reduced MS and LB counts in young children49,50 and was associated with decreased ECC risk in high-risk children. A combination of PVP-I and FV led to a greater reduction in caries incidence than the use of FV alone.51,52 However, most of the studies were performed on permanent or mixed dentitions. Additionally, very few studies incorporated detailed microbiological evaluations to test the efficacy of PVP-I applications.
Our literature search identified 14 clinical trials of “iodine” or “povidone iodine” and “ECC intervention”. We examined eleven studies; 8 trials were excluded due to a lack of microbiological analyses, leaving only 3 studies for the meta-analysis (Table 1). Although 2 studies reported significant reductions of MS (Berkowitz’s study) and LB (El-Housseiny’s study) lasting at least 3 months in the experimental groups treated with 10% PVP-I, including those studies in the meta-analysis model did not improve the overall effects on the cariogenic bacterial reduction (Fig. 2B). Despite the ambiguity in long-term effects of PVP-I on bacterial and ECC reduction, the meta-analysis of ECC outcomes revealed that bi-weekly topical application of PVP-I for 12 months (the Lopez study) significantly increased caries-free outcomes in children at a high risk for ECC compared with other studies in which different antimicrobial agents were used (Fig. 2C).53
4. Effect of a full-mouth comprehensive restoration on the reduction of the oral microbiota
Full-mouth restorative treatment under general anesthesia is used for children with severe ECC, particularly children in low social-economic families.54,55 The regiment generally comprises surgical removal of carious lesions, extraction of un-restorable teeth, and restoration of cavities. Significant reductions in cariogenic bacterial counts in saliva have been reported after comprehensive treatment.46,56–59 Clinicians frequently add an antimicrobial application to the treatment procedure to further reduce the risk of caries recurrence.46,56,57,60 Nevertheless, questions remain regarding the beneficial effects of either full-mouth treatment under general anesthesia alone or in combination with antimicrobial approaches against the total cariogenic microbiota, as well as the outcome of caries incidence in children.46,61,62
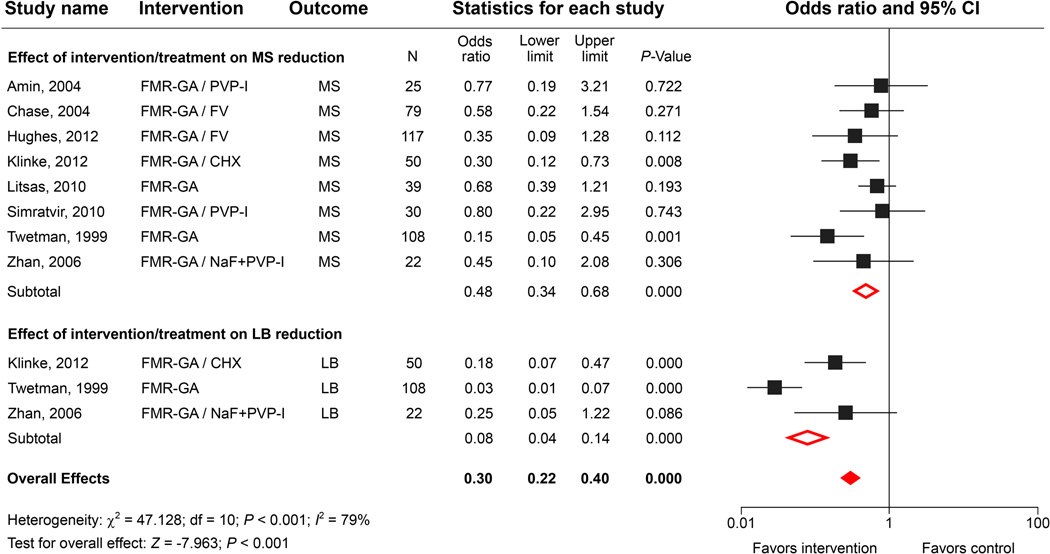
We identified 8 studies that incorporated microbiological evaluations after comprehensive restorative treatment under general anesthesia (Table 2). Two of the 8 studies were observational and did not include antimicrobial therapy. There were 3 observational follow-up studies and 3 randomized clinical trials in which children were given single or combined antimicrobial therapies before or after extensive restorations. The meta-analysis clearly showed a significant overall effect on the reduction of MS levels. Interestingly, 3 reports showed that the extensive treatment was more effective at reducing LB levels compared with MS levels (Fig. 3). It is not clear whether the bacterial reductions were the result of the surgical procedures or the antimicrobial treatments. The combined comprehensive restoration and PVP-I treatment decreased the total bacterial counts, but the reduction was not significant. The meta-analysis further showed that the odds ratio was 0.31 with a 95% confidence interval of 0.23 to 0.41 and that the summary effect was significant when comparing different treatments (P value = < 0.001 (Fig. 3). These findings suggested that full-mouth comprehensive treatment under general anesthesia is an effective approach for dramatically reducing MS and LB levels immediately after treatment. In most cases, however, the bacterial levels in the saliva and plaque increased significantly 6–12 months after the treatment; and 20% to 60% of the treated children developed new carious lesions. The meta-analysis also suggests that pretreatment with CHX, PVP-I or FV has only a limited effect on bacterial reduction and caries relapse rates (Table 2).56,57,60
Table 2.
Effects of full-mouth restorative with antimicrobial treatment on the oral microbiota of ECC children
| Author, Year | Study Design Country |
Sample Size Children Age |
Treatment & Interventions |
Duration | Microbiological Evaluation Method |
Evidence |
|---|---|---|---|---|---|---|
|
Restorations without antimicrobial treatment | ||||||
| Litsas, 201058 |
Prospective observational follow-up study United States |
N=39, ECC 2–5 years |
Full-mouth restoration under general anesthesia |
3 months | Selective culture: Agar plate with bacitracin added |
-The operative procedures under general anesthesia significantly decreased S. mutans for at least three months. - By six months, S. mutans in saliva and plaques increased significantly. |
| Twetman et al., 1999117 |
Prospective observational follow-up study Sweden |
N=108, ECC 2.5–6.0 years |
Full-mouth restoration under general anesthesia |
6 months | Chairside test: - Dentocult-Strip mutans for MS - Dentocult-LB for LB - Dentobuff-strip for salivary pH |
- MS but not LB levels were strongly correlated with caries prevalence, immigrant background, and frequency of night-time meals. - MS and LB post-treatment levels were significantly reduced at the 1- and 6-month recalls. - LB levels were more dramatically reduced compared to MS, but the reduction was not significantly related to the type of treatment. - No difference was found in the saliva buffer capacity between pre- and post-treatment. |
| Restorations with additional antimicrobial treatment | ||||||
| Amin, et al., 200456 |
Randomized clinical trial Canada |
N=25, ECC 2–7 years |
Full-mouth restoration under general anesthesia 10% PVP-I 3 times at 2-month intervals |
12 months | Selective culture: Brucella agar with blood, vitamin K, hemin |
- There was a 49% reduction in S. mutans and a 17% reduction in total bacterial counts at 6 months after the combined treatment. However, the difference between the two groups was not significant. - At the 1-year recall, 63% of the children in the control group and 18% in the experimental group had new caries. |
| Chase, et al., 2004118 |
Prospective observational follow-up study Canada |
N=79, ECC 2.3–7.3 years |
Full-mouth restoration under general anesthesia Topical fluoride application |
6 months | Selective culture: -MSB for MS -SBA for total counts |
- Dental surgery resulted in a statistically significant reduction in salivary MS reservoirs in children treated for ECC. - 37% of the children who returned for follow-up visits had new smooth surface carious lesions. -There were no statistically significant differences in MS levels between the caries relapse and non-relapse groups. |
| Hughes, et al., 201260 |
Prospective observational follow-up study United States |
N=117 2–6 years |
Full mouth restoration under general anesthesia Prophylaxis, Fluoride varnish (Duraphat™) |
12 months | Selective and non- selective culture: TYCSB agar Blood agar Acid agar |
- At baseline, S. mutans and S. sobrinus counts were significantly higher in severe ECC than in caries-free children. - After treatment, S. mutans counts were decreased, particularly in children without caries recurrence. -S. sobrinus counts before treatment, but not S. mutans counts, were correlated with recurrent caries. - Over 70% of the acid-tolerant and 90% of the total microbiota found in severe-ECC were not S. mutans. |
| Klinke et al., 201446 |
Prospective follow-up study Germany |
N=50, ECC 1–5 years |
A 0.2% CHX gel Parents instructed to apply when brushing their children’s teeth twice a day for 2 weeks Followed by full- mouth restoration under general anesthesia |
12 months | Chairside test: CRT Bacteria (Ivoclar Vivadent) for MS and LB CRT ® bacteria Sabouraud/CandiSelect TM) |
-Numbers of MS, LB and Candida albicans were significantly reduced after restorative treatment. The decrease remained significant for 12 months. - At the 12-month visit, pretreatment with CHX had a limited antimicrobial effect for MS and LB, all of the microorganisms showed regrowth, and 34% of the children developed new dentinal lesions. -High scores for LB before treatment predicted caries relapse. -Satisfactory and sustainable success could not be achieved in MB, LB, or Candida colonization or in caries relapse rates. |
| Simratvir, et al., 2010119 |
Randomized clinical Trial Ludhiana, India |
N=30 4.2 years |
Full-mouth restoration under general anesthesia Grp1, 10% PVP-I at 3 months interval for 12 months Grp2, placebo control |
12 months | Selective culture: TYCSB agar selective for S. mutans |
-The application of 10% PVP-I resulted in a significant reduction in the rise of S. mutans levels from baseline and a decrease in the relapse of caries. -Oral rehabilitation coupled with regular application of 10% Povidone Iodine application can be a good alternative to control caries in children affected with ECC. |
| Zhan et al., 200657 |
Randomized clinical trial United States |
N=22, ECC 2–6 years |
Full-mouth restoration under general anesthesia Both groups: Prophylaxis and 1.23% APF gel application (2 min) prior restoration After restoration, -Intervention: 10% PVP-I for 2 min -Control: phosphate saline |
12 months | Selective and non- selective culture: - MSB agar for MS - Rogosa-tomato juice for LB -BHI-blood agar for total counts |
- MS and LB levels in the PVP-I group were significantly reduced at 1 hour, 3 weeks and 3 months. - 60% of the children had new carious lesions. - Complete surgical treatment of caries plus prophylaxis, fluoride gel application at baseline, were insufficient to prevent new caries in more than 60% of the children who had a high risk of caries. |
Figure 3.
5. Effect of children’s xylitol trials on the reduction of MS colonization
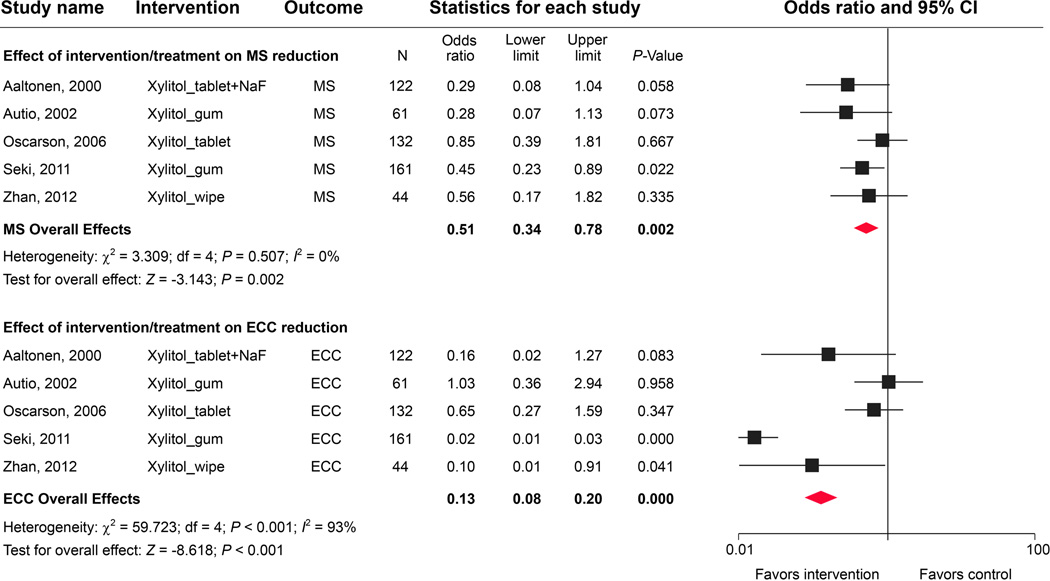
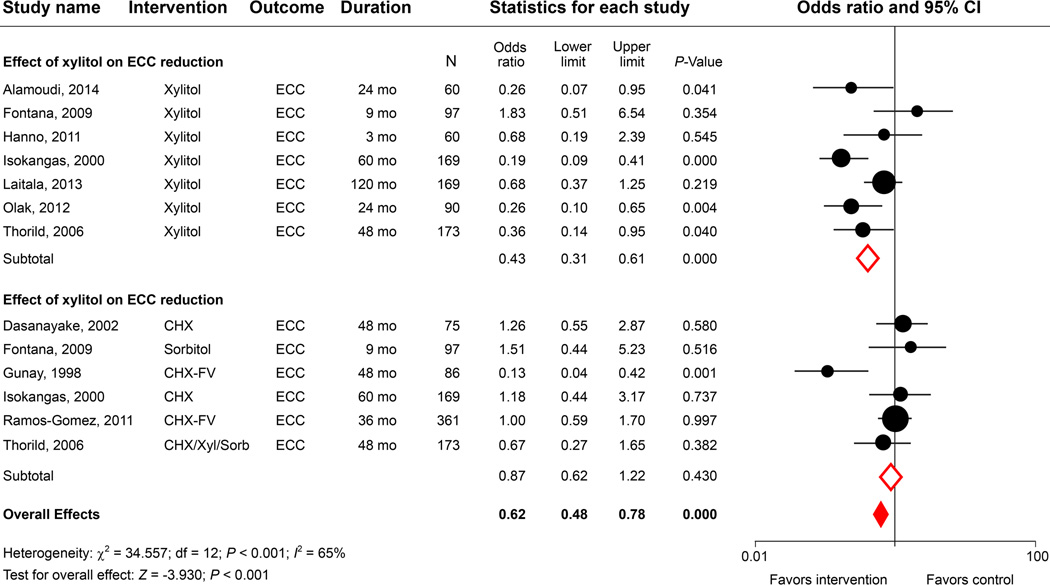
We identified 23 observational studies and clinical trials, but only 5 studies included microbial evaluations and therefore met the inclusion criteria (Table 3). Several xylitol delivery vehicles were used, including chewing gums, tablets, wipes, and combined treatment with NaF. The age of the children studied ranged from 6 months to 5 years. The meta-analysis of xylitol-based interventions indicated an overall significant reduction of MS colonization in young children (Fig. 4). Autio, et al. observed a shift in MS scores from high to low within 3 weeks in children who chewed xylitol gum.63 In contrast, Oscarson, et al. reported no difference in MS levels between test and control groups after a 2-year follow-up observation.64 Seki’s group found that xylitol gum led to reduced MS in dental plaque and also noted that over 10% of the children experienced diarrhea in the experimental group.65 Interestingly, daily xylitol-wipe applications did not lower salivary MS and LB levels over a 12-month observation.66 Notably, the meta-analysis results seem to suggest that xylitol delivered by tablets had the least antimicrobial effect, perhaps due to the lack of a direct interaction with the oral microflora, and was therefore less effective in reducing MS adhesion67 compared with other modes of delivery
Table 3.
Effects of xylitol usage on MS levels and caries in ECC children
| Author Year |
Study Design Country |
Sample Size Children Age |
Treatment & Interventions |
Duration | Microbiological Evaluation Method |
Evidence |
|---|---|---|---|---|---|---|
| Aaltonen, et al., 2000120 |
Prospective cohort study Finland |
N=122 12–14 months |
Fludent tablet containing NaF (0.25 mg F, xylitol (159 mg), sorbitol (153 mg) Grp T, Fludent in pacifier Grp C, Fludent in food |
12 months | Chairside test: Dentocult SM Strip for MS |
-The children in group T developed significantly fewer new lesions than the children in group C when children were between 2 and 3 years of age. -Significantly fewer children in group T were MS-positive compared to group C. -The administration of a NaF-xylitol-sorbitol preparation with FAP proved to be an effective approach in reducing the incidence of caries between children aged 2 and 5 years. |
| Autio, 200263 | Randomized clinical trial United States |
N=61 3–5 years |
Grp 1, Xylitol gum 3x for 3 weeks Grp 2, Control |
3 weeks | Chairside test: Dentocult SM Strip for MS |
-The shift from higher MS scores to lower scores was greater in the xylitol group than in the control group; therefore, chewing xylitol gum may reduce salivary MS and provide a feasible caries prevention method for preschool children. |
| Oscarson, et al., 200664 |
Randomized clinical trial Sweden |
N=132 2 years |
Grp1, Xylitol tablet 0.48 g 1x/day bedtime Grp2, Control |
24 months | Chairside test: Dentocult SM Strip for MS |
-No statistically significant differences in MS levels were detected between the two groups at any of the follow-up visits. -Caries prevalence was low in the xylitol group, but the difference was not statistically significant. -The findings do not support a low-dose xylitol tablet program for caries prevention in preschool children. |
| Seki, et al., 201165 |
Randomized clinical trial Japan |
N=161 3–4 years |
Exp grp = Xylitol gum, 1.8 g (66% xylitol by weight), 3 times/day for 3 months Control = fluoride varnish (5% NaF) every 6 months |
12 months | Chairside test: Dentocult SM Strip for MS |
-Xylitol gum consumption showed a significant negative association with MS levels. -Xylitol gum is effective in avoiding increased plaque MS in young children. -Over 10% of the xylitol group children experienced diarrhea. |
| Zhan, et al. 201266 |
Randomized clinical trial United States |
N=44 6–35 months |
Xylitol-wipe Placebo-wipe |
12 months | Selective culture: MSB agar for MS Rogosa-tomato juice for LB |
-No significant differences between the two groups were observed in levels of MS and LB at all time-points. -Significantly fewer children in the xylitol-wipe group had new caries lesions at 1 year compared with those in the placebo-wipe group. |
Figure 4.
A high degree of heterogeneity was observed in caries outcomes among the 5 studies (I2 statistic = 93%; P < 0.001; Fig. 4). Although 2 out of the 5 studies reported development of significantly fewer new carious lesions in the experimental group, with an overall significant caries reduction, the results should be interpreted cautiously, given (1) the inconsistent effect size (odds ratios ranged from 0.02 to 1.03); (2) the limited number of studies included in the analysis; and (3) the lack of true comparative control groups in the clinical studies. Although there is strong evidence supporting the use of xylitol-containing chewing gum to reduce dental caries in adolescent and adult populations,68 one should not automatically assume that the gum will be as effective for preschool-aged children. Better-designed, placebo-controlled, randomized clinical trials are needed to independently test the antimicrobial properties of xylitol and confirm the caries-preventing effect of xylitol in young children.
6. Effect of maternal xylitol trials on the acquisition of MS in children
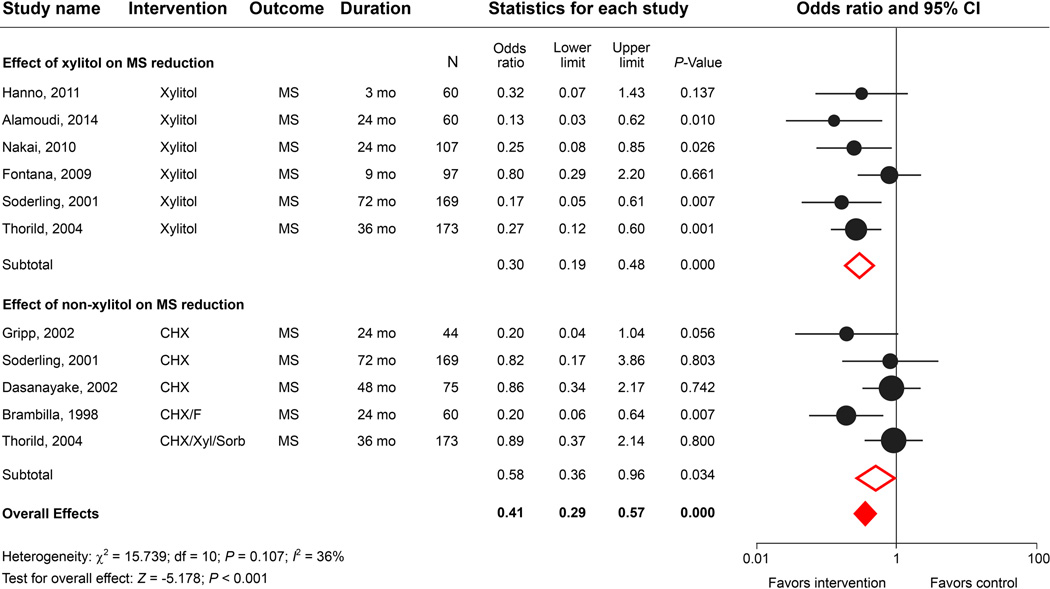
We identified 214 studies using the search key words “clinical trial”, “xylitol”, “mother/maternal”, “antimicrobial”, and “Streptococcus”. Nineteen studies with at least a 3-month follow-up evaluation were analyzed (Table 4). Based on an average of 39-months of observation, most of the studies reported positive correlations between maternal exposure to xylitol or other antimicrobial agents and a delay in MS colonization in young children. Despite some controversy regarding the xylitol dosage needed and the mode of delivery, the meta-analysis indicated that anti-cariogenic-microbe interventions in mothers can not only significantly affect MS acquisition in children (Fig. 5A) but also subsequently lower children’s caries outcomes (Fig. 5B). Xylitol-based interventions show a better caries-protective effect (odds ratio = 0.43, 95% CI = 0.31–0.60; P < 0.001) compared with non-xylitol interventions (odds ratio = 0.71, 95% CI = 0.72–1.20; P = 0.573). In addition, a 10-year follow-up study by Laitala, et al. demonstrated that children who were not colonized by MS at the age of 2 years had a lower caries experience compared with MS-colonized children.69,70 It was hypothesized that the maternal use of xylitol chewing gum can prevent dental caries in children by delaying or prohibiting MS transmission from mother to child. Another 10-year mother-child oral health longitudinal follow-up study led by Thorild, et al. reached a similar conclusion that the children of mothers who used high-content xylitol gums had lower MS counts at 18 months of age and were more likely to have less caries at 10 years of age.71–73 Clearly, more clinical studies will be needed to validate the long-term benefits of maternal xylitol gum exposure on children’s dental health since only marginal differences in caries prevalence were observed between the experimental groups and given the limited sample sizes of those studies.
Figure 5.
7. Effect of silver compounds on the oral microbiota in ECC
For centuries, silver has been known to exhibit antimicrobial effects due to its properties as a heavy metal.74 A recent study suggested that silver ions inhibit microorganism growth by inactivating bacterial DNA replication ability and protein formation.75 Through the use of in vitro bacterial models, silver ions were found to enhance antimicrobial activity against multi-species cariogenic biofilm formation on carious dentin and to reduce demineralization.76–78 Clinically, topical therapeutic application of silver diamine fluoride (SDF), silver fluoride (AgF), Nano-silver fluoride (NSF), and silver nitrate (AgNO3) are highly effective for inhibiting carious lesion progression.76,79 Although the mechanisms by which silver compounds inhibit bacterial growth and arrest carious lesions have not been fully explored, the caries-treatment effects have been reported in a number of epidemiology and clinical studies worldwide.79 We found very few clinical microbiology investigations that adequately examined the antibacterial efficacy of SDF and other silver compounds on ECC treatment outcomes. After an extensive search, we identified 12 ECC-related clinical studies published after 1997, only 7 of which were well-designed randomized control clinical trials using SDF (30%~38% or 44,800 ppm) or NSF (33,990 ppm) as an intervention agent for ECC. However, none of the studies included a microbiological evaluation; therefore, no study was selected for the meta-analysis.
Several additional antimicrobial approaches, other than fluoride, PVP-I, CHX, and xylitol, have been evaluated for managing ECC. Gudipaneni, et al. showed that brushing with toothpaste containing lactoferrin, lysozyme, and lactoperoxidase significantly reduced salivary levels of MS and L. acidophilus in children with severe ECC.80 Lobo, et al. suggested that clinical trials were needed to test the efficacy of Lippia Sidoides Cham (LSO) mouth rinse or gel against ECC.81 A few studies reported the clinical efficacy of different glass ionomers and dental resin adhesive materials with fluoride/xylitol slow-release functions or antibacterial activity.82–85 Yet, none of these studies met the inclusion criteria for the current meta-analysis.
8. Effect of ECC on oral microbial community diversity
We identified 15 reports that investigated the potential correlation between ECC and oral microbial diversity (Table 5). Many studies show differences in the oral microbiota between children with and without ECC. The diversity was either decreased13,18,86 or increased19,87,88 in ECC compared with caries-free status, which depended in part on the microbiological assay used. A high degree of similarity between the oral microbiota of mother and child was observed,89,90 highlighting the mother or primary caregiver as a major source of the bacteria that colonize the oral cavity of young children. Results differed between studies in the microbial composition before and after treatment.90,91 For example, Fontana, et al. reported that the maternal use of xylitol gum had no effect on microbial composition in children.92 Tanner, et al. reported significant microbial changes in children before and after extensive-restorative treatment under general anesthesia using microbiological analyses of a microarray containing 300 oral bacterial probes.59 Tanner’s report demonstrated the feasibility of using this assay and sufficient bacterial probes to detect differences in the caries microbiome and to evaluate successful treatment. Determining which bacteria to target is discussed below, but we propose that the general strategy to achieve a healthy, caries-free-compatible microbiota will be to “reverse” the microbial community that led the alteration from health to disease.93,94
Table 5.
Summary of the oral microbial diversity associated with ECC
| Author Year |
Study Design Country |
Sample Size Children Age |
Microbiology Evaluation Method |
Evidence |
|---|---|---|---|---|
| Cephas, et al., 2011132 |
Exploratory study United States |
N=5 -Mother–child dyads 3–6 years |
454 Genome pyrosequencing |
-The saliva bacterial microbiome was more diverse in adults than in infants. -There is a rich bacterial community in the infant oral cavity prior to tooth eruption. -Streptococcus, Veillonella, and Neisseria are the predominant bacterial genera present in infants. |
| Fontana, et al., 200992 |
Randomized clinical trial United States |
N=97 9–14 months |
Checkerboard DNA/DNA hybridization for species comparisons |
-Maternal use of xylitol gum did not result in statistically significant differences in the microbial plaque composition of 9- to 14-month-oid infants. |
| Gross, et al 201213 |
A combination cross-sectional and longitudinal study United States |
N=72 -36 ECC -36 CF 1–3 years |
16S rRNA gene sequencing analysis |
- Overall, 134 species were identified. Differences in the bacterial community were observed between health and disease (ECC) at all taxonomic levels. -S. mutans was the dominant species in many, but not all, subjects with caries. - Elevated levels of S. salivarius, S. sobrinus, and S. parasanguinis were also associated with caries, especially in subjects with no or low levels of S. mutans -Veillonella was associated with caries. Among children without a previous history of caries, Veillonella, but not S. mutans or other acid-producing species, predicted future caries. - The bacterial community diversity was decreased as caries severity increased compared to the healthy state. |
| Kanasi, et al., 201087,133 |
Exploratory study |
N= 80 -39 ECC -41 CF 2–6 years |
16S rRNA gene cloning and sequencing PCR selected species HOMD* |
-139 different taxa were identified based HOMD. Clonal analysis of the 80 children identified a diverse microbiota that significantly differed between severe caries and caries-free children. -There was an increase in diversity than previously detected in this clonal analysis. -S. mutans and Bifidobacteriaceae species were strongly associated with severe ECC. |
| Li, et al., 200718 |
Exploratory study United States |
N=20 -10 S-ECC -10 CF 2–8 years |
PCR-DGGE** |
-The microbial diversity and complexity of the microbial biota in dental plaque was significantly reduced in S-ECC children compared to CF children. |
| Li, et al., 200790 |
Exploratory study United States |
N=20 -Mother-child dyads 2–8 years |
PCR-DGGE | -There was a high degree of similarity of bacterial compositions between mothers and their children; the two may share as much as 94% of their oral bacterial spectra, including cariogenic species. |
| Luo, et al., 201288 |
Exploratory study China |
N=50 -30 ECC -20 CF 6–8 years |
16S rRNA gene amplification & HOMIM* assay |
-The diversity of microbes within saliva increased in caries active status. -Imbalances in the resident microflora may be the ultimate mechanism underlying the development of dental caries. |
| Palmer, et al 2012134 |
Prospective cohort study United States |
N=5, ECC 3–5 years |
AP-PCR*** | - The number of MS strains was reduced 1 year post-rehabilitation treatment (composite restoration, 0.12% CHX, 1.23% NaF vanish). -The predominant MS strain remained for at least 12 months after the treatment. |
| Qin, et al 2013135 |
Exploratory study China |
N=178 -87 S-ECC -91 CF 3–6 years |
AP-PCR | -The frequency of S. sobrinus detection was significantly higher (18.39%) in SECC children than in caries-free children (3.30%). The presence of S. sobrinus could be a risk factor for high caries activity in severe early childhood caries. -One to three different genotypes of S. sobrinus were detected in each SECC child. Only one genotype was colonized in each caries-free child. The multi-genotypes could be related to different caries susceptibility. |
| Tanner, et al., 201115 |
Exploratory study United States |
N=82 -42 ECC -40 CF 2–6 years |
Anaerobic culture, identifications from 16S rRNA gene sequencing & HOMD |
-The major species associated with severe ECC included S. mutans, Scardovia wiggsiae, Veillonella parvula, S. cristatus, and Actinomyces gerencseriae. S. wiggsiae was significantly associated with severe ECC in the presence and absence of S. mutans detection. |
| Tanner, et al., 201159 |
Exploratory study United States |
N=82 -53 S-ECC -32 CF 2–6 years |
16S rRNA gene PCR amplification & HOMIM* |
-Several bacterial species, including Bifidobacteriaceae, Scardovia wiggsiae, S. mutans with bifidobacteria, and S. mutans with S. wiggsiae, were associated with the etiology of advanced caries. |
| Tao, et al. 201386 |
Prospective cohort study China |
N=12, S-ECC 3 years |
PCR-DGGE | -A total of 21 genera were identified in all subjects. -The onset of S-ECC revealed a decrease in microbial diversity. -The overall composition of the microbiota was highly similar within an individual over a 2-year period. |
| Xu, et al., 201419 |
Exploratory study China |
N=19 -10 ECC -9 CF 1–2 years |
16S rRNA gene pyrosequencing |
-A high bacterial diversity was noted in the plaques of children with ECC but was not significant compared to caries-free children. -Principal component analysis (PCA) showed that caries-related genera included Streptococcus and Veillonella, whereas Leptotrichia, Selenomonas, Fusobacterium, Capnocytophaga and Porphyromonas were more related to the caries-free samples. Neisseria and Prevotella presented numbers that were approximately in between. |
| Zhan, et al. 2012136 |
Randomized clinical trial United States |
N=22 -11 Xylitol-wipe -11 Placebo-wipe 6–35 months |
AP-PCR for MS genotyping |
-No significant differences in the prevalence of xylitol-resistant genotypes or in the biofilm- formation capacity of MS were observed between the two groups. |
HOMD = Human Oral Microbiome Database; HOMIM = Human Oral Microbiome Identification Microarray
PCR-DGGE = polymerase chain reaction-based denaturing gel gradient electrophoresis
AP-PCR = arbitrarily primed-polymerase chain reaction
ECC-ASSOCIATED MICROBIOME
The wide diversity of bacteria in dental caries has been revealed using both culture and molecular microbial methods. Most of the species detected make up a core microbiome, whereas other species in the climax community may be disease associated. It is likely that several species interact with each other to produce the acidic conditions that promote dental caries. Cultured bacteria formed the basis of the ecological plaque hypothesis applied to dental caries95 and its modification.94 Under these models, the biofilm composition changes with the development of carious lesions. As lesions progress, the proportions of acid-producing Streptococcus and Actinomyces species increase, followed by acid-tolerant bacteria such as S. mutans and Lactobacillus species.94
The bacterial diversity of ECC-associated biofilms is supported by molecular studies,94 as well as parallel observations of biofilms in periodontal, endodontic and other oral sites. The major bacterial genera detected in ECC include Streptococcus, Lactobacillus, Actinomyces, Bifidobacterium, Propionibacterium and Scardovia, all of which are Gram positive bacteria. Many species of Gram negative bacteria have also been detected, including Campylobacter, Haemophilus, Aggregatibacter, Fusobacteria, Prevotella, Porphyromonas and Capnocytopaga and Treponema (Spirochetes) species. However, based on molecular methods, the “traditional S. mutans, Lactobacillus Actinomyces and Bifidobacterium species”96 appeared to be less important or missing, which suggests that additional species other than S. mutans and Lactobacillus species may also responsible for ECC. Some of these differences resulted from technical differences between methods, resulting Actinomyces, Bifidobacterium, and Scardovia species being underestimated in molecular studies.97,98 Understanding the microbial diversity of ECC thus requires information from both culture-based and molecular studies.
Cariogenic pathogens in the bacterial microbiome
Several approaches have been used to isolate potential caries pathogens from the microbial complex. Culture studies for ECC have used acidic (low-pH) isolation media to select aciduric bacteria.94 Acidic agar, pH 5–5.2, suppressed 90% of the microbiota60 but enhanced the growth of MS, bifidobacteria and LB, suggesting the successful enrichment of putative caries pathogens. ECC-associated acid-tolerant and acidogenic bacteria cultured from a low-pH broth included S. mutans, Actinomyces israelii and Lactobacillus species.99 The non-MS Streptococcus oralis and Streptococcus intermedius were acid tolerant but were associated with caries-free children rather than ECC children, indicating that acid-tolerance per se is not sufficient to describe a caries pathogen. Using acid agar with anaerobic incubation, the major ECC-associated species were found to be S. mutans, Streptococcus sobrinus, and Parascardovia denticolens, as well as a new species, Scardovia wiggsiae15. S. wiggsiae was associated with ECC in S. mutans-negative samples, suggesting that this new species may be important in ECC that is not associated with MS. S. wiggsiae and Parascardovia denticolens belong to the family/phylum Bifidobacteriaceae, along with Bifidobacterium species. Bifidobacteria were cultured from occlusal lesions of children at similar proportions to those of S. mutans.100 Based on selective isolation, the dominant species in childhood caries were Bifidobacterium dentium and Parascardovia denticolens.
To differentiate bacteria associated with caries progression, several molecular-based studies have compared lesions at different stages. Based on this design, open-ended cloning and sequencing studies compared 3 sites in ECC children: caries-free, white spot lesions (initial caries) and cavities.13,101–103 These studies were instrumental in revealing the wide diversity of bacterial species in both ECC and caries-free children. A recent study that utilized cloning and sequencing strategies reported that S. mutans, S. sobrinus, Streptococcus parasanguinis, Streptococcus vestibularis/salivarius and Veillonella atypica/dispar/parvula increased from healthy regions to cavitated lesions.13 The authors suggested that S. sobrinus, S. salivarius and S. parasanguinis could be alternate ECC pathogens in addition to S. mutans based on their presence in progressing ECC sites that lack S. mutans. Taken together these findings indicate a major role for S. mutans in ECC, but they also suggest that additional species of importance in ECC include Streptococcus sobrinus and Scardovia wiggsiae.
Rapid detection of species and microbial communities in plaque biofilms
Molecular methods have been developed to rapidly detect individual species and multiple species simultaneously, which exhibit great potential for use in clinical studies of ECC. A DNA probe checkerboard study found that Lactobacillus gasseri, Lactobacillus fermentum, Lactobacillus vaginalis, and S. mutans with S. sobrinus were associated with ECC, but not Lactobacillus acidophilus, a probiotic species.87 This suggested specificity among Lactobacillus species with respect to ECC. Probes based on the 16S rRNA have been used in the checkerboard format101–103 and in its successor, the human microbe identification microarray (HOMIM)104, which contains 300 different probes. The HOMIM microarray was used in a treatment study of severe ECC. While the microbiota did not change in children with new lesions (relapse) after therapy, there were changes in the children without disease progression.59 This suggested that major changes had occurred in the biofilm composition, which would require an assay capable of detecting multiple species. PCR-denaturing gradient gel electrophoresis (DGGE) has been used to examine bacterial profiles in ECC18,86,105 and to demonstrate differences in the microbial community between children with and without ECC18, as well as bacterial differences before and after treatment.106
PCR can rapidly detect bacterial species; quantitative PCR (qPCR) can measure bacterial levels and therefore determine DNA amounts and bacterial count equivalents. Genetic assays can be more sensitive than culture methods and improves the detection of S. sobrinus compared with culture.107 Studies using PCR-based methods revealed that detection of S. mutans with S. sobrinus improved predictions of ECC and ECC progression compared with detection of the individual species.59,60,108 In another population, L. fermentum detected by PCR was significantly associated with severe ECC. PCR and qPCR assays have also been developed for many Lactobacillus species and have been used to detect these species in deep dentinal lesions.109,110 PCR assays have also been developed for plaque samples to detect oral Bifidobacterium species,100 and Scardovia wiggsiae.109,110 Using PCR assays, S. mutans, S. sobrinus, S. wiggsiae and Bifidobacterium species were shown to be significantly associated with severe ECC.59
SUMMARY
In this systematic review, we identified 41 clinical studies that incorporated microbiological evaluations of ECC treatments or other interventions. In many studies reductions in salivary MS or LB was observed following the topical application of antimicrobial agents. Perhaps the most significantly effective anti-caries and anti-microbial regimen involved interventions in mothers to influence outcomes in children. Although antimicrobial therapeutic approaches show reductions in MS colonization, bacterial regrowth occurred in most of the studies, with a concomitant high incidence of ECC once the intervention had ceased. These results raise questions regarding the sustainability of the bacterial reductions as well as whether the antimicrobial interventions and treatments used to date produce sustainable reductions in ECC development, caries relapse rates, cariogenic microbial transmission and acquisition, or other microbiological parameters. The meta-analysis highlighted the paucity of high-quality randomized controlled clinical trials that demonstrated the efficacy of commonly used antimicrobial agents and procedures. Many of the tested agents have been evaluated in adult populations and were highly recommended by dental professional organizations and were thus assumed the same agents would provide preventive benefits for young children.
The overall limitations of the studies evaluated included (1) the paucity of good clinical trials evaluating caries outcomes with microbial reductions; (2) the inability of agents to elicit long-term reductions in caries or cariogenic microbiota; (3) the wide variation in the study designs used, some of the which were reflected in the Higgins index (I2 statistics analysis); and (4) the lack of adequate control groups, including in most of the studies that control children were exposed to various forms of fluoride. Thus, the results of those studies should be interpreted with caution. This review also suggests that more well-designed, placebo-controlled randomized clinical trials are needed to individually test specific antimicrobial treatments, particularly to elucidate the critical link between anti-pathogenic mechanisms and caries prevention in young children.
Despite the potential limitations and the risk of bias, this literature review, which combines information from clinical studies for multiple meta-analyses, provides updated evidence on the effectiveness of antimicrobial approaches on the ECC-associated microbiota and ECC management. This information will provide a basis for designing future research studies and clinical interventions.
ACKNOWLEDGMENTS
The authors wish to thank the Office of Continuing Dental Education of the University of Maryland School of Dentistry, Baltimore, Md; the American Academy of Pediatric Dentistry, Chicago, Ill.; DentaQuest Foundation, Boston; the William Bingham 2nd Trust, for their support; and, in part, research grants DE015706, DE019455, DE016937 supported by the National Institute of Dental and Craniofacial Research, Bethesda, Md., USA.
Contributor Information
Yihong Li, Email: yihong.li@nyu.edu.
Anne Tanner, Email: annetanner@forsyth.org.
REFERENCES
- 1.Department of Health and Human Services (US) Indian Health Service. Division of Dental Services. Rockville, MD: United States Department of Health and Human Services; 2002. An oral health survey of American Indian and Alaska Native dental patients: findings, regional differences and national comparisons. Available at: http://dhss.alaska.gov/dph/wcfh/Documents/oralhealth/docs/Oral_Health_1999_IHS_Survey.pdf. [Google Scholar]
- 2.Tinanoff N, Reisine S. Update on early childhood caries since the Surgeon General’s Report. Acad Pediatr. 2009;9:396–403. doi: 10.1016/j.acap.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiboski CH, Gansky SA, Ramos-Gomez F, Ngo L, Isman R, Pollick HF. The association of early childhood caries and race/ethnicity among California preschool children. J Public Health Dent. 2003;63:38–46. doi: 10.1111/j.1752-7325.2003.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 4.Huntington NL, Kim IJ, Hughes CV. Caries-risk factors for Hispanic children affected by early childhood caries. Pediatr Dent. 2002;24:536–542. [PubMed] [Google Scholar]
- 5.Dye BA, Arevalo O, Vargas CM. Trends in paediatric dental caries by poverty status in the United States, 1988–1994 and 1999–2004. Int J Paediatr Dent. 2010;20:132–143. doi: 10.1111/j.1365-263X.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 6.Brown LJ, Wall TP, Lazar V. Trends in untreated caries in primary teeth of children 2 to 10 years old. J Am Dent Assoc. 2000;131:93–100. doi: 10.14219/jada.archive.2000.0027. [DOI] [PubMed] [Google Scholar]
- 7.Dye BA, Tan S, Smith V, et al. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat. 2007:11. [PubMed] [Google Scholar]
- 8.Dye BA, Vargas CM, Lee JJ, Magder L, Tinanoff N. Assessing the relationship between children’s oral health status and that of their mothers. J Am Dent Assoc. 2011;142:173–183. doi: 10.14219/jada.archive.2011.0061. [DOI] [PubMed] [Google Scholar]
- 9.Dye BA, Thornton-Evans G, Li X, Iafolla TJ. NCHS data brief no. 191. Hyattsville, MD: National Center for Health Statistics; 2015. Mar, Dental caries and sealant prevalence in children and adolescents in the United States, 2011–2012. Vol Avaliable at: http://www.cdc.gov/nchs/data/databriefs/db191.pdf. [PubMed] [Google Scholar]
- 10.Batliner T, Wilson AR, Tiwari T, et al. Oral health status in Navajo nation head start children. J Public Health Dent. 2014 doi: 10.1111/jphd.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins-Junior PA, Vieira-Andrade RG, Correa-Faria P, Oliveira-Ferreira F, Marques LS, Ramos-Jorge ML. Impact of early childhood caries on the oral health-related quality of life of preschool children and their parents. Caries Res. 2013;47:211–218. doi: 10.1159/000345534. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz HS. Research issues in early childhood caries. Community Dent Oral Epidemiol. 1998;26:67–81. doi: 10.1111/j.1600-0528.1998.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 13.Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One. 2012;7:e47722. doi: 10.1371/journal.pone.0047722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dige I, Gronkjaer L, Nyvad B. Molecular studies of the structural ecology of natural occlusal caries. Caries Res. 2014;48:451–460. doi: 10.1159/000357920. [DOI] [PubMed] [Google Scholar]
- 15.Tanner AC, Mathney JM, Kent RL, et al. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 2011;49:1464–1474. doi: 10.1128/JCM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falsetta ML, Klein MI, Colonne PM, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obata J, Takeshita T, Shibata Y, et al. Identification of the microbiota in carious dentin lesions using 16S rRNA gene sequencing. PLoS One. 2014;9:e103712. doi: 10.1371/journal.pone.0103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Ge Y, Saxena D, Caufield PW. Genetic profiling of the oral microbiota associated with severe early childhood caries. J Clin Microbiol. 2007;45:81–87. doi: 10.1128/JCM.01622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Hao W, Zhou Q, et al. Plaque bacterial microbiome diversity in children younger than 30 months with or without caries prior to eruption of second primary molars. PLoS One. 2014;9:e89269. doi: 10.1371/journal.pone.0089269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration; 2011. [Google Scholar]
- 21.Whitehead A. Meta-analysis of controlled clinical trials. New York: Willey; 2002. [Google Scholar]
- 22.Gold OG, Jordan HV, Houte Jv. A selective medium for Streptococcus mutans . Arch Oral Biol. 1973;18:1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- 23.Zylber LJ, Jordan HV. Development of a selective medium for detection and enumeration of Actinomyces viscosus and Actinomyces naeslundii in dental plaque. J Clin Microbiol. 1982;15:253–259. doi: 10.1128/jcm.15.2.253-259.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman GH. The isolation and testing of fecal streptococci. Am J Dig Dis. 1946;13:105–107. doi: 10.1007/BF03003570. [DOI] [PubMed] [Google Scholar]
- 25.Kimmel L, Tinanoff N. A modified mitis salivarius medium for a caries diagnostic test. Oral Microbiol Immunol. 1991;6:275–279. doi: 10.1111/j.1399-302x.1991.tb00491.x. [DOI] [PubMed] [Google Scholar]
- 26.Schaeken MJ, van der Hoeven JS, Franken HC. Comparative recovery of Streptococcus mutans on five isolation media, including a new simple selective medium. J Dent Res. 1986;65:906–908. doi: 10.1177/00220345860650060901. [DOI] [PubMed] [Google Scholar]
- 27.Wan AK, Seow WK, Walsh LJ, Bird PS. Comparison of five selective media for the growth and enumeration of Streptococcus mutans . Aust Dent J. 2002;47:21–26. doi: 10.1111/j.1834-7819.2002.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 28.Tanabe Y, Park JH, Tinanoff N, Turng BF, Lilli H, Minah GE. Comparison of chairside microbiological screening systems and conventional selective media in children with and without visible dental caries. Pediatr Dent. 2006;28:363–368. [PubMed] [Google Scholar]
- 29.Rogosa M, Mitchell JA, Wiseman RF. A selective medium for the isolation and enumeration of oral lactobacilli. J Dent Res. 1951;30:682–689. doi: 10.1177/00220345510300051201. [DOI] [PubMed] [Google Scholar]
- 30.Sanders ME, Walker DC, Walker KM, Aoyama K, Klaenhammer TR. Performance of commercial cultures in fluid milk applications. J Dairy Sci. 1996;79:943–955. doi: 10.3168/jds.S0022-0302(96)76445-7. [DOI] [PubMed] [Google Scholar]
- 31.Rogosa M. A selective medium for the isolation and enumeration of the veillonella from the oral cavity. J Bacteriol. 1956;72:533–536. doi: 10.1128/jb.72.4.533-536.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabouraud R. Ann Dermatol Syphilol. 1892;3:1061. [Google Scholar]
- 33.Marinho V, Higgins J, Logan S, Sheiham A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews. 2003:CD002280. doi: 10.1002/14651858.CD002782. Art. No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marinho VC, Worthington HV, Walsh T, Clarkson JE. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews. 2013;7:CD002279. doi: 10.1002/14651858.CD002279.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.dos Santos AP, Nadanovsky P, de Oliveira BH. A systematic review and meta-analysis of the effects of fluoride toothpastes on the prevention of dental caries in the primary dentition of preschool children. Community Dent Oral Epidemiol. 2013;41:1–12. doi: 10.1111/j.1600-0528.2012.00708.x. [DOI] [PubMed] [Google Scholar]
- 36.American Dental Association Council on Scientific A. Professionally applied topical fluoride: evidence-based clinical recommendations. J Am Dent Assoc. 2006;137:1151–1159. doi: 10.14219/jada.archive.2006.0356. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton IR. Biochemical effects of fluoride on oral bacteria. J Dent Res. 1990;69 doi: 10.1177/00220345900690S128. Spec No:660-7; discussion 82-3. [DOI] [PubMed] [Google Scholar]
- 38.American Academy of Pediatric Dentistry. Guideline on fluoride therapy. Pediatr Dent. 2013;35:E165–E168. [PubMed] [Google Scholar]
- 39.Johansen JR, Gjermo P, Eriksen HM. Effect of 2-years’ use of chlorhexidine-containing dentifrices on plaque, gingivitis, and caries. Scand J Dent Res. 1975;83:288–292. doi: 10.1111/j.1600-0722.1975.tb00440.x. [DOI] [PubMed] [Google Scholar]
- 40.Emilson CG. Potential efficacy of chlorhexidine against mutans streptococci and human dental caries. J Dent Res. 1994;73:682–691. doi: 10.1177/00220345940730031401. [DOI] [PubMed] [Google Scholar]
- 41.van Rijkom HM, Truin GJ, van ’t Hof MA. A meta-analysis of clinical studies on the caries-inhibiting effect of chlorhexidine treatment. J Dent Res. 1996;75:790–795. doi: 10.1177/00220345960750020901. [DOI] [PubMed] [Google Scholar]
- 42.Twetman S. Antimicrobials in future caries control? A review with special reference to chlorhexidine treatment. Caries Res. 2004;38:223–229. doi: 10.1159/000077758. [DOI] [PubMed] [Google Scholar]
- 43.Dasanayake AP, Wiener HW, Li Y, Vermund SH, Caufield PW. Lack of effect of chlorhexidine varnish on Streptococcus mutans transmission and caries in mothers and children. Caries Res. 2002;36:288–293. doi: 10.1159/000063922. [DOI] [PubMed] [Google Scholar]
- 44.Twetman S, Grindefjord M. Mutans streptococci suppression by chlorhexidine gel in toddlers. Am J Dent. 1999;12:89–91. [PubMed] [Google Scholar]
- 45.Lobo PL, de Carvalho CB, Fonseca SG, et al. Sodium fluoride and chlorhexidine effect in the inhibition of mutans streptococci in children with dental caries: a randomized, double-blind clinical trial. Oral Microbiol Immunol. 2008;23:486–491. doi: 10.1111/j.1399-302X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- 46.Klinke T, Urban M, Luck C, Hannig C, Kuhn M, Kramer N. Changes in Candida spp., mutans streptococci and lactobacilli following treatment of early childhood caries: a 1-year follow-up. Caries Res. 2014;48:24–31. doi: 10.1159/000351673. [DOI] [PubMed] [Google Scholar]
- 47.Gilmore OJ. A reappraisal of the use of antiseptics in surgical practice. Ann R Coll Surg Engl. 1977;59:93–103. [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez L, Berkowitz R, Zlotnik H, Moss M, Weinstein P. Topical antimicrobial therapy in the prevention of early childhood caries. Pediatr Dent. 1999;21:9–11. [PubMed] [Google Scholar]
- 49.Neeraja R, Anantharaj A, Praveen P, Karthik V, Vinitha M. The effect of povidone-iodine and chlorhexidine mouth rinses on plaque Streptococcus mutans count in 6- to 12-year-old school children: an in vivo study. J Indian Soc Pedod Prev Dent. 2008;26(Suppl 1):S14–S18. [PubMed] [Google Scholar]
- 50.Xu X, Li JY, Zhou XD, Xie Q, Zhan L, Featherstone JD. Randomized controlled clinical trial on the evaluation of bacteriostatic and cariostatic effects of a novel povidone-iodine/fluoride foam in children with high caries risk. Quintessence Int. 2009;40:215–223. [PubMed] [Google Scholar]
- 51.Tut OK, Milgrom PM. Topical iodine and fluoride varnish combined is more effective than fluoride varnish alone for protecting erupting first permanent molars: a retrospective cohort study. J Public Health Dent. 2010;70:249–252. doi: 10.1111/j.1752-7325.2010.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milgrom PM, Tut OK, Mancl LA. Topical iodine and fluoride varnish effectiveness in the primary dentition: a quasi-experimental study. J Dent Child. 2011;78:143–147. [PubMed] [Google Scholar]
- 53.Lopez L, Berkowitz R, Spiekerman C, Weinstein P. Topical antimicrobial therapy in the prevention of early childhood caries: a follow-up report. Pediatr Dent. 2002;24:204–206. [PubMed] [Google Scholar]
- 54.Lee JY, Vann WF, Roberts MW. A cost analysis of treating pediatric dental patients using general anesthesia versus conscious sedation. Pediatr Dent. 2000;22:27–32. [PubMed] [Google Scholar]
- 55.American Academy of Pediatric Dentistry. Guideline on the elective use of minimal, moderate, and deep sedation and general anesthesia for pediatric dental patients. Pediatr Dent. 2005;27:110–118. [PubMed] [Google Scholar]
- 56.Amin MS, Harrison RL, Benton TS, Roberts M, Weinstein P. Effect of povidone-iodine on Streptococcus mutans in children with extensive dental caries. Pediatr Dent. 2004;26:5–10. [PubMed] [Google Scholar]
- 57.Zhan L, Featherstone JD, Gansky SA, et al. Antibacterial treatment needed for severe early childhood caries. J Public Health Dent. 2006;66:174–179. doi: 10.1111/j.1752-7325.2006.tb02576.x. [DOI] [PubMed] [Google Scholar]
- 58.Litsas G. Effect of full mouth rehabilitation on the amount of Streptococcus mutans in children with early childhood caries. Eur J Paediatr Dent. 2010;11:35–38. [PubMed] [Google Scholar]
- 59.Tanner AC, Kent RL, Jr, Holgerson PL, et al. Microbiota of severe early childhood caries before and after therapy. J Dent Res. 2011;90:1298–1305. doi: 10.1177/0022034511421201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hughes CV, Dahlan M, Papadopolou E, et al. Aciduric microbiota and mutans streptococci in severe and recurrent severe early childhood caries. Pediatr Dent. 2012;34:e16–e23. [PMC free article] [PubMed] [Google Scholar]
- 61.Foster T, Perinpanayagam H, Pfaffenbach A, Certo M. Recurrence of early childhood caries after comprehensive treatment with general anesthesia and follow-up. J Dent Child (Chic) 2006;73:25–30. [PubMed] [Google Scholar]
- 62.Almeida AG, Roseman MM, Sheff M, Huntington N, Hughes CV. Future caries susceptibility in children with early childhood caries following treatment under general anesthesia. Pediatr Dent. 2000;22:302–306. [PubMed] [Google Scholar]
- 63.Autio JT. Effect of xylitol chewing gum on salivary Streptococcus mutans in preschool children. ASDC J Dent Child. 2002;69:81–86. 13. [PubMed] [Google Scholar]
- 64.Oscarson P, Lif Holgerson P, Sjostrom I, Twetman S, Stecksen-Blicks C. Influence of a low xylitol-dose on mutans streptococci colonisation and caries development in preschool children. Eur Arch Paediatr Dent. 2006;7:142–147. doi: 10.1007/BF03262555. [DOI] [PubMed] [Google Scholar]
- 65.Seki M, Karakama F, Kawato T, Tanaka H, Saeki Y, Yamashita Y. Effect of xylitol gum on the level of oral mutans streptococci of preschoolers: block-randomised trial. Int Dent J. 2011;61:274–280. doi: 10.1111/j.1875-595X.2011.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhan L, Cheng J, Chang P, et al. Effects of xylitol wipes on cariogenic bacteria and caries in young children. J Dent Res. 2012;91:85S–90S. doi: 10.1177/0022034511434354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soderling EM. Xylitol, mutans streptococci, and dental plaque. Adv Dent Res. 2009;21:74–78. doi: 10.1177/0895937409335642. [DOI] [PubMed] [Google Scholar]
- 68.Deshpande A, Jadad AR. The impact of polyol-containing chewing gums on dental caries: a systematic review of original randomized controlled trials and observational studies. J Am Dent Assoc. 2008;139:1602–1614. doi: 10.14219/jada.archive.2008.0102. [DOI] [PubMed] [Google Scholar]
- 69.Laitala M, Alanen P, Isokangas P, Soderling E, Pienihakkinen K. A cohort study on the association of early mutans streptococci colonisation and dental decay. Caries Res. 2012;46:228–233. doi: 10.1159/000337303. [DOI] [PubMed] [Google Scholar]
- 70.Laitala ML, Alanen P, Isokangas P, Soderling E, Pienihakkinen K. Long-term effects of maternal prevention on children’s dental decay and need for restorative treatment. Community Dent Oral Epidemiol. 2013;41:534–540. doi: 10.1111/cdoe.12057. [DOI] [PubMed] [Google Scholar]
- 71.Thorild I, Lindau B, Twetman S. Salivary mutans streptococci and dental caries in three-year-old children after maternal exposure to chewing gums containing combinations of xylitol, sorbitol, chlorhexidine, and fluoride. Acta Odontol Scand. 2004;62:245–250. doi: 10.1080/00016350410001676. [DOI] [PubMed] [Google Scholar]
- 72.Thorild I, Lindau B, Twetman S. Caries in 4-year-old children after maternal chewing of gums containing combinations of xylitol, sorbitol, chlorhexidine and fluoride. Eur Arch Paediatr Dent. 2006;7:241–245. doi: 10.1007/BF03262559. [DOI] [PubMed] [Google Scholar]
- 73.Thorild I, Lindau B, Twetman S. Long-term effect of maternal xylitol exposure on their children’s caries prevalence. Eur Arch Paediatr Dent. 2012;13:305–307. doi: 10.1007/BF03320831. [DOI] [PubMed] [Google Scholar]
- 74.Berger TJ, Spadaro JA, Chapin SE, Becker RO. Electrically generated silver ions: quantitative effects on bacterial and mammalian cells. Antimicrob Agents Chemother. 1976;9:357–358. doi: 10.1128/aac.9.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 76.Klein U, Kanellis MJ, Drake D. Effects of four anticaries agents on lesion depth progression in an in vitro caries model. Pediatr Dent. 1999;21:176–180. [PubMed] [Google Scholar]
- 77.Kreth J, Kim D, Nguyen M, et al. The antimicrobial effect of silver ion impregnation into endodontic sealer against Streptococcus mutans . Open Dent J. 2008;2:18–23. doi: 10.2174/1874210600802010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mei ML, Li QL, Chu CH, Lo EC, Samaranayake LP. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann Clin Microbiol Antimicrob. 2013;12:4. doi: 10.1186/1476-0711-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chu CH, Lo EC. Promoting caries arrest in children with silver diamine fluoride: a review. Oral Health Prev Dent. 2008;6:315–321. [PubMed] [Google Scholar]
- 80.Gudipaneni RK, Kumar RV, G J, Peddengatagari S, Duddu Y. Short term comparative evaluation of antimicrobial efficacy of tooth paste containing lactoferrin, lysozyme, lactoperoxidase in children with severe early childhood caries: a clinical study. J Clin Diagn Res. 2014;8:ZC18–ZC20. doi: 10.7860/JCDR/2014/8161.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lobo PL, Fonteles CS, de Carvalho CB, et al. Dose-response evaluation of a novel essential oil against Mutans streptococci in vivo. Phytomedicine. 2011;18:551–556. doi: 10.1016/j.phymed.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 82.Featherstone JD. Delivery challenges for fluoride, chlorhexidine and xylitol. BMC Oral Health. 2006;6(Suppl 1):S8. doi: 10.1186/1472-6831-6-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhi QH, Lo EC, Lin HC. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent. 2012;40:962–967. doi: 10.1016/j.jdent.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 84.Zhang K, Li F, Imazato S, et al. Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J Biomed Mater Res B Appl Biomater. 2013;101:929–938. doi: 10.1002/jbm.b.32898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen C, Weir MD, Cheng L, et al. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent Mater. 2014;30:891–901. doi: 10.1016/j.dental.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tao Y, Zhou Y, Ouyang Y, Lin H. Dynamics of oral microbial community profiling during severe early childhood caries development monitored by PCR-DGGE. Arch Oral Biol. 2013;58:1129–1138. doi: 10.1016/j.archoralbio.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Kanasi E, Dewhirst FE, Chalmers NI, et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010;44:485–497. doi: 10.1159/000320158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luo AH, Yang DQ, Xin BC, Paster BJ, Qin J. Microbial profiles in saliva from children with and without caries in mixed dentition. Oral Dis. 2012;18:595–601. doi: 10.1111/j.1601-0825.2012.01915.x. [DOI] [PubMed] [Google Scholar]
- 89.Tanner AC, Milgrom PM, Kent R, Jr, et al. Similarity of the oral microbiota of pre-school children with that of their caregivers in a population-based study. Oral Microbiol Immunol. 2002;17:379–387. doi: 10.1034/j.1399-302x.2002.170608.x. [DOI] [PubMed] [Google Scholar]
- 90.Li Y, Ismail AI, Ge Y, Tellez M, Sohn W. Similarity of bacterial populations in saliva from African-American mother-child dyads. J Clin Microbiol. 2007 doi: 10.1128/JCM.00771-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tanner AC, Milgrom PM, Kent R, Jr, et al. The microbiota of young children from tooth and tongue samples. J Dent Res. 2002;81:53–57. doi: 10.1177/002203450208100112. [DOI] [PubMed] [Google Scholar]
- 92.Fontana M, Catt D, Eckert GJ, et al. Xylitol: effects on the acquisition of cariogenic species in infants. Pediatr Dent. 2009;31:257–266. [PubMed] [Google Scholar]
- 93.Marsh PD. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent Clin North Am. 2010;54:441–454. doi: 10.1016/j.cden.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 95.Marsh PD, Percival RS. The oral microflora--friend or foe? Can we decide? Int Dent J. 2006;56:233–239. doi: 10.1111/j.1875-595x.2006.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 96.van Houte J, Lopman J, Kent R. The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surfaces. J Dent Res. 1996;75:1008–1014. doi: 10.1177/00220345960750040201. [DOI] [PubMed] [Google Scholar]
- 97.Munson MA, Banerjee A, Watson TF, Wade WG. Molecular analysis of the microflora associated with dental caries. J Clin Microbiol. 2004;42:3023–3029. doi: 10.1128/JCM.42.7.3023-3029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tanner ACR. Anaerobic culture to detect periodontal and caries pathogens. Journal of Oral Biosciences. 2014 doi: 10.1016/j.job.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marchant S, Brailsford SR, Twomey AC, Roberts GJ, Beighton D. The predominant microflora of nursing caries lesions. Caries Res. 2001;35:397–406. doi: 10.1159/000047482. [DOI] [PubMed] [Google Scholar]
- 100.Mantzourani M, Gilbert SC, Sulong HN, et al. The isolation of bifidobacteria from occlusal carious lesions in children and adults. Caries Res. 2009;43:308–313. doi: 10.1159/000222659. [DOI] [PubMed] [Google Scholar]
- 101.Aas JA, Griffen AL, Dardis SR, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407–1417. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Becker MR, Paster BJ, Leys EJ, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corby PM, Lyons-Weiler J, Bretz WA, et al. Microbial risk indicators of early childhood caries. J Clin Microbiol. 2005;43:5753–5759. doi: 10.1128/JCM.43.11.5753-5759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Preza D, Olsen I, Aas JA, Willumsen T, Grinde B, Paster BJ. Bacterial profiles of root caries in elderly patients. J Clin Microbiol. 2008;46:2015–2021. doi: 10.1128/JCM.02411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ling Z, Kong J, Jia P, et al. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb Ecol. 2010;60:677–690. doi: 10.1007/s00248-010-9712-8. [DOI] [PubMed] [Google Scholar]
- 106.Li Y, Saxena D, Barnes VM, Trivedi HM, Yao G, Xu T. Polymerase chain reaction-based denaturing gradient gel electrophoresis in the evaluation of oral microbiota. Oral Microbiol Immunol. 2006;21:333–339. doi: 10.1111/j.1399-302X.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- 107.Choi EJ, Lee SH, Kim YJ. Quantitative real-time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood caries. Int J Paediatr Dent. 2009;19:141–147. doi: 10.1111/j.1365-263X.2008.00942.x. [DOI] [PubMed] [Google Scholar]
- 108.Okada M, Soda Y, Hayashi F, et al. PCR detection ofStreptococcus mutans and Ssobrinus in dental plaque samples from Japanese pre-school children. J Med Microbiol. 2002;51:443–447. doi: 10.1099/0022-1317-51-5-443. [DOI] [PubMed] [Google Scholar]
- 109.Byun R, Nadkarni MA, Chhour KL, Martin FE, Jacques NA, Hunter N. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J Clin Microbiol. 2004;42:3128–3136. doi: 10.1128/JCM.42.7.3128-3136.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Callaway A, Kostrzewa M, Willershausen B, et al. Identification of lactobacilli from deep carious lesions by means of species-specific PCR and MALDI-TOF mass spectrometry. Clin Lab. 2013;59:1373–1379. doi: 10.7754/clin.lab.2013.121225. [DOI] [PubMed] [Google Scholar]
- 111.Plonka KA, Pukallus ML, Holcombe TF, Barnett AG, Walsh LJ, Seow WK. Randomized controlled trial: a randomized controlled clinical trial comparing a remineralizing paste with an antibacterial gel to prevent early childhood caries. Pediatric Dentistry. 2013;35:8–12. [PubMed] [Google Scholar]
- 112.Plotzitza B, Kneist S, Berger J, Hetzer G. Efficacy of chlorhexidine varnish applications in the prevention of early childhood caries. Eur J Paediatr Dent. 2005;6:149–154. [PubMed] [Google Scholar]
- 113.Pukallus ML, Plonka KA, Barnett AG, Walsh LJ, Holcombe TF, Seow WK. A randomised, controlled clinical trial comparing chlorhexidine gel and low-dose fluoride toothpaste to prevent early childhood caries. Int J Paediatr Dent. 2013;23:216–224. doi: 10.1111/j.1365-263X.2012.01248.x. [DOI] [PubMed] [Google Scholar]
- 114.Stecksen-Blicks C, Sjostrom I, Twetman S. Effect of long-term consumption of milk supplemented with probiotic lactobacilli and fluoride on dental caries and general health in preschool children: a cluster-randomized study. Caries Res. 2009;43:374–381. doi: 10.1159/000235581. [DOI] [PubMed] [Google Scholar]
- 115.Berkowitz RJ, Koo H, McDermott MP, et al. Adjunctive chemotherapeutic suppression of mutans streptococci in the setting of severe early childhood caries: an exploratory study. J Public Health Dent. 2009;69:163–167. doi: 10.1111/j.1752-7325.2009.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El-Housseiny A, Farsi N. The effectiveness of two antibacterial regimens on salivary mutans streptococci and lactobacilli in children. J Clin Pediatr Dent. 2005;30:145–151. [PubMed] [Google Scholar]
- 117.Twetman S, Fritzon B, Jensen B, Hallberg U, Stahl B. Pre- and post-treatment levels of salivary mutans streptococci and lactobacilli in pre-school children. Int J Paediatr Dent. 1999;9:93–98. doi: 10.1046/j.1365-263x.1999.00108.x. [DOI] [PubMed] [Google Scholar]
- 118.Chase I, Berkowitz RJ, Mundorff-Shrestha SA, Proskin HM, Weinstein P, Billings R. Clinical outcomes for early childhood caries (ECC): the influence of salivary mutans streptococci levels. Eur J Paediatr Dent. 2004;5:143–146. [PubMed] [Google Scholar]
- 119.Simratvir M, Singh N, Chopra S, Thomas AM. Efficacy of 10% Povidone Iodine in children affected with early childhood caries: an in vivo study. J Clin Pediatr Dent. 2010;34:233–238. doi: 10.17796/jcpd.34.3.l552816527xtv122. [DOI] [PubMed] [Google Scholar]
- 120.Aaltonen AS, Suhonen JT, Tenovuo J, Inkila-Saari I. Efficacy of a slow-release device containing fluoride, xylitol and sorbitol in preventing infant caries. Acta Odontol Scand. 2000;58:285–292. doi: 10.1080/00016350050217145. [DOI] [PubMed] [Google Scholar]
- 121.Alamoudi NM, Hanno AG, Almushayt AS, Masoud MI, El Ashiry EA, El Derwi DA. Early prevention of childhood caries with maternal xylitol consumption. Saudi Med J. 2014;35:592–597. [PubMed] [Google Scholar]
- 122.Brambilla E, Felloni A, Gagliani M, Malerba A, Garcia-Godoy F, Strohmenger L. Caries prevention during pregnancy: results of a 30-month study. J Am Dent Assoc. 1998;129:871–877. doi: 10.14219/jada.archive.1998.0351. [DOI] [PubMed] [Google Scholar]
- 123.Gripp VC, Schlagenhauf U. Prevention of early mutans streptococci transmission in infants by professional tooth cleaning and chlorhexidine varnish treatment of the mother. Caries Res. 2002;36:366–372. doi: 10.1159/000065958. [DOI] [PubMed] [Google Scholar]
- 124.Gunay H, Dmoch-Bockhorn K, Gunay Y, Geurtsen W. Effect on caries experience of a long-term preventive program for mothers and children starting during pregnancy. Clin Oral Investig. 1998;2:137–142. doi: 10.1007/s007840050059. [DOI] [PubMed] [Google Scholar]
- 125.Hanno AG, Alamoudi NM, Almushayt AS, Masoud MI, Sabbagh HJ, Farsi NM. Effect of xylitol on dental caries and salivary Streptococcus mutans levels among a group of mother-child pairs. J Clin Pediatr Dent. 2011;36:25–30. doi: 10.17796/jcpd.36.1.d4g77616714w3372. [DOI] [PubMed] [Google Scholar]
- 126.Isokangas P, Soderling E, Pienihakkinen K, Alanen P. Occurrence of dental decay in children after maternal consumption of xylitol chewing gum, a follow-up from 0 to 5 years of age. J Dent Res. 2000;79:1885–1889. doi: 10.1177/00220345000790111201. [DOI] [PubMed] [Google Scholar]
- 127.Soderling E, Isokangas P, Pienihakkinen K, Tenovuo J. Influence of maternal xylitol consumption on acquisition of mutans streptococci by infants. J Dent Res. 2000;79:882–887. doi: 10.1177/00220345000790031601. [DOI] [PubMed] [Google Scholar]
- 128.Soderling E, Isokangas P, Pienihakkinen K, Tenovuo J, Alanen P. Influence of maternal xylitol consumption on mother-child transmission of mutans streptococci: 6-year follow-up. Caries Res. 2001;35:173–177. doi: 10.1159/000047452. [DOI] [PubMed] [Google Scholar]
- 129.Nakai Y, Shinga-Ishihara C, Kaji M, Moriya K, Murakami-Yamanaka K, Takimura M. Xylitol gum and maternal transmission of mutans streptococci. J Dent Res. 2010;89:56–60. doi: 10.1177/0022034509352958. [DOI] [PubMed] [Google Scholar]
- 130.Olak J, Saag M, Vahlberg T, Soderling E, Karjalainen S. Caries prevention with xylitol lozenges in children related to maternal anxiety. A demonstration project. Eur Arch Paediatr Dent. 2012;13:64–69. doi: 10.1007/BF03262846. [DOI] [PubMed] [Google Scholar]
- 131.Ramos-Gomez FJ, Gansky SA, Featherstone JD, et al. Mother and youth access (MAYA) maternal chlorhexidine, counselling and paediatric fluoride varnish randomized clinical trial to prevent early childhood caries. Int J Paediatr Dent. 2012;22:169–179. doi: 10.1111/j.1365-263X.2011.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cephas KD, Kim J, Mathai RA, et al. Comparative analysis of salivary bacterial microbiome diversity in edentulous infants and their mothers or primary care givers using pyrosequencing. PLoS One. 2011;6:e23503. doi: 10.1371/journal.pone.0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kanasi E, Johansson I, Lu SC, et al. Microbial risk markers for childhood caries in pediatricians’ offices. J Dent Res. 2010;89:378–383. doi: 10.1177/0022034509360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Palmer EA, Vo A, Hiles SB, et al. Mutans streptococci genetic strains in children with severe early childhood caries: follow-up study at one-year post-dental rehabilitation therapy. J Oral Microbiol. 2012:4. doi: 10.3402/jom.v4i0.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qin XR, Zhou Q, Qin M. Genotypic diversity and virulence traits of Streptococcus sobrinus isolated from caries-free children and children suffering severe early childhood caries. Chin J Dent Res. 2013;16:63–69. [PubMed] [Google Scholar]
- 136.Zhan L, Featherstone JD, Lo J, et al. Clinical efficacy and effects of xylitol wipes on bacterial virulence. Adv Dent Res. 2012;24:117–122. doi: 10.1177/0022034512449835. [DOI] [PubMed] [Google Scholar]