Abstract
Although angiotensin II (AngII) plays an important role in heart disease associated with pump dysfunction, its direct effects on cardiac pump function remain controversial. We found that, following AngII infusion, the developed pressure and +dP/dtmax in isolated Langendorff-perfused mouse hearts showed a complex temporal response, with a rapid transient decrease followed by an increase above baseline. Similar time-dependent changes in cell shortening and L-type Ca2+ currents were observed in isolated ventricular myocytes. Previous studies have established that AngII signaling involves phosphoinositide 3-kinases (PI3Ks). Dominant-negative inhibition of PI3Kα in the myocardium selectively eliminated the rapid negative inotropic action of AngII while the loss of PI3Kγ had no effect on the response to AngII. Consistent with a link between PI3Kα and PKC, PKC inhibition (with GF 109203X) reduced the negative inotropic effects of AngII by ~50%. Although both PI3Kα and PKC activities are associated with glycogen synthase kinase-3β (GSK3β) and NADPH oxidase, genetic ablation of either GSK3β or p47phox (an essential subunit of NOX2-NADPH oxidase activity) had no effect on AngII’s inotropic actions. Our results establish that AngII has complex temporal effects on contractility and L-type Ca2+ channels in normal mouse myocardium with the negative inotropic effects requiring PI3Kα and PKC activities.
Keywords: Cardiac contraction, Langendorff heart, Patch-clamp, Glycogen synthase kinase-3β, NADPH oxidase
Introduction
Angiotensin II (AngII) plays an important role in cardiovascular physiology and pathology. Circulating AngII levels and the activity of the local cardiac renin-angiotensin system (RAS) are increased in heart disease, including cardiac hypertropy1 and failure.2 Chronic AngII stimulation has been linked to cardiac remodeling (characterized by interstitial fibrosis, myocyte hypertrophy and death, and metabolism alterations, etc.),3, 4 which contributes to depressed mechanical function in heart disease. On the other hand, the acute actions of AngII on cardiac function remain unclear and are controversial with positive,5–8 negative9 or no10, 11 effects on cardiac contractility reported. Moreover, the signaling pathways mediating the inotropic effects of AngII are not fully clear. The positive inotropic effects of AngII have been previously linked to PKC,8 L-type Ca2+ currents (ICa,L),6 and β-Arrestin2,8 as well as secondary endothelin-1 release7 while negative inotropic effects have been associated with PKC9, 12 and p38 MAPK9 activities. AngII has also been shown to activate phosphoinositide 3-kinases (PI3Ks) in cardiac myocytes,13, 14 and vascular smooth muscle.15 Since the Class IA PI3K, PI3Kα, enhances cardiac contraction strength16 and ICa,L,17, 18 while the Class IB PI3K, PI3Kγ, decreases cardiac contractility, accelerates cyclic adenosine monophosphate degradation19, 20 and increases β-adrenergic receptor downregulation,21 we examined the involvement of PI3Kα and PI3Kγ in mediating the inotropic effects of AngII in mouse myocardium. Our results show that AngII has complex effects on mouse cardiac contractility and ICa,L and that PI3Kα, but not PI3Kγ, is required for the negative inotropic effects of AngII. These actions of AngII are independent of GSK3β or NOX2-NADPH oxidase activity.
Materials and Methods
Detailed methods are available in the online supplement (http://hyper.ahajournals.org).
All procedures were approved by the local animal care committee at the University of Toronto. C57BL/6 mice (male, 8–12 week) were obtained from Charles River Laboratories (Montreal, Canada). Mice lacking PI3Kα activity in the myocardium (DN–PI3Kα),22 and mice lacking PI3Kγ activity (PI3Kγ−/−)23 or NOX2-NADPH oxidase activity (p47phox−/−)24 have been described. To generate conditional cardiac-specific GSK3β knockout (GSK3β cKO) mice, GSK3βflox/flox mice25 were crossed with mice expressing tamoxifen-inducible α-myosin MerCreMer.26 α-myosin MerCreMer/GSK3βflox/flox mice (12-week) were treated with tamoxifen citrate (20 mg/kg/day for 4 days), which reduced cardiac GSK3β protein levels by >90%. A total of 90 mice were used in Langendorff studies. In Langendorff studies, hearts were perfused via the aorta with a modified Krebs solution, containing the following (in mmol/L): 118 NaCl, 23 NaHCO3, 3.2 KCl, 1.2 KH2PO4, 2.0 CaCl2, 1.2 MgSO4, 0.5 Na2-EDTA, 11 glucose, and 2 Na-pyruvate (free Ca2+=1.5 mmol/L), which was bubbled with 95% O2–5% CO2 (pH=7.40) and kept at 37 °C. A balloon was inserted into the left ventricle (LV) to record pressure. Single ventricular myocytes were isolated from mouse hearts with collagenase as previously described.17 In cell shortening study myocytes were field-stimulated at 1 Hz in Tyrode solution (containing 1.2 mmol/L Ca2+).27 ICa,L were recorded from myocytes with patch-clamp technique in amphotericin B-perforated configuration. Differences between two means were assessed using paired or unpaired Student’s t-tests. Differences among multiple means were assessed by one-way analysis of variance (ANOVA). A P value<0.05 was considered significant. Group data are expressed as mean±SEM.
Results
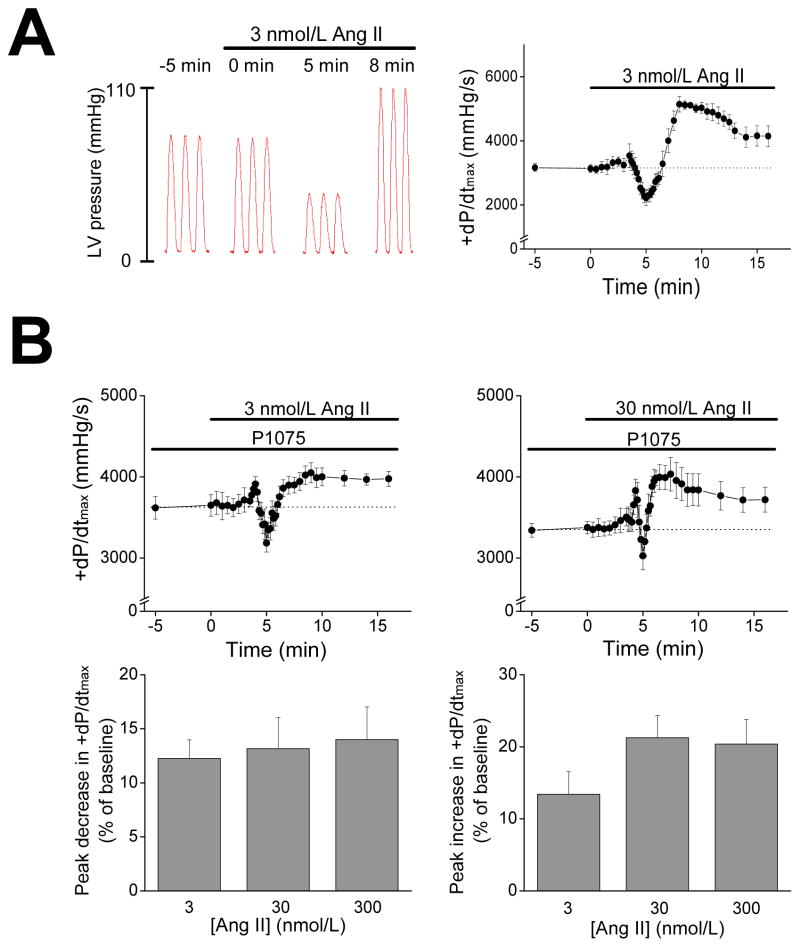
The effects of AngII on cardiac contractility were examined in isolated Langendorff-perfused mouse hearts treated with AngII. For these studies, hearts were initially equilibrated at a constant coronary perfusion pressure of 80 mmHg and ventricular end-diastolic pressures were set at ~5 mmHg (Online Supplement) to establish baseline function. Figure 1A shows typical left ventricular (LV) pressure traces recorded at the indicated times after AngII (3 nmol/L) infusion. AngII caused complex temporal changes in pressure development characterized by rapid reductions (p<0.01, n=4) of the peak rate of LV pressure development (+dP/dtmax) by 32.0±4.7% below baseline (from 3154±175 to 2206±215 mmHg/s) at ~5 min following AngII. After the rapid reduction, +dP/dtmax increased (p<0.01) and peaked at 69.8±4.5% above (p<0.01, n=4) baseline (i.e. 5336±121 mmHg/s) after ~8 min of infusion. The +dP/dtmax declined thereafter to a plateau above (p<0.05, n=4) baseline. Similar patterns of change (p<0.05, n=4) in both peak pressure (Ppeak) and the peak rate of LV pressure decline (−dP/dtmin) were also observed with AngII infusion. As expected from its vasoconstrictor action, AngII infusion caused a decrease of 46.9±4.0% (p<0.01, n=4) in coronary artery flow rate at ~5 min, which returned to baseline levels at ~8 min (Figure S1A).
Figure 1.
A. Representative left ventricle (LV) pressure traces (left) and +dP/dtmax (right, n=4) of mouse hearts during infusion of AngII (3 nmol/L). Hearts were perfused using the Langendorff method at a constant perfusion pressure. B. Top panels: +dP/dtmax time curse during AngII infusion at 3 nmol/L (left, n=5) and 30 nmol/L (right, n=4). Hearts were perfused at a constant coronary flow rate in the presence of a vasodilator (P1075, 100 nmol/L). Lower panels: Summary of peak early-phase decreases (left) and peak late-phase increases (right) in +dP/dtmax of hearts treated with 3, 30, or 300 (n=4) nmol/L AngII.
It is conceivable that the negative inotropic effects of AngII were mediated by changes in coronary vascular resistance possibly leading to metabolic changes or perfusion-related changes in contractility (i.e. “Gregg’s Phenomenon”).28 However, when hearts were perfused at a constant coronary flow rate to achieve a perfusion pressure of ~80 mmHg at baseline, AngII (3 nmol/L) caused early decrease (12.6±2.5%) followed by a late increase (18.9±2.3%) in +dP/dtmax (p<0.01, n=5) over baseline (Figure S1B). Consistent with its vasoconstrictor action, AngII also caused time-dependent increases (p<0.01, n=5) in perfusion pressure when perfusion rate was fixed (Figure S1B). Because vascular effects of AngII could modulate AngII’s inotropic actions, hearts were pretreated with P1075, a vasodilator that opens plasmalemmal KATP channels preferentially (by ~20-fold) in vascular smooth muscle compared to myocardium.29 As expected, pretreatment with P1075 (100 nmol/L), at fixed coronary flows, decreased (p<0.01, n=4) the perfusion pressure from 79.4±1.4 to 64.2±4.5 mmHg, and eliminated the AngII’s effects on coronary perfusion pressure (Figure S1C). Consistent with previous reports showing P1075 dose-dependently affects cardiac function,30, 31 P1075 slightly reduced contractility (i.e. reduction of 9.4±1.5%, p<0.01, n=4), probably as a result of action potential abbreviation.32 More important, P1075 did not influence the actions of AngII. Specifically, AngII (3 nmol/L) infusion in the presence of P1075 still induced (p<0.01, n=5) a rapid decline of 12.3±1.7% in +dP/dtmax relative to baseline followed by an increase that peaked at 13.4±3.1% above (p<0.01) baseline at ~10 min post-AngII infusion and, thereafter (at 16 min post-infusion), remained above (p<0.05) baseline (Figure 1B). In separate experiments using the same conditions, we found that the +dP/dtmax remained elevated (p<0.01, n=6) above baseline for 30 min after AngII treatment. Similar temporal changes in Ppeak (not shown) as well as −dP/dtmin and the time constant (Tau) for pressure relaxation (Figure S2) were also observed. In all remaining studies, hearts were pretreated with P1075 before AngII infusion in order to prevent the effects of AngII on coronary artery constriction.
To further characterize the inotropic actions of AngII, we compared the responses of hearts to different concentrations (3, 30 and 300 nmol/L) of AngII. As shown in Figure 1B and Table S1, the magnitude of changes in +dP/dtmax showed little dose-dependence and a near maximal response was obtained at 30 nmol/L, consistent with previous studies.7 It is notable that +dP/dtmax showed a small, but highly reproducible, increase before the rapid decline phase, while the late-phase increases in +dP/dtmax occurred earlier (p<0.05, n=5) with higher AngII levels. These findings suggest that the positive and negative inotropic actions of AngII involve separate processes with different kinetics. Similar temporal changes were also observed in −dP/dtmin during AngII infusion, as summarized in Table S1.
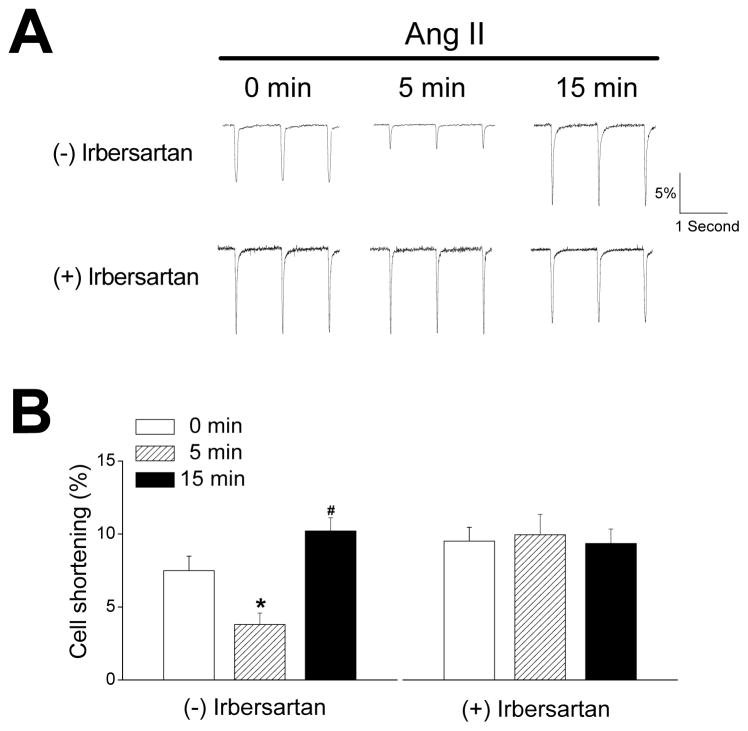
Although the myocardial effects of AngII could be mediated by AngII receptors in non-myocyte cells of the heart, AngII also produced biphasic alterations in cell shortening (CS) amplitudes (Figure 2). Specifically, the percent CS (%CS) was decreased (p<0.01, n=13) by ~50% at 5 min after AngII treatment, followed by a ~40% increase (p<0.05) above control values at 15 min. AngII had no effect (p=0.98, n=14) on %CS in the presence of the type 1 AngII receptor (AT1R) blocker irbersartan (10 μmol/L), supporting the conclusion that AT1Rs in cardiomyocytes mediate AngII’s contractile effects (Figure 2).
Figure 2.
A. Representative cell shortening traces of ventricular myocytes without (0 min) and with (5 and 15 min) AngII treatment (30 nmol/L), in the absence (top panels) and presence (bottom panels) of an AT1 receptor inhibitor (irbersartan, 10 μmol/L). The downward deflections from baselines indicate cell length decrease (shortening). Cell shortening was expressed as a percentage of the baseline diastolic length. Recordings were done at 36 °C and myocytes were stimulated at 1Hz. B. Summary of AngII on cell shortening (n=13–14) in the absence (left) and presence (right) of irbersartan. *p<0.01, #p<0.05 vs. con (0 min).
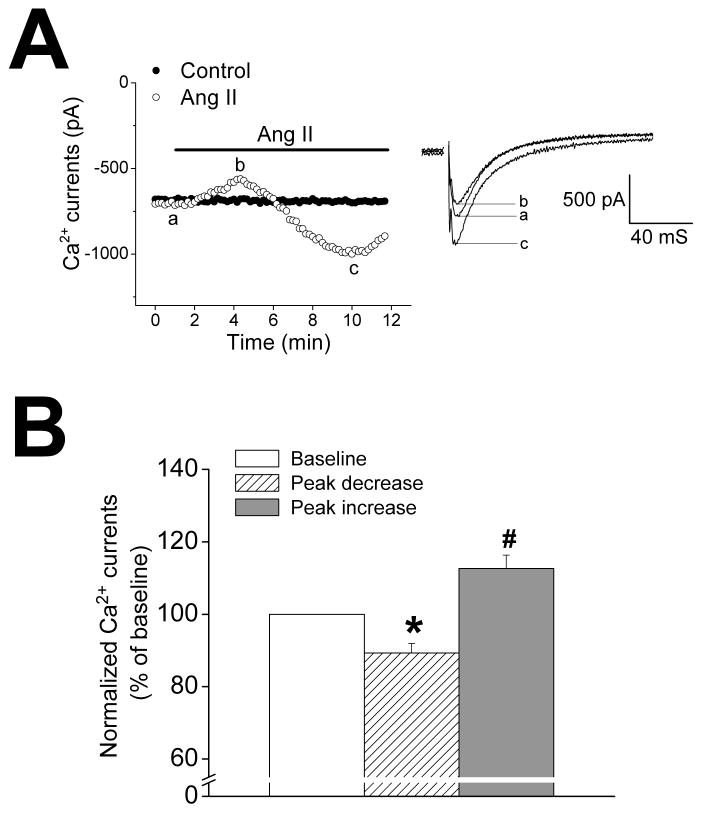
Previous studies have reported that AngII can increase cardiac L-type Ca2+ currents (ICa,L)33, 34 which are key elements in cardiac excitation-contraction coupling and contraction. To examine if ICa,L are affected by AngII in mouse myocardium, we recorded ICa,L using whole-cell patch-clamp technique with the amphotericin B-perforated configuration which appears to be necessary to identify the effects of AngII on ICa,L.33, 34 As shown in Figure 3, AngII perfusion of isolated ventricular myocytes caused (p<0.05, n=7) rapid transient ~10% decreases in ICa,L followed by sustained ~13% increases over baseline values, suggesting that both the negative and positive inotropic effects of AngII are, at least partially, mediated by alterations in ICa,L.
Figure 3.
A. Time course (left) and representative traces (right) of L-type Ca2+ currents recorded at 0 mV from ventricular myocytes with AngII (30 nmol/L) perfusion (open circles) or without AngII (filled circles). Capacitance currents in right panel were removed for clarity. Currents were recorded at 22 °C with patch-clamp technique using amphotericin B-perforated configuration. B. Summary of AngII-induced changes in amplitude of L-type Ca2+ currents recorded at 0 mV (n=7). *p<0.05, #p<0.01 vs. baseline currents.
We next attempted to determine the signaling pathways involved in the biphasic effects of AngII on myocardial contractility. It has been shown that AngII activates G-protein coupled receptors, which can signal through PI3Ks.15, 35 Since PI3Kγ is Gβγ-dependent36 and regulates cardiac contractility,19, 37 we initially examined the effects of AngII on mouse hearts lacking PI3Kγ (PI3Kγ−/−). As expected,37, 38 Figure S3A shows that baseline +dP/dtmax of PI3Kγ−/− hearts was ~30% higher (p=0.01, n=5) than wild-type littermate hearts. However, similar to wild-type hearts, AngII (30 nmol/L) infusion into PI3Kγ−/− hearts induced (p<0.01, n=5) an early-phase 13.1±3.4% reduction in +dP/dtmax followed by a late-phase increase of 27.3±6.5% over baseline levels (Figure S3) with similar changes in −dP/dtmin (not shown). The relative changes in dP/dt induced by AngII in PI3Kγ−/− hearts were not different (p>0.15, n=5) from those observed in wild-type littermate hearts (Figure S3). Thus, PI3Kγ does not mediate the actions of AngII on mouse myocardial contractility.
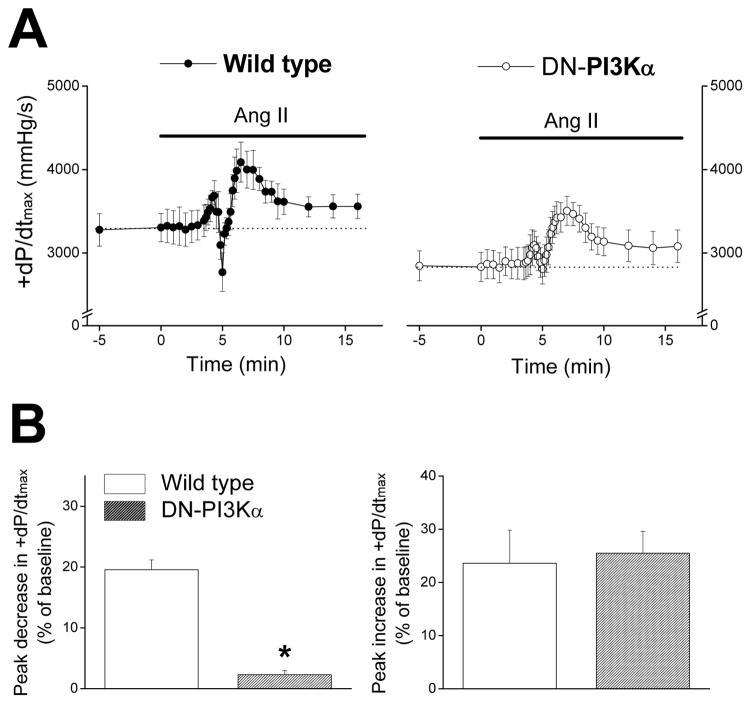
Because AT1Rs are coupled to Gq protein which can regulate PI3Kα,39 we tested the actions of AngII in hearts with cardiac-specific dominant-negative inhibition of PI3Kα (DN-PI3Kα). DN-PI3Kα hearts had slightly lower baseline +dP/dtmax compared to wild-type hearts (Figure 4A), consistent with a recent study.18 More important, AngII infusion resulted in a modest and insignificant 2.3±0.7% decline in +dP/dtmax in DN-PI3Kα hearts (Figure 4), which was far less (p<0.01, n=7) than the 19.6±1.6% decline seen in wild-type littermate hearts. On the other hand, the late-phase increase in +dP/dtmax was unaffected (p=0.80, n=7) by PI3Kα inhibition (Figure 4). AngII infusion of DN-PI3Kα hearts also caused much smaller (p<0.01, n=7) decreases in −dP/dtmin (5.7±1.1%) compared to wild-type littermates (23.8±1.6% decrease), without causing notable differences (p=0.40, n=7) in the late-phase increases of −dP/dtmin (data not shown). These data demonstrate that PI3Kα plays an important role in the early-phase negative inotropic and lusitropic effects in mouse hearts.
Figure 4.
A. Time courses of +dP/dtmax during AngII (30 nmol/L) infusion in wild-type hearts (left, n=5) and DN-PI3Kα hearts (right, n=7). Mouse hearts were perfused at a constant flow rate in the presence of a vasodilator (P1075, 100 nmol/L). B. Summary of peak decreases (left) and peak increases (right) of +dP/dtmax during AngII infusion in wild-type hearts (blank bars) and DN-PI3Kα hearts (filled bars). * p<0.01 vs. wild-type.
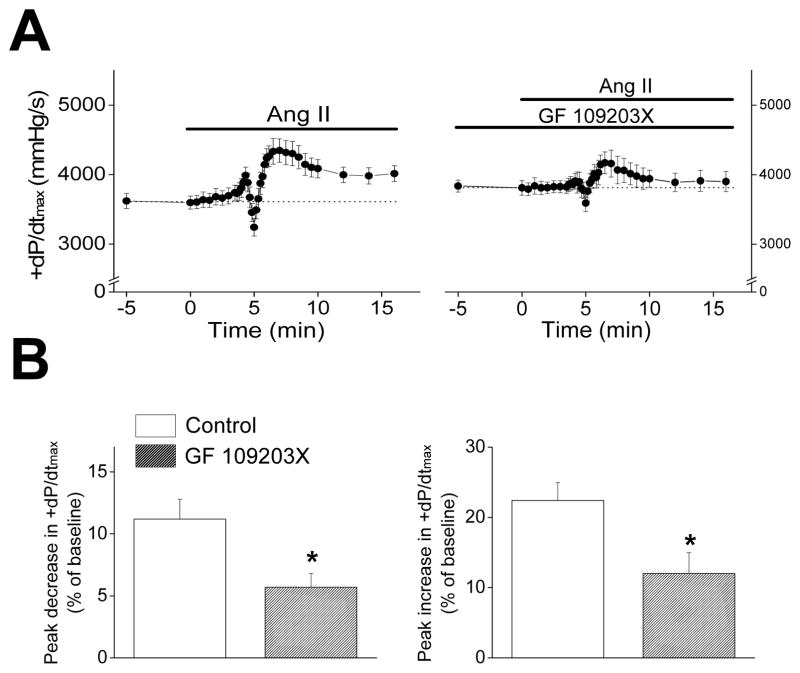
PKC activity has been recently linked to PI3Kα.40 In addition, PKC has been shown to be involved in either the negative9, 12 or positive8 inotropic effects of AngII. Despite these observations, AngII (30 nmol/L) infusion still caused biphasic changes (p<0.01, n=7) in +dP/dtmax in the presence of GF 109203X (1 μmol/L, IC50=32 nmol/L for PKC inhibition), with a rapid transient 5.7±1.1% decline followed by a 12.0±3.0% rise above baseline (Figure 5). However, PKC inhibition reduced (p<0.05, n=7) both the early-phase decreases and the late-phase increases in +dP/dtmax by ~50%, compared to changes caused by AngII in the absence of PKC inhibition (Figure 5). PKC inhibition also reduced (p<0.01, n=7) the early-phase decrease in −dP/dtmin by ~50% without affecting (p=0.91, n=7) the late-phase increase in −dP/dtmin induced by AngII (not shown). These results suggest that PKC plays a role in both the negative and positive inotropic effects of AngII.
Figure 5.
A. Time courses of +dP/dtmax of wild-type C57BL/6 mouse hearts during AngII (30 nmol/L) infusion in the absence (left, n=10) and presence (right, n=7) of a PKC inhibitor (GF 109203X, 1 μmol/L). Hearts were perfused at a constant flow rate in the presence of a vasodilator (P1075, 100 nmol/L). B. Summary of peak decreases (left) and peak increases (right) of +dP/dtmax during AngII infusion in the absence (blank bars) or presence (filled bars) of GF 109203X. *p<0.05, vs. control.
Both PKC41 and PI3Ks42 (particularly PI3Kα)43 have been shown to regulate glycogen synthase kinase-3β (GSK3β), which plays an important role in heart pump function.44 However, AngII infusion into hearts with conditional knockout of GSK3β (GSK3β cKO) induced biphasic changes (p<0.05, n=5) in +dP/dtmax (Figure S4A), which were not different (p>0.19, n=5) from changes induced by AngII in wild-type littermate hearts. These data show that GSK3β does not play a role in AngII’s inotropic effects in mouse hearts.
PKC can be activated by reactive oxygen species (ROS).45 The p47phox-dependent NADPH oxidase (NOX2) is a key enzyme for cardiac generation of ROS46 and can be activated by PI3Ks.47 We, therefore, tested whether NOX2 activity is required for the inotropic effects of AngII by using mice lacking p47phox (p47phox−/−). AngII infusion into p47phox−/− hearts induced biphasic changes (p<0.05, n=5) in +dP/dtmax (Figure S4B), which were not different (p>0.34, n=5) from changes induced by AngII in wild-type littermate hearts. These data establish that p47phox-dependent NADPH oxidase (NOX2) is not required for AngII’s inotropic effects in mouse hearts.
Discussion
Despite AngII being an important regulator of the cardiovascular system, its direct effects on cardiac contractility are unclear with positive,5–8, 48 negative,9, 12, 49 and no10, 11 effects being reported. Our study shows that AngII produces both negative and positive effects on contractility in mouse hearts. While the transient negative inotropic actions of AngII in our studies at constant perfusion pressure could arise from compromised myocardial metabolism resulting from reductions in coronary flow, secondary to vasoconstriction, decreased contractility was also observed when coronary flow was kept constant in the absence or presence of P1075 to dilate the coronary arteries. These findings establish that the contractile effects of AngII on the myocardium are not mediated indirectly by actions of AngII on coronary blood vessels. Consistent with this conclusion, AngII treatment of cardiomyocytes induced a transient impairment of cell shortening amplitude which was followed by a brisk increase in single cell contractility, mimicking the pattern seen in Langendorff hearts. These inotropic effects of AngII were eliminated by AT1R-specific blockade in single cardiomyocytes but were unaffected by pre-treatment with β-adrenergic receptor blockers (propranolol) in isolated hearts (not shown), establishing that enhanced local norepinephrine release from autonomous nerve terminals50 by AngII or receptor cross-talk between AT1R and β-adrenergic receptor49 does not contribute to the actions of AngII.
Taken together, our studies show that AngII produces complex contractile responses by direct actions on myocardial AT1Rs. This conclusion is consistent with the observation that AngII altered ICa,L in ventricular myocytes, as reported previously,33, 34 with a time course that mirrored precisely the changes in heart contractility. The potential mechanistic link between cardiac contractility and ICa,L changes is further supported by our results in DN-PI3Kα hearts as discussed further below. However, it is conceivable that AngII also affects other end-effectors involved in Ca2+ regulation. Support for this possibility is provided by the indices of ventricular relaxation (−dP/dtmin and the time constant for pressure relaxation, Tau, Figure S2B) which also showed biphasic alterations after AngII treatment, consistent with possible changes in sarcoplasmic reticulum Ca2+ ATPase activity or myofilament Ca2+ sensitivity. However, the interpretation of these studies is complicated by complex inter-dependence of the many processes involved in Ca2+ cycling and contractile protein activation/relaxation. Clearly, additional studies will be required to discern the potential role of other functional proteins and processes in transducing the actions of AngII.
Regardless of the functional mechanisms involved in mediating contractility changes induced by AngII, we found that the negative inotropic actions of AngII were largely abolished in DN-PI3Kα hearts, but not in PI3Kγ−/− hearts. The involvement of PI3Kα, but not PI3Kγ (which is Gβγ-dependent36 and a negative regulator of cardiac contractility19, 37), is somewhat surprising since AngII signals through G-protein coupled receptors. These findings, together with observations that genetic inhibition of GSK3β or NADPH oxidase did not affect the inotropic effects of acute AngII stimulation, highlight the divergent mechanisms required for the acute versus long-term regulations of contractility by AngII.4, 19, 37, 44 Regardless, it is not clear whether PI3Kα activity is increased or decreased by elevated AngII levels. It was previously concluded that Gαq-protein, which is linked to AT1Rs, inhibits PI3Kα.39, 51 Thus, since PI3Kα positively regulates cardiac ICa,L17 and contractility,18 our results are consistent with the conclusion that AngII mediates its rapid negative inotropic actions on hearts by inhibiting PI3Kα leading to decreases in cardiac ICa,L. This is supported by the rapid transient reduction in ICa,L after AngII perfusion in our studies. Involvement of ICa,L in the PI3Kα-dependent negative contractility effects of AngII is also consistent with our observation that pre-treatment of heart with a PKC inhibitor abolished a large fraction of the reductions in cardiac contractility induced by AngII because PKC activation can inhibit cardiac ICa,L.52
In addition to interfering with AngII-mediated impairment of contractility, PKC inhibition also blocked ~50% of the positive inotropic actions of AngII. This dual action of PKC is consistent with previous studies showing that PKC activation can have positive53 or negative54 inotropic effects on myocardium. Moreover, since loss of the α isoform of PKC (PKCα) in myocardium enhances contractility and protects against heart failure,55 our data support the possibility that the rapid negative inotropic actions of AngII involve PI3Kα-dependent PKCα activation, leading to ICa,L reductions. On the other hand, global PKC inhibition reduces the positive inotropic actions of AngII, suggesting that AngII may enhance contraction by activating other PKC isoforms (besides PKCα) leading to increased ICa,L as suggested previously.34, 48 This suggestion is consistent with studies showing that the positive effects of AngII on cardiac ICa,L are only observed in perforated-patch voltage-clamp recordings33, 34 when intracellular Ca2+ levels is not buffered thereby allowing activation of Ca2+-dependent isoforms of PKC. PKC activation by AngII could also activate Na+/H+ exchanger56 leading to elevations in contractility via elevated Na+ loading or increases in intracellular pH. Clearly additional studies are required to determine the cellular mechanism whereby AngII alters cardiac contractility.
Perspectives
In contrast to previous studies, our studies establish that AngII activation of AT1Rs leads to complex temporal increases and decreases in both ICa,L and contractility in “healthy” mouse myocardium with the decreases requiring PI3Kα and PKC. The relevance of our studies to larger mammals including humans, wherein elevations in AngII are linked to decreased contractility in advanced heart disease,2 is unclear. Nevertheless, it is conceivable that the PI3Kα- and PKC-dependent reductions in ICa,L and contractility, induced by AngII, may contribute to the impaired function in heart disease. Moreover, these and other potential mechanisms for AngII’s effects on heart function in mice might also be relevant to humans, even though the relative contribution of various downstream factors and signaling pathways may be modified in large mammals. Further studies will be required to assess whether targeting pathways mediating the negative inotropic actions of AngII is a valid strategy for treating patients with heart disease.
Supplementary Material
Acknowledgments
We thank Drs. Steffen-Sebastian Bolz and Rudolf Schubert for assistance. We dedicate this study in the memory of Dr. Michael E. Ward (deceased November 2009).
Source of Funding
This study was funded by a research grant from Canadian Institutes of Health Research (79460) to PHB. PHB holds a Career Investigator Award from the Heart and Stroke Foundation (HSF) of Ontario. WL received a Doctoral Research Award from HSF of Canada.
Footnotes
Disclosures
None.
References
- 1.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 2.Serneri GGN, Boddi M, Cecioni I, Vanni S, Coppo M, Papa ML, Bandinelli B, Bertolozzi I, Polidori G, Toscano T, Maccherini M, Modesti PA. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res. 2001;88:961–968. doi: 10.1161/hh0901.089882. [DOI] [PubMed] [Google Scholar]
- 3.Sadoshima J, Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 4.Gusev K, Domenighetti AA, Delbridge LMD, Pedrazzini T, Niggli E, Egger M. Angiotensin II-mediated adaptive and maladaptive remodeling of cardiomyocyte excitation-contraction coupling. Circ Res. 2009;105:42–50. doi: 10.1161/CIRCRESAHA.108.189779. [DOI] [PubMed] [Google Scholar]
- 5.Moravec C, Schluchter M, Paranandi L, Czerska B, Stewart R, Rosenkranz E, Bond M. Inotropic effects of angiotensin II on human cardiac muscle in vitro. Circulation. 1990;82:1973–1984. doi: 10.1161/01.cir.82.6.1973. [DOI] [PubMed] [Google Scholar]
- 6.Petroff MGV, Aiello EA, Palomeque J, Salas MA, Mattiazzi A. Subcellular mechanisms of the positive inotropic effect of angiotensin II in cat myocardium. J Physiol. 2000;529:189–203. doi: 10.1111/j.1469-7793.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cingolani HE, Villa-Abrille MC, Cornelli M, Nolly A, Ennis IL, Garciarena C, Suburo AM, Torbidoni V, Correa MV, Camilionde Hurtado MC, Aiello EA. The positive inotropic effect of angiotensin II: role of endothelin-1 and reactive oxygen species. Hypertension. 2006;47:727–734. doi: 10.1161/01.HYP.0000208302.62399.68. [DOI] [PubMed] [Google Scholar]
- 8.Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. β-Arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci U S A. 2006;103:16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palomeque J, Sapia L, Hajjar RJ, Mattiazzi A, Vila Petroff M. Angiotensin II-induced negative inotropy in rat ventricular myocytes: role of reactive oxygen species and p38 MAPK. Am J Physiol Heart Circ Physiol. 2006;290:H96–106. doi: 10.1152/ajpheart.00324.2005. [DOI] [PubMed] [Google Scholar]
- 10.Lefroy DC, Crake T, Del Monte F, Vescovo G, Dalla Libera L, Harding S, Poole-Wilson PA. Angiotensin II and contraction of isolated myocytes from human, guinea pig, and infarcted rat hearts. Am J Physiol Heart Circ Physiol. 1996;270:H2060–2069. doi: 10.1152/ajpheart.1996.270.6.H2060. [DOI] [PubMed] [Google Scholar]
- 11.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RAS, Walther T, Touyz RM, Reudelhuber TL. Angiotensin(1–7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008;103:1319–1326. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai K, Norota I, Tanaka H, Kubota I, Tomoike H, Endo M. Negative inotropic effects of angiotensin II, endothelin-1 and phenylephrine in indo-1 loaded adult mouse ventricular myocytes. Life Sci. 2002;70:1173–1184. doi: 10.1016/s0024-3205(01)01497-7. [DOI] [PubMed] [Google Scholar]
- 13.Wenzel S, Abdallah Y, Helmig S, Schafer C, Piper HM, Schluter K-D. Contribution of PI3-kinase isoforms to angiotensin II- and α-adrenoceptor-mediated signalling pathways in cardiomyocytes. Cardiovasc Res. 2006;71:352–362. doi: 10.1016/j.cardiores.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Rabkin SW, Goutsouliak V, Kong JY. Angiotensin II induces activation of phosphatidylinositol 3-kinase in cardiomyocytes. J Hypertens. 1997;15:891–899. doi: 10.1097/00004872-199715080-00014. [DOI] [PubMed] [Google Scholar]
- 15.Quignard J-F, Mironneau J, Carricaburu V, Fournier B, Babich A, Nurnberg B, Mironneau C, Macrez N. Phosphoinositide 3-kinase γ mediates angiotensin II-induced stimulation of L-type calcium channels in vascular myocytes. J Biol Chem. 2001;276:32545–32551. doi: 10.1074/jbc.M102582200. [DOI] [PubMed] [Google Scholar]
- 16.Yano N, Tseng A, Zhao TC, Robbins J, Padbury JF, Tseng Y-T. Temporally controlled overexpression of cardiac-specific PI3Kα induces enhanced myocardial contractility--a new transgenic model. Am J Physiol Heart Circ Physiol. 2008;295:H1690–1694. doi: 10.1152/ajpheart.00531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun H, Kerfant B-G, Zhao D, Trivieri MG, Oudit GY, Penninger JM, Backx PH. Insulin-like growth factor-1 and PTEN deletion enhance cardiac L-type Ca2+ currents via increased PI3Kα/PKB signaling. Circ Res. 2006;98:1390–1397. doi: 10.1161/01.RES.0000223321.34482.8c. [DOI] [PubMed] [Google Scholar]
- 18.Lu Z, Jiang Y-P, Wang W, Xu X-H, Mathias RT, Entcheva E, Ballou LM, Cohen IS, Lin RZ. Loss of cardiac phosphoinositide 3-kinase p110α results in contractile dysfunction. Circulation. 2009;120:318–325. doi: 10.1161/CIRCULATIONAHA.109.873380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerfant B, Rose R, Sun H, Backx P. Phosphoinositide 3-kinase γ regulates cardiac contractility by locally controlling cyclic adenosine monophosphate levels. Trends Cardiovasc Med. 2006;16:250–256. doi: 10.1016/j.tcm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Kerfant B-G, Zhao D, Lorenzen-Schmidt I, Wilson LS, Cai S, Chen SRW, Maurice DH, Backx PH. PI3Kγ is required for PDE4, not PDE3, activity in subcellular microdomains containing the sarcoplasmic reticular calcium ATPase in cardiomyocytes. Circ Res. 2007;101:400–408. doi: 10.1161/CIRCRESAHA.107.156422. [DOI] [PubMed] [Google Scholar]
- 21.Nienaber JJ, Tachibana H, Naga Prasad SV, Esposito G, Wu D, Mao L, Rockman HA. Inhibition of receptor-localized PI3K preserves cardiac β-adrenergic receptor function and ameliorates pressure overload heart failure. J Clin Invest. 2003;112:1067–1079. doi: 10.1172/JCI18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shioi T, Kang P, Douglas P, Hampe J, Yballe C, Lawitts J, Cantley L, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki T, Irie-Sasaki J, Horie Y, Bachmaier K, Fata J, Li M, Suzuki A, Bouchard D, Ho A, Redston M, Gallinger S, Khokha R, Mak T, Hawkins P, Stephens L, Scherer S, Tsao M, Penninger J. Colorectal carcinomas in mice lacking the catalytic subunit of PI(3)Kγ. Nature. 2000;406:897–902. doi: 10.1038/35022585. [DOI] [PubMed] [Google Scholar]
- 24.Tkalcevic J, Novelli M, Phylactides M, Iredale JP, Segal AW, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–210. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- 25.Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR. Tissue-specific role of glycogen synthase kinase 3β in glucose homeostasis and insulin action. Mol Cell Biol. 2008;28:6314–6328. doi: 10.1128/MCB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, Oudit GY, Ramirez RJ, Costantini D, Backx PH. The phosphoinositide 3-kinase inhibitor LY294002 enhances cardiac myocyte contractility via a direct inhibition of Ik,slow currents. Cardiovasc Res. 2004;62:509–520. doi: 10.1016/j.cardiores.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Gregg DE. Effect of coronary perfusion pressure or coronary flow on oxygen usage of the myocardium. Circ Res. 1963;13:497–500. doi: 10.1161/01.res.13.6.497. [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Sasaki N, Seharaseyon J, O’Rourke B, Marban E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal KATP channels in ischemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- 30.Sargent CA, Sleph PG, Dzwonczyk S, Normandin D, Antonaccio MJ, Grover GJ. Cardioprotective effects of the cyanoguanidine potassium channel opener P-1075. J Cardiovasc Pharmacol. 1993;22:564–570. doi: 10.1097/00005344-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Jilkina O, Kuzio B, Grover GJ, Kupriyanov VV. Effects of KATP channel openers, P-1075, pinacidil, and diazoxide, on energetics and contractile function in isolated rat hearts. J Mol Cell Cardiol. 2002;34:427–440. doi: 10.1006/jmcc.2001.1524. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Tsai TD, Lee KS. A specific activator of the ATP-inhibited K+ channels in guinea pig ventricular cells. J Pharmacol Exp Ther. 1993;266:978–984. [PubMed] [Google Scholar]
- 33.Ichiyanagi O, Ishii K, Endoh M. Angiotensin II increases L-type Ca2+ current in gramicidin D-perforated adult rabbit ventricular myocytes: comparison with conventional patch-clamp method. Pflugers Arch. 2002;444:107–116. doi: 10.1007/s00424-002-0808-y. [DOI] [PubMed] [Google Scholar]
- 34.Aiello EA, Cingolani HE. Angiotensin II stimulates cardiac L-type Ca2+ current by a Ca2+- and protein kinase C-dependent mechanism. Am J Physiol Heart Circ Physiol. 2001;280:H1528–1536. doi: 10.1152/ajpheart.2001.280.4.H1528. [DOI] [PubMed] [Google Scholar]
- 35.Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, Tsushima RG, Scholey JW, Khokha R, Penninger JM. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75:29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, Schultz G, Nurnberg B. Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crackower M, Oudit G, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng H, Rybin V, Lembo G, Fratta L, Oliveira-dos-Santos A, Benovic J, Kahn C, Izumo S, Steinberg S, Wymann M, Backx P, Penninger J. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 38.Kerfant B-G, Gidrewicz D, Sun H, Oudit GY, Penninger JM, Backx PH. Cardiac sarcoplasmic reticulum calcium release and load are enhanced by subcellular cAMP elevations in PI3Kγ-deficient mice. Circ Res. 2005;96:1079–1086. doi: 10.1161/01.RES.0000168066.06333.df. [DOI] [PubMed] [Google Scholar]
- 39.Ballou LM, Lin H-Y, Fan G, Jiang Y-P, Lin RZ. Activated Gαq inhibits p110α phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2003;278:23472–23479. doi: 10.1074/jbc.M212232200. [DOI] [PubMed] [Google Scholar]
- 40.Rigor DL, Bodyak N, Bae S, Choi JH, Zhang L, Ter-Ovanesyan D, He Z, McMullen JR, Shioi T, Izumo S, King GL, Kang PM. Phosphoinositide 3-kinase Akt signaling pathway interacts with protein kinase C β2 in the regulation of physiologic developmental hypertrophy and heart function. Am J Physiol Heart Circ Physiol. 2009;296:H566–572. doi: 10.1152/ajpheart.00562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang X, Yu S, Tanyi JL, Lu Y, Woodgett JR, Mills GB. Convergence of multiple signaling cascades at glycogen synthase kinase-3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol Cell Biol. 2002;22:2099–2110. doi: 10.1128/MCB.22.7.2099-2110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3β during preconditioning through a phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 43.Braz JC, Gill RM, Corbly AK, Jones BD, Jin N, Vlahos CJ, Wu Q, Shen W. Selective activation of PI3Kα/Akt/GSK-3β signalling and cardiac compensatory hypertrophy during recovery from heart failure. Eur J Heart Fail. 2009;11:739–748. doi: 10.1093/eurjhf/hfp094. [DOI] [PubMed] [Google Scholar]
- 44.Michael A, Haq S, Chen X, Hsich E, Cui L, Walters B, Shao Z, Bhattacharya K, Kilter H, Huggins G, Andreucci M, Periasamy M, Solomon RN, Liao R, Patten R, Molkentin JD, Force T. Glycogen synthase kinase-3β regulates growth, calcium homeostasis, and diastolic function in the heart. J Biol Chem. 2004;279:21383–21393. doi: 10.1074/jbc.M401413200. [DOI] [PubMed] [Google Scholar]
- 45.Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 47.Yamamori T, Inanami O, Nagahata H, Kuwabara M. Phosphoinositide 3-kinase regulates the phosphorylation of NADPH oxidase component p47phox by controlling cPKC/PKC δ but not Akt. Biochem Biophys Res Commun. 2004;316:720–730. doi: 10.1016/j.bbrc.2004.02.108. [DOI] [PubMed] [Google Scholar]
- 48.Salas M, Vila-Petroff M, Palomeque J, Aiello E, Mattiazzi A. Positive inotropic and negative lusitropic effect of angiotensin II: intracellular mechanisms and second messengers. J Mol Cell Cardiol. 2001;33:1957–1971. doi: 10.1006/jmcc.2001.1460. [DOI] [PubMed] [Google Scholar]
- 49.Meissner A, Min J, Simon R. Effects of angiotensin II on inotropy and intracellular Ca2+ handling in normal and hypertrophied rat myocardium. J Mol Cell Cardiol. 1998;30:2507–2518. doi: 10.1006/jmcc.1998.0813. [DOI] [PubMed] [Google Scholar]
- 50.Schumann H, Starke K, Werner U. Interactions of inhibitors of noradrenaline uptake and angiotensin on the sympathetic nerves of the isolated rabbit heart. Br J Pharmacol. 1970;39:390–397. doi: 10.1111/j.1476-5381.1970.tb12902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Z, Jiang Y-P, Ballou LM, Cohen IS, Lin RZ. Gαq inhibits cardiac L-type Ca2+ channels through phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:40347–40354. doi: 10.1074/jbc.M508441200. [DOI] [PubMed] [Google Scholar]
- 52.McHugh D, Sharp EM, Scheuer T, Catterall WA. Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the N-terminal domain. Proc Natl Acad Sci U S A. 2000;97:12334–12338. doi: 10.1073/pnas.210384297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pi Y, Sreekumar R, Huang X, Walker JW. Positive inotropy mediated by diacylglycerol in rat ventricular myocytes. Circ Res. 1997;81:92–100. doi: 10.1161/01.res.81.1.92. [DOI] [PubMed] [Google Scholar]
- 54.Yuan SH, Sunahara FA, Sen AK. Tumor-promoting phorbol esters inhibit cardiac functions and induce redistribution of protein kinase C in perfused beating rat heart. Circ Res. 1987;61:372–378. doi: 10.1161/01.res.61.3.372. [DOI] [PubMed] [Google Scholar]
- 55.Liu Q, Chen X, MacDonnell SM, Kranias EG, Lorenz JN, Leitges M, Houser SR, Molkentin JD. Protein kinase Cα, but not PKCβ or PKCγ, regulates contractility and heart failure susceptibility: implications for ruboxistaurin as a novel therapeutic approach. Circ Res. 2009;105:194–200. doi: 10.1161/CIRCRESAHA.109.195313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunasegaram S, Haworth RS, Hearse DJ, Avkiran M. Regulation of sarcolemmal Na+/H+ exchanger activity by angiotensin II in adult rat ventricular myocytes: opposing actions via AT1 versus AT2 Receptors. Circ Res. 1999;85:919–930. doi: 10.1161/01.res.85.10.919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.