Abstract
Childhood cancer survivors treated with anthracycline chemotherapy or chest radiation are at an increased risk of developing congestive heart failure (CHF). In this population, CHF is well-recognized as a progressive disorder, with a variable period of asymptomatic cardiomyopathy which precedes signs and symptoms. As a result, a number of practice guidelines have been developed to facilitate detection and treatment of asymptomatic cardiomyopathy. These guidelines differ with regards to definitions of at risk populations, surveillance modality and frequency, and recommendations for interventions. These differences may hinder the effective implementation of these recommendations. We report on the results of an international collaboration to harmonize existing cardiomyopathy surveillance recommendations, using an evidence-based approach that relied on standardized definitions for outcomes of interest and transparent presentation of the quality of the evidence. The resultant recommendations were graded according to the quality of the evidence and the potential benefit gained from early detection and intervention.
INTRODUCTION
Advances in treatment strategies for childhood cancer have resulted in marked improvements in survival, with current 5-year survival rates approaching 80%.1 However this improvement in outcome is has been compromised by the occurrence of long term morbidities of therapy. The cumulative incidence of severe or life-threatening chronic health conditions exceeds 40% for childhood cancer survivors surviving 30 years after primary diagnosis.2, 3 These conditions include second malignant neoplasms, endocrine disorders, cardiopulmonary dysfunction, renal dysfunction, and neurosensory impairment.2, 3
Cardiovascular complications (such as coronary artery disease, and stroke, but especially congestive heart failure [CHF]) have emerged as a leading cause of morbidity and mortality in long-term survivors of childhood cancer.4 In fact, childhood cancer survivors are at a 15-fold increased risk of developing CHF2 and are at 7-fold higher risk of premature death due to cardiac causes,5 when compared with the general population. There is a strong dosedependent relation between anthracycline chemotherapy exposure and CHF risk, and the risk is higher among those exposed to chest radiation.4 The incidence of CHF is <5% with cumulative anthracyclines exposure of <250 mg/m2; approaches 10% at doses between 250 and 600 mg/m2; and exceeds 30% for doses >600 mg/m2.4, 6–8 Of note, nearly 60% of all childhood cancer survivors carry a history of prior anthracycline and/or chest radiation exposure.9, 10
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the diagnosis and management of CHF describe heart failure as a progressive disorder, with a variable period of asymptomatic cardiac dysfunction which precedes clinically overt signs and symptoms.11 For anthracycline-exposed survivors, the asymptomatic stage is often characterized by thinning of the left ventricular (LV) wall, enlargement of LV diameter, and subsequent increase in LV wall stress, a clinical picture similar to dilated cardiomyopathy.4, 12 These subclinical changes can result in impairment of LV systolic function, manifesting as decreased ejection fraction (EF) and/or shortening fraction (SF).4, 12 It is important to recognize, however, that anthracycline-exposed survivors could, over time, also develop restrictive cardiomyopathy, resulting in abnormal E/A ratio (peak early atrial divided by peak late atrial velocities), or prolonged isovolumic relaxation time (IVRT) in the setting of preserved EF/SF.4, 12 Individuals who receive chest radiation may be at an especially high risk of developing combined dilated and restrictive cardiomyopathy that results from myocardial fibrosis primarily due to radiation effects on the supporting vasculature.4, 12
In childhood cancer survivors, there is often a long latency between cardiotoxic exposure and clinically evident disease.4, 12 As a result, a number of clinical practice guidelines have been developed to facilitate early detection and treatment of asymptomatic cardiomyopathy.13–16 These guidelines were developed by various North American and European groups and they differ with regards to definitions of at risk populations, surveillance modality and frequency, and recommendations for interventions. These differences may, in turn, hinder the effective implementation of screening across a wide spectrum of clinical settings. Recognizing the importance for collaboration, an international effort was organized to harmonize existing late effects screening recommendations for survivors of childhood cancer.17 The current effort represents the summary of the evidence and recommendations for cardiomyopathy surveillance in childhood cancer survivors treated with anthracyclines and/or chest radiation.
METHODS
A description of the international guideline harmonization effort and methodology has been provided elsewhere.17 The cardiomyopathy surveillance recommendations were prepared by representatives from the North American Children’s Oncology Group (COG),13 the Dutch Childhood Oncology Group (DCOG),14 the Scottish Intercollegiate Guidelines Network (SIGN),16 and the United Kingdom Children’s Cancer and Leukaemia Group (UKCCLG).15 The current effort encompassed published guidelines that were developed following systematic evaluation of the quality of the late effects literature, linking therapeutic interventions with adverse outcomes. The expert membership included pediatric and adult cardiologists, pediatric oncologists, radiation oncologists, epidemiologists, methodologists, nurses and other survivorship care providers.
The initial step of the cardiomyopathy harmonization effort involved identifying areas of concordance and discordance across the COG, DCOG, SIGN, and UKCCLG guidelines. In order to achieve consensus, clinical questions were devised to address areas of discordance for cardiomyopathy surveillance. Systematic literature searches were performed to update previous systematic searches for asymptomatic18 and symptomatic19 cardiomyopathy (search strategy through December 2012: Appendix 1), and evidence summaries were formed to address areas of discordance. When evidence was lacking for childhood cancer survivors, we extrapolated information from other populations. In the case of concordance, we extracted and evaluated the evidence cited by the guidelines.
Given the heterogeneity in definitions used to describe relevant therapeutic exposures, surveillance strategies, and cardiovascular outcomes, we proposed standardized definitions which were incorporated into our literature review and final formulation of recommendations. Childhood cancer survivors included individuals treated for cancer up to 21 years of age, regardless of current age. Anthracyclines chemotherapy consisted of: doxorubicin, daunorubicin, epirubicin, idarubicin; the anthraquinone mitoxantrone was also included due to its similar cardiotoxic profile. Chest radiation included any radiation in which the heart was in the field of treatment (mediastinal, thoracic, spinal, left or whole upper abdominal or total body irradiation [TBI]). Asymptomatic cardiomyopathy was defined as a decline in LV systolic function (abnormal EF, SF, wall stress)20–22 or diastolic dysfunction (abnormal E/A ratio, prolonged IVRT)22, 23 in the context of preserved EF, without corresponding symptoms of heart failure. CHF was defined per the ACC/AHA guidelines,11 and corresponded to symptomatic cardiomyopathy with evidence of cardiac dysfunction on imaging studies. The current effort does not address screening for other known therapy-associated cardiovascular complications (coronary artery disease, carotid artery disease, pericardial fibrosis, conduction abnormalities, or valvular stenosis/insufficiency); these will be addressed by future collaborations.
The quality of the evidence and the strength of the recommendations were determined according to criteria that were based on modified Grading of Recommendations Assessment Development and Evaluation (GRADE) and the ACC/AHA classification for recommendations (Appendix 2).24, 25 Final recommendations relied on this scientific knowledge combined with other considerations such as clinical judgements, decisions about thresholds, costs, and potential harms from excessive screening. The harmonized cardiomyopathy surveillance recommendations were critically appraised by two external experts (K.O. and J.B.) in the field.
RESULTS
Discordances and concordances among the cardiomyopathy surveillance recommendations are provided in Table 1. There was concordance across guidelines for the following statements:
Childhood cancer survivors treated with anthracyclines (including mitoxantrone) or chest radiation are at increased risk of cardiomyopathy.
Surveillance using echocardiography should be lifelong and performed at a minimum of every five years.
Given the increased cardiometabolic demand on the heart of the mother during pregnancy, closer monitoring of survivors during pregnancy is warranted.
Survivors with documented asymptomatic cardiomyopathy should be referred to a cardiologist for further diagnostic work-up and possible treatment.
At risk cancer survivors should be regularly screened for traditional cardiovascular risk factors (i.e.: hypertension, diabetes, dyslipidemia, overweight/obesity) and should be counseled against smoking and physical inactivity.
Levels of evidence to support concordant areas are included in Table 2.
Table 1.
Concordances and discordances among cardiomyopathy surveillance recommendations
| Who needs cardiomyopathy surveillance? | |||||
| At risk | |||||
| Anthracyclines | Yes | Yes | Yes | Yes | Concordance |
| Mitoxantrone | Yes | Yes | Yes | Yes | Concordance |
| Differing risk by anthracycline analogues | Yes | Not stated | Not stated | Not stated | Discordance |
| Chest Radiation* | Yes | Yes | Yes | Yes | Concordance |
| CV risk factors | Yes | Yes | Yes | Yes | Concordance |
| Highest risk | ≥300 mg/m2 anthracyclines ≥30 Gy RT involving heart Anthracyclines + chest RT Younger age at treatment Pregnancy | ≥300 mg/m2 anthracyclines ≥30 Gy RT involving heart Anthracyclines + chest RT Pregnancy | >250 mg/m2 anthracyclines Anthracyclines + chest RT Hx of transient cardiomyopathy during treatment Pregnancy | >250 mg/m2 anthracyclines ≥30 Gy RT involving heart Anthracyclines + chest RT | Discordance |
| What surveillance modality should be used? | |||||
| Screening for cardiomyopathy | |||||
| Echocardiography | Yes | Yes | Yes | Yes | Concordance |
| Radionuclide angiography | Yes | Yes | No | No | Discordance |
| At what frequency and for how long should cardiomyopathy surveillance be performed? | |||||
| Screening begins | ≥2 yrs after treatment or ≥5 yrs after dx (whichever is first) | ≥5 yrs after diagnosis | 1–3 months after treatment | ≥5 yrs after completion of treatment | Discordance |
| Screening frequency | Every 1 –5 yrs | Every 2–5 years | Every 3–5 yrs | Every 2–5 yrs | Discordance |
| Duration of screening | Lifelong | Lifelong | Not stated | Not stated | Discordance |
| Closer monitoring during pregnancy | Yes | Yes | Yes | Yes | Concordance |
| Refer to cardiologist | Yes | Yes | Yes | Yes | Concordance |
| Consider ACE-inhibitors | Not stated | Yes | Not stated | Yes | Discordance |
Radiation therapy (RT) involving the heart: mediastinal, thoracic, spinal, left or whole upper abdominal or total body irradiation (TBI)
Abbreviations: Hx, History; CV, cardiovascular; Gy, Gray; yrs, years; ACE, angiotensin converting enzyme; Dx, diagnosis.
Table 2.
Conclusions of evidence for cardiomyopathy surveillance in childhood cancer survivors
| Who needs cardiomyopathy surveillance? | Level of evidence |
|---|---|
| Risk by anthracycline dose | |
| - Exponential increase in risk for symptomatic cardiomyopathy with increasing lifetime cumulative dose | Level A6, 19, 26, 27 |
| - Childhood cancer survivors treated with cumulative anthracycline dose ≥250 mg/m2 are at highest risk for symptomatic cardiomyopathy | Level A6, 19, 26, 27 |
| - Increased risk for asymptomatic cardiomyopathy with increasing cumulative dose | Level A18, 21, 38, 90 |
| Risk by age at anthracycline exposure | |
| - Increased risk for symptomatic cardiomyopathy with younger age at exposure | Conflicting evidence6, 8, 26, 33 |
| - Increased risk for asymptomatic cardiomyopathy with younger age at exposure | Conflicting evidence18, 90, 91 |
| Risk by anthracycline derivatives (including mitoxantrone) | |
| - Cardiomyopathy has been associated with all anthracycline derivatives | Level A92 |
| - Daunorubicin is as cardiotoxic as doxorubicin when given at an equieffective dose | Level C6, 26, 92 |
| - Epirubicin is less cardiotoxic than doxorubicin when given at an equieffective dose | No evidence |
| - Idarubicin is more cardiotoxic than doxorubicin when given at an equieffective dose | No evidence |
| - Mitoxantrone is more cardiotoxic than doxorubicin when given at an equieffective dose | No evidence |
| Risk by chest radiation dose | |
| - Increased risk for symptomatic cardiomyopathy with increasing radiation dose to cardiac tissues | Level A6, 8, 26, 28, 29 |
| - Childhood cancer survivors treated with chest radiation dose ≥35 Gy are at highest risk for symptomatic cardiomyopathy | Level B6, 26 |
| - Increased risk for asymptomatic cardiomyopathy with increasing radiation dose to cardiac tissues | Level B90, 93, 94 |
| Risk following anthracycline and chest radiation exposure | |
| - Increased risk after anthracycline and chest radiation exposure | Level A8, 19, 26 |
| Risk following conditioning with total body irradiation (TBI) | |
| - There is no increased risk following conditioning with TBI | Level B31, 95, 96 |
| Risk due to modifiable cardiovascular risk factors | |
| - Increased risk in anthracycline- and/or radiation- exposed survivors who develop modifiable cardiovascular risk factors (hypertension, diabetes, dyslipidemia, obesity) | Level B74, 97 |
| What surveillance modality should be used? | |
| Diagnostic value of echocardiography | |
| - Good diagnostic value of 2D echocardiography for detection of asymptomatic cardiomyopathy in childhood cancer survivors | Level B41, 98–100 |
| Diagnostic value of cardiac magnetic resonance imaging (CMR) | |
| - Good diagnostic value of CMR for detection of asymptomatic cardiomyopathy in childhood cancer survivors | Level B41 |
| Diagnostic value of radionuclide angiography | |
| - Good diagnostic value for detection of asymptomatic cardiomyoathy in childhood cancer survivors | Level C101, 102 |
| Diagnostic value of blood biomarkers of cardiac injury and remodeling | |
| - Poor diagnostic value of cardiac troponins (Troponin-T) for detection of asymptomatic cardiomyopathy in childhood cancer survivors | Level B45–47 |
| - Poor diagnostic value of cardiac troponins (Troponin-I) for detection of asymptomatic cardiomyopathy in childhood cancer survivors | Level C48 |
| - Poor diagnostic value of natriuretic peptides (ANP, BNP, NT Pro-BNP) for detection of asymptomatic cardiomyopathy in childhood cancer survivors | Level B45, 93, 103, 104 |
| Cost-benefit of surveillance in childhood cancer survivors | |
| - Screening for asymptomatic cardiomyopathy using conventional imaging or blood biomarkers is cost-effective. | No evidence |
| Cost-benefit of surveillance in other populations | |
| - Screening for asymptomatic cardiomyopathy using conventional imaging or blood biomarkers is cost-effective. | Level B50 |
| At what frequency and for how long should surveillance for cardiomyopathy be performed? | |
| - High risk childhood cancer survivors have a more rapid rate of deterioration in cardiac function when compared to moderate/low-risk survivors | No evidence |
| - There is a more rapid rate of deterioration in cardiac function during puberty | No evidence |
| - Female childhood cancer survivors who have asymptomatic cardiomyopathy at the time of becoming pregnant are at risk for symptomatic cardiomyopathy during pregnancy/delivery | Level C56 |
| - Female childhood cancer survivors treated with anthracyclines or radiation who have normal LV systolic function at the time of becoming pregnant are not at increased risk for deterioration in cardiac function during pregnancy/delivery | Level C56, 57 |
| - The risk for deterioration in cardiac function continues to increase with longer follow-up | Level B6, 8, 19, 26, 90 |
| What should be done when abnormalities are detected during surveillance? | |
| Utility of medical interventions in childhood cancer survivors | |
| - ACE-inhibitors are effective for improving cardiac function in survivors with asymptomatic cardiomyopathy | No evidence105 |
| - Beta-blockers are effective for improving cardiac function in survivors with asymptomatic cardiomyopathy | No evidence105 |
| - Other interventions such as angiotensin II receptor blockers or placement of ICD can be effective for improving cardiac function for prevention of sudden arrhythmic cardiac death in survivors with asymptomatic cardiomyopathy | No evidence105 |
| Utility of medical interventions in other populations | |
| - ACE-inhibitors are effective for improving cardiac function in individuals with asymptomatic cardiomyopathy | Level A60, 80–82 |
| - Beta-blockers are effective for improving cardiac function in individuals with asymptomatic cardiomyopathy | Level C60, 106–109 |
| - Other interventions such as angiotensin II receptor blockers or placement of ICD can be effective for improving cardiac function or for prevention of arrhythmic cardiac death in survivors with asymptomatic cardiomyopathy | Level C60, 109, 110 |
| What are the limitations for physical activity? | |
| Role of physical activity in childhood cancer survivors | |
| - Regular physical exercise, as recommended by the AHA and ESC, is beneficial for childhood cancer survivors with normal LV systolic function | Level C66 |
| - Regular physical exercise, as recommended by the AHA and ESC, is beneficial for childhood cancer survivors with asymptomatic cardiomyopathy | No evidence |
| - Participation in high intensity exercise increases the risk for cardiac functional deterioration in childhood cancer survivors | No evidence |
| Role of physical activity in other populations | |
| - Regular physical exercise, as recommended by the AHA and ESC, is beneficial for individuals who have normal cardiac function | Level A62, 63 |
| - Regular physical exercise, as recommended by the AHA and ESC, is beneficial for individuals who have normal cardiac function, but at risk for cardiomyopathy due to genetic susceptibility | Level B67, 68 |
| - Participation in high intensity exercise increases the risk for cardiac functional deterioration in individuals with asymptomatic cardiomyopathy | Level B63 |
A, high level of evidence (i.e. consistent evidence from well performed and high quality studies or systematic reviews with a low risk of bias, and direct, consistent and precise results); B, moderate to low level of evidence (i.e. evidence from studies or systematic reviews with few important limitations); and C, very low level of evidence (i.e. evidence from studies with serious flaws, only expert opinion or standards of care).
Abbreviations: Gy, Gray; LV, left ventricular; ACE, angiotensin converting enzyme; ICD, implantable cardioverter defibrillator; AHA, American Heart Association; ESC, European Society of Cardiology.
As illustrated by Table 1, there were also areas of discordance that required more detailed investigation of the available literature. The evidence summaries for the following areas of discordance are presented in Appendix 3: cardiomyopathy risk by anthracycline dose, chest radiation dose, combination of anthracycline and radiation exposure, TBI alone, and age at cancer treatment; differences in risk by anthracycline analogues, including mitoxantrone; utility of radionuclide angiography, cardiac magnetic resonance imaging (CMR), and cardiac blood biomarkers for surveillance of asymptomatic cardiomyopathy; frequency of screening in survivors treated with higher dose anthracyclines or radiation; risk of deterioration in cardiac function during puberty; effect of pharmacologic therapy in survivors with asymptomatic cardiomyopathy; limitations for physical activity following cardiotoxic exposure.
The conclusions of the evidence and the final recommendations are summarized in Tables 2 and 4, respectively. The rationale for the grading of the evidence and resultant recommendations are provided below.
Table 4.
Harmonized recommendations for cardiomyopathy surveillance for childhood cancer survivors.
| General recommendation |
| Survivors treated with anthracyclines and/or chest radiation and their providers should be aware of the risk of cardiomyopathy. |
| Who needs cardiomyopathy surveillance? Anthracyclines |
| Cardiomyopathy surveillance is recommended for survivors treated with high dose (≥ 250 mg/m2) anthracyclines. |
| Cardiomyopathy surveillance is reasonable for survivors treated with moderate dose (≥ 100 to < 250 mg/m2) anthracyclines. |
| Cardiomyopathy surveillance may be reasonable for survivors treated with low dose (< 100 mg/m2) anthracyclines. |
| Who needs cardiomyopathy surveillance? Chest radiation |
| Cardiomyopathy surveillance is recommended for survivors treated with high dose (≥ 35 Gy) chest radiation. |
| Cardiomyopathy surveillance may be reasonable for survivors treated with moderate dose (≥ 15 to < 35 Gy) chest radiation. |
| No recommendation can be formulated for cardiomyopathy surveillance for survivors treated with low dose (< 15 Gy) chest radiation with conventional fractionation. |
| Who needs cardiomyopathy surveillance? Anthracyclines + Chest radiation |
| Cardiomyopathy surveillance is recommended for survivors treated with moderate-high dose anthracyclines (≥ 100 mg/m2) and moderate-high dose chest radiation (≥ 15 Gy). |
| What surveillance modality should be used? |
| Echocardiography is recommended as the primary cardiomyopathy surveillance modality for assessment of left ventricular systolic function in survivors treated with anthracyclines and/or chest radiation. |
| Radionuclide angiography or cardiac magnetic resonance imaging (CMR) may be reasonable for cardiomyopathy surveillance in at risk survivors for whom echocardiography is not technically feasible/optimal. |
| Assessment of cardiac blood biomarkers (e.g., natriuretic peptides) in conjunction with imaging studies may be reasonable in instances where symptomatic cardiomyopathy is strongly suspected or in individuals who have borderline cardiac function during primary surveillance. |
| Assessment of cardiac blood biomarkers is not recommended as the only strategy for cardiomyopathy surveillance in at risk survivors. |
| Cardiomyopathy surveillance is recommended for High Risk survivors to begin no later than 2 years after completion of cardiotoxic therapy, repeated at 5 years after diagnosis and continued every 5 years thereafter. |
| More frequent cardiomyopathy surveillance is reasonable for High Risk survivors. |
| Lifelong cardiomyopathy surveillance may be reasonable for High Risk survivors. |
| At what frequency should surveillance be performed for Moderate/Low Risk survivors? |
| Cardiomyopathy surveillance is reasonable for Moderate/Low Risk survivors to begin no later than 2 years after completion of cardiotoxic therapy, repeated at 5 years after diagnosis and continue every 5 years thereafter. |
| More frequent cardiomyopathy surveillance may be reasonable for Moderate/Low Risk survivors. |
| Lifelong cardiomyopathy surveillance may be reasonable for Moderate/Low Risk survivors. |
| At what frequency should surveillance be performed for survivors who are pregnant or planning to become pregnant? |
| Cardiomyopathy surveillance is reasonable prior to pregnancy or in the first trimester for all female survivors treated with anthracyclines and/or chest radiation |
| No recommendations can be formulated for the frequency of ongoing surveillance in pregnant survivors who have normal LV systolic function immediately prior to or during the first trimester of pregnancy. |
| What should be done when abnormalities are identified? |
| Cardiology consultation is recommended for survivors with asymptomatic cardiomyopathy following treatment with anthracyclines and/or chest radiation. |
| What advice should be given regarding physical activity and other modifiable cardiovascular risk factors? |
| Regular exercise, as recommended by the AHA and ESC, offers potential benefits to survivors treated with anthracyclines and/or chest radiation. |
| Regular exercise is recommended for survivors treated with anthracyclines and/or chest radiation who have normal LV systolic function. |
| Cardiology consultation is recommended for survivors with asymptomatic cardiomyopathy to define limits and precautions for exercise. |
| Cardiology consultation may be reasonable for High Risk survivors who plan to participate in high intensity exercise to define limits and precautions for physical activity. |
| Screening for modifiable cardiovascular risk factors (hypertension, diabetes, dyslipidemia, obesity) is recommended for all survivors treated with anthracyclines and/or chest radiation so that necessary interventions can be initiated to help avert the risk of symptomatic cardiomyopathy. |
Green represents a strong recommendation, with a low degree of uncertainty (high quality evidence). Yellow (moderate quality evidence) and orange (weak quality evidence) represent moderate level recommendations. Red represents a recommendation against a particular intervention, with harms outweighing benefits.
Who needs cardiomyopathy surveillance?
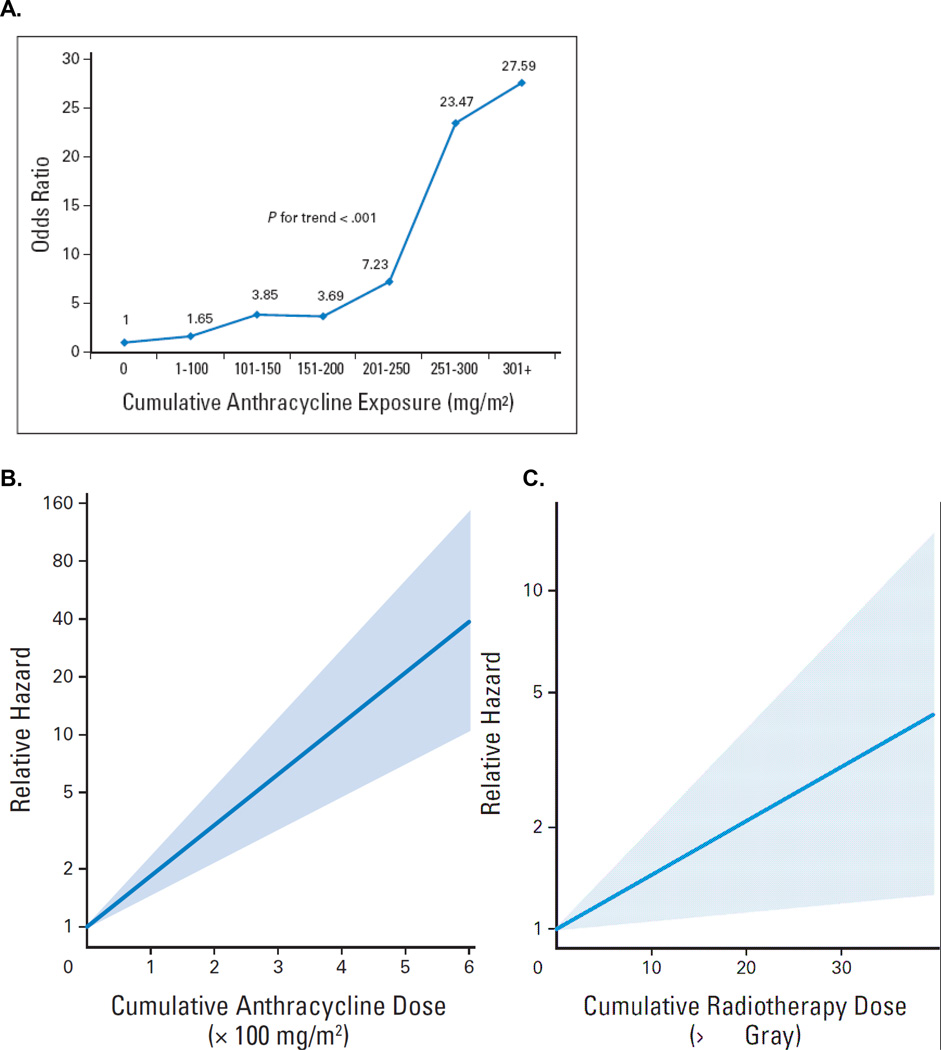
Children and adolescents treated with anthracyclines or radiation are at increased risk of developing cardiomyopathy. These individuals and their providers should be aware of their risk after completion of therapy (strong recommendation). There is an exponential increase in risk of cardiomyopathy with increasing lifetime cumulative dose (Figure 1A, B).19, 26, 27 The risk is especially high in children treated with ≥250 mg/m2 and is lowest among those treated with <100 mg/m2.6, 19, 26, 27 Importantly, there appears to be no clear cut-off for a safe anthracycline dose as symptomatic cardiomyopathy has been reported in survivors who received doses well-below 250 mg/m2.6, 26, 27 Individuals treated with ≥35 Gy of chest radiation are also at high risk of developing CHF (Figure 1C), and this risk remains elevated for those treated with moderate doses (15 Gy-<35Gy).6, 8, 26, 28, 29 On the other hand, there is lack of evidence to suggest that children treated with lower doses (<15 Gy in <2 Gy daily fractions) of chest radiation, including TBI, are at increased risk of CHF.6, 29–31 Survivors treated with a combination of chest radiation and anthracyclines are at an especially high risk for developing CHF due to the combined myocardial injury and dysfunction that result from these two therapeutic approaches.8, 26, 32
Figure 1. Risk of cardiomyopathy and CHF by cumulative lifetime anthracycline (A and B) and radiotherapy dose (C).
1A: Dose-response relationship between cumulative anthracycline exposure and risk of cardiomyopathy. Patients with no exposure to anthracyclines served as the referent group. Magnitude of risk is expressed as odds ratio, which was obtained using conditional logistic regression adjusting for age at diagnosis, sex, and chest radiation.
Blanco JG, Sun CL, Landier W, et al: Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group. J Clin Oncol 30:1415–21, 2012.
1B, C: Association between cumulative anthracycline dose and hazard ratio, and cumulative radiotherapy dose and hazard ratio (in equivalent 2-Gray [Gy] fractions) for congestive heart failure, based on the Cox model that also included sex, age at diagnosis, cisplatin, vincristine, cyclophosphamide, ifosfamide, and congenital heart disease. No cardiotoxic treatment (dose = 0) was the reference value. For cardiac events, effect of anthracycline dose is shown for zero irradiation dose and effect of irradiation dose is shown for zero dose of anthracycline.
van der Pal HJ, van Dalen EC, van Delden E, et al: High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol 30:1429–37, 2012.
Based on the available evidence, anthracycline and/or chest radiation-exposed survivors who have a four-fold or greater risk of CHF when compared to those without these exposures should undergo routine surveillance for cardiomyopathy (strong recommendation). Surveillance may be recommended for survivors who have a greater than 1.5-fold increase in CHF risk (moderate recommendation). The resultant risk stratification (High, Moderate, Low) by anthracycline and/or chest radiation dose is presented in Table 3, and specific risk-based recommendations are presented in Table 4.
Table 3.
Cardiomyopathy risk group definitions.
| Risk Group | Anthracycline dose (mg/m2) |
Chest radiation dose (Gy) |
Anthracycline (mg/m2) + Chest radiation (Gy) |
|---|---|---|---|
| High | ≥ 250 | ≥ 35 | ≥ 100 (Anthracycline) + ≥ 15 (Radiation) |
| Moderate | 100 to < 250 | ≥ 15 to < 35 | -- |
| Low | < 100 | -- | -- |
While some studies have reported an increased risk of CHF in individuals treated with anthracyclines at a younger age (<5 years old),6, 8 others have found no association with age at exposure.7, 26, 33 As a result, no recommendations could be made regarding surveillance intensity by age at exposure. In addition, no recommendations could be made regarding the risk for cardiotoxicity by different anthracycline analogues, as the doxorubicin-equivalent conversion scores utilized by certain guidelines are based on hematologic toxicity and not cardiotoxicity.34 Cardioprotectants such as dexrazoxane have been shown to minimize cardiac injury and remodeling shortly after anthracycline administration without compromising its anti-tumor efficacy.35, 36 However, long-term data on efficacy of dexrazoxane is lacking, and certain subgroups, particularly children who have the greatest potential number of life years following cancer therapy, remain understudied.35 As a result, no recommendations can be made regarding surveillance intensity in survivors treated with cardioprotectant such as dexrazoxane.
What surveillance modality should be used?
Comprehensive history and physical examination with specific emphasis on cardiac symptoms such as dyspnea, chest pain, palpitations, or exertion intolerance, should be performed during routine follow-up in all childhood cancer survivors treated with cardiotoxic therapies. Detailed two-dimensional (2D) echocardiography is the recommended surveillance modality for these survivors (strong recommendation), and should be performed per the AHA/ACC task force practice guidelines for the clinical application of echocardiography.37 Several echocardiographic parameters including EF, SF, LV wall stress, decreased LV mass, velocity of shortening corrected for heart rate, LV thickness to dimension ratio, and diastolic dysfunction, have been used to describe asymptomatic cardiac dysfunction in childhood cancer survivors treated with anthracyclines or radiation.18, 21, 38 In this population, EF, SF, and wall stress are the most frequently used and readily reproducible parameters of LV systolic function, while E/A ratio and IVRT are commonly used to describe diastolic function.18 The long-term implications of many of the other early echocardiographic changes on future cardiomyopathy risk are not known. It is important to acknowledge that chronic ventricular remodeling and cardiac functional impairment could result from several conditions associated with radiation exposure to the heart, including asymptomatic coronary artery stenosis, progressive valvular dysfunction, or constrictive pericarditis.4, 12 As such, in these patients, routine surveillance should not be limited to assessment of ventricular function alone; healthcare providers should maintain a low threshold for evaluating coronary artery disease in survivors who have received high dose radiation therapy that included the coronaries.
Radionuclide angiography has been a well-established alternative to echocardiography in adult non-oncology populations.39 However it is not readily available across all treatment centers, and does not provide detailed information regarding cardiac structure and diastolic function,39 limiting its application as a primary surveillance modality in cancer survivors. CMR has emerged as a sensitive and reproducible alternative to echocardiography for assessment of cardiac structure and function (systolic and diastolic) in non-oncology populations and cancer survivors.40, 41 CMR is noninvasive and unlike radionuclide angiography, does not involve exposure to ionizing radiation. As in radionuclide angiography, CMR may not be readily accessible and its costs too prohibitive for population-based screening in at risk childhood cancer survivors. Current recommendations are to consider either radionuclide angiography or CMR in individuals for whom echocardiography is not technically feasible/optimal (moderate recommendation). In instances where both of these alternative imaging modalities are available, preference should be given to CMR due to its lack of ionizing radiation exposure and potential for additional information regarding cardiac structure and function.
Serum cardiac troponins T (cTnT) and I (cTnl) are specific and sensitive biomarkers for myocardial cell injury, and have established diagnostic and prognostic value in acute coronary syndrome.42 However, while cTn’s have successfully been used as biomarkers to monitor acute anthracycline-related cardiotoxicity,43, 44 studies have failed to demonstrate a clear association between cTn and LV dysfunction in childhood cancer survivors in part due to the low-sensitivity of conventional testing kits;.45–48 it remains to be seen what role, if any, newer high-sensitivity Troponin assays49 may play in predicting late-occurring LV dysfunction. Serum natriuretic peptides ([NP]: NT-Pro-BNP, BNP, ANP) are released in response to myocardial wall stress, and have become established biomarkers for the diagnosis of symptomatic heart failure.42 There is emerging evidence to suggest that persistent elevation of NPs during treatment with anthracyclines may be a predictor of cardiac dysfunction years after completion of therapy.43 However, data on the diagnostic accuracy of NPs for routine surveillance of cardiac dysfunction in asymptomatic cancer survivors has been mixed, as studies have reported high negative predictive values (63%–100%), but low sensitivity (0%–32%) and positive predictive values (12.5%–37.5%; Appendix Table 4), making them unreliable for use as the only surveillance strategy in this population. We acknowledge the growing body of literature in adult oncology4, 49 and non-oncology50, 51 populations supporting the complementary role of cardiac biomarkers and imaging studies for detection of cardiomyopathy. As such, it may be reasonable to consider blood biomarkers in individuals who may be symptomatic but have preserved systolic function, or in those with borderline cardiac function during primary surveillance (moderate recommendation).
At what frequency and for how long should surveillance be performed?
Due to lack of data, recommendations regarding initiation and frequency of surveillance are largely based on consensus. Consideration was given to the relative risk of CHF as well as to the potential difference in rate of cardiac function deterioration between risk groups during follow-up. There was consensus that surveillance should begin no later than 2 years after completion of cardiotoxic therapy and continue for a minimum of every 5 years thereafter, since pharmacologic interventions in individuals with asymptomatic cardiomyopathy can delay the onset of CHF and decrease mortality.11 These were strong and moderate recommendations for high and moderate/low-risk survivors, respectively. With regards to frequency of screening, there is no data to suggest that high risk survivors have a more rapid rate of deterioration when compared to moderate/low-risk survivors. However, given the higher prevalence of asymptomatic disease in high risk survivors, we believe more frequent surveillance is reasonable for high risk patients, and may be reasonable for moderate/low-risk survivors. On the other hand, there was no data to support higher risk of deterioration in cardiac function during the pubertal growth spurt.
During pregnancy, there is an overall increase in plasma volume of up to 50% that begins soon after gestation and peaks at 24–26 weeks.52 This change in volume contributes to an increase in cardiac output and compensatory increase in heart rate that lasts through the third trimester.52 Studies in non-oncology populations with pre-existing cardiomyopathy have reported a high risk of cardiac decompensation that is due to the added hemodynamic challenges of pregnancy,53, 54 and there are established guidelines for diagnosis and management of heart failure in this population.55 The limited experience in childhood cancer survivors suggests that women with compromised LV systolic function (SF<30%) prior to pregnancy are more likely to have further reduction in cardiac function post-partum, irrespective of lifetime anthracycline dose.56 As such, cardiomyopathy surveillance is reasonable prior to pregnancy or in the first trimester for all female survivors treated with anthracyclines and/or chest radiation (moderate recommendation). On the other hand, due to the paucity of data on cardiac outcomes, no recommendations can be formulated for the frequency of ongoing cardiomyopathy surveillance in pregnant survivors who have normal LV systolic function immediately prior to or during the first trimester of pregnancy.56, 57 Health care providers should maintain a high index of suspicion for cardiomyopathy in survivors treated with anthracyclines and/or radiation who present with symptoms such as shortness of breath, fatigue, and ankle swelling, as these are commonly reported during pregnancy.55
There is evidence from large cohort studies that the incidence of CHF in cancer survivors treated with anthracyclines and/or radiation increases with follow-up, and that this risk is greater in survivors treated with higher dose (≥250 mg/m2) anthracyclines.6, 7, 26 It is important to note that these cohort studies represent survivors who are relatively young (median age at CHF diagnosis: 25 to 27 years), and that there is limited data to inform us of the incidence of CHF >30 years after cancer diagnosis. However, emerging data in survivors with longer follow-up (median 25 years from diagnosis)3 show a substantially higher incidence of severe and life-threatening cardiovascular complications when compared to age- and sex-matched controls, decades after completion of therapy. Recognizing the increasing background risk of CHF with older age in the general population,11 we believe lifelong surveillance may be reasonable (moderate recommendation) for childhood cancer survivors treated with anthracyclines and/or radiation.
What should be done when abnormalities are identified?
The recommendations outlined in the current paper are for primary surveillance and do not address all the investigative steps necessary for the diagnosis and appropriate management of cardiomyopathy. As such, cardiology consultation is recommended for individuals who have abnormal cardiac function detected during surveillance (strong recommendation). The only randomized trial58 (ACE inhibitors vs. placebo) in anthracycline-exposed childhood cancer survivors with a history of transient or persistent cardiac dysfunction failed to demonstrate a clinically detectable difference in overall survival, mortality due to CHF, development of CHF or quality of life.58 As such, any recommendations for management of cardiomyopathy are based on findings from studies conducted in non-oncology populations at risk for CHF. That being said, when possible, pharmacologic intervention following diagnosis of cardiomyopathy should be personalized, taking into consideration available age-appropriate (pediatric59 vs. adult onset60, 61 CHF) treatment guidelines which take into consideration the physiology of the cardiomyopathy (systolic, diastolic, or both), severity of the disease, and the individual’s tolerance of the intervention.
What are the limitations for physical activity?
There is considerable evidence supporting the advantages derived from regular moderate exercise and fitness in the general population.62, 63 The current joint guidelines from the AHA and the American College of Sports Medicine (ACSM) recommend 30 to 40 minutes of aerobic exercise five times per week and strength training twice per week.62 Studies in limited numbers of childhood cancer survivors have found that despite having lower exercise capacity, evidenced by lower peak myocardial oxygen consumption,64, 65 survivors can attain significant improvements in muscle strength and flexibility, cardiopulmonary fitness, and overall physical function when engaged in routine aerobic activity.66 Given the well-documented benefits of exercise in the general population as well as in non-oncology populations at risk for CHF due to genetic disorders, regular exercise is recommended for survivors treated with anthracyclines and/or chest radiation who have normal cardiac function (strong recommendation). Individuals initiating an exercise regimen should be encouraged to promptly report to their primary healthcare providers any symptoms such as difficulty breathing or unusual tiredness.
With regards to limitations in the intensity of exercise, the AHA67 and the ESC68 provide no restrictions in activity for individuals who are at risk for cardiac decompensation due to genetic disorders (i.e.: familial dilated cardiomyopathy, hypertrophic cardiomyopathy) but have normal cardiac function (abnormal genotype, normal phenotype). However, for individuals with asymptomatic cardiac dysfunction, there are specific recommendations by the AHA and ESC regarding allowable activities (high, moderate, low-intensity; Appendix 4) that are based on severity of existing cardiac dysfunction.67 Cardiology consultation is recommended for survivors with asymptomatic cardiomyopathy to define limits and precautions for exercise (strong recommendation). Due to unpublished anecdotal reports of cardiac deterioration in childhood cancer survivors during intensive isometric exercise, cardiology consultation may be reasonable for high risk survivors who plan to be engaged in high intensity exercise (i.e. body building, rock climbing, windsurfing), as defined by the AHA and ESC (moderate recommendation).67, 68
Role of modifiable cardiovascular risk factors and cardiomyopathy risk
In general, healthcare providers are asked to educate and counsel all childhood cancer survivors regarding the importance of maintaining a heart-healthy lifestyle, including recommended five portions of fresh fruit and vegetables a day.69 Extensive studies conducted in non-oncology populations support the benefits of interventions to reduce modifiable risk factors, such as obesity, smoking, hypertension, diabetes and dyslipidemia.70, 71 Childhood cancer survivors are at a higher risk of developing many of these and other conditions such as growth hormone deficiency and abnormal body composition when compared to the general population, placing them at increased risk of developing premature cardiovascular disease later in life.72, 73 In fact, survivors who have hypertension or diabetes in addition to past exposure to anthracyclines and/or radiation are at an especially high risk of developing CHF.74 While there have been no studies conducted to demonstrate a rate reduction in cardiovascular events after risk factor modification in cancer survivors, findings from studies in non-oncology populations strongly suggest that routine screening for these risk factors can be beneficial, setting the stage for interventions (lifestyle modification, pharmacologic therapy) to mitigate adverse cardiovascular outcomes (strong recommendation).
DISCUSSION
The growing population of long-term childhood cancer survivors has brought to the forefront a host of chronic health-related conditions that can significantly impact the overall quality and quantity of survival.75 Cardiovascular complications such as CHF contribute increasingly to the long-term morbidity and mortality from these health conditions.4 We present the international harmonized cardiomyopathy surveillance recommendations for childhood cancer survivors treated with anthracyclines and/or chest radiation. The resultant recommendations are derived from knowledge gained from extensive scientific review of the available literature and strict standards used to grade the supporting evidence. Importantly, we have identified key gaps in knowledge (Table 5) that may serve as the impetus for collaborative research aimed at improving cardiovascular health of at risk childhood cancer survivors.
Table 5.
Gaps in knowledge and future directions for research.
|
It is abundantly clear that childhood cancer survivors treated with anthracyclines and/or chest radiation are at increased risk of CHF, and that the risk increases with treatment dose and duration of follow-up.19, 26, 27 Less is known regarding the dose-specific magnitudes of risk due to combined anthracycline and chest radiation exposure, or the risk due to lower-dose (<15 Gy) chest radiation exposure alone. Significant advances in systemic treatment and radiotherapy techniques during the past three decades have allowed reduction of radiation volume and dose delivered to healthy tissues such as the heart,76 resulting in decreased risk of non-myocardial infarction cardiac death in survivors of adult-onset cancers.76, 77 It remains to be seen if similar improvements in cardiovascular outcomes can be demonstrated in survivors of childhood cancer. With regards to anthracycline chemotherapy, there is virtually no information on the comparative cardiotoxicity of anthracycline analogues in children,34 nor is there evidence to support the long-term efficacy of cardioprotectants such as dexrazoxane in children with cancer.35 As a result, the current recommendations do not advocate different surveillance strategies based on anthracycline analogue or dexrazoxane exposure. Studies are needed to address these gaps in knowledge, setting the stage for more comprehensive characterization of CHF risk in these survivors.
Traditionally, monitoring of anthracycline-related cardiotoxicity has relied upon serial 2D echocardiography using resting LV EF or SF.13–16 These measurements are load-dependent, demonstrate intra-patient and inter-observer variability, and may not detect more subtle changes in cardiac systolic function.4 Studies in non-oncology populations4, 78 have shown that many of these limitations can be overcome if these measurements are performed in centralized core echocardiography laboratories. When possible, routine screening should incorporate load-independent parameters such as LV wall thickness, atrial and ventricular chamber dimensions, or M-mode-based stress velocity index, which can be calculated from the velocity of fiber shortening and corrected for heart rate and wall stress.4, 79 Further, routine surveillance should include measures of diastolic function, as survivors can develop restrictive cardiomyopathy in setting of normal systolic function.4 While there is no data to support that intervention after identification of abnormal early indices can delay the onset of symptomatic CHF in childhood cancer survivors, studies in non-oncology populations strongly support the use of pharmacologic intervention in individuals with asymptomatic cardiac dysfunction (regardless of etiology or physiology),80–82 and provide the basis for the early screening advocated in the current harmonized recommendations.
More novel imaging approaches for early detection of asymptomatic cardiac dysfunction include tissue Doppler imaging, CMR, “speckle tracking”, and 3D echocardiography.83 In fact, there is emerging evidence that 3D echocardiography, where technically feasible, has the lowest interobserver and serial variability for measurement of LV systolic function in survivors of childhood41 and adult-onset84 cancer. These newer imaging approaches have helped shed additional insight into the pathophysiology of cardiac injury after cancer treatment and may provide important prognostic utility in at risk survivors. However, these imaging modalities are not uniformly available across cancer follow-up centers, and lack of longitudinal follow-up studies in childhood cancer survivors precludes their routine use for primary cardiomyopathy surveillance at the current time. Data from adult oncology and non-oncology populations suggest that these imaging modalities may be used in individuals for whom routine 2D echocardiography is not technically feasible.39, 85
There is agreement across the COG, DCOG, SIGN, and UKCCLG guidelines that cardiomyopathy screening should begin no later than two years after completion of therapy, and to continue for a minimum of every five years thereafter. The harmonized recommendations for more frequent screening in higher risk survivors is consensus based, and they balance the potential benefit gained from early detection with the harms associated with increased cost and false positive testing. Given the long latency of disease and large numbers needed for follow-up, clinical trials evaluating efficacy of different screening frequencies would be cost-prohibitive. In addition, the paucity of information on efficacy of interventions to prevent progression of asymptomatic cardiomyopathy to CHF may temper the enthusiasm for aggressive surveillance in these survivors. Recognizing these limitations, studies have utilized decision-modeling to estimate the economic and health impact of different screening strategies and interventions in childhood cancer survivors at risk for CHF.86, 87 These studies have found that routine screening for cardiac dysfunction can be cost-effective when compared to no screening, and that survivors at highest risk of developing CHF may benefit from more frequent screening than those in the lowest risk categories,86, 87 a strategy advocated in the current harmonized recommendations.
Lastly, although the lifetime cumulative dose likely remains the single most important factor in influencing anthracycline or radiation-related related cardiotoxicity, some patients can develop CHF at relatively low doses while others do not appear to be affected despite very high doses, suggesting the importance of host-specific factors. There is emerging data to suggest that genetic susceptibility could play a role in modifying individual response to therapeutic exposures.27, 88, 89 Using a biologically plausible candidate gene approach, investigators have begun to identify polymorphisms that could alter metabolic pathways of therapeutic agents associated with specific adverse events, including CHF.23, 77, 78 Many of these genomic variables, when fully established, could advance our understanding of the pathogenesis of therapy-related CHF, and facilitate the implementation of targeted primary prevention strategies (individualized therapy in future cancer populations), as well as secondary prevention strategies (targeted screening, behavior modification, and chemoprevention in long-term survivors).
The cardiomyopathy screening harmonization effort was strengthened by our evidence-based approach, reliance on standardized definitions for outcomes of interest, transparent presentation of the quality of the available evidence and the strength of the recommendation, and the multidisciplinary approach necessary to derive a consensus for screening. We performed a critical appraisal of published guidelines13–16 that were developed following systematic evaluation of the quality of the late effects literature. In order to avoid duplication of effort, our literature review and resultant grading of the evidence primarily focused on areas of discordance. While we recognize that this may have introduced a risk of bias for the concordant recommendations, we do not believe the adopted strategy compromised the integrity of the resultant recommendations. When evidence was lacking for childhood cancer survivors, we extrapolated information from other populations at risk of CHF. Importantly, we have identified key gaps in knowledge pertaining to frequency of screening in different risk groups, role of CMR, myocardial strain, 3D echocardiography as well as cardiac blood biomarkers in primary surveillance, prognostic utility changes in intermediate echocardiographic indices of LV systolic and diastolic function, and efficacy of early intervention strategies for CHF prevention. These gaps can be filled only by approaching these problems in a systematic, comprehensive manner that not only helps identify those at highest risk of these adverse outcomes but also modifies the natural history of their disease. This approach requires multidisciplinary and international collaborations and access to large patient populations. The current international harmonization initiative will help set the stage for collaborative research to minimize the burden of cardiovascular disease in survivors of pediatric malignancies.
Supplementary Material
Acknowledgements
S. Armenian is supported by the National Institues of Health (2 K12 CA001727-14, 1 U10 CA098543). M.M. Hudson is supported by the Cancer Center Support (CORE) grant CA 21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC). R. L. Mulder is supported by the Dutch Cancer Society, Amsterdam, the Netherlands (UVA 2011–4938). G. Levitt and R. Skinner are supported in part by the 7th Framework Program of the EU, PanCareSurfUp (257505). J. Steinberger is supported by NCI/NIDDK: 1R01CA113930, NIDDK: 1R01DK072124. E. van Dalen is supported by Stichting Kinderen Kankervrij, the Netherlands. H. van der Pal is supported by the Tom Voûte Foundation, Amsterdam, the Netherlands.
We thank Kevin Oeffinger and Jako Burgers for critically appraising the recommendations and the manuscript as external reviewers. We would like to thank the experts of the International Late Effects of Childhood Cancer Guideline Harmonization Group and members of the PanCareSurfUp Consortium for their participation in the international guideline harmonization process: Smita Bhatia, Wendy Landier, Edit Bárdi, Eva Frey, Riccardo Haupt, Claudia Kühni, Gisela Michel, Flora van Leeuwen, Cecile Ronckers, Berthe Aleman,Gregory Armstrong, Eric Chow, Richard Cohn, Junichiro Fujimoto, Satomi Funaki, Daniel Green, Tara Henderson, Lars Hjorth, David Hodgson, Hiroyuki Ishiguro, Shunichi Kato, Chikako Kiyotani, Miho Maeda,Michael Schaapveld, Jane Skeen, Charles Sklar.
Appendix 1
Search Medline/PubMed for studies published (January 2007 to December 2012)
Working Group 1
-
Anthracyclines:
(anthracyclines OR anthracyclin* OR idarubicin OR idarubic* OR epirubicin OR epirubic* OR adriamycin OR doxorubicin OR doxorubic* OR adriamyc* OR daunorubicin OR daunorubic* OR daunoxome OR doxil OR caelyx OR myocet)
-
Mitoxantrone:
(mitoxantrone OR mitoxantr*)
-
Radiotherapy:
(Radiotherapy OR radiation OR radiat* OR irradiation OR X-ray therapy)
-
Cancer:
(Cancer OR neoplasm OR tumor OR tumour OR carcinoma OR malignancy OR Childhood cancer)
-
Survivors:
(surviv* OR survivor OR survivors)
-
(A)symptomatic cardiac dysfunction:
(ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right OR shortening fraction OR ejection fraction OR LVEF OR LVSF OR systolic OR myocardial contraction OR contract* OR cardiomyopathy OR heart failure, congestive OR heart failure OR cardiomyopathy congestive)
(anthracyclines OR anthracyclin* OR idarubicin OR idarubic* OR epirubicin OR epirubic* OR adriamycin OR doxorubicin OR doxorubic* OR adriamyc* OR daunorubicin OR daunorubic* OR daunoxome OR doxil OR caelyx OR myocet OR mitoxantrone OR mitoxantr* OR Radiotherapy OR radiation OR radiat* OR irradiation OR X-ray therapy) AND (age at treatment OR younger age OR age at exposure)
Working group 2:
Question 1: (Cancer OR neoplasm OR tumor OR tumour OR carcinoma OR malignancy OR Childhood cancer) AND (surviv* OR survivor OR survivors) AND (echocardiography OR echocardiogr*) AND (radionuclide angiography OR radionuclide ventriculography OR gated blood-pool imaging OR blood pool scintigraphy OR gated radionuclide ventriculography OR ventriculogr* OR scintigr* OR MUGA OR angiocardiography OR angio*) AND (ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right OR shortening fraction OR ejection fraction OR LVEF OR LVSF OR systolic OR myocardial contraction OR contract*)
-
Question 2: (Cancer OR neoplasm OR tumor OR tumour OR carcinoma OR malignancy OR Childhood cancer) AND (surviv* OR survivor OR survivors) AND (echocardiography OR echocardiogr*) AND (Atrial natriuretic factor OR ANP OR ANF OR atrial natriuretic peptides OR Brain natriuretic peptide OR BNP OR Pro-brain natriuretic peptide OR N-terminal pro-BNP OR NT-proBNP OR NT-proBNP OR proBNP) AND (ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right OR shortening fraction OR ejection fraction OR LVEF OR LVSF OR systolic OR myocardial contraction OR contract*)
(Cancer OR neoplasm OR tumor OR tumour OR carcinoma OR malignancy OR Childhood cancer) AND (surviv* OR survivor OR survivors) AND (echocardiography OR echocardiogr*) AND (troponin T OR troponin I OR ctnt OR ctni) AND (ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right OR shortening fraction OR ejection fraction OR LVEF OR LVSF OR systolic OR myocardial contraction OR contract*)
Question 3: (echocardiography OR echocardiogr*) AND (Atrial natriuretic factor OR ANP OR ANF OR atrial natriuretic peptides OR Brain natriuretic peptide OR BNP OR Pro-brain natriuretic peptide OR N-terminal pro-BNP OR NT-proBNP OR NT-proBNP OR proBNP) AND (ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right OR shortening fraction OR ejection fraction OR LVEF OR LVSF OR systolic OR myocardial contraction OR contract*) Limits: Meta-Analysis, Review, Adult: 19–44 years, Middle Aged: 45–64 years, Aged: 65+ years, 80 and over: 80+ years
Question 4: (Cancer OR neoplasm OR tumor OR tumour OR carcinoma OR malignancy OR Childhood cancer) AND (Survivor OR survivors OR surviv*) AND (echocardiography OR echocardiogr*) AND (Magnetic resonance imaging OR NMR imaging OR MR tomography OR NMR tomography OR MRI OR MRI scan OR MRI scan*) AND (ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right OR shortening fraction OR ejection fraction OR LVEF OR LVSF OR systolic OR myocardial contraction OR contract*)
Question 5: (Cancer OR neoplasm OR tumor OR tumour OR carcinoma OR malignancy OR Childhood cancer) AND (Survivor OR survivors OR surviv*) AND (Cost-benefit analyses OR cost benefit analyses OR cost-benefit analysis OR cost benefit analysis OR cost effectiveness OR Cost-Benefit Data OR Cost Benefit Data OR Cost Benefit OR Benefits and Costs OR Costs and Benefits) AND (ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right OR shortening fraction OR ejection fraction OR LVEF OR LVSF OR systolic OR myocardial contraction OR contract*)
Working Group 3
(anthracyclines OR anthracyclin* OR idarubicin OR idarubic* OR epirubicin OR epirubic* OR adriamycin OR doxorubicin OR doxorubic* OR adriamyc* OR daunorubicin OR daunorubic* OR daunoxome OR doxil OR caelyx OR myocet OR mitoxantrone OR mitoxantr* OR Radiotherapy OR radiation OR radiat* OR irradiation OR X-ray therapy) AND (ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right OR shortening fraction OR ejection fraction OR LVEF OR LVSF OR systolic OR myocardial contraction OR contract* OR cardiomyopathy OR heart failure, congestive OR heart failure OR cardiomyopathy, congestive OR echocardiography OR echocardiogr* OR radionuclide angiography OR radionuclide ventriculography OR gated blood-pool imaging OR blood pool scintigraphy OR gated radionuclide ventriculography OR ventriculogr* OR scintigr* OR MUGA OR angiocardiography OR angio*) AND (surviv* OR survivor OR survivors)
Working Group 4
In short
(Anthracyclines OR Mitoxantrone OR Radiotherapy) AND Cancer AND Survivors AND (A)symptomatic cardiac dysfunction AND therapy AND RCT/CCT
Complete
(anthracyclines OR anthracyclin* OR idarubicin OR idarubic* OR epirubicin OR epirubic* OR adriamycin OR doxorubicin OR doxorubic* OR adriamyc* OR daunorubicin OR daunorubic* OR daunoxome OR doxil OR caelyx OR myocet OR mitoxantrone OR mitoxantr* OR Radiotherapy OR radiation OR radiat* OR irradiation OR X-ray therapy) AND (Cancer OR neoplasm OR tumor OR tumour OR carcinoma OR malignancy OR Childhood cancer) AND (surviv* OR survivor OR survivors) AND (ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right OR shortening fraction OR ejection fraction OR LVEF OR LVSF OR systolic OR myocardial contraction OR contract* OR cardiomyopathy OR heart failure, congestive OR heart failure OR cardiomyopathy, congestive)
(ace inhibitor OR ace-inhibitor OR ace inhibitor*OR ace-inhibitor* OR Angiotensin-Converting Enzyme Inhibitors OR Angiotensin- Converting Enzyme Inhibitors[Pharmacological Action] OR Angiotensin Converting Enzyme Inhibitors OR Angiotensin-Converting Enzyme Antagonists OR Angiotensin Converting Enzyme Antagonists OR Enzyme Antagonists, Angiotensin-Converting OR Antagonists, Angiotensin-Converting Enzyme OR Antagonists, Angiotensin Converting Enzyme OR Antagonists, Kininase II OR Inhibitors, Kininase II OR Inhibitors, ACE OR ACE Inhibitors OR Kininase II Inhibitors OR Kininase II Antagonists OR Angiotensin I Converting Enzyme Inhibitors OR Angiotensin I Converting Enzyme Inhibitors OR Inhibitors, Angiotensin-Converting Enzyme OR Enzyme Inhibitors, Angiotensin-Converting OR Inhibitors, Angiotensin Converting Enzyme OR Angiotensin-Converting Enzyme Inhibitor* OR Angiotensin Converting Enzyme Inhibitor* OR Angiotensin-Converting Enzyme Antagonist* OR Angiotensin Converting Enzyme Antagonist* OR Kininase II Inhibitor* OR Kininase II Antagonist* OR Angiotensin I-Converting Enzyme Inhibitor* OR Angiotensin I Converting Enzyme Inhibitor* OR captopril OR enalapril OR fosinopril) OR (peptidyl dipeptidase OR Peptidyl Dipeptidase A OR Angiotensin I-Converting Enzyme OR Angiotensin I Converting Enzyme OR Carboxycathepsin OR Kininase A OR CD143 Antigen OR CD143 Antigens OR Dipeptidyl Peptidase A OR Antigens, CD143 OR Angiotensin Converting Enzyme OR Kininase II)
(angiotensin receptor blocker OR angiotensin receptor blockers OR angiotensin receptor blocker* OR Angiotensin II Type 1 Receptor Blockers OR Angiotensin II Type 1 Receptor Antagonists OR Type 1 Angiotensin Receptor Antagonists OR Type 1 Angiotensin Receptor Blockers OR Selective Angiotensin II Receptor Antagonists OR Sartans OR Angiotensin II OR Angiotensin Receptors/ antagonists & inhibitors OR Angiotensin II Type 1 Receptor Blocker* OR Type 1 Angiotensin Receptor Antagonist* OR Type 1 Angiotensin Receptor Blocker* OR Selective Angiotensin II Receptor Antagonist* OR losartan OR valsartan)
(beta blocker OR beta blockers OR beta-blockers OR beta-blocker OR beta-blocker* OR beta blocker* OR Adrenergic beta Antagonists OR adrenergic beta-antagonists OR adrenergic beta-antagonists[Pharmacological Action] OR beta-Antagonists, Adrenergic OR Adrenergic beta-Receptor Blockaders OR Adrenergic beta Receptor Blockaders OR Blockaders, Adrenergic beta-Receptor OR beta-Receptor Blockaders, Adrenergic OR beta-Adrenergic Receptor Blockaders OR Blockaders, beta-Adrenergic Receptor OR Receptor Blockaders, beta-Adrenergic OR beta Adrenergic Receptor Blockaders OR beta-Adrenergic Blocking Agents OR Agents, beta-Adrenergic Blocking OR Blocking Agents, beta-Adrenergic OR beta Adrenergic Blocking Agents OR beta-Adrenergic Blockers OR Blockers, beta-Adrenergic OR beta Adrenergic Blockers OR beta-Blockers, Adrenergic OR Adrenergic beta-Blockers OR beta Blockers, Adrenergic OR Sympatholytics OR Sympatholytics [Pharmacological Action] OR Sympathetic-Blocking Agents OR Agents, Sympathetic-Blocking OR Sympathetic Blocking Agents OR Sympatholytic Agents OR Agents, Sympatholytic OR Sympatholytic Drugs OR Drugs, Sympatholytic OR Sympatholytic* OR Adrenergic beta Antagonist* OR Adrenergic beta-Receptor Blockader* OR Adrenergic beta Receptor Blockader* OR beta-Adrenergic Receptor Blockader* OR beta Adrenergic Receptor Blockader* OR beta-Adrenergic Blocking Agent* OR beta Adrenergic Blocking Agent* OR beta Adrenergic Blocker* OR beta-Adrenergic Blocker* OR Adrenergic beta-Blocker* OR Sympathetic-Blocking Agent* OR Sympathetic Blocking Agent* OR Sympatholytic Agent* OR Sympatholytic Drug* OR carvedilol OR atenolol OR metoprolol OR propranolol)
(calcium channel blocker OR calcium channel blockers OR calcium channel blockers[Pharmacological Action] OR calcium channel blocker* OR Exogenous Calcium Antagonists OR Antagonists, Exogenous Calcium OR Calcium Antagonists, Exogenous OR Exogenous Calcium Blockaders OR Blockaders, Exogenous Calcium OR Calcium Inhibitors, Exogenous OR Calcium Channel Blocking Drugs OR Exogenous Calcium Inhibitors OR Inhibitors, Exogenous Calcium OR Calcium Blockaders, Exogenous OR Channel Blockers, Calcium OR Blockers, Calcium Channel OR Exogenous Calcium Antagonist* OR Exogenous Calcium Blockader* OR Calcium Channel Blocking Drug* OR Exogenous Calcium Inhibitor* OR Exogenous Calcium Blockader* OR Calcium Channel Blocking Drug* OR Exogenous Calcium Inhibitor* OR diltiazem OR nifedipine)
(digoxin OR digoxin* OR Lanoxin)
(vasodilator OR vasodilators OR vasodilator* OR vasodilator agents OR vasodilator agents[Pharmacological Action] OR Agents, Vasodilator OR Vasodilator Drugs OR Drugs, Vasodilator OR Vasoactive Antagonists OR Antagonists, Vasoactive OR Vasoactive Antagonist* OR vasodilator agent* OR Vasodilator Drug* OR nitroglycerin OR Glyceryl Trinitrate OR Trinitrate, Glyceryl OR Nitroglycerin* OR diazoxide OR adenosine)
(diuretic OR diuretics OR diuretic* OR diuretics[Pharmacological Action] OR furosemide)
(aldosteron antagonist OR aldosteron antagonists OR aldosterone antagonist OR aldosterone antagonists OR aldosterone antagonist* OR aldosteron antagonist* OR “Aldosterone antagonists”[Pharmacological Action] OR Antagonists, Aldosterone OR spironolactone)
(antihypertensiva OR anti-hypertensive OR anti hypertensive OR anti hypertensive drugs OR antihypertensive drugs OR antihypertensive agents OR antihypertensive agents[Pharmacological Action] OR Agents, Antihypertensive OR Anti-Hypertensive Agents OR Agents, Anti-Hypertensive OR Anti Hypertensive Agents OR Anti-Hypertensive Drugs OR Anti Hypertensive Drugs OR Drugs, Anti-Hypertensive OR Anti-Hypertensives OR Anti Hypertensives OR Antihypertensive Drugs OR Drugs, Antihypertensive OR Antihypertensives OR antihypertensiv* OR antihypertensive drug* OR anti hypertensive drug* OR antihypertensive agent* OR anti hypertensive agent* OR clonidine)
(inotropics OR inotropic OR inotropic* OR dopamine OR dobutamine OR epinephrine OR norepinephrine)
(growth hormone OR Growth Hormone, Pituitary OR Pituitary Growth Hormone OR Somatotropin OR Growth Hormone, Recombinant OR Growth Hormones Pituitary, Recombinant OR Pituitary Growth Hormones, Recombinant OR Recombinant Pituitary Growth Hormones OR Somatotropin, Recombinant OR Recombinant Somatotropin OR Recombinant Growth Hormone OR Recombinant Growth Hormones OR Growth Hormones, Recombinant OR Recombinant Somatotropins OR Somatotropins, Recombinant OR growth hormon* OR Somatotropin* OR Pituitary Growth Hormon* OR Recombinant Pituitary Growth Hormon* OR Recombinant Somatotropin* OR Recombinant Growth Hormon*)
((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) AND humans[mh])
2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12
Appendix 2
Criteria for grading the levels of evidence for conclusions (based on modified GRADE)
| Conclusions of evidence |
Study quality | Study findings | Wording in conclusions |
|---|---|---|---|
|
A High level of evidence |
Evidence from well performed and high quality studies or systematic reviews (low risk of bias, direct,* consistent, precise) | If a risk factor is significantly associated with the outcome in ≥95% of studies | ‘There is evidence that…’ |
|
B Moderate/Low level of evidence |
Evidence from studies or systematic reviews with few important limitations | If a risk factor is significantly associated with the outcome in ≥50% of the studies reporting on this risk factor, and in the remaining studies this association is not significant | ‘Evidence suggests that…’ |
|
C Very low level of evidence |
Evidence from studies with serious flaws (high risk of bias, inconsistent, indirect*, imprecise) | If a risk factor is significantly associated with the outcome in 1 study | ‘Some evidence suggests that…’ |
| If a risk factor is significantly associated with the outcome in <50% of the studies, while in the remaining studies this association is not significant | |||
| If a risk factor is significantly (either positively or negatively) associated with the outcome in >50% of the studies, while the remaining studies show the opposite association of the risk factor and outcome. | |||
| Conflicting evidence | N/A | If a risk factor is significantly (both positively and negatively) associated with the outcome in the same number of studies of comparable quality. | ‘There is conflicting evidence…’ |
| No evidence | N/A | If no studies reported on a risk factor | ‘No studies reported on…’ |
Abbreviations: GRADE, Grading of Recommendations Assessment Development and Evaluation; N/A, not applicable.
Direct evidence comes from research that directly compares the interventions in which we are interested when applied to the populations in which we are interested and measures outcomes important to patients. Studies are indirect if there are differences in study population (our population of interest is childhood cancer survivors), interventions, or outcome measures, or if there are indirect comparisons of interventions.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004; 328(7454): 1490.
Strength of the Recommendation (based on modified AHA/ACC criteria)
| Strong recommendation to do |
| Benefits >>> risks & burdens |
| Based on high quality evidence, using anchor terms usch as ‘is recommended’, and with low degree of uncertainty. |
| Moderate recommendation to do |
| Benefits >> risks & burdens |
| Based on moderate quality of evidence, using anchor terms such as ‘is reasonable’, with higher degree of uncertainty. |
| Weak recommendation to do |
| Benefits >= risks & benefits |
| Based on weak quality of evidence, using anchor terms such as ‘may be reasonable’, with high degree of uncertainty; other factors such as patient preferences and costs need to be considered in the decision making process. |
| Recommendation not to do |
| No benefit/Potentially harm |
Abbreviations: AHA/ACC, American Heart Association/American College of Cardiology
Gibbons RJ, Smith S, Antman E. American College of Cardiology/American Heart Association clinical practice guidelines: Part I: where do they come from? Circulation. 2003; 107(23): 2979–86.
Appendix 3
Recommendations for the Acceptability of Recreational (Noncompetitive) Sports Activties and Exercise in Patients With GCVDs*
Appendix 3.
| Intensity Level | HCM† | LQTS† | Marfan Syndrome‡ |
ARVC | Brugada Syndrome |
|---|---|---|---|---|---|
| High | |||||
| Basketball | |||||
| Full court | 0 | 0 | 2 | 1 | 2 |
| Half court | 0 | 0 | 2 | 1 | 2 |
| Body building§ | 1 | 1 | 0 | 1 | 1 |
| Ice hockey§ | 0 | 0 | 1 | 0 | 0 |
| Racquetball/squash | 0 | 2 | 2 | 0 | 2 |
| Rock climbing§ | 1 | 1 | 1 | 1 | 1 |
| Running (sprinting) | 0 | 0 | 2 | 0 | 2 |
| Skllng (downhill)§ | 2 | 2 | 2 | 1 | 1 |
| Skllng (cross-country) | 2 | 3 | 2 | 1 | 4 |
| Soccer | 0 | 0 | 2 | 0 | 2 |
| Tennis (singles) | 0 | 0 | 3 | 0 | 2 |
| Touch (flag) football | 1 | 1 | 3 | 1 | 3 |
| Windsurfing‖ | 1 | 0 | 1 | 1 | 1 |
| Moderate | |||||
| Baseball/softball | 2 | 2 | 2 | 2 | 4 |
| Biking | 4 | 4 | 3 | 2 | 5 |
| Modest hiking | 4 | 5 | 5 | 2 | 4 |
| Motorcycling§ | 3 | 1 | 2 | 2 | 2 |
| Jogging | 3 | 3 | 3 | 2 | 5 |
| Sailing‖ | 3 | 3 | 2 | 2 | 4 |
| Surfing‖ | 2 | 0 | 1 | 1 | 1 |
| Swimming (lap)‖ | 5 | 0 | 3 | 3 | 4 |
| Tennis (doubles) | 4 | 4 | 4 | 3 | 4 |
| Treadmill/stationary bicycle | 5 | 5 | 4 | 3 | 5 |
| Weightlifting (free weights)§¶ | 1 | 1 | 0 | 1 | 1 |
| Hiking | 3 | 3 | 3 | 2 | 4 |
| Low | |||||
| Bowling | 5 | 5 | 5 | 4 | 5 |
| Golf | 5 | 5 | 5 | 4 | 5 |
| Horseback riding§ | 3 | 3 | 3 | 3 | 3 |
| Scuba diving‖ | 0 | 0 | 0 | 0 | 0 |
| Skating# | 5 | 5 | 5 | 4 | 5 |
| Snorkeling‖ | 5 | 0 | 5 | 4 | 4 |
| Weights (non–free weights) | 4 | 4 | 0 | 4 | 4 |
| Brisk walking | 5 | 5 | 5 | 5 | 5 |
Abbreviations: HCM, hypertrophic cardiomyopathy; LQTS, prolonged QT-syndrome
Maron BJ, Chaitman BR, Ackerman MJ, et al: Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation 109:2807–16, 2004
Appendix 4: Working Group Evidence Summaries
Working Group 1: “Who needs cardiomyopathy surveillance?”
| 1. what is the evidence behind the conversion score for different derivates for anthracyclines (including mitoxantrone) | ||||||
|---|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks | |
| van der Pal1 2012 | Retrospective cohort 1966–1996 22.2 yrs (5.0–44.5) |
5-yr survivors (N=1362) |
Conversion score: Doxorubicin : 1.0 Daunorubicin: 1.0 Epirubicin: 0.67 |
Refs: Mertens (2008): late mortality Le Deley (2003): SMN after solid CA Perez (1991): Breast CA (epi vs.dox) |
||
| Mulrooney2 2009 | Retrospective cohort 1970–1986 27.0 yrs (8–51) |
5-yr Survivors (N=14, 358) Siblings (N=3899) |
Conversion score: Doxorubicin = Daunorubicin Idarubicin = 3× doxorubicin |
Conversion score based on a review paper recommendations (Pai Nahata 2000) | ||
| Blanco3 2012 | Case-Control 1966–2008 Cases: 9.2 (0.1–35.1) Controls: 12.3 (0.4–40) |
Case (CHF) – N=170 Control (none) – N=317 |
Conversion score: Guidelines Doxorubicin: 1.0 Daunorubicin: 0.75 0.83 Epirubicin: 0.75 Idarubicin: 3 Mitoxantrone: 3 |
COG LTFU Doxorubicin: 1.0 Daunorubicin: Epirubicin: 0.67 Idarubicin: 5 Mitoxantrone: 4 |
Conversion score based on: Lehmann (2000), which is based on sited review literature with 1 in vivo model of acute toxicity | |
| Temming4 2011 | Retrospective cohort N=124, 86 1987–2004 7.3 yrs (0–21.7) |
124/158 available for Cardiotox analysis 86 data for late cardiotox | AML 10 and 12 trials Anthracyclines: Dauno and Mitox (1:5 conversion) 550–610 mg/m2 |
Anthracycline dose range similar across AML 10 and 12, unable to assess dose-association No discussion on conversion factor |
||
| Creutzig5 2007 | Retrospective cohort 1993–2003 BFM98: 3.6ys (0.8–7.0) BFM93: 7.5ys (1.1–11) |
Eligible: N=1207 Late Cartox eval: N=547 (45%) 76% of echo w/in first 5yrs |
AML BFM 93 98 Dauno : Ida 1:5 Dauno : Mitox 1:5 |
|||
| van Dalen6 2010 | Systematic review Meta-analysis 1966–2009 RCT’s: children, adults |
Different anthracycline derivatives | Dox Epi Lipo-Dox |
Epi vs. Dox (5 RCTs) = 1036 pts Clinical: RR=0.36, NS Lipo- vs. Dox (2 RCTs) = 521 pts Clinical: RR=0.2 (0.02–0.75) Subclinical: RR=0.38 (0.24–0.59) |
For other possible combinations of different anthracycline derivatives, only 1 RCT or no RCT was identified Inconclusive evidence for children | |
| Le Deley7 2003 | Case-control 1980–1999 |
Secondary leukemias after treatment of solid ca in childhood | Doxorubicin 50 mg/m2 = 75 mg/m2 epirubicin 60 mg/m2 dauno 12.5 mg/m2 mitox |
Conversion based on leukemogenic potential of anthracyclines -NO ref for basis of anthracycline dose calculation |
||
| Neri8 1989 | Observational ?Tx era: 1980’s |
Doxorubicin N=9 Epirubicin N=13 Authors propose:
|
Dox 60 mg/m2 (Max 540) Vs. Epi 60 mg/m2 (Max 720) |
Blood biomarker measurements, Echo’s Epirubicin less CK-MB elevation VO2 changes: Dox vs. Epi: 44% vs. 13% reduction Incidence of CHF: Dox vs. Epi: 67% vs. 23% Conclusion: “Epi-related cardiotoxicity 40% less than that produced by doxorubicin..” |
Small numbers, not controlled for risk factors, older treatment era Non-random assignment tBreast CA, non-pediatric Acute cardiotoxicity |
|
| 2. What is the risk of (a)symptomatic cardiac systolic dysfunction in childhood and young adult cancer survivors of TBI that is above and beyond the risk due to pre-HCT anthracycline and chest radiation? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Uderzo9 2007 | Prospective cohort 1994–1997 5 yrs. |
N= 162, Age: 0–18 y.o. at HCT |
Allogeneic HCT 67% anthracyclines 58% TBI 80% HCT for malignancy |
Decline in FS over time Univariate: TBI alone, p=0.04 TBI + Anthracyclines, p=0.004 Multivariate No association with TBI and FS decline |
In addition, no differences seen by gender or age at HCT. TBI fractionated (12Gy) in nearly all except 2. |
| Lonnerholm10 1999 | Prospective cohort 1985–1996 1–10 years (median 5) |
N= 45, Age: 1.2–16.2 at dx |
Autologous HCT 53% TBI Pre-HCT anthr: 150–450 |
Standard echo: 1y-, 3y-and 5-post LVDD/SD, EF, FS No difference in LV dimensions by TBI No discussion of anthracycline dose and changes in LV parameters |
|
| Eames11 1997 | Cross-sectional 1994–1995 Mean f-up 4.1 yrs |
N=63 Age: 2y–32 y at partic. |
Allo HCT: 82% Auto HCT: 18% TBI: 65% HD-Cy: 95% Anth: 63.5% Anth dose: 308 (60–450) |
Comprehensive cardiac echo: NYHA grading of all participants Normal FS (>=29%): 98% No regression analysis for risk factors for abn EF/FS TBI (fractionated or not) NOT predictive of cardiotoxicity |
Selection bias 22% of HCT population included Treadmill exercise testing Abnormal: 48.4% |
| Armenian12 2011 | Retrospective cohort 1970–1986 CCSS 1974–1998 BMTSS CCSS: 16 yrs (+/−5) BMTSS: 13 yrs (+/−5.6) |
Heme malign CCSS: N=7207Age: 8.9 yrs at dx 25 yrs at partic. BMTSS: N=145 Age: 10.9 yrs at dx 24 yrs at partic. Sibling N=4020 Age: 26. yrs at partic. |
BMTSS Chemo + TBI: 76.6% Autologous HCT: 28% Anthracycline: None −8.3% 1–249 – 50.3% >=250 – 41.4% Chest Radiation: 5.5% CCSS Anthracycline: None – 61.0% 1–249 – 19.3% >=250 – 19.7% Chest radiation: 23.1% |
CTCAE graded chronic health conditions Grade 3–5 cardiac disease Multivariate regression adjusting for: Age, gender, race, insurance, treatment era, time from dx, diagnosis, chest radiation, anthracycline dose BMTSS vs. siblings: RR 12.7 p<0.01 BMTSS vs. CCSS: RR 0.5, p=NS |
After adjusting for pre-HCT treatment-related exposures, no differences in CV outcomes seen, Sub-analysis of specific HCT-related exposures (TBI, HD Cytoxan) did not reveal a difference |
| Armenian 200813 | Case-control 1981–2003 6.4 yrs (1.3–22.1) |
1+year survivors Allo and auto HCT Case (CHF): 60 Control: 166 Age 43 yrs (+/−13) |
Mean Anthracycline: 261 vs. 171 mg/m2 Chest XRT: 10% vs. 8% TBI: 65.0% vs. 65.7% HD-Cy: 75.0% vs. 75.3% |
Clinical CHF per AHA/ACC def. Anthracyclines as the only treatment-related predictor of post-HCT CHF. TBI, HD-Cy not significant in univariate or multivariate models. |
Mostly adults, only included late-occurring events. |
| Armenian 201114 | Retrospective cohort Nested case-control 1988–2002 5.3 yrs (0.1–20.5 yrs) |
Autologous HCT Cohort: N=1244 CHF: N=88 peds + adults 7200 person-yrs |
TBI (12 Gy Frax): 59.2% (60% vs. 59%) HD-CY: 85.9% (87% vs. 86%) Anthracycline mg/m2: 309 vs. 237, p<0.01 |
Clinical CHF per AHA/ACC def. Multivariate Condit. regression: Female: RR 2.4, p<0.01 Lymphoma dx: 1.5, p=0.05 Age: RR↑ wth age TBI, HD-Cy NOT associated with risk |
Pre-HCT anthracycline dose, and post-HCT CV risk factors, gender, most significant predictors of post-HCT risk. CI of CHF 15% at 15 yrs in female lymphoma survivors. |
| Chow15 2011 | Retrospective cohort 1985–2006 |
2+year survivors Allo and auto HCT N=1491 Gen pop (by age) matching N=4352 |
Autologous: 43.7% Allogeneic: 56.3% TBI: 76.7% HD-Cy: 48.1% |
CV outcomes, ICD-9 coding, hospital records: MI, DCM, CHF, stroke, other vascular dz. Multivariate regression Risk of DCM, CHF: Post HCT relapse: RR 1.9 (1.1–3.3) TBI: RR 1.0 (0.6–1.8) Allo HCT: 0.8 (0.5–1.4) |
No anthracycline in models Hosp ICD-9 codes, not validated outcomes Post-HCT CV risk factors as significant predictors of DCM or CHF. |
| Tichelli162008 | Retrospective cohort 1990–1995 9 yrs (1–16 yrs) |
1+-year survivors Allogeneic HCT Adult HCT N=548 |
Hem. Malign: 85% TBI: 58% |
Limited to clinically validated arterial events TBI: 70% (arterial dz), 57% (no dz), NS Multivariate model: Older age at HCT and CVRFs as the only independent predictors of dz. |
No anthracycline in models Post-HCT risk factors as predictors of post-HCT CV outcomes |
| 3. What is the risk for different anthracycline doses for developing (a)symptomatic cardiac systolic dysfunction in childhood and young adult cancer survivors? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Symptomatic cardiomyopathy and anthracycline dose | |||||
| van der Pal1 2012 | Retrospective cohort 1966–1996 22.2 yrs (5.0–44.5) |
5-yr survivors (N=1362) Age at Dx: 5.9 (0–18) |
Anthracyclines: 33.6% Cardiac XRT: 19.5% Anth+XRT: 7.9% Median Anth: 250 (25–775) |
Symptomatic cardiac events (CE) Grading: CTCAE v 3.0 50 CEs in 42 CS (CHF in 27/50) Median time to event: 18.6 yrs Multivariate regression (Model 1) Anthracycline (per 100 mg/m2) HR 1.8 (1.5–2.3) Multivariate regression (Model 2) Anthracycline (Yes/No) vs. no cardiotoxic therapy HR 33.5 (4.4–254) |
Clinically validated outcomes Long follow-up, large cohort |
| Blanco3 2012 | Case-Control 1966–2008 Cases: 9.2 (0.1–35.1) Controls: 12.3 (0.4–40) |
Case (CHF) – N=170 Control (none) – N=317 Matching criteria: Diagnosis Year of Dx (+/−5 yrs) Race/ethnicity Follow-up (controls) |
Cases vs. controls: Anthracyclines 291 vs. 168, p<0.01 Chest XRT 25% vs. 14%, p<0.01 |
Clinically validated DCM, CHF Multivariate (CHF): Referent group – no anthracycline P for trend p<0.001; Odds Ratios 1–100: 1.65 101–150: 3.85 151–200: 3.69 201–250: 7.23 251–300: 23.5 >300: 27.6 |
Genetic susceptibility Matching based on diagnosis Differences in mean anthracycline dose between Ca-Co’s |
| Temming4 2011 | Retrospective cohort 1987–2004 7.3 yrs (0–21.7) |
124/158 available for Cardiotox analysis 86 data for late cardiotox Age at Dx: 2.9 (0.1–12.9) |
AML 10 and 12 trials Anthracyclines: Dauno and Mitox (1:5 conversion) 550–610 mg/m2 |
Subclinical cardiotox (SF<28%) Clinical CHF per AHA Anthracycline dose-relationship not determined |
Not a very wide distribution of age due to Dx., likely reason for no anth-dose association |
| Armenian14 2011 | Retrospective cohort Nested case-control 1988–2002 5.3 yrs (0.1–20.5 yrs) |
Autologous HCT Cohort: N= 1244 CHF: N=88 peds + adults 7200 person-yrs Clinical CHF per AHA/ACC def. |
Regression: Anthr Dose <150 (ref) 150–249: RR 3.5 250–349: RR 9.9, >349: RR 19.8, <0.01 |
CV Risk factors and HD (≥250 Anth) No HTN, No HD-Anth: Ref HTN, no HD-Anth: 3.5 (NS) HTN + HD Anth: 35.3, <0.01 |
No Diab, No HD-Anth: Ref Diab, no HD-Anth: 5.1, <0.01 Diab + HD Anth: 26.8, <0.01 |
| Rathe17 2010 | Prospective cohort 1986–2000 8.2 yrs (1.1–30.6) |
1-yr survivors ALL N=116, 36 excluded Screening echo: At Diagnosis 2yrs after completion 5-year intervals |
Median age at Dx: 4.0 yrs (0.8–13.4) Median age at f/up: 13.o yrs (2.0–30.5) Median anth dose: 250 mg/m2 (120–300) |
1 patient with EF<55% None with clinical CHF Evidence of cardiac remodelling over time, but no symptoms. No association with gender, age. |
Looking specifically at cardiotoxicity at lower doses of anthracyclines (<300) |
| Mulrooney2 2009 | Retrospective cohort 1970–1986 27.0 yrs (8–51) |
5-yr Survivors (N=14, 358) Age at Dx: 0–4 yrs: 40.1% 5–9 yrs: 22.3% 10–14 yrs: 20.3% 15–20 yrs: 17.3% Siblings (N=3899) |
Anthracyclines: 33.1% No Cardiac XRT: 29% <5 Gy: 34% 5–15 Gy: 5.8% 15–35Gy: 9.7% >=35Gy: 6.9% |
Self-reported CV outcomes Graded per CTCAE v. 3.0 CHF (N=248) – HR 5.9 (3.4–9.6) Multivariate (CHF): Anthracycline vs. none <250 mg/m2 – HR 2.4 (1.5–3.9) >=250 mg/m2 – HR 5.2 (3.6–7.4) |
Self-reported Large sample size Long-term follow-up |
| Creutzig5 2007 | Retrospective cohort 1993–2003 BFM98: 3.6ys (0.8–7.0) BFM93: 7.5ys (1.1–11) Median F/up late cartox: 5.3 (0.8–11.5) |
Eligible: N=1207 Late Cartox evaluated: N=547 (45%) 76% of echo evaluations done within first 5yrs |
AML BFM 93 and 98 Dauno : Ida – 1:5 Dauno : Mitox – 1:5 Anth dose: B 93: 300–400 mg/m2 B 98: 420–450 mg/m2 |
CI of late cardiotoxicity: 5% +/1 % (includes subset with early cardiotoxicity) No difference by randomization: Dauno vs. Ida Cox Regression: Age, early crtox, FAB Early cartox only predictor of late |
Early and late cardiotoxicity. Study summary only presents data on late cardiotoxicity. Sig. #’s lost to follow-up Homogeneous pop: Age Anthracycline dose |
| van Dalen18 2006 | Retrospective cohort 1976–2001 8.5 yrs (0.01–28.4) F/up on prev 2001 JCO study |
830 Children treated with anthracyclines Age at Anth exposure: <2 – 9.2% 2–6 – 30.9% 7–11 – 27% 12–16 – 30.2% >16 – 2.7% |
Anthracyclines: Mean – 288 (15–900) Chest XRT: 21.2% Mitoxantrone: Any 4.1% |
CI and risk factors for A-CHF Univariate (CHF): Cumulative anthracycline ≥300 RR: 8.66 (2.01–37.35), p<0.01 Multivariate (CHF): Cumulative anthracycline ≥300 RR: 7.78 (1.76–34.27), p<0.01 |
Not limited to long-term survivors |
| Pein19 2004 | Retrospective cohort 1968–1982 18 yrs |
Original cohort: 447 218 (48.8%) not evaluated 229 (51.2%) echo’s 15+year survivors Age at treatment: 6.2 yrs (0–21) |
Anthracycline: 344 mg/m2 (40–600) Radiotherapy: 245 (55%) |
Cardiac abnormality: Multivariate regression Cardiac failure, FS<25, EF<50, or ESWS>100 Cumulative anthracycline: 1–150 (Ref) >150–250: RR 2.0 (0.44–9.5) >250–400: RR 4.0 (0.95–17) >400: RR 3.3 (0.78–14) P<0.001 (trend) |
High proportion with XRT exposure. Potential survival bias due to participation rate XRT included in regression model |
| Green20 2001 | Retrospective cohort Case-Control Through 1998 |
NWTS 1–4 Cohort 1: 1–4 received dox N=2,843 Cohort 2: 1–3, dox as part of salvage only N=228 |
Anthracyclines Chest XRT – mostly due to lung XRT |
CI and risk factors for CHF Nested Case-Control Multivariate Cumulative Doxorubicin: 1–199 mg/m2 (Referent) 200–299 mg/m2: 1.1 (0.3–5.1), NS ≥300 mg/m2: 6.0 (1.5–24), p=0.01 |
Homogeneous population due to diagnosis, the vast majority were exposed before 7 yo |
| Kremer21 2002 | Review of Frequency and Risk Factors of anthracycline-induced clinical heart failure Medline search: 1966–2000 |
71 articles reviewed Limitations in many studies evaluated: Missing info Lack of RF analysis Non-rep. populations |
Assess RR of possible Risk factors in 10 studies |
Univariate (CHF): Risk with anthracycline dose in 5 out of 10 studies Goorin (1981), N=382 ≤500 mg/m2 (Ref) >500 mg/m2: RR 4.8 (1.6–14) Dearth (1984), N=112 ≤400 mg/m2 (Ref) >400 mg/m2: RR 26.1 (3.2–210) Sallan (1984), N=379 Maximal dose/wk <45 mg/m2 (Ref) Maximal dose/wk ≥45 mg/m2 RR: 7.7 (2.1–28.1) Godoy (1997), N=120 ≤300 mg/m2 (Ref) >300 mg/m2 – HR 1.5 (0.3–3.9), NS Krischer (1997) <500 mg/m2 (Ref) ≥500 mg/m2: RR 2.6 (1.1–6) |
Multivariate regression showed type of anthracycline and maximal dose of anthracycline within 1 week were independent predictors of frequency of CHF. |
| Asymptomatic cardiomyopathy and anthracycline dose (Abnormal EF, SF) | |||||
| Brouwer22 2011 | Cross-sectional 1976–1999 17.7 years |
5-yr survivors 401 eligible 277 (69%) participated 8 (3%) on cardiac meds for CHF/ renal |
Anthracycline Median: 183 (50–600) Radiation 63%?? |
Multivariate Logistic Regression SF<29% Anthracycline ≥183 mg/m2: OR 2.2, 1.25–3.8, p<0.01 Mediast RT: 3.0, 1.4–6.7, p<0.01 TBI: 1.9, 0.6–5.6 |
Good participation rates Comprehensive echo screen Long term follow-up Handful with clinical HF included in analysis |
| van der Pal23 2010 | Prospective cohort-Survivorship clinic 1966–1997 15.4 yrs (5.1–4.3) |
5-yr survivors 735 anthracycline-treated 601 Eligible for study 525 Had echocardiogram Age at Dx: 8.9 (0.1–17.8) |
Anthracycline: Med – 250 (33–720) Chest XRT: 36.4% |
Asymptomatic cardiac dysf. Graded per CTCAE LVSF as primary outcome (1st echo) Multivariate regression (SF<30%): 1–150 mg/m2 (Ref) 151–300: OR 3.98 (1.58–10.01) 301–450: OR 7.77 (2.85–21.22) >450: OR 10.58 (3.35–33.40) |
|
| Abosoudah24 2010 | Prospective cohort -Survivorship clinic 1995–2003 3.0 yrs (1–10) |
4-year survivors 896 anthracycline-treated 603 eligible for study 469 >=1 screening echo Age at Dx: 7.7 (SD 4.6) |
Anthracycline: Mean – 205 (114.7) Chest XRT: 34% |
Screening echo per COG LTFU Guidelines Not limited to abn EF/FS Multivariate regression: <200 mg/m2 (Ref) 200–300: HR 1.32 (0.61–2.85) >300: HR 3.0 (1.51–5.98) |
Time to first abnormal echocardiogram Unclear for transients Screening frequency driven by age and anthracycline dose, so unclear implication |
| Hudson25 2007 | Cross-sectional 9.0 (3.0–18.0) |
223 anthracycline-treated Vs. 55 – not at risk Age at Dx: 5.5 (0–23.6) |
Anthracycline (AR) Med: 202 (25–510) Chest XRT: 29% Anth + XRT: 26.9% |
Screening echo. LVSF, Wall stress Multivariate regression (SF<28%): Anthracycline dose 50 unit increase: 1.19 (1.01–1.39) |
Asymptomatic One time-point |
| Paulides26 2006 | Prospective cohort 1992–2004 3 yrs (+/−1 yr) |
LESS - sarcoma 1066 non-relapse cohort 564 excluded 502 eligible 265 with echo Age at tx: 13 +/5 yrs |
Anthracycline: Mean – 290 +/−91 Chest XRT: 6.8% |
Subclinical FS<29% × 2 Clinical CHF – per AHA 4/265 Clinical CHF 16/265 subclinical DCM No regression analyses |
|
| Lipshultz27 2005 | Prospective cohort DF consortium: 72 – 85-01 11.8 years |
ALL survivors N=115 Serial echos N=499 |
Median anth: 352 mg/m2 (45–550) |
Fig 2, dose-breakdown of FS Z-score: Clear delineation between <300 mg/m2, 300–400 mg/m2, >400 |
No multivariate regression analysis |
| Sorensen28 2003 | Prospective cohort 1970–1990 6.2–6.7 years from Dx |
ALL survivors – N=101 Age dx: 4.8 +/−2.7 Wilm;s – N=83 Age dx: 4.1 +/−2.3 2 Echo’s mean 4 years apart. |
Anthracycline: ALL – 180 +/−73 WT – 301 +/−78 |
Comprehensive echo. Intermediate indices + FS Multivariate linear regression FS timepoint 2: Dose × 100 mg: B −1.77 (−2.7, −0.9) Diff FS (time 1–2): Dose × 100 mg: B −1.48 (−2.4, −0.5) |
Homogeneous populations: ALL and Wilm’s Essentially comparing high dose vs. low-dose anthracycline with no heterogeneity in age |
| Kremer29 2002 | Review of Frequency and Risk Factors of anthracycline-induced subclinical cardiotoxicity Medline: 1966–2001 >50 children/study |
58 articles reviewed Limitations in many: Missing info Non-rep. populations Non-original research Validity evaluated in 25 studies 10 studies with RF analyses 6 studies which defined an abnormal SF with validity score>5 |
Risk Factor analysis: Steinherz (1991) Lipshutz (1991) Silber (1993) Sorensen (1995) Lipshultz (1995) Pihkala (1996) Sorensen (1997) Nysom (1998) Lanzarini (2000) Bossi (2001) |
4 Studies with anthracyline dose as predictor (limited to FS or EF abn) Risk Factor analysis: Steinherz (1991) N=201: Anth – median 450 (200–1275) >cumulative dose × f/up Silber (1993) N=150: Anth – mean 307 (50–750) >anthracycline dose Lipshultz (1995) N=87: Anth- median 390 (224–550) >dosage in w3 wks × diagnosis >cumulative dose Nysom (1998) N=189: Anth range 0–550 >cumulative dose |
6 with validity score >5 Frequency of abnormal SF <300 mg/m2 (0–15.2%) >300 mg/m2 (15.5%–27.8%) |
| 4. What is the risk for different cardiac RT doses for developing (a)symptomatic cardiac systolic dysfunction in childhood and young adult cancer survivors? | |||||
|---|---|---|---|---|---|
| Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Symptomatic cardiomyopathy and radiation dose | |||||
| van der Pal1 2012 | Retrospective cohort 1966–1996 22.2 yrs (5.0–44.5) |
5-yr survivors (N=1362) Age at Dx: 5.9 (0–18) |
Anthracyclines: 33.6% Anth+XRT: 7.9% Median Anth: 250 mg/m2 (25–775) Cardiac irradiation: None (80.4%) Any (19.5%) Localization of XRT: Thorax (31.6%) Abdomen (24.4%) Spine (33.5%) TBI (10.5%) Cardiac XRT (EQD2): Thorax: 24 (9.5–88.5) Abd: 26.9 (3.7–57) Spine: 30.14 (8–50) TBI: 15.8 (14–21.6) |
Symptomatic cardiac events (CE) Grading: CTCAE v 3.0 50 CEs in 42 CS (CHF in 27/50) Median time to event: 18.6 yrs CI of CHF: Radiotherapy only: 0.7% at 30-yrs XRT + Anth: 7.9% at 30yrs Multivariate regression (Model 1) Radiotherapy (per 10 Gy) HR 1.4 (1.1–2.0) Multivariate regression (Model 2) Radiotherapy (Yes vs. No) HR 6.6 (0.6–73), p=0.13 Anth + Radiotherapy (Yes vs. No) HR 55.9 (6.6–470), p<0.001 |
Clinically validated outcomes Long follow-up, large cohort XRT dose conversion: Fractions of 2 Gy (EQD2) – includes both fractionation size and total dose Model 2 removes mutually exclusive cardiotoxic treatments. Radiotherapy alone not significant for CHF, but is predictive of other cardiac events |
| Schellong30 2010 | Prospective cohort 1978–1995 15.1 yrs (3.1–29.4) |
Hodgkin lymphoma: All pts. treated on German HD-78 to HD90 studies XRT field/dose reduction Uniform anth. dose Age at Dx:12.8 (2.5–17.9) Cardiac screening recs: Every 2–3 yrs up to 10 yrs Every 5 years thereafter In person +questionnaire |
1132 eligible survivors Anthracyclines: 160mg/m2 everyone Mediastinal XRT: Median 25Gy (8–50) Mediast RT (MedRT) ≥36 Gy: 248 (21.9%) 30 Gy: 133 (11.7%) 25 Gy: 282 (24.9%) 20 Gy: 171 (15.1%) None: 298 (26.3%) |
Cardiac grading per ACC/AHA 50/1132 (4.4%) w/ cardiac dz 14/1132 (1.2%) w/myocardial dz. 10/14 (71%) – MedRD-36 3/14 – MedRD20–30 25-yr CI of non-valvular cards dz ≥36 Gy: 4%, 30 Gy: 9%, 25 Gy: 4%, 20 Gy: 5%, None: 3%; p=0.2 Cox-regression: MedRD only predictor |
Low prevalence/ incidence of myocardial disease likely due to low dose of anthracycline. Large study, long f/up, XRT is the only modified cardiotoxic exposure Unable to look at anth+XRT Non-valvular card dz includes CADz, valvular, conduction Homogeneous patient pop (age) |
| Mulrooney2 2009 | Retrospective cohort 1970–1986 27.0 yrs (8–51) |
5-yr Survivors (N=14, 358) Age at Dx: 0–4 yrs: 40.1% 5–9 yrs: 22.3% 10–14 yrs: 20.3% 15–20 yrs: 17.3% Siblings (N=3899) |
Anthracyclines: 33.1% No Cardiac XRT: 29% <5 Gy: 34% 5–15 Gy: 5.8% 15–35Gy: 9.7% >=35Gy: 6.9% |
CV outcomes Graded per: CTCAE v. 3.0 CHF (N=248) – HR 5.9 (3.4–9.6) Multivariate (CHF): No cardiac radiation (Ref) <5 Gy: HR 0.9 (0.6–1.4) 5–15 Gy: HR 1.3 (0.7–2.5) 15–35Gy: HR 2.2 (1.4–3.5) ≥35Gy: HR (4.5 (2.8–7.2) Dose-dependent increase in cumulative incidence of CHF |
Self-reported Large sample size Long-term follow-up Cardiac XRT dosimetry calculations (Stovall et al.) Significance emerges at 15–35Gy XRT data not mutually exclusive of anthracycline exposure. |
| Blanco3 2012 | Case-Control 1966–2008 |
Case (CHF) – N=170 Control (none) – N=317 Matching criteria: Diagnosis Year of Dx (+/−5 yrs) Race/ethnicity Follow-up (controls) |
Cases vs. controls: Anthracyclines 291 vs. 168, p<0.01 Chest XRT 25% vs. 14%, p<0.01 |
Clinically validated DCM, CHF Genetic susceptibility Multivariate (CHF): Chest radiation None (Ref) Any: OR 4.29 (1.9–9.6), p<0.001 |
Largest pop of clinically validated DCM, CHF XRT prevalence difference, but no info on dosimetry. |
| Aleman31 2007 | Retrospective cohort 1965–1995 8.7 yrs (28 669 person-years for cohort) |
5-year survivors of HL Age at treatment: <20 yo (21.3%) 20–35 yo (63.4%) >35 yo (15.3%) Age at f/up: <35 yo (16.6%) >55 yo (20.1%) |
RT only 27.5% Chemo (CT) only 4.8% RT + CT, anth 29.5% RT + CT, no anth 38% Unknown 0.2% 17% recent smokers 10% HTN 5% diabetes 8.5% Dyslipidemia |
Cumulative incidence of CHF 25y: No RT 0.4% Mediastinal RT only 6.8% Mediast RT + CT, no anth 4.9% Mediast RT + CT, anth 7.9% Multivariate regression (CHF): Model 2 Mediastinal RT only (Ref) Med. RT + CT, no anthracycline: RR 1.3 (0.79–2.24) Med. RT + CT, anthracycline: RR 2.81 (1.44–5.49) |
Large pop of adult lymphoma survivors (most <35 yo at Dx) Very long follow-up Critical role of cardiovascular risk factors Suggest that RT alone no inc. risk for CHF? Ref group is RT No dosimetry for cardiac XRT Includes older treatment era |
| van Dalen18 2006 | Retrospective cohort 1976–2001 8.5 yrs (0.01–28.4) F/up on prev 2001 JCO study |
830 Children treated with anthracyclines Age at Anth exposure: <2 - 9.2% 2–6 – 30.9% 7–11 – 27% 12–16 – 30.2% >16 – 2.7% |
Anthracyclines: Mean – 288 (15–900) Chest XRT: Any 21.2% None 78.7% Unknown 0.1% |
CI and risk factors for A-CHF Univariate (CHF): RT on heart: RR 0.67 (0.2–2.3), NS Multivariate (CHF): No association with chest RT reported. |
Not limited to long-term survivors No XRT dosimetry reported |
| Guldner32 2006 | Retrospective cohort Cross-sectional eval 1968–1985 5.4 yrs |
447 eligible based on anthracycline exposure No XRT alone pop. 245 (N=55%) participated in study Age at Dx: 6.2 (0–21 yrs) |
Anthracyclines: Median: 300 mg/m2 Entire cohort XRT heart dose: Mean 8.1 (15.6) |
140 examined and healthy 24 with cardiac failure 65 with other cardiac disorders Heart radiation dose: Healthy vs. heart failure: 0.6 Gy vs. 17.8 Gy, p<0.001 Dose-dependent increase in HF risk by radiation dose |
No XRT heart dosimetry, dosing estimated |
| Pein19 2004 | Retrospective cohort 1968–1982 18 yrs |
Original cohort: 447 218 (48.8%) not evaluated 229 (51.2%) echo’s 15+year survivors Age at treatment: 6.2 yrs (0–21) |
Anthracycline: 344 mg/m2 (40–600) Radiotherapy: 245 (55%) XRT dose to heart: Mean 6.7 Gy (0–91) Max 31.3 Gy (0–125) |
Clear increase incidence w/time Multivariate regression: Cardiac failure, FS<25, EF<50, or ESWS>100 (not limited to CHF) Avg. XRT dose to heart, p<0.001 0 No XRT (Ref) >0–5 Gy: 1.63 (0.82–3.26) >5–20 Gy: 6.48 (2.76–15.20) >20 Gy: 4.40 (1.11–17.48) |
High proportion treated with chest radiation Very long term follow-up One of the earlier studies to demonstrate dose-resposne with XRT |
| Adams33 2004 | Cross-sectional 1970–1991 14.3 (5.9–27.5) |
Hodgkin Lymphoma 24% participation rate Age at diagnosis: Median 16.5 (6.3–25.0) Age at study visit: Median 31.9 (18–49) |
Anthracycline: 4/48 (8.3%) Mediastinal XRT dose: Median 40 Gy (27–52) |
Comprehensive echo evaluation and stress testing No discussion of CHF Very few had systolic dysfunction Most with indices of diastolic dysfunction |
Very long-term follow-up One of few studies to evaluate XRT without anthracyclines Homogeneous population with not much variance in XRT dose Poor participation rate |
| Green20 2001 | Retrospective cohort Case-Control Through 1998 |
NWTS 1–4 Cohort 1: 1–4 received dox N=2,843 Cohort 2: 1–3, dox as part of salvage only (N=228) Age at Dx: 80% <8 y.o. |
Anthracyclines Chest XRT – mostly due to lung XRT |
CI and risk factors for CHF Risk of CHF est. to increase by factor of 1.6 for every 10 Gy of lung XRT, 1.8 for every 10Gy of left abd. XRT (no effect for Right) Multivariate regression (inclanth) Lung XRT: None (Ref) 10–19.9 Gy: RR 1.5 (0.6–3.9), p-0.4 ≥20 Gy: 4.3 (0.8–24), p=0.1 L. Abd XRT: None or right (Ref) Left: RR 4.0 (1.4–11.6) |
Homogeneous population due to diagnosis, the vast majority were exposed before 7 yo Results approach sig at high dose lung XRT |
| Van der Pal34 2005 | Systematic review of risk of morbidity and mortality from cardiovascular disease for childhood cancer Lit Review: 1966–2002 |
Criteria for review:
|
Many studies include arterial events (ie: MI) and CHF as CVE. For CVE: 9 studies selected based on validity and inclusion criteria. 8/9 studies, outcome well-defined 3/9 risk estimation well-defined and adequate |
Relative Risk for CVE: Cardiac event, matched for anthracycline, time at risk, cohort Continuous tx. Variables (RR): Female/Male: 4.5, p<0.01 Anth, 100 mg/m2: 3.2, p<0.01 Lung RT, 10 Gy: 1.6, p=0.06 Left abd, 10 Gy: 1.8, p=0.02 Right abd. 10 Gy: 0.94, p=0.77 Categorical tx. Variables (RR): Female/Male: 3.7, p<0.01 Anth,>300 mg/m2: 5.0, p<0.02 Lung RT >20Gy: 3.1, p=0.21 Left abd. RT: 3.5, p=0.02 |
Older treatment eras For many, no clear delineation between RT-related systolic heart failure vs. CHF due to coronary artery disease, or MI alone. Dose-dependent Risk |
| Kremer21 2002 | Review of Frequency and Risk Factors of anthracycline-
induced clinical heart failure Medline: 1966–2000 |
71 articles reviewed Limitations in many: issing info Lack of RF analysis Non-rep. populations |
Assess RR of possible Risk factors in 10 studies |
Univariate (CHF): Risk with XRT reported in 4 out of 10 studies (3 out of 4 significant) Gilladoga (1976) N=50 XRT to heart: RR 5.2 (1.6–16.8) Dearth (1984) N=116 XRT to heat: RR13.5 (3.4–53.3) Bu’Lock (1996) N=226 XRT to heart: 11.1 (3.7–33.5) Krischer (1997) N=6493 XRT to heart: RR 0.7 (0.3–1.9) |
Review is driven by anthracycline exposure Few with XRT dose quantification and none with careful heart dosimetry calculation |
| Asymptomatic cardiomyopathy and radiation dose (Abnormal EF, SF). | |||||
| Brouwer22 2011 | Cross-sectional 1976–1999 17.7 years |
5-yr survivors 401 eligible 277 (69%) participated 8 (3%) on cardiac meds for CHF/renal |
Anthracycline Median: 183 (50–600) Radiation 63%?? |
No breakdown by dose Multivariate LogisticRegression SF<29% Anthracycline ≥183: OR 2.2, 1.25–3.8, p<0.01 Mediast RT: 3.0, 1.4– 6.7,p<0.01 TBI: 1.9, 0.6–5.6 |
Good participation rates Comprehensive echo screen Long term follow-up Handful with clinical HF included in analysis |
| van der Pal23 2010 | Prospective cohort-Survivorship clinic 1966–1997 15.4 yrs (5.1–4.3) |
5-yr survivors 735 anthracycline-treated 601 Eligible for study 525 Had echocardiogram Age at Dx: 8.9 (0.1–17.8) |
Anthracycline: Med – 250 (33–720) Chest XRT: 36.4% Cumm. XRT dose: ≤30 Gy 10.8% >30 Gy 23.2% |
Asymptomatic cardiac dysf. Graded per CTCAE LVSF as primary outcome (1st echo) LVSF<30% XRT ≤30 vs. >30 Gy: 12.5% vs. 31% Multivariate regression (SF<30%): No Radiotherapy (Ref) Odds Ratio Thorax: 3.49 (1.6–7.6) Abdomen: 2.66 (1.0–7.05) Spine: 0.64 (0.23–1.74) TBI: 0.53 (0.10–2.87) |
|
| Abosoudah24 2011 | Prospective cohort -Survivorship clinic 1995–2003 3.0 yrs (1–10) |
4-year survivors 896 anthracycline-treated 603 eligible for study 469 >=1 screening echo Age at Dx: 7.7 (SD 4.6) |
Anthracycline: Mean – 205 (114.7) Chest XRT: 34% No dose in modelField involving heart |
Screening echo per COG LTFU GuidelinesNot limited to abn EF/FS Multivariate regression: No radiation (Ref) RT to heart: HR 1.7 (1.1–2.8) |
Time to first abnormal echocardiogram Screening frequency driven by age, anthracycline dose, and XRT so unclear implication |
| Hudson25 2007 | Cross-sectional 9.0 (3.0–18.0) |
223 anthracycline-treated Vs. 55 – not at risk Age at Dx: 5.5 (0–23.6) |
Anthracycline (AR) Med: 202 (25–510) Anth + XRT: 26.9% Chest XRT: 2.7% |
Screening echo. LVSF, Wall stress Univariate regression (SF<28%): No Cardiac RT (Ref) RT: OR 0.9 (0.4–2.05) |
Asymptomatic One time-point No cardiac dose quantification |
| Kremer29 2002 | Review of Frequency and Risk Factors of anthracycline-induced subclinical cardiotoxicity Medline: 1966–2001 >50 children/study |
58 articles reviewed Limitations in many: Missing info Non-rep. populations Non-original research Validity evaluated in 25 studies 10 studies w/RF analyses 6 studies which defined an abnormal SF with validity score>5 |
Risk Factor analysis: Steinherz (1991) Lipshutz (1991) Silber (1993) Sorensen (1995) Lipshultz (1995) Pihkala (1996) Sorensen (1997) Nysom (1998) Lanzarini (2000) Bossi (2001) |
1 Study with chest radiation dose as predictor (limited to FS or EF abn)
Risk Factor analysis: Steinherz (1991), N=201 >cumulative anth dose × f/up >mediastinal radiation No dose-effect calculations |
Not all 10 studies had populations that would have received chest radiation (ie: ALL, AML) |
| 5. What is the additional effect of age at treatment on developing (a)symptomatic cardiac systolic dysfunction | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Symptomatic cardiomyopathy and age | |||||
| van der Pal1 2012 | Retrospective cohort 1966–1996 22.2 yrs (5.0–44.5) |
5-yr survivors (N=1362) Age at Dx: 5.9 (0–18) |
Anthracyclines: 33.6% Cardiac XRT: 19.5% Anth+XRT: 7.9% Median Anth: 250 (25–775) |
Symptomatic cardiac events (CE) Grading: CTCAE v 3.0 50 CEs in 42 CS (CHF in 27/50) Median time to event: 18.6 yrs Multivariate (CHF): Age at Dx (per year): HR 0.98, NS |
Clinically validated outcomes |
| Mulrooney2 2009 | Retrospective cohort 1970–1986 27.0 yrs (8–51) |
5-yr Survivors (N=14, 358) Age at Dx: 0–4 yrs: 40.1% 5–9 yrs: 22.3% 10–14 yrs: 20.3% 15–20 yrs: 17.3% Siblings (N=3899) |
Anthracyclines: 33.1% No Cardiac XRT: 29% <5 Gy: 34% 5–15 Gy: 5.8% 15–35Gy: 9.7% >=35Gy: 6.9% |
Self-reported CV outcomes Graded per CTCAE v. 3.0 CHF (N=248) – HR 5.9 (3.4–9.6) Multivariate (CHF): Age at Dx: 0–4 yrs – HR 3.9 (2.1–7.3) 5–9 yrs – HR 2.3 (1.3–4.0) 10–14 yrs – HR 1.2 (0.8–1.9) 15–20 yrs – Ref |
Self-reported Large sample size Long-term follow-up |
| Blanco3 2012 | Case-Control 1966–2008 Cases: 9.2 (0.1–35.1) Controls: 12.3 (0.4–40) |
Case (CHF) – N=170 Control (none) – N=317 Matching criteria: Diagnosis Year of Dx (+/−5 yrs) Race/ethnicity Follow-up (controls) |
Cases vs. controls: Anthracyclines 291 vs. 168, p<0.01 Chest XRT 25% vs. 14%, p<0.01 |
Clinically validated DCM, CHF Genetic susceptibility Multivariate (CHF): Age at dx (per year): 0.99, NS |
Largest pop of clinically validated DCM, CHF Ca-Co matched on diagnosis, by default would have also matched on Age at diagnosis (exposure) |
| Temming4 2011 | Retrospective cohort 1987–2004 7.3 yrs (0–21.7) |
124/158 available for Cardiotox analysis 86 data for late cardiotox Age at Dx: 2.9 (0.1–12.9) |
AML 10 and 12 trials Anthracyclines: Dauno and Mitox (1:5 conversion) 550–610 mg/m2 |
Subclinical cardiotox (SF<28%) Clinical CHF per AHA Multivariate (CHF): Age <4 yrs: 0.76 (0.20–2.94) Age >=4 (Ref) |
Not a very wide distribution of age due to Dx. |
| Creutzig5 2007 | Retrospective cohort 1993–2003 BFM98: 3.6ys (0.8–7.0) BFM93: 7.5ys (1.1–11) Median F/up late cartox: 5.3 (0.8–11.5) |
Eligible: N=1207 Late Cartox evaluated: N=547 (45%) 76% of echo evaluations done within first 5yrs Age at diagnosis not provided, all <18 y.o. |
AML BFM 93 and 98 Dauno : Ida – 1:5 Dauno : Mitox – 1:5 Anth dose: B 93: 300–400 mg/m2 B 98: 420–450 mg/m2 |
CI of late cardiotoxicity: 5% +/1 % (includes subset with early cardiotoxicity) No difference by randomization: Dauno vs. Ida Cox Regression: Age, early cartox, FAB Early cartox only predictor of late |
Early and late cardiotoxicity. Study summary only presents data on late cardiotoxicity. Sig. #’s lost to follow-up Homogeneous pop: Age, Anthracycline dose ??Role of HCT |
| van Dalen18 2006 | Retrospective cohort 1976–2001 8.5 yrs (0.01–28.4) F/up on prev 2001 JCO study |
830 Children treated with anthracyclines Age at Anth exposure: <2 – 9.2% 2–6 – 30.9% 7–11 – 27% 12–16 – 30.2% >16 – 2.7% |
Anthracyclines: Mean –288 (15–900) Chest XRT: 21.2% Mitoxantrone: Any 4.1% |
CI and risk factors for A-CHF Univariate (CHF): Age <=2 yrs = RR 0.28 (0.04–2.1) Multivariate (CHF): No association with age |
Not limited to long-term survivors |
| Pein19 2004 | Retrospective cohort 1968–1982 18 yrs |
Original cohort: 447 218 (48.8%) not evaluated 229 (51.2%) echo’s 15+year survivors Age at treatment: 6.2 yrs (0–21) |
Anthracycline: 344 mg/m2 (40–600) Radiotherapy: 245 (55%) |
Clear increase CHD incidence over time Univariate regression: Cardiac failure, FS<25, EF<50, or ESWS>100 (not limited to clinical CHF) >=8 yrs (Ref) 0–7 years: RR 2.63 (0.87–7.96) P-Value 0.08?? |
High proportion treated with chest radiation Very long term follow-up No mention if age was significant in multivariate regression model |
| Green20 2001 | Retrospective cohort Case-Control Through 1998 |
NWTS 1–4 Cohort 1: 1–4 received dox N=2,843 Cohort 2: 1–3, dox as part of salvage only (N=228) Age at Dx: 80% <8 y.o. |
Anthracyclines Chest XRT – mostly due to lung XRT |
CI and risk factors for CHF Age not included in multivariate model |
Homogeneous population due to diagnosis, the vast majority were exposed before 7 yo |
| Kremer21 2002 | Review of Frequency and Risk Factors of anthracycline-induced clinical heart failure Medline: 1966–2000 |
71 articles reviewed Limitations in many: Missing info Lack of RF analysis Non-rep. populations |
Assess RR of possible Risk factors in 10 studies | 1 out of 10 studies: Age <4 years as predictor of CHF Godoy (1997), N=69 RR = 11.7 (1.4–96.4) |
Unclear If lack of association with age in the other 9 studies b/c age not evaluated or non-significant. |
| Asymptomatic cardiomyopathy and age (Abnormal EF, SF) | |||||
| van der Pal23 2010 | Prospective cohort-Survivorship clinic 1966–1997 15.4 yrs (5.1–4.3) |
5-yr survivors 735 anthracycline-treated 601 Eligible for study 525 Had echocardiogram Age at Dx: 8.9 (0.1–17.8) |
Anthracycline: Med – 250 (33–720) Chest XRT: 36.4% |
Asymptomatic cardiac dysf. Graded per CTCAE LVSF as primary outcome (1st echo) Multivariate regression (SF<30%): Age at dx 0–5yr – OR 2.94 (1.08–8.02) >5–10 – OR 1.64 (0.67–4.01) >10–15 – (0.64–3.28) >15 – Ref |
|
| Abosoudah24 2010 | Prospective cohort-Survivorship clinic 1995–2003 3.0 yrs (1–10) |
4-year survivors 896 anthracycline-treated 603 eligible for study 469 >=1 screening echo Age at Dx: 7.7 (SD 4.6) |
Anthracycline: Mean – 205 (114.7) Chest XRT: 34% |
Screening echo per COG LTFU Guidelines Not limited to abn EF/FS Multivariate regression: Age at tx: 1–4 yrs – 1.89 (1.1–3.3); Ref >=5 |
Time to first abnormal echocardiogram Unclear for transients Screening frequency driven by age, so unclear implication |
| Hudson25 2007 | Cross-sectional 9.0 (3.0–18.0) |
223 anthracycline-treated Vs. 55 – not at risk Age at Dx: 5.5 (0–23.6) |
Anthracycline (AR) Med: 202 (25–510) Chest XRT: 29% Anth + XRT: 26.9% |
Screening echo. LVSF, Wall stress Multivariate regression (SF<28%): Age at dx >=5 yrs – OR 2.41 (0.9–6.4), p0.08 <5 Ref |
Asymptomatic One time-point |
| Paulides26 2006 | Prospective cohort 1992–2004 3 yrs (+/−1 yr) |
LESS -sarcoma 1066 non-relapse cohort 564 excluded (addt’l anth) Age at tx: 13 +/5 yrs |
Anthracycline: Mean – 290+/−91 Chest XRT: 6.8% |
Subclinical FS<29%×2 Clinical CHF – per AHA 4/265 Clinical CHF 16/265 subclinical DCM No regression analyses |
Clinical and subclinical DCM Homogeneous cohort, similar age, so not as clear Short follow-up |
| Sorensen28 2003 | Prospective cohort 1970–1990 6.2–6.7 years from Dx |
ALL survivors – N=101 Age dx: 4.8 +/−2.7 Wilm;s – N=83 Age dx: 4.1 +/−2.3 2 Echo’s mean 4 years apart. |
Anthracycline: ALL – 180 +/−73 WT – 301 +/−78 |
Comprehensive echo. Intermediate indices + FS Multivariate linear regression FS at second timepoint (FS2) Age (yrs): −0.09 (−0.35, +0.16) Difference in FS over time Age (yrs): +0.18 (−0.09, +0.45) |
Homogeneous populations: ALL and Wilm’s Essentially comparing high dose vs. low-dose anthracycline with no heterogeneity in age |
| Kremer29 2002 | Review of Frequency and Risk Factors of anthracycline-induced subclinical cardiotoxicity Medline: 1966–2001 >50 children/study |
58 articles reviewed Limitations in many: Missing info Non-rep. populations Non-original research Validity evaluated in 25 studies RF analyses in 10 |
Steinherz (1991) Lipshutz (1991) Silber (1993) Sorensen (1995) Lipshultz (1995) Pihkala (1996) Sorensen (1997) Nysom (1998) Lanzarini (2000) Bossi (2001) |
Studies with age as predictor (limited to FS or EF abn) Silber 1993 -<age at tx Lipshultz 1995 -<age at dx Sorensen 1997 ->age at tx |
Several studies with associations with age and other indices (ie: ESWS, SVI, wall thickness) |
| 6. What is the risk of (a)symptomatic cardiac systolic dysfunction in childhood and young adult cancer survivors treated with mitoxantrone? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow- up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Temming4 2011 | Retrospective cohort N=124, 86 1987–2004 7.3 yrs (0–21.7) |
124/158 available for Cardiotox analysis 86 data for late cardiotox Age at Dx: 2.9 (0.1–12.9) |
AML 10 and 12 trials Anthracyclines: Dauno and Mitox (1:5 conversion) 550–610 mg/m2 Amsacrine 100 mg/m2 in AML 10/12 |
Late cardiotoxicity prevalence: 17.4% (10.9–26.8%) Non-relapse pts: 4.5% (1.5–12%) Time to CHF: 1.75 yrs (0.6–8.3) Unclear role of potentiating cardiotoxicity amsacrine Regression analysis does not include Mitox dose comparison |
Not a very wide distribution of age due to Dx. Anthracycline dose range similar across AML 10 and 12, unable to assess dose-association |
| O’Brien35 2008 | Prospective Cohort Down synd.: N=57 Vs. Non DS: N=565 1995–1999 Long-term f/up not clear (chart review) |
Down syndrome 42% with CHDz Age at Dx <2y: 67% AML M7: 79% Daunorubicin 135 mg/m2 Mitox 80 mg/m2 Cumulative: 535 mg/m2 5:1 conversion Mitox:Dauno Study echo reqmt’s while on study and at end of therapy |
POG 9421 No Mitox randomization |
Symptomatic CHF 10/57: 17.5% Includes during and after tx 5/10 with CHF had hx of CHDz 9/10 with sx’s during therapy Anecdotal report of CHF 1.1% in non-DS cohort (not validated) Historic DS studies: POG 8821 (dauno 135 mg/m2): 5/34 – 15% CCG 2891 (dauno 350 mg/m2): 1% (vs. 2% without DS) BFM-93–98 (220–240 mg/m2) 2.7% early, 4% late CHF |
Small numbers Disproportionate number with CHDz Nearly all events occurred while on tx Long-term follow-up for cardiac outcomes not complete Non DS population with low prevalence of CHF (Host vs. treatment vs. study methodology) Suggestion of high Cardiotox but likely due to combination of factors |
| Aviles36 2005 | Randomized clinical trial ABVD (N=191) vs. EBVD (N=182) vs. MBVD (N=103) 1988–1996 11.5 yrs (7.5–14.8) |
Hodgkin lymphoma III–IV Adults-onset Median age: 38.5–40.1 yrs. MBVD arm closed early due to low efficacy |
A-Doxorubicin (400 mg/m2) E-Epirubicin (560 mg/m2) M-Mitoxantrone (160 mg/m2) No chest XRT |
Clinical CHF and subclinical dz Clinical CHF: Mitox (17%), Epi (6%), Dox (9%) SMR for clinical cardiac event: Mitox: 67.8 (39.8–89.4) Epi: 19.4 (11.6–36.8) Dox: 46.4 (28.9–70.1) |
Adult data, Stages III-IV HL 33–38% smokers Long term follow-up Unbalanced accrual due to early Mitox arm closure No multivariate regression Groups similar in characteristics |
| van Dalen37 2004 | Systematic Review 17 studies included
1966–2002 |
Krischer (1997) only study to assess risk factors
|
CI and risk factors for mitoxantrone-induced cardiotoxicity in children Sympt. Cardiotox (16/17 articles): 0–6.7% (7/16 no symptomatic CHF) Asympt. Cardiotox (11/17 articles) 0–80% (2/11 no Cardiotox) Risk Factor (Krischer): Univariate analysis: Mitox >40 mg/m2 (RR 5.08, p<0.05) Multivariate analysis: Non-sig |
Children treated with Mitox at risk, but difficult to quantify CI and risk factors due to methodologic limitations of studies. Difficult to find attribution to Mitox alone due to mixed use |
|
| Smith38 2010 | Systematic Review and meta-analysis 55 RCTs Majority women with advanced breast CA 1988–2008 |
15 studies comparing anthracycline vs. Mitox
|
Meta-analysis: Clinical cardiotoxicity Mitoxantrone: OR 2.88 (1.29–6.44, p=0.01) Subclinical cardiotoxicity: OR 1.09 (0.74–1.61, p=0.67) |
?Conversion scores of meta-analyses Adult population |
|
| 7. What is the additional effect of radiotherapy on developing (a)symptomatic cardiac systolic dysfunction in childhood and young adult cancer survivors treated with anthracyclines? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow- up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| van der Pal1 2012 | Retrospective cohort 1966–1996 22.2 yrs (5.0–44.5) |
5-yr survivors (N=1362) Age at Dx: 5.9 (0–18) |
Anthracyclines: 33.6% Anth+XRT: 7.9% Median Anth: 250 mg/m2 (25–775) Cardiac irradiation: None (80.4%) Any (19.5%) Localization of XRT: Thorax (31.6%) Abdomen (24.4%) Spine (33.5%) TBI (10.5%) Cardiac XRT (EQD2): Thorax: 24 (9.5–88.5) Abd: 26.9 (3.7–57) Spine: 30.14 (8–50) TBI: 15.8 (14–21.6) |
Symptomatic cardiac events (CE) Grading: CTCAE v 3.0 50 CEs in 42 CS (CHF in 27/50) Median time to event: 18.6 yrs CI of CHF: Radiotherapy only: 0.7% at 30-yrs XRT + Anth: 7.9% at 30yrs Multivariate regression (Model 1) Radiotherapy (per 10 Gy) HR 1.4 (1.1–2.0) Multivariate regression (Model 2) Radiotherapy (Yes vs. No) HR 6.6 (0.6–73), p=0.13 Anth + Radiotherapy (Yes vs. No) HR 55.9 (6.6–470), p<0.001 |
Clinically validated outcomes Long follow-up, large cohort XRT dose conversion: Fractions of 2 Gy (EQD2) – includes both fractionation size and total dose Model 2 removes mutually exclusive cardiotoxic treatments. Radiotherapy alone not significant for CHF, but is predictive of other cardiac events |
| Aleman31 2007 | Retrospective cohort 1965–1995 8.7 yrs (28 669 person-years for cohort) |
5-year survivors of HL Age at treatment: <20 yo (21.3%) 20–35 yo (63.4%) >35 yo (15.3%) Age at f/up: <35 yo (16.6%) >55 yo (20.1%) |
RT only 27.5% Chemo (CT) only 4.8% RT + CT, anth 29.5% RT + CT, no anth 38% Unknown 0.2% 17% recent smokers 10% HTN 5% diabetes 8.5% Dyslipidemia |
Cumulative incidence of CHF 25y: No RT 0.4% Mediastinal RT only 6.8% Mediast RT + CT, no anth 4.9% Mediast RT + CT, anth 7.9% Multivariate regression (CHF): Model 2 Mediastinal RT only (Ref) Med. RT + CT, no anthracycline: RR 1.3 (0.79–2.24) Med. RT + CT, anthracycline: RR 2.81 (1.44–5.49) |
Large pop of adult lymphoma survivors (most <35 yo at Dx) Very long follow-up Critical role of cardiovascular risk factors Suggest that RT alone no inc. risk for CHF? Ref group is RT Includes older treatment era |
| Pein19 2004 Br J Ca |
Retrospective cohort 1968–1982 18 yrs |
Original cohort: 447 218 (48.8%) not evaluated 229 (51.2%) echo’s 15+year survivors Age at treatment: 6.2 yrs (0–21) |
Anthracycline: 344 mg/m2 (40–600) Radiotherapy: 245 (55%) XRT dose to heart: Mean 6.7 Gy (0–91) Max 31.3 Gy (0–125) |
Clear increase incidence w/time Multivariate regression: Cardiac failure, FS<25, EF<50, or ESWS>100 (not limited to CHF) <250 mg/m2 Dox <5Gy to the heart (Ref) ≥5 Gy: RR 4.9 (1.3–18) ≥250 mg/m2 Dox <5Gy + <250 anth (Ref) <5Gy: RR 5.1 (1.8–14.5) ≥5 Gy: RR 6.6 (2.1–20.6) |
High proportion treated with chest radiation Very long term follow-up One of the earlier studies to demonstrate dose-response with XRT Potential interaction with anthracycline, with highest risk among those exposed to HD-anth and XRT |
Working group 2 “What surveillance modality should be used?”
| 1. What is the diagnostic value (i.e. sensitivity, specificity and/or inter-observer variability) of radionuclide angiography as compared to echocardiography (or vice versa) for screening of asymptomatic cardiac systolic dysfunction in childhood and young adult cancer survivors? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Diagnostic tests | Main outcomes~ | Addt’l remarks |
| Postma39 1996 | Single-center cohort study (the Netherlands). Treatment era: 1977–1990*. Years of follow-up since last doxorubicin dose: mean 8.7 years~ (range 2.3–14.1). |
22 long-term survivors of a malignant bone tumor#. 17 men/5 women; mean age at diagnosis tumor 15.8 years~ (range 10–21.3). Treatment based on Rosen’s T5 and T10 protocols: doxorubicin median cumulative dose 360 mg/m2 (range 225–550); cyclophosphamide median cumulative dose 4800 mg/m2 (range 500–9600); no mediastinal irradiation*. |
Two-dimensional M-mode and color Doppler echocardiography (single observer to exclude interobserver variability); an abnormal test result was defined as LVSF<0.29 (n=6; prevalence 27.3%). Equilibrium gated radionuclide angiography (LVEF was calculated with a semi-automatic software program); an abnormal test result was defined as LVEF<55% (n=2; prevalence 9.1%). Time between tests: nm. |
When the echocardiographic result is used as the reference standard^: Sensitivity: 16.7% (95% CI 0.9 to 32.4) Specificity: 93.8% (95% CI 87.8 to 99.7) Positive predictive value: 50% (95% CI 2.7 to 97.3) Negative predictive value: 75% (95% CI 70.3 to 79.7) Agreement between tests (i.e. either both abnormal or both normal): 16/22 (72.7%). |
At time of testing clinical symptoms (fatigue and/or palpitations) were mentioned by 6 patients, of which 1 had physical signs of congestive heart failure*. Selection bias cannot be ruled out (31 out of 37 (84%) consecutive patients still alive at the time of this study: 3 lost to follow-up, 2 refused participation and 1 excluded because of pregnancy*). The risk of detection bias is unclear; nm if outcome assessors were blinded. Low risk of outcome/attrition bias: all 22 patients had both tests. |
| Pihkala40 1994 | Single-center cohort study (Finland). Treatment era: November 1974 through January 1992. Years of follow-up after transplant: Median 4.8 years (range 0.5 to 10.7). |
30 bone marrow transplant survivors (20 allogeneic, 9 autologous and 1 peripheral blood stem cells) for ALL (n=9), AML (n=7), neuroblastoma (n=8), retinoblastoma (n=1) or aplastic anemia (n=5). 15 men/15 women; mean age at transplant 8.1 years~ (range 1.1 to 16.4); median age at time of study 9 years (range 1 to 25). Treatment: High-dose therapy preparative for transplant: cyclophosphamide (n=4); cyclophosphamide and TBI (n=12); ara- C and TBI (n=3); ara-C, VP-16 and TBI (n=2); VP-16, cisplatin, melphalan and TBI (n=9). Mean TBI dose 1097CGy~ (range 970 to 1200); mean number of fractions 4.46 (range 1 to 6). Previous anthracyclines (n=25): cumulative 1 dose unclear1. |
Two-dimensional M-mode echocardiography (number of observers nm); an abnormal test result was defined contractility <-2SD (SD according to Colan) (n=4; prevalence 14.8%). ECG-gated radionuclide cineangiography (number of observers nm); an abnormal test result was defined as LVEF<50% (n=7; prevalence 25.9%). Time between tests: nm. |
When the echocardiographic result is used as the reference standard^: Sensitivity: 0% (95% CI 0.00 to 55.8) Specificity: 69.6% (95% CI 69.6 to 79.3) Positive predictive value: 0% (95% CI 0.00 to 31.9) Negative predictive value: 80% (95% CI 80.0 to 91.2) Agreement between tests (i.e. either both abnormal or both normal): 16/27 (59.3%). |
At time of testing none of the patients had symptomatic cardiac disease. Selection bias cannot be ruled out (30 out of 41 (73%) consecutive patients still alive at the time of this study: reasons for not participating nm). The risk of detection bias is unclear; nm if outcome assessors were blinded. Outcome/attrition bias cannot be ruled out (for 3 out of 30 participants (10%) no radionuclide cineangiography results were available). |
LVSF: left ventricular shortening fraction; LVEF: left ventricular ejection fraction; nm: not mentioned; CI: confidence interval; N: number; ALL: acute lymphoblastic leukaemia; AML: acute myeloid leukaemia; TBI: total body irradiation
In this study not only 22 childhood and young adult cancer survivors (i.e. tumor diagnosis ≤21 years) were included, but also 9 adult cancer survivors (i.e. tumor diagnosis ≥22 years). In this table only data for the childhood and young adult cancer survivors is included, unless otherwise stated.
For all 31 patients combined.
Since echocardiography is most often used to assess cardiac function in clinical practice, we have chosen the echocardiographic results as reference standard.
Calculated by the guideline developers based on information provided in the article (for the main outcomes we used the calculator on http://statpages.org/ctab2x2.html).
In the text of the article it was stated that the median cumulative dose was 140 mg/m2 (range 90 to 450), while in the table the range was 60 to 400 mg/m2 (median nm, mean 167 mg/m2~).
| 2. What is the diagnostic value (i.e. sensitivity and/or specificity) of biomarker ANP, BNP, NT-pro-BNP, troponin-T, and troponin-I to detect asymptomatic cardiac systolic dysfunction as measured by echocardiography in childhood and adult cancer survivors? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Diagnostic tests | Main outcomes~ | Addt’l remarks |
| Krawczuk-Rybak41 2011 | Single-center cohort study (Poland). Treatment era: Nm. Years of follow-up after treatment completion: mean 5.91 years (range 1.6 to 13.8). |
44 childhood cancer survivors treated with anthracyclines (doxorubicin, daunorubicin) for ALL (n=37) or Hodgkin lymphoma (n=7). 30 males/ 14 females; mean age at diagnosis nm; mean age at study 14.7 years (range 6 to 23). Treatment: Cumulative anthracycline dose for ALL 180 to 540 mg/m2; for Hodgkin lymphoma 120 to 240 mg/m2; patients with Hodgkin lymphoma received 15 Gy of radiotherapy to the upper mediastinum (no information on number of fractions). |
Doppler and colour flow visualization echocardiography; M-mode for heart structures and Teicholz method for contractility and LVEF (number of observers nm); an abnormal test result was defined as indexed stroke volume < 40 ml/m2 (n=16; prevalence 36.4%). NT-pro-BNP; an abnormal test result was defined as > 115 ng/ml (n=6; prevalence 13.6%). Time between tests: nm. |
When the echocardiographic result is used as the reference standard^: Sensitivity: 12.5% (95% CI 2.3 to 27.9) Specificity: 85.7% (95% CI 79.9 to 94.5) Positive predictive value: 33.3% (95% CI 6.1 to 74.4) Negative predictive value: 63.2% (95% CI 58.9 to 69.6) Agreement between tests (i.e. either both abnormal or both normal): 26/44 (59.1%). |
Patients had no history of heart disease and no signs of cardiac failure. The risk of selection bias is unclear: not stated if all eligible patients or a random sample thereof were included. The risk of detection bias is unclear; nm if outcome assessors were blinded. Low risk of outcome/attrition bias: all 44 patients had both tests. |
| Brouwer22 2011 | Single-center cross-sectional study (the Netherlands). Treatment era: between 1976 and 1999; current tests between August 2004 and April 2007. Years of follow-up post-treatment: median 18.2 years (range 5.4 to 30.8). |
277 childhood cancer survivors ≥ 18 years treated with potential cardiotoxic therapy (i.e. anthracyclines, platinum analogues or radiotherapy on mediastinum (including mantle field, spine or total body) for leukemia (n=113), malignant lymphoma (n=56), sarcoma (n=48), brain tumor (n=32), nephro/neuroblastoma (n=23) or germ cell tumor (n=5) and surviving at least 5 years after diagnosis. 155 males/122 females; median age at diagnosis 8.8 years (range 0 to 20.1); median age at cardiac evaluation 27.5 years (range 18.1 to 48.2). Treatment: Median cumulative anthracycline dose (doxorubicin, daunorubicin) 183 mg/m2 (range 50–600); median dose of mediastinal radiotherapy 25 Gy (no information on number of fractions); no further information on treatment doses provided; all patients received anthracyclines, platinum analogues or radiotherapy as described above. |
2D echocardiography, colour flow mapping 2D guided M-mode blood pool and tissue velocity imaging (performed by a single skilled technician masked to treatment versus control group to exclude interobserver variability); an abnormal test result was defined as LVSF < 29% (n=97; prevalence 37%) or WMSI > 1.00 (n=38; prevalence 14.5%). NT-pro-BNP; an abnormal test result was defined as > 125 ng/ml (n=32; prevalence 12.2%). Time between tests: nm. |
When the echocardiographic result of the LVSF is used as the reference standard^: Sensitivity: 16.5% (95% CI 10.9 to 22.1) Specificity: 90.3% (95% CI 87.0 to 93.6) Positive predictive value: 50% (95% CI 33.1 to 66.8) Negative predictive value: 64.8% (95% CI 62.4 to 67.1) Agreement between tests (i.e. either both abnormal or both normal): 165/262 (63.0%). When the echocardiographic result of the WMSI is used as the reference standard^: Sensitivity: 31.6% (95% CI 19.2 to 45.1) Specificity: 91.1% (95% CI 89.0 to 93.4) Positive predictive value: 37.5% (95% CI 22.7 to 53.6) Negative predictive value: 88.7% (95% CI 86.6 to 90.9) Agreement between tests (i.e. either both abnormal or both normal): 216/262 (82.4%). |
Patients with current treatment for a relapse or secondary malignant disease or with mental incapacity were excluded. At time of study 263 out of 274 patients had NYHA class I and 11 out of 274 NYHA class II; for 3 patients no data mentioned. 17 out of 275 patients used cardioactive medications (ACE-inhibitor, β-blocker or diuretic); for 2 patients this was unknown; nm if all patients receiving medication did for cardiac causes. Selection bias cannot be ruled out (277 out of 401 eligible patients (69%) participated in this study). The risk of detection bias is low; the echocardiographic outcome assessor was blinded. Outcome/attrition bias cannot be ruled out (only for 262 out of 277 patients (95%) both test were available). The authors stated that the high prevalence of abnormal LVSF in apparently healthy sibling controls suggests (22%) the possibility of false-positive findings and challenges the appropriateness of LVSF as a reliable marker of systolic function in adults. |
| Mavinkurve-Groothuis42 2009 | Single-center cohort study (the Netherlands). Treatment era: Nm (current study executed between May 2006 and October 2007). Median years of follow-up: 13.8 years (range 5 to 28.7). |
122 long-term survivors of childhood cancer treated with anthracyclines for ALL (n=38), AML (n=8), ependymoma (n=1), Ewing sarcoma (n=6), hepatoblastoma (n=3), Hodgkin lymphoma (n=13), neuroblastoma (n=6), NHL (n=30), osteosarcoma (n=3), rhabdomyosarcoma (n=4) or Wilms tumor (n=10). 62 males/60 females; median age at diagnosis 5.7 years (range 0.03 to 14.4); median age at study 21 years (range 5 to 39.4 years). Treatment: Median cumulative anthracycline dose (doxorubicin and/or daunorubicin) 180 mg/m2 (range 50–542); 7 patients also received mediastinal irradiation (no further information provided). |
Transthoracic M-mode echocardiography (performed by experienced echocardiographic technicians and supervised by 2 (pediatric) cardiologists who were unaware of the cumulative chemotherapy dose and levels of NT-pro-BNP); an abnormal test result was defined as LVEF < 55% (n=9; prevalence 7.4%). NT-pro-BNP; an abnormal test result was defined as males <10 pmol/L, females <18 pmol/L and for children age dependent reference values by Albers et al (n=16; prevalence 13.1%). Both tests were performed at the same time. |
When the echo result is used as the reference standard^: Sensitivity: 22.2% (95% CI 4.0 to 57.0) Specificity: 87.6% (95% CI 86.2 to 90.4) Positive predictive value: 12.5% (95% CI 2.3 to 32.1) Negative predictive value: 93.4% (95% CI 91.8 to 96.3) Agreement between tests (i.e. either both abnormal or both normal): 101/122 (82.8%). |
At time of testing none of the patients had symptomatic cardiac disease (defined as < NYHA class II) or a history of cardiovascular disease or chronic renal insufficiency. The risk of selection bias is unclear: all consecutive patients who visited the Late Effects Clinic during the study period were included, but it is not stated if those patients represented a random sample of the complete cohort of survivors. The risk of detection bias is low; echocardiographic outcome assessors were blinded. Low risk of outcome/attrition bias: all 122 patients had both tests. |
| Mavinkurve-Groothuis42 2009 | Single-center cohort study (the Netherlands). Treatment era: nm (current study executed between May 2006 and October 2007). Median years of follow-up: 13.8 years (range 5 to 28.7). |
122 long-term survivors of childhood cancer treated with anthracyclines for ALL (n=38), AML (n=8), ependymoma (n=1), Ewing sarcoma (n=6), hepatoblastoma (n=3), Hodgkin lymphoma (n=13), neuroblastoma (n=6), NHL (n=30), osteosarcoma (n=3), rhabdomyosarcoma (n=4) or Wilms tumor (n=10). 62 males/60 females; median age at diagnosis 5.7 years (range 0.03 to 14.4); median age at study 21 years (range 5 to 39.4 years). Treatment: Median cumulative anthracycline dose (doxorubicin and/or daunorubicin) 180 mg/m2 (range 50–542); 7 patients also received mediastinal irradiation (no further information provided). |
Transthoracic M-mode echocardiography (performed by experienced echocardiographic technicians and supervised by 2 (pediatric) cardiologists who were unaware of the cumulative chemotherapy dose and levels of cardiac troponin T); an abnormal test result was defined as LVEF < 55% (n=9; prevalence 7.4%) or as LVSF < 29% (n=4; prevalence 3.3%). Cardiac troponin T; an abnormal test result was defined as ≥ 0.010 ng/ml (n=0%; prevalence 0%) Both tests were performed at the same time. |
When the echocardiographic result of the LVEF is used as the reference standard^: Sensitivity: 0% (95% CI 0 to 0) Specificity: 100% (95% CI 100 to 100) Positive predictive value: NaN Negative predictive value: 92.6% (95% CI 92.6 to 92.6) Agreement between tests (i.e. either both abnormal or both normal): 113/122 (92.6%). When the echocardiographic result of the LVSF is used as the reference standard^: Sensitivity: 0% (95% CI 0 to 0) Specificity: 100% (95% CI 100 to 100) Positive predictive value: NaN Negative predictive value: 96.7% (95% CI 96.7 to 96.7) Agreement between tests (i.e. either both abnormal or both normal): 118/122 (96.7%). |
At time of testing none of the patients had symptomatic cardiac disease (defined as < NYHA class II) or a history of cardiovascular disease or chronic renal insufficiency. The risk of selection bias is unclear: all consecutive patients who visited the Late Effects Clinic during the study period were included, but it is not stated if those patients represented a random sample of the complete cohort of survivors. The risk of detection bias is low; echocardiographic outcome assessors were blinded. Low risk of outcome/attrition bias: all 122 patients had both tests. |
| Sherief44 2012 | Single-center cohort study (Egypt). Treatment era: nm. Mean years of follow-up: not completely clear from manuscript, but most likely 3.75 years (range 1.5 to 6). |
50 survivors of childhood acute leukemia (n=39 ALL; n=11 AML) treated with anthracyclines. 30 males/20 females; mean age at diagnosis 8.4 years (range 3 to 15); mean age at evaluation 11.63 years (range 8 to 16). Treatment: n=18 cumulative anthracycline dose <150–300 mg/m2; n=32 cumulative anthracycline dose > 300 mg/m2 (but elsewhere in the manuscript n=19 < 300mg/m2 and n=31 > 300 mg/m2 was mentioned). |
Conventional echocardiography (no further information provided; number of observers nm); an abnormal test result was defined as LVEF < 55% or a LVSF < 29% (n=8 subclinical cardiotoxicity in the form of increase of left ventricular dimension and EF; prevalence 16%). Cardiac troponin T; an abnormal test result was defined as > 0.010 ng/ml (n=0; prevalence 0%). Time between tests: Nm. |
When the echocardiographic result is used as the reference standard^: Sensitivity: 0% (95% CI 0 to 0) Specificity: 100% (95% CI 100 to 100) Positive predictive value: NaN Negative predictive value: 84% (95% CI 84 to 84) Agreement between tests (i.e. either both abnormal or both normal): 42/50 (84%). |
At time of testing all survivors were asymptomatic (i.e. no signs and symptoms of cardiac impairment); patients with renal or hepatic impairment were excluded as were patients with a history of cardiac disease and hypertension. The risk of selection bias is unclear; not clear if these 50 patients were all eligible patients or a random sample thereof. The risk of detection bias is unclear; nm if outcome assessors were blinded. Low risk of outcome/attrition bias: all 50 patients had both tests. |
| Kismet45 2004 | Multi-center cohort study (Turkey). Treatment era: June 1982 to August 2000. Median time from last doxorubicin dose: 12 months (range 1 to 168). |
24 childhood cancer patients who received doxorubicin for treatment of Hodgkin disease (n=4), rhabdomyosarcoma (n=4), Ewing sarcoma (n=3), osteosarcoma (n=3), malignant mesenchymal tumor (n=3). Wilms tumor (n=2), neuroblastoma (n=1), hepatoblastoma (n=1), clear cell sarcoma (n=1), malignant mesothelioma (n=1) and primitive neuroectodermal tumor (n=1). 14 males/10 females; median age at diagnosis nm; median age at study 14 years (range 3–31). Treatment: Median cumulative doxorubicin dose 480 mg/m2 (range 400 to 840); 4 patients also received mediastinal irradiation (no further information provided). |
Two-dimensional, M-mode and Doppler echocardiography performed by pediatric cardiologists (number of observers nm); an abnormal test result was defined as LVEF < 55% and LVSF < 29% (n=2; prevalence 8.3%). Cardiac troponin T; an abnormal test result was defined as ≥ 0.010 ng/ml (n=3; prevalence 12.5%). Time between tests: within 24 hours. |
When the echocardiographic result is used as the reference standard^: Sensitivity: 50% (95% CI 2.7 to 97.2) Specificity: 90.9% (95% CI 86.6 to 95.2) Positive predictive value: 33.3% (95% CI 1.8 to 64.8) Negative predictive value: 95.2% (95% CI 90.7 to 99.7) Agreement between tests (i.e. either both abnormal or both normal): 21/24 (87.5%). |
None of the patients had clinical evidence of abnormal cardiac functions; patients with evidence of renal disease were excluded from the study. The risk of selection bias is unclear; not clear if these 24 patients were all eligible patients or a random sample thereof. The risk of detection bias is unclear; nm if outcome assessors were blinded. Low risk of outcome/attrition bias: all 24 patients had both tests. |
| Soker46 2005 | Single-center study (Turkey). Treatment era: October 2000 and December 2004. Mean follow-up after the last anthracycline dose 9.39 months (range 1 to 42). |
31 childhood cancer patients who received doxorubicin for treatment of ALL (n=27), AML (n=2), Hodgkin disease (n=1), NHL (n=1). 14 males/17 females; median age at diagnosis nm; median age at study 8.16 years (range 4 to 15). Treatment: Median cumulative doxorubicin dose 240 mg/m2 (range 30–600). |
Two-dimensional, pulse-wave Doppler and M-mode echocardiography (performed by 1 experienced pediatric cardiologist); an abnormal test result was defined as LVEF < 60% and LVSF < 30% (n=4; prevalence 12.9%). Cardiac troponin I; an abnormal test result was defined as ≥ 0.50 ng/ml (n=0; prevalence 0%). Time between tests: performed simultaneously. |
When the echocardiographic result is used as the reference standard^: Sensitivity: 0% (95% CI 0 to 0) Specificity: 100% (95% CI 100 to 100) Positive predictive value: NaN Negative predictive value: 87.1% (95% CI 87.1 to 87.1) Agreement between tests (i.e. either both abnormal or both normal): 27/31 (87.1%). |
Two of the 4 patients with systolic dysfunction had clinical findings; patients who received mediastinal irradiation or had other illnesses such as infections were excluded. The risk of selection bias is unclear; not clear if these 31 patients were all eligible patients or a random sample thereof. The risk of detection bias is unclear; nm if outcome assessors were blinded. Low risk of outcome/attrition bias: all 31 patients had both tests. |
Nm: not mentioned; ALL: acute lymphoblastic leukaemia; n: number; LVEF: left ventricular ejection fraction; CI: confidence interval; LVSF: left ventricular shortening fraction; WMSI: wall motion score index; NYHA: New York Heart Association; AML: acute myeloid leukaemia; NHL: non-Hodgkin lymphoma; NaN: not a number (data type)
Since echocardiography is most often used to assess cardiac function in clinical practice, we have chosen the echocardiographic results as reference standard
Calculated by the guideline developers based on information provided in the article (for the main outcomes we used the calculator on http://statpages.org/ctab2x2.html)
It was unclear if both or only one of the two markers should have been abnormal for this definition
| 3. What is the diagnostic value (i.e. sensitivity and/or specificity) of biomarker ANP, BNP, NT-pro-BNP to detect asymptomatic cardiac systolic dysfunction as measured by echocardiography in adult non-cancer populations? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design | Participants | Diagnostic tests | Main outcomes# | Addt’l remarks |
| Hill47 2008 | Systematic review of RCTs and observational studies (published between 1989 and February 2005). For screening studies general populations with no known symptomatic heart failure were included. 6 studies were addressing our question* (n=2 cross sectional study, n=4 cohort study). |
Setting: population-based cohort study (n=1; males and females reported separately), GP sample (n=1), population samples (n=3), cohort with stable coronary artery disease (n=1). Sample size: range 293–2042 participants (1 study presented males (1470) en females (1707) separately: 3177 in total). Males: range 43–49.6% (n=3), results presented for males and females separately (46.3% males) (n=1), nm (n=2). Age: range mean age 58–75 years (n=3), >45 years (n=1), range 50–90 years (n=1), nm (n=1). Prevalence cardiac dysfunction: 1–16%. |
Index test: BNP (n=5) or NT-pro-BNP (n=2)¶. Reference standard: LVSD based on LVEF (n=5) or a combination of LV mass, LVEF<50% and moderate to severe LVSD (LVEF<40%) (n=1). Time between tests: Nm. Cutoff points: BNP: range 21->115 pg/mL. NTproBNP: range >338–850 pg/mL. Reference test: LVEF range 35–55%. |
BNP: Sensitivity: range 26–93% Specificity: range 47–89% NT-pro-BNP: Sensitivity: range 70–80% Specificity: range 63–85% |
Risk of bias assessment of included studies: nm. |
| Ewald48 2008 | Systematic review of prospective studies (published up to June 2005). 7 studies were addressing our question*. |
Setting: population-based cohort studies (n=2; 1 study reporting males and females separately), GP samples (n=2), population samples (n=3). Sample size: range 203–1997 participants (1 study presented males (1470) and females (1707) separately: 3177 in total). Males: range 43–56% (n=6), results presented for males and females separately (46.3% males) (n=1). Median/average age: range 58–75 years. Prevalence cardiac dysfunction: 0.6–6.9%. |
Index test: BNP (n=5) or NT-pro-BNP (n=3)¶. Reference standard: LVSD based on LVSF (n=1), LVEF (n=4), wall motion index (n=2). Time between tests: nm for each study separately, but it was stated that the quality of studies was generally adequate, except for 1 study with delays up to one year between both tests. Cutoff points: BNP: range 6.9–19.2 pM/L (n=4); >54.5 pg/ml (n=1). NTproBNP: range 37.7–48.9 pM/L (n=2), nm (n=1). Reference test: LVSF: 28% (n=1); LVEF: range 40–50% (n=4); wall motion index: >2 (n=1) and < 1.7 (equates LVEF < 40%) (n=1). |
BNP: Sensitivity: range 55–90%~ Specificity: range 77–90%~ NT-pro-BNP: Sensitivity: range 76–92% Specificity: range 67–81% |
Risk of bias assessment of included studies was based on (1) blinding of outcome assessor for other test result, (2) detailed description of methods and criteria for both tests, and (3) performance of both tests on same day. The quality of included studies was generally adequate, but in 1 study delays of up to 1 year occurred between the echocardiography and the peptide estimation (no further information provided); a sensitivity analysis taking into account the quality score was done, but not presented in the paper |
| Wang49 2003 | Systematic review of studies of patients with asymptomatic LVSD (published between 1975 and November 2002). 13 studies were addressing our question* (n=5 community based studies, n=6 referral series). | Setting: population-based cohort studies (n=3; 1 study reporting males and females separately), GP sample (n=1), population sample (n=1), referral series (not further specified) (n=6). Sample size: Community based: range 126–1707 participants (1 study presented males (1470) and females (1707) separately: 3177 in total); Referral series: range 75–466 participants. Males: Community based: only men (n=1), results presented for males and females separately (46.3% males) (n=1), nm (n=3). Referral series: nm (n=6) Age: Nm. Prevalence cardiac dysfunction: Nm. |
Index test#: Community based: BNP (n=3), NT-ANP (n=2). Referral series: BNP (n=5), NT-ANP (n=1). Reference standard: Community based: LVSD based on LVSF (n=1), LVSF or mild or greater reduction in LVEF on visual estimation (n=1) or LVEF (n=3). Referral series: LVSD based on LVEF alone (n=4), LVEF in rest or exercise (n=1) or LVEF or wall-motion abnormalities (n=1) Time between tests: Nm. Cutoff points: Community based: BNP: range 17.9–34 ng/L. NT-ANP: range 398–800 pmol/L. Reference test: LVSF: range 0.28–0.29 (no further information provided on combination with LVEF reduction); LVEF: range 0.30–0.45. Referral series: BNP: range 13.8–87 ng/L. NT-ANP: 54 pmol/L Reference test: LVEF: range 0.35–0.55 (LVEF at rest or during exercise: resting LVEF<0.45 or exercise LVEF<0.55; no further information provided on combination with wall motion abnormalities). |
Community based: BNP: Sensitivity: range 26–77% Specificity: range 84–89% NT-ANP: Sensitivity: range 43–86% Specificity: range 75–89% Referral series: BNP: Sensitivity: range 58–100% Specificity: range 58–81% NT-ANP: Sensitivity: 90% Specificity: 92% |
Risk of bias assessment of included studies: nm. |
RCT: randomized controlled trial; n: number; nm: not mentioned; GP: general practitioner; LVSD: left ventricular systolic dysfunction; LVEF: left ventricular ejection fraction; LV: left ventricular; LVSF: left ventricular shortening fraction
We only included studies that used a measure of asymptomatic cardiac systolic dysfunction as the reference standard. Studies comparing biomarkers with measures of diastolic dysfunction, a qualitative assessment, a clinical assessment or studies that did not report the reference test were excluded. We included all studies reporting LVEF as a reference test, although in the different systematic reviews it was not reported if in the individual studies LVEF was measured by echocardiography or radionuclide angiography. Only studies for which sensitivity and/or specificity were available were eligible. Please note that there is overlap in included studies between the different systematic reviews.
Some studies presented results for different cutoff points for either one or both diagnostic tests and/or for males and females separately; we have included all available information in this evidence table
one study assessed both tests
For one of the included studies sensitivity and specificity were calculated by the guideline developers based on information provided in the systematic review
Only results for the better performing biomarker (if applicable, i.e. either BNP or NT-ANP) were presented in the systematic review
| 4. What is the diagnostic value (i.e. sensitivity, specificity and/or inter-observer variability) of MRI as compared to echocardiography (or vice versa) for detection of asymptomatic cardiac systolic dysfunction in childhood and young adult cancer survivors? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Diagnostic tests | Main outcomes | Addt’l remarks |
| Armstrong50 2012 | Single-center cohort study (USA). Treatment era: nm. Years of follow-up since cancer diagnosis: mean 27.7 years (range 18.4–38.3). |
134 adult childhood cancer survivors (cancer diagnosed before age 21 years) treated with chestdirected radiotherapy and/or anthracyclines for ALL (n=44), Hodgkin’s lymphoma (n=37), osteosarcoma (n=11), non-Hodgkin’s lymphoma (n=8), AML (n=6), neuroblastoma (n=3), Ewing sarcoma (n=2). Wilms tumour (n=2) and soft tissue sarcoma (n=1). 47 men / 67 women; mean age at diagnosis tumour 10.5 years (range 0.02–19); mean age at time of study 38.3 years (range 22.7–53.7). Treatment: Mean cumulative anthracycline dose 186 mg/m2 (range 0–803); 97 patients received anthracyclines. 37 patients received chest-directed radiotherapy (n=16 1–30 Gy and n=21 > 30Gy; no information on number of fractions). |
Cardiac magnetic resonance imaging (analysis was supervised and/or performed by a single investigator); an abnormal test result was defined as LVEF<50% (n=16; prevalence 14%). 3D as well as a 2D echocardiogram with Doppler and time-motion mode (M-mode) (analysis was performed by a single investigator); an abnormal test result was defined as LVEF<50% (n=22/prevalence 19.3% with 3D echocardiography; n=6/prevalence 5.3% with biplane 2D echocardiography; n=8/prevalence 7% with apical 4-Chamber 2D echocardiography and n=24/prevalence 21.1% with Teichholz 2D echocardiography). Time between tests: within a 48-hour period. |
Screening performance of echocardiography compared with cardiac magnetic resonance imaging (reference standard) for detection of an LVEF<50%: 3D echocardiography: Sensitivity 53% Specificity 86% Positive predictive value 36% Negative predictive value 92% Biplane 2D echocardiography: Sensitivity 25% Specificity 98% Positive predictive value 67% Negative predictive value 89% Apical 4-Chamber 2D echocardiography: Sensitivity 25% Specificity 96% Positive predictive value 50% Negative predictive value 89% Teichholz 2D echocardiography: Sensitivity 29% Specificity 79% Positive predictive value 17% Negative predictive value 88% Bland-Altman measures of agreement with cardiac magnetic resonance imaging: For 3D echocardiography (bias, 1%; Bland-Altman limits of agreement [± 1.96 standard deviation], −11.8% to 14.0%); For 2D echocardiography: 2D biplane (bias, −5.2%; −19.0% to 8.69%), 2D apical 4-chamber (bias, −5.4%; −22.1% to 11.4%), Teichholz M-mode (bias, −3.1%; −28.3% to 22.1%). |
This study is an analysis of data from 5 pilot studies, convenience sampled from the larger St. Jude Lifetime Cohort Study (SJLIFE). Patients with an implanted medical device or a history of congenital heart disease were excluded. Of the 114 patients that completed the evaluation, 108 were previously undiagnosed with cardiomyopathy. Selection bias cannot be ruled out (692 survivors enrolled in the SJLIFE cohort were exposed to anthracyclines and/or chest radiotherapy of which 134 participated in the study). The risk of detection bias is unclear; nm if outcome assessors were blinded. Outcome/attrition bias cannot be ruled out (for 20 out of 134 survivors that agreed to participate (15%) cardiac magnetic resonance imaging could not be completed*). |
Nm: not mentioned; ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; Gy: Gray; LVEF: left ventricular ejection fraction
information provided in this table is for the 114 participants with results for all tests unless otherwise stated.
| 5. What is the cost-benefit ratio of screening for asymptomatic cardiac systolic dysfunction in childhood and young adult cancer survivors? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design | Participants | Diagnostic tests | Main outcomes | Addt’l remarks |
| No studies identified | |||||
| 6. What is the cost-benefit ratio of screening for asymptomatic cardiac systolic dysfunction in adult non-oncology populations? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design | Participants | Diagnostic tests | Main outcomes | Addt’l remarks |
| Heidenreich51 2004 | Cost-benefit analysis using published data from community cohorts (gender-specific BNP test characteristics, prevalence of depressed LVEF) and randomized trials (benefit from treatment). | Men and women age 60 years with no history of heart failure (hypothetical cohorts). Prevalence of depressed LVEF: 3.5% in men; 0.45% in women. |
Four screening strategies:
Threshold BNP: 21ng/dl for men; 34 ng/dl for women. |
Screening 1,000 asymptomatic patients with BNP followed by echocardiography in those with an abnormal test increased the lifetime cost of care (176,000 US dollars for men, 101,000 US dollars for women) and improved outcome (7.9 QALYs for men, 1.3 QALYs for women), resulting in a cost per QALY of 22,300 US dollars for men and 77,700 US dollars for women. The number of men needed to screen with BNP was 44 to identify one with depressed LVEF, 133 to gain one year of life, and 127 to gain one QALY. The number of women needed to screen with BNP was 278 to identify one with depressed LVEF, 909 to gain one year of life, and 769 to gain one QALY. Screening with BNP followed by echocardiography in those with an abnormal test was economically attractive for 60-year-old men and possibly for women. Screening all patients with echocardiography was expensive, and relying on BNP alone to decide treatment led to higher cost and worse outcome compared to the sequential BNP-echocardiography strategy. In general, screening with BNP followed by echocardiography is likely to be economically attractive for patient groups with at least a 1% prevalence of moderate or greater LV systolic dysfunction (i.e. increased outcome at a cost < 50,000 US dollars per QALY gained). Screening would not be attractive if a diagnosis of left ventricular dysfunction led to significant decreases in quality of life or income |
Possible limitations as reported in the article:
|
BNP: B-type natriuretic peptide; LVEF: left ventricular ejection fraction; QALY: quality-adjusted life years.
Working Group 3: At what frequency should cardiomyopathy surveillance be performed?
| 1. Is there evidence for a difference in deterioration of cardiac systolic dysfunction between high or standard risk groups of childhood and young adult cancer survivors treated with anthracyclines and/or radiation involving the heart? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| No studies identified | |||||
| 2. Does the risk of cardiac deterioration cease after a certain follow-up time? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| van der Pal1 2012 | Retrospective cohort 1966–1996 22.2 yrs (5.0–44.5) |
5-yr survivors (N=1362) Age at Dx: 5.9 (0–18) |
Anthracyclines: 33.6% Anth+XRT: 7.9% Median Anth: 250 mg/m2 (25–775) |
Symptomatic cardiac events (CE); Grading: CTCAE v 3.0 CI of CHF: Radiotherapy: 0.7% at 30-yrs XRT + Anth: 7.9% at 30yrs |
Clinically validated outcomes Long follow-up, large cohort |
| Lipshutz27 2005 | Observational prospective longitudinal cohort | 115 survivors at a median of 11.8 (8.3–15) years off therapy | Median anthracycline 360 mg/m2 (280–550), no radiation | 5 late CHF, LV contractility fell significantly over time and was depressed at last f/u in those who received >300mg/m2 | With median f/u of 11.8 years, thinned ventricular wall by 6 years, depressed LV contractility by 12 years, depressed SF over time |
| Mulrooney2 2009 | Prospective longitudinal cohort study – questionnaire based | 14,358 survivors and 3,899 siblings | Mix of anthracycline treated/not treated | 1.7% risk of CHF in survivors. Increasing incidence over time with no plateau. Longest follow-up was 30 years. | |
| Roodpeyma52 2008 | Cross-sectional | 58 survivors of pediatric cancer plus health controls | Various anthracyclines | SF/EF reduced in survivors compared with controls. | With a median follow-up of 9 years (5–22), significant association between length of follow-up and risk for abnormal SF/EF. |
| Pein19 2004 | Cross-sectional | 447 treated for solid tumor in single institution | Anthracyclines +/− radiation therapy | Risk for CHF increased without plateau over time. Increased risk with increasing dose. | Last case occurred at ~25 years from exposure |
| Sorensen28 2003 | Prospective longitudinal cohort study | 101 ALL survivors; 83 Wilms tumor survivors | Range of anthracyclines | Decreased contractility in both groups. Anthracycline dose most important risk factor. | Significant decrease in wall thickness and SF in Wilms tumor survivors in echocardiograms performed at a mean of 11.9 years and 16.3 years. |
| Van Dalen18 2006 | Retrospective medical record review – cross sectional | 830 children at a single institution | Mean cumulative anthracycline dose 288 mg/m2 | At a mean follow up of 8.5 years, 2.5% risk of CHF. Authors calculated 10% risk of CHF at 20-years after treatment in survivors treated with ≥300 mg/m2 | |
| Van der Pal23 2010 | Retrospective medical record review and prospective cardiac screening (cross sectional) | 525 survivors seen in an outpatient clinic with echocardiogram | 361/525 received an anthracycline | At average age of assessment=23.1 (18.0–47.1) years, 27% had an abnormal LVSF (<30%). Risk greatest in those with >25 year follow up and anthracycline dose ≥450 mg/m2 | |
| 3. Is there an increased risk of deterioration during puberty? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| No studies identified | |||||
| 4. Is there an increased risk of deterioration during pregnancy and delivery? | |||||
|---|---|---|---|---|---|
| Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Bar53 2003 | Single centre cohort | 37 females treated with anthracyclines b/w 1973–1982 who had a pregnancy between 1986–2003 | Median doxorubicin 400 mg/m2 (150–500) | No change in average FS through pregnancy. Among 8 women with FS<30%, pregnancy outcome was worse. More hospitalizations, ICU stays, induction. Two had admission for cardiac deterioration. Non-significant decrease in FS in women who started <30% | |
| Van Dalen 200654 | Single centre prospective cohort study | 206 females >17 y.o. who had survived >5 yrs after a childhood malignancy. 53 had delivered 1 or more children | Among 53, mean anthracycline 267 mg/m2 (60–552). | No peripartum CHF after 83 deliveries pregnancies in 53 women | Upper limit of 95% CI is 5.7% |
Working group 4: What should be done when abnormalities are found? What are the limitations in physical activity?
| 1. What is the effect of treatment with ACE-inhibitors in childhood and young adult cancer survivors with asymptomatic cardiomyopathy? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Silber55 2004 | RCT (double-blinded) Unknown treatment era (probably end ’70 – mid ’90) Median (range) follow-up time was 2.80 years (2 weeks to 6.1 years). | 135 childhood cancer survivors (aged 8.3 to 30.6 years, 78 males, at least 4 years from diagnosis and 2 years off treatment) with asymptomatic decline of cardiac function at some time after anthracycline exposure, detected with echocardiography, resting or exercise GNA, MCI at peak exercise and / or resting ECG. Median (range) time since cancer diagnosis 9 (4.2 to 22.3) years in the enalapril group and 9.6 (4.3 to 25.8) years in the placebo group |
Oral enalapril once daily (n = 69) or oral placebo once daily (n = 66). Dosing of study medication was as follows: at start 0.05 mg/kg/day, escalation after 14 days to 0.10 mg/ kg/day and escalation at 3 months visit to 0.15 mg/kg/day if no side effects occurred | Overall survival, mortality due to heart failure, development of clinical heart failure and quality of life: no (statistically) significant differences between treatment and control group. Cardiac function: a post-hoc analysis showed a decrease (i.e. improvement) in one measure (left ventricular end systolic wall stress (LVESWS): −8.62%change) compared with placebo (+1.66% change) in the first year of treatment (P = 0.036), but not afterwards. Adverse events: patients treated with enalapril had a higher risk of dizziness or hypotension (RR 7.17, 95% CI 1.71 to 30.17) and fatigue (Fisher’s exact test, P = 0.013). |
Median (range) follow-up time was 2.80 years (2 weeks to 6.1 years). Loss of follow-up was not mentioned. Since the authors did not present dichotomous outcomes, we were not able to define RRs for the outcome change in cardiac function; we therefore describe the outcomes as presented in the original study. The study had a low/moderate risk of selection bias, performance bias and detection bias. For most outcomes there was a low risk of attrition bias, but for some outcomes (the post-hoc analysis of LVESWS, other parameters of cardiac function (shortening fraction and stress-velocity index), the change in quality of life and the risk of adverse events) intention-to-treat analysis was not possible or it was unclear if follow-up was complete, leading to a possible risk of attrition bias for these other outcomes. |
| 2. What is the effect of treatment with beta-blockers in childhood and young adult cancer survivors with asymptomatic cardiomyopathy? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| No studies identified | A Cochrane systematic review assessed if a study on beta-blockers in children with heart failure included anthracycline-treated patients (Shaddy 2007)56: patients with anthracycline-induced cardiomyopathy were included in the trial, but it was not possible to separate the data of these patients from the data of all included patients. | ||||
| 3. What is the effect of other medical interventions in childhood and young adult cancer survivors with asymptomatic cardiomyopathy? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| No studies identified | |||||
| 4. What is the effect of treatment with ACE-inhibitors in non-oncology populations with asymptomatic cardiomyopathy? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| SOLVD investigators57 1992 | Double-blind, placebo-controlled RCT Mean: 37.4 (range: 14.6 – 62) months |
4228 asymptomatic patients with EF <35%, and no medication for heart failure | Enalapril: N=2111 Placebo: N=2117 |
All-cause mortality: Enalapril: 313 (14.8%) Placebo: 334 (15.8%) Risk reduction: 8% (95% CI −8% to +21%) Clinical heart failure or all cause mortality: Enalapril: 630 (29.8%) Placebo: 818 (38.6%) Risk reduction: 29% (95% CI 21% to 36%) |
Flather 2000: 74% of all SOLVD-patients (including another RCT with symptomatic patients) had a previous MI. Exner 1999: one third of the SOLVD prevention trial was in NYHA II EF was determined by echocardiography |
| Pfeffer58 1992 | Double-blind, Placebo controlled RCT Mean: 42 (range: 24 – 60) months |
2231 asymptomatic patients with EF ≤40%, 3 – 16 days after MI | Captopril: N=1115 Placebo: N=1116 |
All-cause mortality: Captopril: 20% versus placebo 25% (RR 19%, 3 – 32%, P=0.014) Development of clinical heart failure: Captopril: 11% versus placebo 16%, RR 37% (20– 50%, P<0.001) | EF was determined by RNA |
| Jong59 2003 | Cohort study after RCT 11.2 years (IQR: 10.3 – 12.1) since randomization |
3581 patients of the SOLVD prevention trial (asymptomatic patients with EF <35%), treated previously with enalapril or placebo during a mean of 37.4 months, who survived the time of the trial | Enalapril group: N=1798 Placebo group: N=1783 |
All-cause mortality: Enalapril: 1074 (50.9%) Placebo: 1195 (56.4%) HR: 0.86 (95% CI 0.77 – 0.93) Increased life expectancy (median): 9.2 months (95% CI 0 – 19.2 months) | Patients with a lower EF had more benefit of treatment EF was determined by echocardiography |
| Kober60 1995 | Double-blind, Placebo controlled RCT 24 – 50 months clinical follow-up | 1749 patients with an MI in the previous week and EF ≤35% | Trandopril: N=876 Placebo: N=873 |
All-cause mortality: Trandopril versus placebo: RR 0.78 (0.67 – 0.91) Clinical heart failure: Trandopril versus placebo: RR 0.71 (0.56 – 0.89) | 41% of patients was in NYHA I EF was determined by echocardiography |
| Hunt61,62 AHA/ACC Guideline (2005 and 2009) | Angiotensin converting enzyme inhibitors can be useful to prevent HF in patients at high risk for developing HF | Stage A * with a history of atherosclerotic vascular disease, diabetes mellitus, or hypertension with associated cardiovascular risk factors | Perindopril Ramipril |
Class of recommendation IIa Level of evidence A | |
| Hunt61,62 AHA/ACC Guideline (2005 and 2009) | Angiotensin converting enzyme inhibitors should be used in patients with a reduced EF and no symptoms of HF, even if they have not experienced MI | Stage B* | Enalapril | Class of recommendation I Level of evidence A | |
| Dickstein63 2008 ESC Guideline | Recommendation to treat with beta-blockers based upon the patients enrolled in the RCTs | LVEF ≤40% Mild to severe symptoms (NYHA II–IV)** and patients with asymptomatic LV systolic dysfunction after MI | Bisoprolol Carvedilol Metoprolol succinate Nebivolol |
Class of recommendation I Level of evidence A | CIBIS-II 1999 MERIT-HF 1999 & 2000 Packer 2001 COPERNICUS 2002 SENIORS 2005 BBEST 2001 COMET 2003 |
| 5. What is the effect of treatment with beta-blockers in non-oncology populations with asymptomatic cardiomyopathy? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Designh Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Dargie64 2001 | Double-blind, placebo-controlled RCT 1.3 years clinical follow-up |
1959 patients with MI 3–21 days before randomization, EF≤ 40% or wall-motion score index ≤ 1.3 and at least 24 hours on a stable dose of ACE-inhibitor treatment. | Carvedilol: N=975 Placebo: N=984 |
All-cause mortality: Carvedilol: 116 (12%) Placebo: 141 (15%) HR: 0.77 (0.60 – 0.98) Hospitalization for heart failure: Carvedilol: 118 (12%) Placebo: (138 (14%) HR 0.86 (0.67 – 1.09) |
Eligible patients had LV dysfunction with or without heart failure, but patients with severe heart failure were excluded. EF was determined by echocardiography, RNA or ventriculography |
| Exner65 1999 | Retrospective analysis of RCT Mean followup 35 months |
4228 patients participating in the SOLVD prevention trial | Patients that used a beta blocker at the start of the trial, in addition to study medication: N=1015 (24%) Patients that did not use a beta blocker at the start of the trial, in addition to study medication: N=3213 (76%) |
All-cause mortality: Using a beta blocker: IR 4.3/100 person-years No beta blocker: IR 5.6/100 person-years Multivariate model, using a beta blocker in addition to ACE inhibitor allocation: * All-cause mortality RR 0.70 * All-cause mortality or hospitalization for CHF: RR 0.64 (0.49 – 0.83) |
|
| Vantrimpont66 1997 | Retrospective analysis of RCT Mean clinical follow-up of surviving patients: 42 months (+/−10 months) |
2231 patients participating in the SAVE trial | Patients that used captopril at the start of the trial, in addition to study medication: N=789 (35%) Patients that did not use captopril at the start of the trial, in addition to study medication: N=1442 (65%) |
Cardiovascular mortality: Captopril: 13.1% No captopril: 22.1% (RR 0.58, 0.43 – 0.79) Severe heart failure: Captopril: 16.5% No captopril: 22.6% (RR 0.68, 0.55 – 0.83) Multivariate model (including captopril use): * CV mortality RR 0.70 * Severe CHF RR 0.79 |
|
| Hunt61,62 AHA/ACC Guideline (2005 and 2009) | Beta-blockers are indicated in all patients without a history of MI who have a reduced LVEF with no HF symptoms | Stage B* | Class of recommendation I Level of evidence C | ||
| 6. What is the effect of other medical interventions in other groups of patients with asymptomatic cardiomyopathy? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Konstam67 2000 | Double-blind, placebo-controlled RCT Median follow-up 555 days. |
3152 patients aged 60 years or older with New York Heart Association class II–IV heart failure and LVEF ≤40% | losartan (n=1578) titrated to 50 mg once daily or captopril (n=1574) titrated to 50 mg three times daily | all-cause mortality: 11·7 vs 10·4% average annual mortality rate HR 1·13 [95·7% CI 0·95–1·35], p=0·16 sudden death or resuscitated arrests: 9·0 vs 7·3% HR 1·25 [95% CI 0·98–1·60], p=0·08 |
Significantly fewer patients in the losartan group (excluding those who died) discontinued study treatment because of adverse effects (9·7 vs 14·7%, p<0·001), including cough (0·3 vs 2·7%) |
| Hunt61,62 AHA/ACC Guideline (2005 and 2009) | Angiotensin II receptor blockers can be useful to prevent HF in patients at high risk for developing HF | Stage A* who have a history of atherosclerotic vascular disease, diabetes mellitus, or hypertension with associated cardiovascular risk factors | Angiotensin II receptor blockers | Class of recommendation IIa Level of evidence C | |
| Hunt61,62 AHA/ACC Guideline (2005 and 2009) | Angiotensin II receptor blockers can be beneficial in patients with low EF and no symptoms of HF who are intolerant of ACEIs. | Stage B* | Angiotensin II receptor blockers | Class of recommendation IIa Level of evidence C | |
| Hunt61,62 AHA/ACC Guideline (2005 and 2009) | Placement of an ICD might be considered in patients without HF | Stage B* who have non-ischemic cardiomyopathy and an LVEF ≤30% who are in NYHA I with chronic optimal medical therapy and have a reasonable expectation of survival with good functional status for >1 year. | ICD | Class of recommendation IIb Level of evidence C | |
| Dickstein63 2008 | Recommendation to treat with angiotensin receptor blockers (ARB) based upon the patients enrolled in the RCTs | LVEF ≤40% and either
|
Candesartan Valsartan | Treatment reduces the risk of death from cardiovascular causes Class of recommendation I Level of evidence A 1. An ARB is recommended as an alternative in patients intolerant of an ACEI Class of recommendation IIa Level of evidence B 2. in patients with persistent symptoms (NYHA II–IV) despite treatment with an ACE-Inhibitor and beta-blocker Class of recommendation I Level of evidence B |
Cohn 2001 CHARM-Added trial 2003 CHARM-Alternative trial 2003 Pfeffer 2003 OPTIMAAL trial 2002 McMurray 2004 |
| Dickstein68 2010 | Recommendation cardiac resynchronization therapy with defibrillator function in patients with heart failure in NYHA I/II | NYHA function class II LVEF ≤35%, QRS ≥150 ms, SR Optimal medical therapy | CRT preferentially by CRT-D is recommended to reduce morbidity or to prevent disease progression*** | Class of recommendation I Level of evidence A | Abraham 2004 Moss 2009 Linde 2009 Daubert 2009 |
| 7. Is there evidence that exercise increases the risk of deterioration of cardiac systolic function in childhood cancer survivors who received potentially cardiotoxic therapies? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Huang69 2011 | Systematic review. 15 studies identified including 4 RCTs |
Mostly ALL patients during and after treatment | Different exercise training schedules | Different in all studies. Positive effects of physical training on organ system function, fatigue and physical well-being | However, the optimal intervention modality and the intensity, timing, and duration of the intervention are difficult to determine. |
| 8. Is there evidence that exercise increases the risk of deterioration of cardiac systolic function in adult-onset cancer survivors and non- oncology at-risk populations? | |||||
|---|---|---|---|---|---|
| First Author Year |
Study Design Treatment era Years of follow-up |
Participants | Treatment | Main outcomes | Addt’l remarks |
| Schmitz70 2010 | Guideline-expert opinion- American College of Sports Medicine | Only ADULT cancer studies reviewed | Physical activity is strongly recommended with the exception of activities resulting in rapid BP elevation (eg isometric exercise) | ||
| Pellicia71 2006 | Guideline-expert opinion- European Society of Cardiology | Recommendation is for physical activity in individuals with genetic susceptibility to CHF, but with normal systolic function. | |||
| Dickstein63 2008 | Guideline – review of published evidence, expert panel; European Society of Cardiology | Recommendations – Weight reduction should be considered in obese persons with heart failure In moderate to severe heart failure, weight reduction should not be recommended routinely |
No supporting evidence supplied Level of evidence C |
||
| Maron72 2004 | Consensus document; expert international panel of clinical cardiovascular specialists and molecular biologists; American Heart Association | Young people (<40 years age) with genetic cardiovascular diseases including hypertrophic cardiomyopathy but not specifically including dilated cardiomyopathy. | Not specifically considered. Considered recommendations for physical activity and recreational sports participation. Childhood cancer survivors (CCS) not included. |
Recommendations: Can safely participate in most low or moderate-intensity recreational exercise Some activities should be avoided, eg burst exertion, extremely adverse environmental conditions, exercise programmes with systematic / progressive levels of exertion and aiming at higher levels of conditioning, intense isometric exertion, extreme sports, performance-enhancing substances |
|
| Riegel73 2009 | Review / scientific statement; expert panel; American Heart Association | Persons with heart failure | Not specifically considered. CCS not mentioned specifically. |
Statements In moderate heart failure, exercise improves certain physiological parameters including VO2max, ventilatory response, heart rate variability. Can also reduce depression. Effect on mortality not clear. Cites Pina et al 2003. Individually tailored exercise programme based on results of formal exercise testing may benefit patients with severe symptomatic LV dysfunction. Cites Fletcher et al 2001. Exercise is a beneficial adjunctive treatment in patients with current or prior heart failure symptoms and reduced LVEF. Cites Hunt et al 2005 (states this is level 1B evidence). Modest benefit in HF-Action RCT (Flynn et al, 2009, see below) |
|
| Flynn74 2009 | HF-Action Randomised controlled trial Randomised 2003-7 Median FU 2.5 years | 2331 stable out-patients with heart failure (LVEF ≤35%) 82 centres in USA, Canada, France | Randomised to Usual care + aerobic exercise training (initially supervised, subsequently home-based) vs usual care + recommendation for regular physical activity. Usual care included optimal medical therapy. | At 3 months, usual care + exercise training group showed statistically greater improvement in Kansas City Cardiomyopathy Questionnaire (KCCQ – a 23 item disease-specific questionnaire) score than usual care group. Improvement was maintained. Also modest but significant improvement in quality of life and non-significant reduction in all-cause mortality and hospitalisation in usual care + exercise training group. |
|
| Piepoli75 2004 | Meta-analysis (individual patient data) 1990–2002 Individual median F/U 5–75mths, overall 23mths |
9 studies, total 395 training to 406 control 87% males, 59% with IHD, mean LVEF <28%, 73% on ACE inhibitors | All RCTs, usual care vs addition of exercise training (mostly supervised) | Outcome of mortality in favour of exercise – 0.65 (0.46–0.92) Outcome of death or admission to hospital also in favour of exercise – 0.72 (0.56–0.93) |
Intensity generally set at 60–80% peak oxygen consumption. These trials are designed to be “safe” first and foremost. Question of whether differing aetiologies of systolic dysfunction/heart failure have differing responses to physical activity not yet answered. |
| Davies76 2010 | Meta-analysis (publication data) 2001-Jan2008 Individual median F/U 5 mths-60mths., overall 11mths | 19 trials, total 3647 patients (HF-ACTION trial contributed 60%) Only one trial 57% femaies, others 72–100% male; age 58 | All RCTs, usual care vs addition of exercise training (mostly supervised) Only 4 trials F/U longer than 12 mths. |
All cause mortality <12 mth F/U outcome in favour of usual care – 1.03 (0.70–1.53), but >12mth F/U favoured exercise – 0.91 (0.78–1.06) All hospital admissions both < and >12 mths favoured exercise. HRQoL measurements also favoured exercise. |
If HF-ACTION trial excluded, significant reduction longer-term mortality seen (0.62 (0.39–0.98). Issues of mix of endurance and resistance training starting to be addressed. |
References
- 1.van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 2.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco JG, Sun CL, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group. J Clin Oncol. 2012;30:1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temming P, Qureshi A, Hardt J, et al. Prevalence and predictors of anthracycline cardiotoxicity in children treated for acute myeloid leukaemia: retrospective cohort study in a single centre in the United Kingdom. Pediatr Blood Cancer. 2011;56:625–630. doi: 10.1002/pbc.22908. [DOI] [PubMed] [Google Scholar]
- 5.Creutzig U, Diekamp S, Zimmermann M, et al. Longitudinal evaluation of early and late anthracycline cardiotoxicity in children with AML. Pediatr Blood Cancer. 2007;48:651–662. doi: 10.1002/pbc.21105. [DOI] [PubMed] [Google Scholar]
- 6.van Dalen EC, Michiels EM, Caron HN, et al. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2010:CD005006. doi: 10.1002/14651858.CD005006.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Le Deley MC, Leblanc T, Shamsaldin A, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case-control study by the Societe Francaise d'Oncologie Pediatrique. J Clin Oncol. 2003;21:1074–1081. doi: 10.1200/JCO.2003.04.100. [DOI] [PubMed] [Google Scholar]
- 8.Neri B, Cini-Neri G, Bandinelli M, et al. Doxorubicin and epirubicin cardiotoxicity: experimental and clinical aspects. Int J Clin Pharmacol Ther Toxicol. 1989;27:217–221. [PubMed] [Google Scholar]
- 9.Uderzo C, Pillon M, Corti P, et al. Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation: a prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone Marrow Transplant. 2007;39:667–675. doi: 10.1038/sj.bmt.1705652. [DOI] [PubMed] [Google Scholar]
- 10.Lonnerholm G, Arvidson J, Andersson LG, et al. Myocardial function after autologous bone marrow transplantation in children: a prospective long-term study. Acta Paediatr. 1999;88:186–192. doi: 10.1080/08035259950170376. [DOI] [PubMed] [Google Scholar]
- 11.Eames GM, Crosson J, Steinberger J, et al. Cardiovascular function in children following bone marrow transplant: a cross-sectional study. Bone Marrow Transplant. 1997;19:61–66. doi: 10.1038/sj.bmt.1700600. [DOI] [PubMed] [Google Scholar]
- 12.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS) Blood. 2011;118:1413–1420. doi: 10.1182/blood-2011-01-331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armenian SH, Sun CL, Francisco L, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26:5537–5543. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armenian SH, Sun CL, Shannon T, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118:6023–6029. doi: 10.1182/blood-2011-06-358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155:21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 16.Tichelli A, Passweg J, Wojcik D, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 17.Rathe M, Carlsen NL, Oxhoj H, et al. Long-term cardiac follow-up of children treated with anthracycline doses of 300 mg/m2 or less for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54:444–448. doi: 10.1002/pbc.22302. [DOI] [PubMed] [Google Scholar]
- 18.van Dalen EC, van der Pal HJ, Kok WE, et al. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Pein F, Sakiroglu O, Dahan M, et al. Cardiac abnormalities 15 years and more after adriamycin therapy in 229 childhood survivors of a solid tumour at the Institut Gustave Roussy. Br J Cancer. 2004;91:37–44. doi: 10.1038/sj.bjc.6601904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green DM, Grigoriev YA, Nan B, et al. Congestive heart failure after treatment for Wilms' tumor: a report from the National Wilms' Tumor Study group. J Clin Oncol. 2001;19:1926–1934. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 21.Kremer LC, van Dalen EC, Offringa M, et al. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Ann Oncol. 2002;13:503–512. doi: 10.1093/annonc/mdf118. [DOI] [PubMed] [Google Scholar]
- 22.Brouwer CA, Postma A, Vonk JM, et al. Systolic and diastolic dysfunction in long-term adult survivors of childhood cancer. Eur J Cancer. 2011;47:2453–2462. doi: 10.1016/j.ejca.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 23.van der Pal HJ, van Dalen EC, Hauptmann M, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170:1247–1255. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 24.Abosoudah I, Greenberg ML, Ness KK, et al. Echocardiographic surveillance for asymptomatic late-onset anthracycline cardiomyopathy in childhood cancer survivors. Pediatr Blood Cancer. 2011;57:467–472. doi: 10.1002/pbc.22989. [DOI] [PubMed] [Google Scholar]
- 25.Hudson MM, Rai SN, Nunez C, et al. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25:3635–3643. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 26.Paulides M, Kremers A, Stohr W, et al. Prospective longitudinal evaluation of doxorubicin-induced cardiomyopathy in sarcoma patients: a report of the late effects surveillance system (LESS) Pediatr Blood Cancer. 2006;46:489–495. doi: 10.1002/pbc.20492. [DOI] [PubMed] [Google Scholar]
- 27.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 28.Sorensen K, Levitt GA, Bull C, et al. Late anthracycline cardiotoxicity after childhood cancer: a prospective longitudinal study. Cancer. 2003;97:1991–1998. doi: 10.1002/cncr.11274. [DOI] [PubMed] [Google Scholar]
- 29.Kremer LC, van der Pal HJ, Offringa M, et al. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13:819–829. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 30.Schellong G, Riepenhausen M, Bruch C, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55:1145–1152. doi: 10.1002/pbc.22664. [DOI] [PubMed] [Google Scholar]
- 31.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 32.Guldner L, Haddy N, Pein F, et al. Radiation dose and long term risk of cardiac pathology following radiotherapy and anthracyclin for a childhood cancer. Radiother Oncol. 2006;81:47–56. doi: 10.1016/j.radonc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 34.van der Pal HJ, van Dalen EC, Kremer LC, et al. Risk of morbidity and mortality from cardiovascular disease following radiotherapy for childhood cancer: a systematic review. Cancer Treat Rev. 2005;31:173–185. doi: 10.1016/j.ctrv.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien MM, Taub JW, Chang MN, et al. Cardiomyopathy in children with Down syndrome treated for acute myeloid leukemia: a report from the Children's Oncology Group Study POG 9421. J Clin Oncol. 2008;26:414–420. doi: 10.1200/JCO.2007.13.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aviles A, Neri N, Nambo JM, et al. Late cardiac toxicity secondary to treatment in Hodgkin's disease. A study comparing doxorubicin, epirubicin and mitoxantrone in combined therapy. Leuk Lymphoma. 2005;46:1023–1028. doi: 10.1080/10428190500063229. [DOI] [PubMed] [Google Scholar]
- 37.van Dalen EC, van der Pal HJ, Bakker PJ, et al. Cumulative incidence and risk factors of mitoxantrone-induced cardiotoxicity in children: a systematic review. Eur J Cancer. 2004;40:643–652. doi: 10.1016/j.ejca.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and metaanalysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postma A, Bink-Boelkens MT, Beaufort-Krol GC, et al. Late cardiotoxicity after treatment for a malignant bone tumor. Med Pediatr Oncol. 1996;26:230–237. doi: 10.1002/(SICI)1096-911X(199604)26:4<230::AID-MPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 40.Pihkala J, Saarinen UM, Lundstrom U, et al. Effects of bone marrow transplantation on myocardial function in children. Bone Marrow Transplant. 1994;13:149–155. [PubMed] [Google Scholar]
- 41.Krawczuk-Rybak M, Dakowicz L, Hryniewicz A, et al. Cardiac function in survivors of acute lymphoblastic leukaemia and Hodgkin's lymphoma. J Paediatr Child Health. 2011;47:455–459. doi: 10.1111/j.1440-1754.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 42.Mavinkurve-Groothuis AM, Groot-Loonen J, Bellersen L, et al. Abnormal NT-pro-BNP levels in asymptomatic long-term survivors of childhood cancer treated with anthracyclines. Pediatr Blood Cancer. 2009;52:631–636. doi: 10.1002/pbc.21913. [DOI] [PubMed] [Google Scholar]
- 43.Hayakawa H, Komada Y, Hirayama M, et al. Plasma levels of natriuretic peptides in relation to doxorubicin-induced cardiotoxicity and cardiac function in children with cancer. Med Pediatr Oncol. 2001;37:4–9. doi: 10.1002/mpo.1155. [DOI] [PubMed] [Google Scholar]
- 44.Sherief LM, Kamal AG, Khalek EA, et al. Biomarkers and early detection of late onset anthracycline-induced cardiotoxicity in children. Hematology. 2012;17:151–156. doi: 10.1179/102453312X13376952196412. [DOI] [PubMed] [Google Scholar]
- 45.Kismet E, Varan A, Ayabakan C, et al. Serum troponin T levels and echocardiographic evaluation in children treated with doxorubicin. Pediatr Blood Cancer. 2004;42:220–224. doi: 10.1002/pbc.10368. [DOI] [PubMed] [Google Scholar]
- 46.Soker M, Kervancioglu M. Plasma concentrations of NT-pro-BNP and cardiac troponin-I in relation to doxorubicin-induced cardiomyopathy and cardiac function in childhood malignancy. Saudi Med J. 2005;26:1197–1202. [PubMed] [Google Scholar]
- 47.Hill SA, Balion CM, Santaguida P, et al. Evidence for the use of B-type natriuretic peptides for screening asymptomatic populations and for diagnosis in primary care. Clin Biochem. 2008;41:240–249. doi: 10.1016/j.clinbiochem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Ewald B, Ewald D, Thakkinstian A, et al. Meta-analysis of B type natriuretic peptide and N-terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J. 2008;38:101–113. doi: 10.1111/j.1445-5994.2007.01454.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang TJ, Levy D, Benjamin EJ, et al. The epidemiology of"asymptomatic" left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003;138:907–916. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 50.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heidenreich PA, Gubens MA, Fonarow GC, et al. Cost-effectiveness of screening with B-type natriuretic peptide to identify patients with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2004;43:1019–1026. doi: 10.1016/j.jacc.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 52.Roodpeyma S, Moussavi F, Kamali Z. Late cardiotoxic effects of anthracycline chemotherapy in childhood malignancies. J Pak Med Assoc. 2008;58:683–687. [PubMed] [Google Scholar]
- 53.Bar J, Davidi O, Goshen Y, et al. Pregnancy outcome in women treated with doxorubicin for childhood cancer. Am J Obstet Gynecol. 2003;189:853–857. doi: 10.1067/s0002-9378(03)00837-8. [DOI] [PubMed] [Google Scholar]
- 54.van Dalen EC, van der Pal HJ, van den Bos C, et al. Clinical heart failure during pregnancy and delivery in a cohort of female childhood cancer survivors treated with anthracyclines. Eur J Cancer. 2006;42:2549–2553. doi: 10.1016/j.ejca.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22:820–828. doi: 10.1200/JCO.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 56.Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. Jama. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 57.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 58.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 59.Jong P, Yusuf S, Rousseau MF, et al. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. Lancet. 2003;361:1843–1848. doi: 10.1016/S0140-6736(03)13501-5. [DOI] [PubMed] [Google Scholar]
- 60.Kober L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 61.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 62.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 63.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 65.Exner DV, Dries DL, Waclawiw MA, et al. Beta-adrenergic blocking agent use and mortality in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a post hoc analysis of the Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1999;33:916–923. doi: 10.1016/s0735-1097(98)00675-5. [DOI] [PubMed] [Google Scholar]
- 66.Vantrimpont P, Rouleau JL, Wun CC, et al. Additive beneficial effects of beta-blockers to angiotensin-converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) Study. SAVE Investigators. J Am Coll Cardiol. 1997;29:229–236. doi: 10.1016/s0735-1097(96)00489-5. [DOI] [PubMed] [Google Scholar]
- 67.Konstam MA, Neaton JD, Poole-Wilson PA, et al. Comparison of losartan and captopril on heart failure-related outcomes and symptoms from the losartan heart failure survival study (ELITE II) Am Heart J. 2005;150:123–131. doi: 10.1016/j.ahj.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 68.Dickstein K, Vardas PE, Auricchio A, et al. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Europace. 2010;12:1526–1536. doi: 10.1093/europace/euq392. [DOI] [PubMed] [Google Scholar]
- 69.Huang TT, Ness KK. Exercise interventions in children with cancer: a review. Int J Pediatr. 2011;2011:461512. doi: 10.1155/2011/461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 71.Pelliccia A, Corrado D, Bjornstad HH, et al. Recommendations for participation in competitive sport and leisure-time physical activity in individuals with cardiomyopathies, myocarditis and pericarditis. Eur J Cardiovasc Prev Rehabil. 2006;13:876–885. doi: 10.1097/01.hjr.0000238393.96975.32. [DOI] [PubMed] [Google Scholar]
- 72.Maron BJ, Chaitman BR, Ackerman MJ, et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation. 2004;109:2807–2816. doi: 10.1161/01.CIR.0000128363.85581.E1. [DOI] [PubMed] [Google Scholar]
- 73.Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 74.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. Jama. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piepoli MF, Davos C, Francis DP, et al. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328:189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12:706–715. doi: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
SHA, MMH, RM, GL, and LCMK contributed to the conception and design of the study. SHA, MMH, RLM, MHC, LSC, MD, PCN, WT, SS, ES, RS, JS, EvD, HvdP, WHW, GL, LCMK contributed to the search strategy, data extraction, interpretation of the data, and formulation of the recommendations. SHA, MMH, RM, GL, and LCMK drafted the manuscript, and MHC, LSC, MD, PCN, WT, SS, ES, RS, JS, EvD, HvdP, WHW critically revised the manuscript. All authors approved the final version.
Conflicts of interest:
The authors have no conflicts of interest to declare.
References
- 1.Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, et al. National Cancer Institute. Bethesda, MD: 2008. Cancer Statistics Review, 1975–2005. http://seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. The New England journal of medicine. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32(12):1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term Cardiovascular Toxicity in Children, Adolescents, and Young Adults Who Receive Cancer Therapy: Pathophysiology, Course, Monitoring, Management, Prevention, and Research Directions: A Scientific Statement From the American Heart Association. Circulation. 2013;128(17):1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL, et al. Late Mortality Among 5-Year Survivors of Childhood Cancer: A Summary From The Childhood Cancer Survivor Study. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42(18):3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Pein F, Sakiroglu O, Dahan M, Lebidois J, Merlet P, Shamsaldin A, et al. Cardiac abnormalities 15 years and more after adriamycin therapy in 229 childhood survivors of a solid tumour at the Institut Gustave Roussy. British journal of cancer. 2004;91(1):37–44. doi: 10.1038/sj.bjc.6601904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. Jama. 2013;309(22):2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landier W, Armenian SH, Lee J, Thomas O, Wong FL, Francisco L, et al. Yield of screening for long-term complications using the children's oncology group long-term follow-up guidelines. J Clin Oncol. 2012;30(35):4401–4408. doi: 10.1200/JCO.2012.43.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. Journal of the American College of Cardiology. 2009;53(15):e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Adams MJ, Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatric blood & cancer. 2005;44(7):600–606. doi: 10.1002/pbc.20352. [DOI] [PubMed] [Google Scholar]
- 13.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers. 3.0 ed. Arcadia, CA: Children's Oncology Group; 2008. [Google Scholar]
- 14.Sieswerda E, Postma A, van Dalen EC, van der Pal HJ, Tissing WJ, Rammeloo LA, et al. The Dutch Childhood Oncology Group guideline for follow-up of asymptomatic cardiac dysfunction in childhood cancer survivors. Ann Oncol. 2012;23(8):2191–2198. doi: 10.1093/annonc/mdr595. [DOI] [PubMed] [Google Scholar]
- 15.United Kingdom Children's Cancer Study Group Late Effects Group. Therapy based long term follow up practice statement. [Available online, 2013];2005 http://www.cclg.org.uk. [cited; Available from. [Google Scholar]
- 16.Wallace WH, Thompson L, Anderson RA. Long term follow-up of survivors of childhood cancer: summary of updated SIGN guidance. BMJ. 2013;346:f1190. doi: 10.1136/bmj.f1190. [DOI] [PubMed] [Google Scholar]
- 17.Kremer LC, Mulder RL, Oeffinger KC, Bhatia S, Landier W, Levitt G, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatric blood & cancer. 2013;60(4):543–549. doi: 10.1002/pbc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer LC, van der Pal HJ, Offringa M, van Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13(6):819–829. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 19.Kremer LC, van Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Ann Oncol. 2002;13(4):503–512. doi: 10.1093/annonc/mdf118. [DOI] [PubMed] [Google Scholar]
- 20.Lauer MS, Larson MG, Levy D. Gender-specific reference M-mode values in adults: population-derived values with consideration of the impact of height. Journal of the American College of Cardiology. 1995;26(4):1039–1046. doi: 10.1016/0735-1097(95)00275-0. [DOI] [PubMed] [Google Scholar]
- 21.Hudson MM, Rai SN, Nunez C, Merchant TE, Marina NM, Zalamea N, et al. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25(24):3635–3643. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 22.Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008;21(8):922–934. doi: 10.1016/j.echo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Fischer M, Baessler A, Hense HW, Hengstenberg C, Muscholl M, Holmer S, et al. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. European heart journal. 2003;24(4):320–328. doi: 10.1016/s0195-668x(02)00428-1. [DOI] [PubMed] [Google Scholar]
- 24.Gibbons RJ, Smith S, Antman E. American College of Cardiology/American Heart Association clinical practice guidelines: Part I: where do they come from? Circulation. 2003;107(23):2979–2986. doi: 10.1161/01.CIR.0000063682.20730.A5. [DOI] [PubMed] [Google Scholar]
- 25.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Pal HJ, van Dalen EC, van Delden E, van Dijk IW, Kok WE, Geskus RB, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30(13):1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 27.Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group. J Clin Oncol. 2012;30(13):1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schellong G, Riepenhausen M, Bruch C, Kotthoff S, Vogt J, Bolling T, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatric blood & cancer. 2010;55(6):1145–1152. doi: 10.1002/pbc.22664. [DOI] [PubMed] [Google Scholar]
- 29.van der Pal HJ, van Dalen EC, Kremer LC, Bakker PJ, van Leeuwen FE. Risk of morbidity and mortality from cardiovascular disease following radiotherapy for childhood cancer: a systematic review. Cancer Treat Rev. 2005;31(3):173–185. doi: 10.1016/j.ctrv.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Green DM, Grigoriev YA, Nan B, Takashima JR, Norkool PA, D'Angio GJ, et al. Congestive heart failure after treatment for Wilms' tumor: a report from the National Wilms' Tumor Study group. J Clin Oncol. 2001;19(7):1926–1934. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 31.Armenian SH, Sun CL, Kawashima T, Arora M, Leisenring W, Sklar CA, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS) Blood. 2011;118(5):1413–1420. doi: 10.1182/blood-2011-01-331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van 't Veer MB, Baaijens MH, de Boer JP, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 33.Temming P, Qureshi A, Hardt J, Leiper AD, Levitt G, Ancliff PJ, et al. Prevalence and predictors of anthracycline cardiotoxicity in children treated for acute myeloid leukaemia: retrospective cohort study in a single centre in the United Kingdom. Pediatric blood & cancer. 2011;56(4):625–630. doi: 10.1002/pbc.22908. [DOI] [PubMed] [Google Scholar]
- 34.van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane database of systematic reviews (Online) 2010;(5):CD005006. doi: 10.1002/14651858.CD005006.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane database of systematic reviews (Online) 2008;(2):CD003917. doi: 10.1002/14651858.CD003917.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. The lancet oncology. 2010;11(10):950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) Journal of the American College of Cardiology. 2003;42(5):954–970. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 38.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(12):2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 39.Janardhanan R, Beller GA. Radionuclide imaging in stage B heart failure. Heart failure clinics. 2012;8(2):191–206. doi: 10.1016/j.hfc.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Ylanen K, Poutanen T, Savikurki-Heikkila P, Rinta-Kiikka I, Eerola A, Vettenranta K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. Journal of the American College of Cardiology. 2013;61(14):1539–1547. doi: 10.1016/j.jacc.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30(23):2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braunwald E. Biomarkers in heart failure. The New England journal of medicine. 2008;358(20):2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 43.Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30(10):1042–1049. doi: 10.1200/JCO.2010.30.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. The New England journal of medicine. 2004;351(2):145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 45.Mavinkurve-Groothuis AM, Groot-Loonen J, Bellersen L, Pourier MS, Feuth T, Bokkerink JP, et al. Abnormal NT-pro-BNP levels in asymptomatic long-term survivors of childhood cancer treated with anthracyclines. Pediatric blood & cancer. 2009;52(5):631–636. doi: 10.1002/pbc.21913. [DOI] [PubMed] [Google Scholar]
- 46.Kismet E, Varan A, Ayabakan C, Alehan D, Portakal O, Buyukpamukcu M. Serum troponin T levels and echocardiographic evaluation in children treated with doxorubicin. Pediatric blood & cancer. 2004;42(3):220–224. doi: 10.1002/pbc.10368. [DOI] [PubMed] [Google Scholar]
- 47.Sherief LM, Kamal AG, Khalek EA, Kamal NM, Soliman AA, Esh AM. Biomarkers and early detection of late onset anthracycline-induced cardiotoxicity in children. Hematology. 2012;17(3):151–156. doi: 10.1179/102453312X13376952196412. [DOI] [PubMed] [Google Scholar]
- 48.Soker M, Kervancioglu M. Plasma concentrations of NT-pro-BNP and cardiac troponin-I in relation to doxorubicin-induced cardiomyopathy and cardiac function in childhood malignancy. Saudi medical journal. 2005;26(8):1197–1202. [PubMed] [Google Scholar]
- 49.Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008;130(5):688–695. doi: 10.1309/AJCPB66LRIIVMQDR. [DOI] [PubMed] [Google Scholar]
- 50.Heidenreich PA, Gubens MA, Fonarow GC, Konstam MA, Stevenson LW, Shekelle PG. Cost-effectiveness of screening with B-type natriuretic peptide to identify patients with reduced left ventricular ejection fraction. Journal of the American College of Cardiology. 2004;43(6):1019–1026. doi: 10.1016/j.jacc.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 51.Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PW, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. Jama. 2002;288(10):1252–1259. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 52.Murali S, Baldisseri MR. Peripartum cardiomyopathy. Critical care medicine. 2005;33(10 Suppl):S340–S346. doi: 10.1097/01.ccm.0000183500.47273.8e. [DOI] [PubMed] [Google Scholar]
- 53.Grewal J, Siu SC, Ross HJ, Mason J, Balint OH, Sermer M, et al. Pregnancy outcomes in women with dilated cardiomyopathy. Journal of the American College of Cardiology. 2009;55(1):45–52. doi: 10.1016/j.jacc.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 54.Stergiopoulos K, Shiang E, Bench T. Pregnancy in patients with pre-existing cardiomyopathies. Journal of the American College of Cardiology. 2011;58(4):337–350. doi: 10.1016/j.jacc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–778. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 56.Bar J, Davidi O, Goshen Y, Hod M, Yaniv I, Hirsch R. Pregnancy outcome in women treated with doxorubicin for childhood cancer. American journal of obstetrics and gynecology. 2003;189(3):853–857. doi: 10.1067/s0002-9378(03)00837-8. [DOI] [PubMed] [Google Scholar]
- 57.van Dalen EC, van der Pal HJ, van den Bos C, Kok WE, Caron HN, Kremer LC. Clinical heart failure during pregnancy and delivery in a cohort of female childhood cancer survivors treated with anthracyclines. Eur J Cancer. 2006;42(15):2549–2553. doi: 10.1016/j.ejca.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Silber JH, Cnaan A, Clark BJ, Paridon SM, Chin AJ, Rychik J, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22(5):820–828. doi: 10.1200/JCO.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 59.Rosenthal D, Chrisant MR, Edens E, Mahony L, Canter C, Colan S, et al. International Society for Heart and Lung Transplantation: Practice guidelines for management of heart failure in children. J Heart Lung Transplant. 2004;23(12):1313–1333. doi: 10.1016/j.healun.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 60.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10(10):933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 62.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 63.Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA, 3rd, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 64.De Caro E, Smeraldi A, Trocchio G, Calevo M, Hanau G, Pongiglione G. Subclinical cardiac dysfunction and exercise performance in childhood cancer survivors. Pediatric blood & cancer. 2011;56(1):122–126. doi: 10.1002/pbc.22606. [DOI] [PubMed] [Google Scholar]
- 65.Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31(22):2799–2805. doi: 10.1200/JCO.2012.47.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang TT, Ness KK. Exercise interventions in children with cancer: a review. International journal of pediatrics. 2011;2011:461512. doi: 10.1155/2011/461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maron BJ, Chaitman BR, Ackerman MJ, Bayes de Luna A, Corrado D, Crosson JE, et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation. 2004;109(22):2807–2816. doi: 10.1161/01.CIR.0000128363.85581.E1. [DOI] [PubMed] [Google Scholar]
- 68.Pelliccia A, Corrado D, Bjornstad HH, Panhuyzen-Goedkoop N, Urhausen A, Carre F, et al. Recommendations for participation in competitive sport and leisure-time physical activity in individuals with cardiomyopathies, myocarditis and pericarditis. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2006;13(6):876–885. doi: 10.1097/01.hjr.0000238393.96975.32. [DOI] [PubMed] [Google Scholar]
- 69.Gidding SS, Lichtenstein AH, Faith MS, Karpyn A, Mennella JA, Popkin B, et al. Implementing American Heart Association pediatric and adult nutrition guidelines: a scientific statement from the American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular Disease in the Young, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Council on Epidemiology and Prevention, and Council for High Blood Pressure Research. Circulation. 2009;119(8):1161–1175. doi: 10.1161/CIRCULATIONAHA.109.191856. [DOI] [PubMed] [Google Scholar]
- 70.Smith SC, Jr, Collins A, Ferrari R, Holmes DR, Jr, Logstrup S, McGhie DV, et al. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke) Journal of the American College of Cardiology. 2012;60(22):2343–2348. doi: 10.1016/j.jacc.2012.08.962. [DOI] [PubMed] [Google Scholar]
- 71.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122(25):e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 72.Meacham LR, Chow EJ, Ness KK, Kamdar KY, Chen Y, Yasui Y, et al. Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19(1):170–181. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oeffinger KC, Adams-Huet B, Victor RG, Church TS, Snell PG, Dunn AL, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27(22):3698–3704. doi: 10.1200/JCO.2008.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oeffinger KC, Nathan PC, Kremer LC. Challenges after curative treatment for childhood cancer and long-term follow up of survivors. Hematology/oncology clinics of North America. 2010;24(1):129–149. doi: 10.1016/j.hoc.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 76.Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ, Allen AM, et al. Radiation dose-volume effects in the heart. International journal of radiation oncology, biology, physics. 2010;76(3 Suppl):S77–S85. doi: 10.1016/j.ijrobp.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 77.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. Jama. 1993;270(16):1949–1955. [PubMed] [Google Scholar]
- 78.Colan SD, Shirali G, Margossian R, Gallagher D, Altmann K, Canter C, et al. The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy. J Am Soc Echocardiogr. 2012;25(8):842–854. e6. doi: 10.1016/j.echo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colan SD, Parness IA, Spevak PJ, Sanders SP. Developmental modulation of myocardial mechanics: age- and growth-related alterations in afterload and contractility. Journal of the American College of Cardiology. 1992;19(3):619–629. doi: 10.1016/s0735-1097(10)80282-7. [DOI] [PubMed] [Google Scholar]
- 80.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. The New England journal of medicine. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 81.Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113(24):2851–2860. doi: 10.1161/CIRCULATIONAHA.105.600437. [DOI] [PubMed] [Google Scholar]
- 82.Flather MD, Yusuf S, Kober L, Pfeffer M, Hall A, Murray G, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355(9215):1575–1581. doi: 10.1016/s0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- 83.Monsuez JJ. Detection and prevention of cardiac complications of cancer chemotherapy. Archives of cardiovascular diseases. 2012;105(11):593–604. doi: 10.1016/j.acvd.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 84.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. Journal of the American College of Cardiology. 2013;61(1):77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 85.Partington SL, Cheng S, Lima JA. Cardiac magnetic resonance imaging for stage B heart failure. Heart failure clinics. 2012;8(2):179–190. doi: 10.1016/j.hfc.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 86.Wong FL, Bhatia S, Landier W, Francisco L, Leisenring W, Hudson MM, et al. Cost-effectiveness of the children's oncology group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Annals of internal medicine. 2014;160(10):672–683. doi: 10.7326/M13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeh JM, Nohria A, Diller L. Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects. Annals of internal medicine. 2014;160(10):661–671. doi: 10.7326/M13-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Armenian SH, Ding Y, Mills G, Sun C, Venkataraman K, Wong FL, et al. Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation. British journal of haematology. 2013;163(2):205–213. doi: 10.1111/bjh.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Visscher H, Ross CJ, Rassekh SR, Barhdadi A, Dube MP, Al-Saloos H, et al. Pharmacogenomic Prediction of Anthracycline-Induced Cardiotoxicity in Children. J Clin Oncol. 2012;30(13):1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 90.van der Pal HJ, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van den Bos C, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Archives of internal medicine. 2010;170(14):1247–1255. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 91.Sorensen K, Levitt GA, Bull C, Dorup I, Sullivan ID. Late anthracycline cardiotoxicity after childhood cancer: a prospective longitudinal study. Cancer. 2003;97(8):1991–1998. doi: 10.1002/cncr.11274. [DOI] [PubMed] [Google Scholar]
- 92.van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane database of systematic reviews (Online) 2006;(4):CD005006. doi: 10.1002/14651858.CD005006.pub2. [DOI] [PubMed] [Google Scholar]
- 93.Brouwer CA, Postma A, Vonk JM, Zwart N, van den Berg MP, Bink-Boelkens MT, et al. Systolic and diastolic dysfunction in long-term adult survivors of childhood cancer. Eur J Cancer. 2011;47(16):2453–2462. doi: 10.1016/j.ejca.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 94.Abosoudah I, Greenberg ML, Ness KK, Benson L, Nathan PC. Echocardiographic surveillance for asymptomatic late-onset anthracycline cardiomyopathy in childhood cancer survivors. Pediatric blood & cancer. 2011;57(3):467–472. doi: 10.1002/pbc.22989. [DOI] [PubMed] [Google Scholar]
- 95.Uderzo C, Pillon M, Corti P, Tridello G, Tana F, Zintl F, et al. Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation: a prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone marrow transplantation. 2007;39(11):667–675. doi: 10.1038/sj.bmt.1705652. [DOI] [PubMed] [Google Scholar]
- 96.Lonnerholm G, Arvidson J, Andersson LG, Carlson K, Jonzon A, Sunnegardh J. Myocardial function after autologous bone marrow transplantation in children: a prospective long-term study. Acta Paediatr. 1999;88(2):186–192. doi: 10.1080/08035259950170376. [DOI] [PubMed] [Google Scholar]
- 97.Armenian SH, Sun CL, Vase T, Ness KK, Blum E, Francisco L, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120(23):4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Erbel R, Schweizer P, Krebs W, Meyer J, Effert S. Sensitivity and specificity of two-dimensional echocardiography in detection of impaired left ventricular function. European heart journal. 1984;5(6):477–489. doi: 10.1093/oxfordjournals.eurheartj.a061694. [DOI] [PubMed] [Google Scholar]
- 99.Habash-Bseiso DE, Rokey R, Berger CJ, Weier AW, Chyou PH. Accuracy of noninvasive ejection fraction measurement in a large community-based clinic. Clinical medicine & research. 2005;3(2):75–82. doi: 10.3121/cmr.3.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rovai D, Morales MA, Di Bella G, Prediletto R, De Nes M, Pingitore A, et al. Echocardiography and the clinical diagnosis of left ventricular dysfunction. Acta cardiologica. 2008;63(4):507–513. doi: 10.2143/AC.63.4.2033051. [DOI] [PubMed] [Google Scholar]
- 101.Postma A, Bink-Boelkens MT, Beaufort-Krol GC, Kengen RA, Elzenga NJ, Schasfoort-van Leeuwen MJ, et al. Late cardiotoxicity after treatment for a malignant bone tumor. Medical and pediatric oncology. 1996;26(4):230–237. doi: 10.1002/(SICI)1096-911X(199604)26:4<230::AID-MPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 102.Pihkala J, Saarinen UM, Lundstrom U, Salmo M, Virkola K, Virtanen K, et al. Effects of bone marrow transplantation on myocardial function in children. Bone marrow transplantation. 1994;13(2):149–155. [PubMed] [Google Scholar]
- 103.Krawczuk-Rybak M, Dakowicz L, Hryniewicz A, Maksymiuk A, Zelazowska-Rutkowska B, Wysocka J. Cardiac function in survivors of acute lymphoblastic leukaemia and Hodgkin's lymphoma. Journal of paediatrics and child health. 2011;47(7):455–459. doi: 10.1111/j.1440-1754.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 104.Hayakawa H, Komada Y, Hirayama M, Hori H, Ito M, Sakurai M. Plasma levels of natriuretic peptides in relation to doxorubicin-induced cardiotoxicity and cardiac function in children with cancer. Medical and pediatric oncology. 2001;37(1):4–9. doi: 10.1002/mpo.1155. [DOI] [PubMed] [Google Scholar]
- 105.Sieswerda E, van Dalen EC, Postma A, Cheuk DK, Caron HN, Kremer LC. Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer. Cochrane database of systematic reviews (Online) 2011;(9):CD008011. doi: 10.1002/14651858.CD008011.pub2. [DOI] [PubMed] [Google Scholar]
- 106.Exner DV, Dries DL, Waclawiw MA, Shelton B, Domanski MJ. Beta-adrenergic blocking agent use and mortality in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a post hoc analysis of the Studies of Left Ventricular Dysfunction. Journal of the American College of Cardiology. 1999;33(4):916–923. doi: 10.1016/s0735-1097(98)00675-5. [DOI] [PubMed] [Google Scholar]
- 107.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357(9266):1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 108.Vantrimpont P, Rouleau JL, Wun CC, Ciampi A, Klein M, Sussex B, et al. Additive beneficial effects of beta-blockers to angiotensin-converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) Study. SAVE Investigators. Journal of the American College of Cardiology. 1997;29(2):229–236. doi: 10.1016/s0735-1097(96)00489-5. [DOI] [PubMed] [Google Scholar]
- 109.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. Circulation. 2005;112(12):e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 110.Konstam MA, Neaton JD, Poole-Wilson PA, Pitt B, Segal R, Sharma D, et al. Comparison of losartan and captopril on heart failure-related outcomes and symptoms from the losartan heart failure survival study (ELITE II) American heart journal. 2005;150(1):123–131. doi: 10.1016/j.ahj.2004.10.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.