Abstract
The current study investigates both gray and white matter changes in non-demented Parkinson’s disease (PD) patients with varying degrees of mild cognitive deficits and elucidates the relationships between the structural changes and clinical sequelae of PD. Twenty-six PD patients and 15 healthy controls (HCs) were enrolled in the study. Participants underwent T1-weighted and diffusion tensor imaging (DTI) scans. Their cognition was assessed using a neuropsychological battery. Compared with HCs, PD patients showed significant cortical thinning in sensorimotor (left pre- and postcentral gyri) and cognitive (left dorsolateral superior frontal gyrus [DLSFG]) regions. The DLSFG cortical thinning correlated with executive and global cognitive impairment in PD patients. PD patients showed white matter abnormalities as well, primarily in bilateral frontal and temporal regions, which also correlated with executive and global cognitive impairment. These results seem to suggest that both gray and white matter changes in the frontal regions may constitute an early pathological substrate of cognitive impairment of PD providing a sensitive biomarker for brain changes in PD.
Keywords: Parkinson’s disease, Cognition, Executive dysfunction, Cortical thickness, White matter, Diffusion tensor imaging
Introduction
Parkinson’s disease (PD) is a multisystem neurodegenerative disease, affecting not only dopaminergic nerve cells of the substantia nigra but also other brain regions and neurotransmitters (Braak et al. 2006). PD presents with the cardinal motor symptoms such as tremor, rigidity, bradykinesia, and loss of postural stability along with a set of non-motor symptoms (Bonnet et al. 2012) such as cognitive impairment, depression, sleep disturbances, and autonomic dysfunction (Barnum and Tansey 2012; Chaudhuri et al. 2011; Ferrer et al. 2011). For this reason, PD research has expanded its investigation beyond the nigrostriatal region to the whole brain in order to characterize the different symptoms. In particular, neuroimaging investigation of cognitive impairment is a topic of a growing interest (Christopher and Strafella 2013). It is prevalent and present regardless of the disease stage (Litvan et al. 2011). It ranges from mild deficits demonstrable by means of comprehensive neuropsychological testing, to dementia (Jellinger 2012), and mild cognitive impairment (MCI) increases risk of developing dementia (Janvin et al. 2006). The neural substrate of motor and cognitive symptoms has been investigated using non-invasive brain imaging such as structural MRI, which can offer the opportunity of identifying early biomarkers. In fact, different MRI techniques are providing mounting evidence of both gray and white matter changes in PD (Cochrane and Ebmeier 2013; Pan et al. 2012).
Whole-brain gray matter changes have been investigated using voxel-based morphometry (VBM) as well as surface-based analyses including cortical thickness and surface analyses combined with subcortical volumetric analysis. PD patients with dementia (PDD) typically show bilateral diffuse gray matter changes (Beyer et al. 2007; Compta et al. 2012; Melzer et al. 2012; Song et al. 2011; Zarei et al. 2013). On the other hand, non-demented PD patients show regional gray matter changes in areas such as frontal (Biundo et al. 2011; Burton et al. 2004; Ibarretxe-Bilbao et al. 2010; Jubault et al. 2011), temporal and/or limbic regions (Feldmann et al. 2008; Ibarretxe-Bilbao et al. 2009; Pellicano et al. 2012; Tinaz et al. 2011; Wattendorf et al. 2009), or posterior regions including the parieto-occipital cortex (Pereira et al. 2012). Furthermore, non-demented PD patients show faster progression of gray matter changes including atrophy and cortical thinning than healthy controls (HCs) in diffuse areas including frontal, temporal and parietal regions (Hu et al. 2001; Ibarretxe-Bilbao et al. 2012), restricted regions to cortical motor areas and cerebellum (Ibarretxe-Bilbao et al. 2010), or limbic, paralimbic, and temporo-occipital regions (Ramirez-Ruiz et al. 2007). Among PD patients, variability in cortical thickness and/or subcortical volume is also associated with cognitive measures (Biundo et al. 2011; Camicioli et al. 2009; Ibarretxe-Bilbao et al. 2009; Melzer et al. 2012; Pellicano et al. 2012; Zarei et al. 2013), facial emotion recognition (Baggio et al. 2012), duration of disease (Hanganu et al. 2013; Jubault et al. 2011; Lyoo et al. 2011), motor severity (Lyoo et al. 2011; Melzer et al. 2012; Zarei et al. 2013) and stage (Zarei et al. 2013) as well as dopamine (DA) non-responsive symptoms (Brenneis et al. 2003; Camicioli et al. 2009).
White matter changes have been investigated using diffusion tensor imaging (DTI). The two most common indices derived from DTI are fractional anisotropy or FA and mean diffusivity or MD (Cochrane and Ebmeier 2013). FA estimates the degree of anisotropic directionality of water diffusion while MD estimates the magnitude/size of water diffusion. FA and MD in the whole-brain white matter can be assessed using voxel-based analysis (VBA) or tract-based statistical analysis (TBSS). Contrary to gray matter findings, in which regional changes are more common in non-demented PD, white matter changes are reported in more diffuse brain areas even in non-demented PD (Hattori et al. 2012; Kim et al. 2013; Melzer et al. 2013; Theilmann et al. 2013; Zheng et al. 2014), and the frontal region was one of the most consistently reported regions for white matter changes (Agosta et al. 2013b; Deng et al. 2013; Gattellaro et al. 2009; Rae et al. 2012; Zhan et al. 2012; Zhang et al. 2011). Moreover, variability in FA and/ or MD was primarily associated with cognitive measures (Agosta et al. 2013b; Gallagher et al. 2013; Rae et al. 2012; Theilmann et al. 2013; Zheng et al. 2014.)
Despite the evidence of structural abnormalities and variability associated with clinical and cognitive manifestations in non-demented PD, whether structural abnormalities account for specific clinical sequelae in PD patients is still unclear. This is largely due to the lack of imaging studies investigating both structural group differences, and relationships between these differences and various clinical and cognitive measures. For example, brain regions displaying significant group differences in structure have not been specifically investigated for correlation analyses (Camicioli et al. 2009; Hanganu et al. 2013; Jubault et al. 2011; Tinaz et al. 2011). Even though brain regions demonstrating significant group differences have been shown to correlate with cognition, PD patients did not show impairment on cognitive tasks (Theilmann et al. 2013). Furthermore, only correlations were demonstrated in PD without comparing structural data of PD with those of HCs (Lyoo et al. 2011; Zheng et al. 2014). To date, only a handful of studies have addressed which structural abnormalities account for different PD symptoms by investigating whole-brain gray and white matter changes (Agosta et al. 2013a, 2013b; Hattori et al. 2012). Using both VBM and TBSS analyses, these studies have consistently concluded that white matter and not gray matter changes underlie cognitive impairment in PD (Agosta et al. 2013a, b). This discrepancy may be due to the fact that VBM may not be highly sensitive for detecting subtle cortical atrophy in early stages of PD (Agosta et al. 2013b; Jubault et al. 2011). In fact, cortical thickness analysis appeared more prone to detect cortical gray matter changes in PD than VBM when both analysis methods were compared (Pereira et al. 2012). Thus based on these preliminary observations, the current study aimed at (1) investigating both gray and white matter changes using cortical thickness analysis and TBSS, and thereby also demonstrating which MRI technique can be a promising biomarker for PD and (2) further elucidating the relationships between observed structural abnormalities and clinical and cognitive manifestations in non-demented PD patients.
Materials and methods
Participants
Twenty-six patients meeting UK Brain Bank criteria for the diagnosis of idiopathic PD (Defer et al. 1999; Langston et al. 1992) and 15 HCs participated in the study. PD patients were recruited from the Movement Disorders Clinic of the Toronto Western Hospital. The HCs were recruited from friends and spouses of the patients or through advertisements posted at the hospital and the affiliated university. Exclusion criteria included (1) history of a head injury, psychiatric, neurological or major medical diseases, (2) dementia assessed by a modified disability assessment for dementia (DAD) with an additional question regarding whether any reported impairment was related to cognitive difficulties or the physical impairments of PD, (3) contraindications for MRI scanning, and (4) for HCs evidence of cognitive impairment as assessed by a neuropsychological test battery. All participants underwent a cognitive assessment by means of an extended neuropsychological test battery and the Montreal Cognitive Assessment (MoCA; Nasreddine et al. 2005; Tison et al. 1995) as well as the Beck depression inventory (Beck et al. 1961) that assesses levels of depression. PD patients were additionally evaluated for motor severity of the disease using the motor subset of the Unified Parkinson Disease Rating Scale (UPDRS-III). All participants underwent structural MRI scans and 35 participants had additional DTI scans. Among the 35 participants, five subjects (four PD patients and one HC) were excluded due to motion artifacts resulting in 16 PD patients and 14 HCs included in the DTI analysis. PD patients underwent all the study procedures in an “on-medication” state. All participants gave informed consent following full explanation of the study procedures. This study was approved by the Institutional Ethics Committee of the Centre for Addiction and Mental Health and the University Health Network.
Neuropsychological assessment
Cognitive function in the domains of executive function, attention/working memory, language, visuospatial function, and memory was assessed using the following neuropsychological tests (Litvan et al. 2012). For executive function: Visual Verbal Test total number of shifts (Wicklund et al. 2004), Delis-Kaplan Executive Function System (D-KEFS) Color-Word Interference (Stroop task); time to complete condition 3: inhibition, and D-KEFS Verbal Fluency, total score for category fluency (Delis et al. 2001). For attention and working memory: Wechsler Memory Scale-III (WMS-III) Digit Span and Letter-Number Sequencing total score (Wechsler 1997), Delis-Kaplan Executive Function System (D-KEFS) Color-Word Interference (Stroop task); time to complete condition 1: color naming (attention). For language: the category fluency from the Verbal Fluency subtest (Delis et al. 2001). For visuospatial function: Judgment of Line Orientation (JLO) total score (Benton et al. 1994). For memory: California Verbal Learning Test-II (CVLT-II) long delay free recall score (Delis et al. 2001). Global composite z scores were calculated for each domain of cognition. When the cognitive domain had only one test, the individual test score was used. For executive function, the average of the z scores from the visual verbal test, inhibition segment of the Stroop task, and verbal fluency was used, while for attention/working memory, the average of the z scores from the digit span, letter-number sequencing, and color-naming segment of the Stroop was used.
MRI acquisition
Whole-brain T1-weighted and diffusion-weighted images were acquired using a 3.0 T GE Signa HD × MRI system (General Electric, Milwaukee, WI) equipped with an eight-channel phased array head coil. For gray matter analysis, a high-resolution three-dimensional (3D) anatomical scan was acquired with a T1-weighted 3D IR-FSPGR sequence (TR/TE/TI, 7.8/min full/450 ms; matrix, 256 × 256; voxel size, 1 × 1 × 1 mm; filed of view, 256 × 256 mm; flip angle, 15°; 180 axial slices). For white matter analysis, a DWI scan was acquired with spin-echo single-shot echo planar imaging with diffusion encoding in 60 noncolinear directions (TR/TE, 17,000/min ms; field of view, 230 × 230 mm; matrix, 128 × 128, voxel size, 1.8 × 1.8 × 2.4 mm; b value, 1,000 s/mm2; 64 slices). Parallel imaging was employed using the Array Spatial Sensitivity Encoding Technique (ASSET) with an acceleration factor of two. DWI images were acquired in the axial plane. In addition, ten non-diffusion-weighted scans were acquired at the beginning of each scan. The DWI scans were repeated three times to increase signal-to-noise ratio. Two PD patients and two HCs underwent only two DWI scans due to inability to lie down in the scanner for an extended period of time.
MRI processing
Cortical thickness processing
Cortical thickness (CTh) analysis was performed using the FreeSurfer image analysis suite (version 5.1; available at http://surfer.nmr.mgh.harvard.edu). The processing of T1 high-resolution images for the cortical surface reconstruction involved several steps (Dale et al. 1999): automated Talairach transformation, intensity normalization (Sled et al. 1998), skull stripping, white matter segmentation, tessellation of the gray/white matter boundary, automated topology correction (Fischl et al. 2001; Segonne et al. 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location (Dale and Sereno 1993; Dale et al. 1999; Fischl and Dale 2000). All surface models were visually inspected for accuracy. Cortical thickness was calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl and Dale 2000).
Subcortical volume processing
Segmentation of brain volume was obtained based on the automatic procedure included in FreeSurfer (version 5.1; available at: http://surfer.nmr.harvard.edu; Fischl et al. 2002). The labels were created using an automated subcortical labeling algorithm based on a probabilistic atlas obtained from a manually labeled training set. The image was rigid body registered to the probabilistic brain atlas, followed by non-linear morphing to the atlas. Then, an automated segmentation procedure assigned a label to each voxel in a dataset based on signal intensity information and the spatial relationship of the subcortical labels in the training sets. Volumetric measures from 18 structures in each hemisphere as well as intracranial volume (ICV) were automatically obtained. Among these structures, we selected the thalamus, putamen, caudate nucleus, pallidum, nucleus accumbens, hippocampus, amygdala and brainstem for our regions of interests (ROIs).
TBSS processing
Individual FA and MD images were generated to conduct TBSS analysis using FSL tools from the FMRIB software library (FSL version 4.1.5, http://www.fmrib.ox.ac.uk/; Smith et al. 2004; Woolrich et al. 2009). First, each volume upsampled to create isotropic voxel size (2.4 × 2.4 × 2.4 mm) was affine registered to the nineth b0 volume using FLIRT to correct motion and eddy current distortion (Jenkinson and Smith 2001; Jenkinson et al. 2002). Then, an averaged DWI image was generated from the three sets of DWI images. Non-brain tissue of the averaged DWI image was removed using brain extraction tool (BET) (Smith 2002), and FA and MD images were derived using DTIfit from FMRIB’s Diffusion Toolbox. The FA maps of all participants were warped to the FMRIB58_FA template using FNIRT (Andersson et al. 2007a,b). A mean FA map of all subjects was created and thinned to generate a mean FA skeleton, which represents the centers of white matter tracts common to all subjects included in the present study. The mean skeleton was thresholded and binarized at FA value of 0.2 to minimize partial voluming. The individual FA and MD maps were projected onto the mean skeleton resulting in a skeletonised FA and MD maps.
Statistical analysis
The cortical thickness analysis was performed using Freesurfer’s QDEC application that fits a general linear model (GLM) at each surface vertex. CTh maps were smoothed using a circularly symmetric Gaussian kernel across the surface with a full width at half maximum (FSHM) of 15 mm. Z Monte Carlo simulations with 10,000 iterations were applied to CTh maps to provide clusterwise correction for multiple comparisons, and the results were thresholded at a corrected P value of 0.05 (Z = 1.3). Mean thickness was calculated for each significant cluster.
Voxelwise statistics were performed on the skeletonised FA and MD maps using the Threshold-Free Cluster Enhancement (TFCE, Smith and Nichols 2009). A P <0.05 voxelwise correction for multiple comparisons was considered significant. The JHU DTI-based white matter atlases including the Johns Hopkins University WM tractography atlas and the ICBM-DTI WM labels (Hua et al. 2008; Mori et al. 2005; Wakana et al. 2007) were used to identify white matter tracts that showed significant group differences and correlations in FA and MD values.
Age, years of education, MoCA, BDI and neuropsychological tests were compared between groups using independent samples t tests. Gender and handedness were compared using Pearson’s Chi square tests. Subcortical volume in a priori ROIs (thalamus, putamen, caudate nucleus, pallidum, nucleus accumbens, hippocampus, amygdala, and brainstem) in each hemisphere was compared between groups using independent t test or Analysis of Variance (ANOVA) after testing the effects of intracranial volume (ICV) on the subcortical volume in each ROI.
From the clusters displaying significant group differences, mean individual values were extracted to investigate the relationships between structural changes (cortical thickness, subcortical volume, and DTI) and (1) cognitive data (MoCA and neuropsychological test scores) and (2) clinical data (UPDRS-III scores and duration of disease). Prior to the correlation analyses, bivariate correlation was performed among all relevant covariates including demographic (age, gender, years of education, and handedness), clinical (symptom-dominant side, UPDRS-III scores, disease duration, LEDD, and BDI), cognitive (MoCA, visual verbal test, JLO, and global and executive composite z) and MRI findings from the group analysis using Pearson’s correlation tests to determine the effects of nuisance variables, which would be then controlled for in the correlations, if necessary. All statistical analyses were two-sided and statistical significance was set at P <0.05 corrected for multiple comparisons. All of the statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS 13.0).
Results
Demographic, clinical, and cognitive characteristics
Table 1 shows the demographic and clinical characteristics as well as neuropsychological data of all of the patients with Parkinson’s disease (PD) and healthy controls (HC). There was no difference between the two groups regarding age, gender, education, BDI, and handedness (P >0.05). However, they were significant differences on MoCA (P = 0.006), global composite z (P = 0.007) and executive composite z (P = 0.001), visual verbal test (P <0.001), and judgement of line orientation (P = 0.027).
Table 1.
Demographic, clinical, and cognitive characteristics of patients with Parkinson’s disease (PD) and healthy controls (HC)
| PD (n = 26) | HC (n = 15) | |
|---|---|---|
| Age (years) | 70.5 (5.6) | 67.13 (5.1) |
| Sex (male/female) | 13/13 | 4/11 |
| Handedness (right/left) | 24/2 | 14/1 |
| Education (years) | 15.6 (2.1) | 17.0 (2.5) |
| MoCAa | 25.2 (2.8) | 27.6 (2.2)** |
| BDIb | 6.5 (5.5) | 3.8 (3.6) |
| Disease duration (years) | 6.7 (4.2) | – |
| Symptom-dominant side (right/left) | 17/9 | |
| UPDRS-IIIc (on-medication) | 25.3 (15.3) | – |
| Total LEDDd (mg/day) | 731.3 (459.8) | – |
| Neuropsychological tests | ||
| Global composite z | −0.22 (0.60) | 0.30 (0.50)** |
| Attention/WM composite z | 0.12 (0.63) | 0.27 (0.70) |
| Executive composite z | −0.64 (0.89) | 0.25 (0.60)** |
| Digit span forward | 0.31 (0.73) | 0.46 (1.02) |
| California verbal test | 0.19 (1.06) | 0.23 (0.92) |
| Letter-number sequencing | 0.20 (0.77) | 0.15 (0.68) |
| Visual verbal test | −2.21 (1.93) | −0.380 (0.48)** |
| Judgement of line orientation | −0.78 (1.57) | 0.24 (0.88)* |
| STROOP | ||
| Color naming | −1.44 (0.86) | 0.19 (1.17) |
| Inhibition | 0.13 (0.72) | 0.29 (1.00) |
| Category fluency | 0.21 (1.21) | 0.86 (0.83) |
Data are presented in mean (standard deviation) and neuropsychological data are presented in group based mean Z scores (standard deviation)
WM working memory
Montreal cognitive assessment
Beck depression inventory
Unified Parkinson’s Disease Rating Scale III
Levodopa Equivalent Daily Dose: 1-L dopa dose + 1-L dopa-CR × 0.75 + pramipexole (mg) × 67 (Evans et al. 2004)
P <0.03,
P <0.01
Cortical thickness
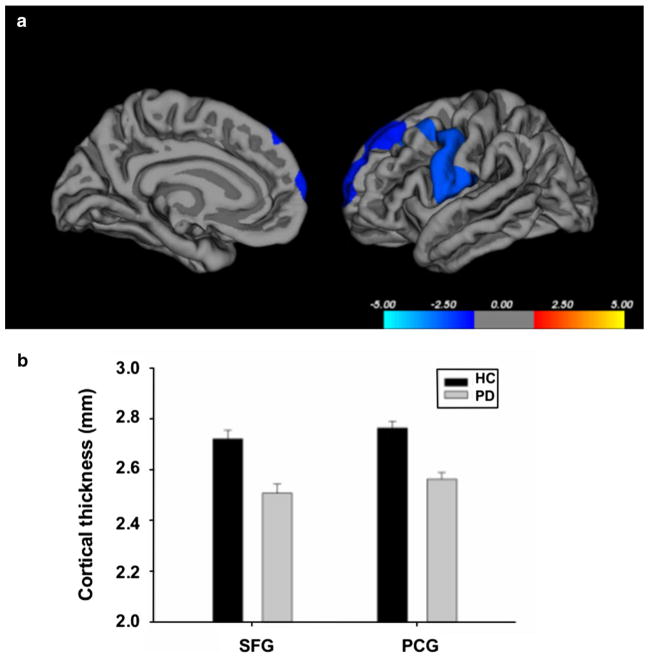
We found that the PD patients showed significant reduction in cortical thickness in the left superior frontal gyrus (SFG) (cluster size: 1,585 mm2; P = 0.03) and the left precentral gyrus extending back into the postcentral gyrus (PCG) (cluster size: 2,555.42 mm2; P = 0.01) compared with the HCs (Fig. 1). The PD patients did not show significant increase in cortical thickness in any brain region compared with the HCs.
Fig. 1.
a Cortical areas showing significant cortical thinning in patients with Parkinson’s disease compared to healthy controls. Color bar indicates the significance levels in the clusters in Z values. b Bar graphs on extracted cortical thickness values (mm) from the significant clusters in the left superior frontal gyrus (SFG) and left precentral gyrus (PCG) between healthy controls (HC) and PD patients (PD). Error bars represent sem
We further investigated whether the cortical thickness in these two significant clusters was correlated with clinical sequelae. To this end, first, we ran bivariate correlations among different covariates including the cortical thickness values in the SFG and PCG clusters and demographic, clinical and cognitive measures to determine the effects of nuisance variables. In particular, age showed significant negative correlation both with SFG cortical thickness (r = −0.497, P = 0.01) and cognitive scores including MoCA (r = −0.423, P = 0.031), visual verbal test (r = −0.499, P = 0.009), and executive composite z scores (r = −0.541, P = 0.004). Thus controlling for the effect of age, we found significant positive correlations between the SFG thickness and global composite z (r = 0.597, P = 0.002), and executive composite z (r = 0.430, P = 0.032) scores (Fig. 2). In HCs, we found a significant positive correlation between the SFG thickness and visual verbal test scores (r = 0.607, P = 0.016).
Fig. 2.
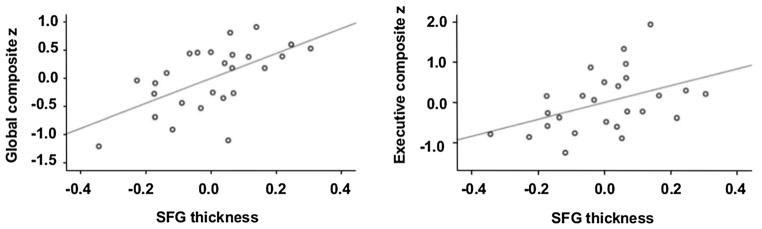
Partial correlations with age as a covariate between superior frontal gyrus (SFG) thickness (extracted from the significant cluster) and global composite z (left) and executive composite z (right) in 26 patients with Parkinson’s disease showing the significant positive correlations
Subcortical volume
We found no significant group differences in any ROIs and therefore, no correlation analysis was pursued.
White matter
We found that PD patients showed white matter changes with significantly higher MD values compared to HCs. The significant cluster with increased MD was detected in widespread regions including the frontal, temporal, parietal and occipital regions (Fig. 3). These changes were located primarily in a larger area of bilateral frontal and temporal regions and smaller areas of the left parietal and occipital regions. More specifically, white matter tracts with MD changes included forceps minor, cingulum, anterior thalamic radiation, superior corona radiata, external capsule, body of corpus callosum, uncinate fasciculus, inferior fronto-occipital fasciculus, superior and inferior longitudinal fasciculi, and forceps major. PD patients did not show any significantly lower MD values in any white matter tracts compared with HCs.
Fig. 3.
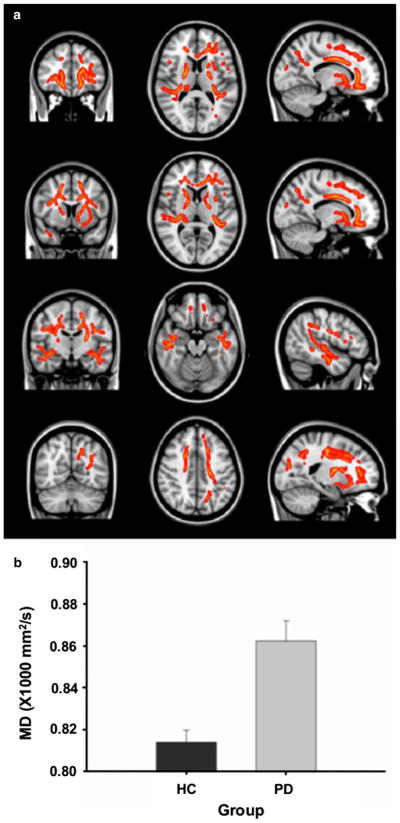
a Clusters of significantly increased mean diffusivity (MD) in 16 patients with Parkinson’s disease (PD) compared with 15 healthy controls in tract-based spatial statistics. Result images are overlaid on the MNI152 template. b Bar graphs on mean MD values derived from the significant clusters in tract-based spatial statistics between healthy control and PD groups. Error bars represent sem
We extracted mean MD values from the significant cluster resulting from the group analysis for each subject and investigated correlations between the MD values and (1) cognitive measures that showed significant group differences (i.e., visual verbal test, P <0.01; global composite z, P <0.03; and executive composite z, P <0.01) in both PD patients and HCs as well as clinical measures (duration of disease and UPDRS-III scores) in PD patients. We first ran bivariate correlations among all relevant covariates. In PD patients we found a significant negative correlation between age and executive z scores (r = −0.622, P = 0.01) and a positive correlation between age and the mean MD values in the significant cluster (r = 0.525, P = 0.037). In this group of patients, we also observed a significant correlation between gender and cognitive measures (scores of global composite z (r = 0.667, P = 0.005) and executive composite z (r = 0.583, P = 0.018). Thus, controlling for the effects of age and gender, we found significant negative correlations between the MD values and scores of global composite z (r = −0.39, P <0.05) and executive composite z (r = −0.44, P = 0.018) in PD patients, suggesting that white matter damage (mainly in frontal and temporal regions) was associated with cognitive impairment. In HCs, we found no correlations among any of the considered variables.
Discussion
The present study corroborated structural changes in PD patients and demonstrated specific relationships between gray and white matter damages and cognitive deficits in PD, suggesting that these abnormalities may represent a sensitive biomarker for detecting brain changes associated with cognitive changes.
Our results provided evidence of gray matter changes at the cortical level in PD. PD patients showed significant cortical thinning in the left superior frontal, caudal middle frontal, precentral and postcentral gyri compared to HCs. Gray matter changes in these regions have previously been reported in non-demented PD patients (Kostic et al. 2010; Melzer et al. 2012; Pereira et al. 2012; Zarei et al. 2013). In addition, the superior frontal, caudal middle frontal gyrus and precentral sulcus were among the areas showing significantly greater progression of cortical thinning in PD patients compared to HCs (Ibarretxe-Bilbao et al. 2012).
We further investigated whether these cortical abnormalities would explain the clinical sequelae including motor and cognitive manifestations in PD and found that cortical thinning in the dorsolateral SFG was associated with global and executive cognitive measures. The dorsolateral SFG including BA8 and BA9 that showed significant thinning, is involved in a variety of cognitive functions including working memory (Levy and Goldman-Rakic 2000; Owen et al. 1998), attention (Corbetta et al. 2008), episodic memory (Desgranges et al. 1998) and spatial cognition (Courtney et al. 1998; du Boisgueheneuc et al. 2006). In particular, the left SFG is involved in higher levels of cognitive processing (du Boisgueheneuc et al. 2006) or executive functions. This may well explain the significant correlation between the SFG thinning and global composite and executive z scores. We also found a significant positive correlation between the left SFG thickness and visual verbal test scores in HCs but not in PD patients. This is most likely due to a lack of variability in the scores of the PD patients.
We did not find any correlation between the reduced cortical thickness in the sensorimotor area and UPDRS-III scores or duration of disease. These observations seem to be consistent with previous studies where cortical thinning was found in motor areas including the left medial SMA and right dorsal pre-SMA without any correlation with UPDRS-III scores or disease duration in those regions (Jubault et al. 2011). On the other hand, correlations between cortical gray matter measures in the sensorimotor area and motor symptoms (Lyoo et al. 2011; Rosenberg-Katz et al. 2013) as well as between cortical thickness in several cortical areas and duration of disease (Lyoo et al. 2011; Rosenberg-Katz et al. 2013) have been reported in other studies. However, it is unknown whether PD patients had cortical abnormalities in those areas compared to HCs, as those studies did not include control data. The lack of a significant correlation between cortical abnormality in the sensorimotor area and UPDRS-III scores in our patients may well be due to the fact that UPDRS evaluations were performed during an on-medication state (instead of during an off-medication state), and this could have very likely diminished the possibility of detecting a significant relationship with the cortical thinning in those regions.
We did not find any changes in subcortical volume between groups. Previous studies using the same method also failed to find significant group differences (Tinaz et al. 2011; Zarei et al. 2013). These consistent observations suggest that current imaging analysis may lack sensitivity for detecting subtle gray matter changes.
Our TBSS analysis revealed that PD patients showed white matter damage in multiple white matter tracts in widespread areas, more extensively in the bilateral frontal and temporal regions compared to HCs. White matter changes in these regions were consistently reported in previous TBSS findings (Agosta et al. 2013a, b; Deng et al. 2013; Hattori et al. 2012; Matsui et al. 2007; Melzer et al. 2013; Rae et al. 2012). We detected group differences with an increase in MD (but not in FA) in our patients. MD appears to be more sensitive in detecting subtle white matter changes than FA as suggested in other studies in early PD (Melzer et al. 2013), early Alzheimer patients (Acosta-Cabronero et al. 2010) and individuals with concussion (Cubon et al. 2011).
We further found that the frontotemporal white matter damage significantly correlated with the global and executive cognitive measures. Our findings combined with previous studies (Agosta et al. 2013b; Gallagher et al. 2013; Hattori et al. 2012; Melzer et al. 2013) suggest that frontal white matter damage is a core pathological substrate of mild cognitive deficits in PD. White matter damage did not correlate UPDRS-III scores. This is very likely because UPDRS evaluation was representative of the on-medication state for the same explanation provided above.
Our gray and white matter data consistently showed structural changes in the frontal region in PD. The frontal cortex includes part of the frontostriatal loops (Alexander et al. 1986), which have important implications for motor and non-motor symptoms of PD. Prefrontostriatal dysfunction is thought to underlie the basis for the most prominent executive impairment in PD (Nagano-Saito et al. 2013; Owen 2004; Pagonabarraga and Kulisevsky 2012; Zgaljardic et al. 2006). Abnormality in the SFG in particular can be an early indicator for further decline of cognitive function. For example, PD patients who converted to dementia showed cortical thinning in the frontal regions including the SFG, PCG, and anterior cingulate at a baseline assessment and showed wider areas of cortical thinning in temporal, parietal and occipital regions at a follow-up assessment (Compta et al. 2013). The white matter underlying the prefrontal cortex contains projections from striatum via thalamus, to the striatum via thalamus, and directly to striatum. Although the TBSS does not allow for specific identification of the frontostriatal pathway, it reveals that the white matter comprising the frontostriato-thalamic loop was affected in our PD patients. For example, the anterior thalamic radiation includes white matter tracts from thalamus to prefrontal cortex and vice versa. The lateral anterior ventral and medial dorsal nuclei of thalamus, in particular, receive input from the basal ganglia. White matter damage found in our study extended beyond the frontal region and thus, it is still to be determined whether white matter changes in the prefrontal region alone can contribute to cognitive impairment. However, prefrontal white matter alone appears to be able to contribute to executive cognitive functions in PD (Gallagher et al. 2013).
Our PD patients also showed extensive white matter changes in bilateral temporal regions. A few studies consistently showed that PD with MCI may be associated with gray matter atrophy in limited regions of frontal and temporal regions (Hanganu et al. 2013; Melzer et al. 2012; Song et al. 2011) while one of them additionally showed parietal volume loss (Melzer et al. 2012) and others, occipital volume loss (Hanganu et al. 2013; Song et al. 2011). A longitudinal study also demonstrated higher rates of cortical thinning in the frontal and temporal regions, extending to parietal cortex (Ibarretxe-Bilbao et al. 2012). Although our PD patients did not show gray matter abnormalities in the temporal region, the white matter changes may have preceded gray matter changes. The topography of white matter changes in our study is similar to the previous gray matter findings mentioned above with extensive frontal and temporal abnormalities as core changes and unilateral focal parietal and occipital changes. Abnormality in the temporal region may be associated with developing dementia in PD patients. For example, compared to the PD with normal cognition, PD-MCI and PDD had significantly smaller hippocampal volumes, and PDD additionally showed the medial temporal lobe atrophy (Weintraub et al. 2011). Furthermore, PDD showed significant atrophy in the entorhinal cortex compared with PD with normal cognition (Goldman et al. 2012).
Our findings in gray and white matter changes in PD are in line with previous studies showing widespread white matter abnormalities but limited (Agosta et al. 2013a) or absent (Agosta et al. 2013b; Hattori et al. 2012) cortical gray matter changes. However, differently from those studies using VBM, the significant gray matter abnormalities reported here seem to suggest that cortical thickness analysis may be a better approach. Thus, the combination of DTI and cortical thickness analyses appear to be sensitive approaches for detecting subtle white and gray matter changes associated with PD.
Although the MRI techniques used in the present study are validated methods to assess structural changes, the biological underpinnings of these changes are not fully understood. Subtle cortical thinning may reflect changes in size of cell bodies, dendritic arborisation, and/or presynaptic terminals (Morrison and Hof 1997; Pellicano et al. 2012). Changes in DTI indices can result from a number of processes including neuronal loss and gliosis, as well as disturbances in axonal membranes, myelin sheath, microtubules, and neurofilaments (Shenton et al. 2012). PD has been associated with cytoskeletal damage of various neuronal cells including dopaminergic, glutamatergic, cholinergic, tryptaminergic, GABAergic, noradrenergic and adrenergic neurons (Braak et al. 1994, 1995, 1998; Foley and Riederer 1999; Jellinger 1991). The cytoskeletal damage leads to Lewy pathologies including Lewy bodies and Lewy neuritis mostly located in presynaptic terminals and in axons of affected nerve cells, respectively (Braak et al. 2004). The major components of Lewy pathologies include aggregations of misfolded alpha synuclein (Braak et al. 2004) and abnormally phosphorylated neurofilaments (Braak and Braak 2000). Thus, MRI changes may reflect the Lewy pathologies and/or neuronal degeneration secondary to the Lewy pathologies.
The current study has a few potential limitations. First, PD patients included in the present study had been taking parkinsonian medications for quite some time. The effects of chronic dopaminergic medication on brain structures remain to be determined. Second, our PD patients underwent all study procedures in an on-medication state. It is well known that dopaminergic medications in general can influence cognition (Kehagia et al. 2010). For example, while they can ameliorate certain cognitive deficits (e.g. executive functions), dopaminergic medications can also worsen other cognitive abilities (Kehagia et al. 2010; MacDonald et al. 2013; Ryterska et al. 2013). Our decision not to study them in an off-medication state was justified by the risk of worsening their motor symptoms increasing the risk of motion artifacts during MRI acquisitions.
Conclusions
The present study further demonstrates that both gray and white matters are affected in PD and these anatomical changes may represent the neural substrate underlying mild cognitive deficits in non-demented PD patients. The structural changes in the frontal region in particular may be an early pathological substrate of cognitive impairment of PD, and may represent a sensitive biomarker for brain changes in PD.
Acknowledgments
This study was supported by Canadian Institutes of Health Research (MOP 117891). A.P.S. is supported by the Canada Research Chair program. Yuko Koshimori was supported by a scholarship from Parkinson Society Canada.
Footnotes
Conflict of interest There are no actual or potential conflicts of interest.
Contributor Information
Yuko Koshimori, Division of Brain, Imaging and Behaviour–Systems Neuroscience, Toronto Western Research Institute, UHN, University of Toronto, 399 Bathurst St, Toronto, ON M5T 2S8, Canada, Research Imaging Centre, Centre for Addiction and Mental Health (CAMH), University of Toronto, 250 College St, Toronto, ON M5T 1R8, Canada.
Barbara Segura, Research Imaging Centre, Centre for Addiction and Mental Health (CAMH), University of Toronto, 250 College St, Toronto, ON M5T 1R8, Canada, Department of Psychiatry and Clinical Psychobiology, Faculty of Medicine, University of Barcelona, Casanova 146, 08035 Barcelona, Spain.
Leigh Christopher, Division of Brain, Imaging and Behaviour–Systems, Neuroscience, Toronto Western Research Institute, UHN, University of Toronto, 399 Bathurst St, Toronto, ON M5T 2S8, Canada, Research Imaging Centre, Centre for Addiction and Mental Health (CAMH), University of Toronto, 250 College St, Toronto, ON M5T 1R8, Canada.
Nancy Lobaugh, Research Imaging Centre, Centre for Addiction and Mental Health (CAMH), University of Toronto, 250 College St, Toronto, ON M5T 1R8, Canada.
Sarah Duff-Canning, Morton and Gloria Shulman Movement Disorder Unit and E.J. Safra Parkinson Disease Program, Toronto Western Hospital, UHN, University of Toronto, 399 Bathurst St, Toronto, ON M5T 2S8, Canada, Department of Neuropsychology, Toronto Western Hospital, 399 Bathurst St, Toronto, ON M5T 2S8, Canada.
Romina Mizrahi, Research Imaging Centre, Centre for Addiction and Mental Health (CAMH), University of Toronto, 250 College St, Toronto, ON M5T 1R8, Canada.
Clement Hamani, Division of Neurosurgery, Toronto Western Hospital, UHN, University of Toronto, 399 Bathurst St, Toronto, ON M5T 2S8, Canada.
Anthony E. Lang, Morton and Gloria Shulman Movement Disorder Unit and E.J. Safra Parkinson Disease Program, Toronto Western Hospital, UHN, University of Toronto, 399 Bathurst St, Toronto, ON M5T 2S8, Canada
Kelly Aminian, Division of Brain, Imaging and Behaviour–Systems, Neuroscience, Toronto Western Research Institute, UHN, University of Toronto, 399 Bathurst St, Toronto, ON M5T 2S8, Canada, Research Imaging Centre, Centre for Addiction and Mental Health (CAMH), University of Toronto, 250 College St, Toronto, ON M5T 1R8, Canada.
Sylvain Houle, Research Imaging Centre, Centre for Addiction and Mental Health (CAMH), University of Toronto, 250 College St, Toronto, ON M5T 1R8, Canada.
Antonio P. Strafella, Division of Brain, Imaging and Behaviour–Systems, Neuroscience, Toronto Western Research Institute, UHN, University of Toronto, 399 Bathurst St, Toronto, ON M5T 2S8, Canada, Research Imaging Centre, Centre for Addiction and Mental Health (CAMH), University of Toronto, 250 College St, Toronto, ON M5T 1R8, Canada, Morton and Gloria Shulman Movement Disorder Unit and E.J. Safra Parkinson Disease Program, Toronto Western Hospital, UHN, University of Toronto, 399 Bathurst St, Toronto, ON M5T 2S8, Canada, Toronto Western Hospital and Institute, CAMH-Research Imaging Centre, University of Toronto, Toronto, ON, Canada
References
- Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain. 2010;133:529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- Agosta F, Canu E, Stefanova E, Sarro L, Tomic A, Spica V, Comi G, Kostic VS, Filippi M. Mild cognitive impairment in Parkinson’s disease is associated with a distributed pattern of brain white matter damage. Hum Brain Mapp. 2013a doi: 10.1002/hbm.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Canu E, Stojkovic T, Pievani M, Tomic A, Sarro L, Dragasevic N, Copetti M, Comi G, Kostic VS, Filippi M. The topography of brain damage at different stages of Parkinson’s disease. Hum Brain Mapp. 2013b;34:2798–2807. doi: 10.1002/hbm.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB technical report TR07JA1. 2007a from www.fmrib.ox.ac.uk/analysis/techrep.
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. 2007b from www.fmrib.ox.ac.uk/analysis/techrep.
- Baggio HC, Segura B, Ibarretxe-Bilbao N, Valldeoriola F, Marti MJ, Compta Y, Tolosa E, Junque C. Structural correlates of facial emotion recognition deficits in Parkinson’s disease patients. Neuropsychologia. 2012;50:2121–2128. doi: 10.1016/j.neuropsychologia.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Tansey MG. Neuroinflammation and non-motor symptoms: the dark passenger of Parkinson’s disease? Curr Neurol Neurosci Rep. 2012;12:350–358. doi: 10.1007/s11910-012-0283-6. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benton AB, Sivan KD, Hamsher NR, Varney NR, Spreen O. Contributions to neuropsychological assessment. 2. Psycholog Assess Resour; Orland: 1994. [Google Scholar]
- Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biundo R, Formento-Dojo P, Facchini S, Vallelunga A, Ghezzo L, Foscolo L, Meneghello F, Antonini A. Brain volume changes in Parkinson’s disease and their relationship with cognitive and behavioural abnormalities. J Neurol Sci. 2011;310:64–69. doi: 10.1016/j.jns.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Bonnet AM, Jutras MF, Czernecki V, Corvol JC, Vidailhet M. Nonmotor symptoms in Parkinson’s disease in 2012: relevant clinical aspects. Parkinsons Dis. 2012 doi: 10.1155/2012/198316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247(Suppl 2):3–10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J, Jellinger K. Amygdala pathology in Parkinson’s disease. Acta Neuropathol. 1994;88:493–500. doi: 10.1007/BF00296485. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, Schultz C, de Vos RA, Jansen EN. Nigral and extranigral pathology in Parkinson’s disease. J Neural Transm Suppl. 1995;46:15–31. [PubMed] [Google Scholar]
- Braak H, de Vos RA, Jansen EN, Bratzke H, Braak E. Neuropathological hallmarks of Alzheimer’s and Parkinson’s diseases. Prog Brain Res. 1998;117:267–285. doi: 10.1016/s0079-6123(08)64021-2. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K. Stanley Fahn lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21:2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- Brenneis C, Seppi K, Schocke MF, Muller J, Luginger E, Bosch S, Loscher WN, Buchel C, Poewe W, Wenning GK. Voxel-based morphometry detects cortical atrophy in the Parkinson variant of multiple system atrophy. Mov Disord. 2003;18:1132–1138. doi: 10.1002/mds.10502. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Gee M, Bouchard TP, Fisher NJ, Hanstock CC, Emery DJ, Martin WR. Voxel-based morphometry reveals extranigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism Relat Disord. 2009;15:187–195. doi: 10.1016/j.parkreldis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson’s disease: the non-motor issues. Parkinsonism Relat Disord. 2011;17:717–723. doi: 10.1016/j.parkreldis.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Christopher L, Strafella AP. Neuroimaging of brain changes associated with cognitive impairment in Parkinson’s disease. J neuropsychol. 2013;7:225–240. doi: 10.1111/jnp.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane CJ, Ebmeier KP. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology. 2013;80:857–864. doi: 10.1212/WNL.0b013e318284070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Ibarretxe-Bilbao N, Pereira JB, Junque C, Bargallo N, Tolosa E, Valldeoriola F, Munoz E, Camara A, Buongiorno M, Marti MJ. Grey matter volume correlates of cerebrospinal markers of Alzheimer-pathology in Parkinson’s disease and related dementia. Parkinsonism Relat Disord. 2012;18:941–947. doi: 10.1016/j.parkreldis.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Compta Y, Pereira JB, Rios J, Ibarretxe-Bilbao N, Junque C, Bargallo N, Camara A, Buongiorno M, Fernandez M, Pont-Sunyer C, Marti MJ. Combined dementia-risk biomarkers in Parkinson’s disease: a prospective longitudinal study. Parkinsonism Relat Disord. 2013;19:717–724. doi: 10.1016/j.parkreldis.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2011;28:189–201. doi: 10.1089/neu.2010.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD) Mov Disord. 1999;14:572–584. doi: 10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Examiner’s manual for the Delis-Kaplan executive function system. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Deng B, Zhang Y, Wang L, Peng K, Han L, Nie K, Yang H, Zhang L, Wang J. Diffusion tensor imaging reveals white matter changes associated with cognitive status in patients with Parkinson’s disease. Am J Alzheimers Dis Other Demen. 2013;28:154–164. doi: 10.1177/1533317512470207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, Eustache F. The functional neuro-anatomy of episodic memory: the role of the frontal lobes, the hippocampal formation, and other areas. Neuroimage. 1998;8:198–213. doi: 10.1006/nimg.1998.0359. [DOI] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- Evans AH, Katzenschlager R, Paviour D, O’Sullivan JD, Appel S, Lawrence AD, Lees AJ. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- Feldmann A, Illes Z, Kosztolanyi P, Illes E, Mike A, Kover F, Balas I, Kovacs N, Nagy F. Morphometric changes of gray matter in Parkinson’s disease with depression: a voxel-based morphometry study. Mov Disord. 2008;23:42–46. doi: 10.1002/mds.21765. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Martinez A, Blanco R, Dalfo E, Carmona M. Neuropathology of sporadic Parkinson disease before the appearance of Parkinsonism: preclinical Parkinson disease. J Neural Transm. 2011;118:821–839. doi: 10.1007/s00702-010-0482-8. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Foley P, Riederer P. Pathogenesis and preclinical course of Parkinson’s disease. J Neural Transm Suppl. 1999;56:31–74. doi: 10.1007/978-3-7091-6360-3_2. [DOI] [PubMed] [Google Scholar]
- Gallagher C, Bell B, Bendlin B, Palotti M, Okonkwo O, Sodhi A, Wong R, Buyan-Dent L, Johnson S, Wilette A, Harding S, Ninman N, Kastman E, Alexander A. White matter microstructural integrity and executive function in Parkinson’s disease. J Int Neuropsychol Soc. 2013;19:349–354. doi: 10.1017/S1355617712001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattellaro G, Minati L, Grisoli M, Mariani C, Carella F, Osio M, Ciceri E, Albanese A, Bruzzone MG. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30:1222–1226. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JG, Stebbins GT, Bernard B, Stoub TR, Goetz CG, deToledo-Morrell L. Entorhinal cortex atrophy differentiates Parkinson’s disease patients with and without dementia. Mov Disord. 2012;27:727–734. doi: 10.1002/mds.24938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu A, Bedetti C, Jubault T, Gagnon JF, Mejia-Constain B, Degroot C, Lafontaine AL, Chouinard S, Monchi O. Mild cognitive impairment in patients with Parkinson’s disease is associated with increased cortical degeneration. Mov Disord. 2013;28:1360–1369. doi: 10.1002/mds.25541. [DOI] [PubMed] [Google Scholar]
- Hattori T, Orimo S, Aoki S, Ito K, Abe O, Amano A, Sato R, Sakai K, Mizusawa H. Cognitive status correlates with white matter alteration in Parkinson’s disease. Hum Brain Mapp. 2012;33:727–739. doi: 10.1002/hbm.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MT, White SJ, Chaudhuri KR, Morris RG, Bydder GM, Brooks DJ. Correlating rates of cerebral atrophy in Parkinson’s disease with measures of cognitive decline. J Neural Transm. 2001;108:571–580. doi: 10.1007/s007020170057. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N, Junque C, Tolosa E, Marti MJ, Valldeoriola F, Bargallo N, Zarei M. Neuroanatomical correlates of impaired decision-making and facial emotion recognition in early Parkinson’s disease. Eur J Neurosci. 2009;30:1162–1171. doi: 10.1111/j.1460-9568.2009.06892.x. [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N, Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Bargallo N, Juanes S, Tolosa E. Differential progression of brain atrophy in Parkinson’s disease with and without visual hallucinations. J Neurol Neurosurg Psychiatry. 2010;81:650–657. doi: 10.1136/jnnp.2009.179655. [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N, Junque C, Segura B, Baggio HC, Marti MJ, Valldeoriola F, Bargallo N, Tolosa E. Progression of cortical thinning in early Parkinson’s disease. Mov Disord. 2012;27:1746–1753. doi: 10.1002/mds.25240. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtyptes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14:153–197. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neurobiology of cognitive impairment in Parkinson’s disease. Expert Rev Neurother. 2012;12:1451–1466. doi: 10.1586/ern.12.131. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jubault T, Gagnon JF, Karama S, Ptito A, Lafontaine AL, Evans AC, Monchi O. Patterns of cortical thickness and surface area in early Parkinson’s disease. Neuroimage. 2011;55:462–467. doi: 10.1016/j.neuroimage.2010.12.043. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9:1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim SJ, Kim HS, Choi CG, Kim N, Han S, Jang EH, Chung SJ, Lee CS. Alterations of mean diffusivity in brain white matter and deep gray matter in Parkinson’s disease. Neurosci Lett. 2013;550:64–68. doi: 10.1016/j.neulet.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Kostic VS, Agosta F, Petrovic I, Galantucci S, Spica V, Jecmenica-Lukic M, Filippi M. Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology. 2010;75:857–863. doi: 10.1212/WNL.0b013e3181f11c1d. [DOI] [PubMed] [Google Scholar]
- Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Tröster AI, Weintraub D. MDS task force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord. 2011;26:1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo CH, Ryu YH, Lee MS. Cerebral cortical areas in which thickness correlates with severity of motor deficits of Parkinson’s disease. J Neurol. 2011;258:1871–1876. doi: 10.1007/s00415-011-6045-6. [DOI] [PubMed] [Google Scholar]
- MacDonald AA, Monchi O, Seerqobin KN, Ganjavi H, Tamjeedi R, MacDonald PA. Parkinson’s disease duration determines effect of dopaminergic therapy on ventral striatum function. Mov Disord. 2013;28:153–1560. doi: 10.1002/mds.25152. [DOI] [PubMed] [Google Scholar]
- Matsui H, Nishinaka K, Oda M, Niikawa H, Komatsu K, Kubori T, Udaka F. Depression in Parkinson’s disease. Diffusion tensor imaging study. J Neurol. 2007;254:1170–1173. doi: 10.1007/s00415-006-0236-6. [DOI] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, Dalrymple-Alford JC, Anderson TJ. Grey matter atrophy in cognitively impaired Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:188–194. doi: 10.1136/jnnp-2011-300828. [DOI] [PubMed] [Google Scholar]
- Melzer TR, Watts R, Macaskill MR, Pitcher TL, Livingston L, Keenan RJ, Dalrymple-Alford JC, Anderson TJ. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology. 2013;80:1841–1849. doi: 10.1212/WNL.0b013e3182929f62. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI Atlas of Human White Matter. Elsevier; Amsterdam: 2005. [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Habak C, Mejia-Constain B, Degroot C, Monetta L, Jubault T, Bedetti C, Lafontaine AL, Chouinard S, Soland V, Ptito A, Strafella AP, Monchi O. Effect of mild cognitive impairment on the patterns of neural activity in early Parkinson’s disease. Neurobiol Aging. 2013;35:223–231. doi: 10.1016/j.neurobiolaging.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10:525–537. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci USA. 1998;95:7721–7726. doi: 10.1073/pnas.95.13.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagonabarraga J, Kulisevsky J. Cognitive impairment and dementia in Parkinson’s disease. Neurobiol Dis. 2012;46:590–596. doi: 10.1016/j.nbd.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Pan PL, Song W, Shang HF. Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson’s disease. Eur J Neurol. 2012;19:199–206. doi: 10.1111/j.1468-1331.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- Pellicano C, Assogna F, Piras F, Caltagirone C, Pontieri FE, Spalletta G. Regional cortical thickness and cognitive functions in non-demented Parkinson’s disease patients: a pilot study. Eur J Neurol. 2012;19:172–175. doi: 10.1111/j.1468-1331.2011.03465.x. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Ibarretxe-Bilbao N, Marti MJ, Compta Y, Junque C, Bargallo N, Tolosa E. Assessment of cortical degeneration in patients with Parkinson’s disease by voxel-based morphometry, cortical folding, and cortical thickness. Hum Brain Mapp. 2012;33:2521–2534. doi: 10.1002/hbm.21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Correia MM, Altena E, Hughes LE, Barker RA, Rowe JB. White matter pathology in Parkinson’s disease: the effect of imaging protocol differences and relevance to executive function. Neuroimage. 2012;62:1675–1684. doi: 10.1016/j.neuroimage.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ruiz B, Marti MJ, Tolosa E, Gimenez M, Bargallo N, Valldeoriola F, Junque C. Cerebral atrophy in Parkinson’s disease patients with visual hallucinations. Eur J Neurol. 2007;14:750–756. doi: 10.1111/j.1468-1331.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Katz K, Herman T, Jacob Y, Giladi N, Hendler T, Hausdorff JM. Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology. 2013;80:1476–1484. doi: 10.1212/WNL.0b013e31828cfaa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryterska A, Jahanshahi M, Osman M. What are people with Parkinson’s disease really impaired on when it comes to making decisions? A meta-analysis of the evidence. Neurosci Biobehav Rev. 2013;37:2836–2846. doi: 10.1016/j.neubiorev.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, Vu MA, Purohit MP, Helmer K, Koerte I, Lin AP, Westin CF, Kikinis R, Kubicki M, Stern RA, Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson’s disease according to cognitive status. Mov Disord. 2011;26:289–296. doi: 10.1002/mds.23477. [DOI] [PubMed] [Google Scholar]
- Theilmann RJ, Reed JD, Song DD, Huang MX, Lee RR, Litvan I, Harrington DL. White-matter changes correlate with cognitive functioning in Parkinson’s disease. Front Neurol. 2013;4:37. doi: 10.3389/fneur.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinaz S, Courtney MG, Stern CE. Focal cortical and subcortical atrophy in early Parkinson’s disease. Mov Disord. 2011;26:436–441. doi: 10.1002/mds.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tison F, Dartigues JF, Auriacombe S, Letenneur L, Boller F, Alperovitch A. Dementia in Parkinson’s disease: a population-based study in ambulatory and institutionalized individuals. Neurology. 1995;45:705–708. doi: 10.1212/wnl.45.4.705. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattendorf E, Welge-Lussen A, Fiedler K, Bilecen D, Wolfensberger M, Fuhr P, Hummel T, Westermann B. Olfactory impairment predicts brain atrophy in Parkinson’s disease. J Neurosci. 2009;29:15410–15413. doi: 10.1523/JNEUROSCI.1909-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memeory Scale. 3. The Psychological Corporation; New York: 1997. [Google Scholar]
- Weintraub D, Doshi J, Koka D, Davatzikos C, Siderowf AD, Duda JE, Wolk DA, Moberg PJ, Xie SX, Clark CM. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol. 2011;68:1562–1568. doi: 10.1001/archneurol.2011.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund AH, Johnson N, Weintraub S. Preservation of reasoning in primary progressive aphasia: further differentiation from Alzheimer’s disease and the behavioral presentation of frontotemporal dementia. J Clin Exp Neuropsychol. 2004;26:347–355. doi: 10.1080/13803390490510077. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(Suppl 1):173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Zarei M, Ibarretxe-Bilbao N, Compta Y, Hough M, Junque C, Bargallo N, Tolosa E, Marti MJ. Cortical thinning is associated with disease stages and dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2013;84:875–881. doi: 10.1136/jnnp-2012-304126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ, Gordon MF, Feigin A, Eidelberg D. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson’s disease. J Clin Exp Neuropsychol. 2006;28:1127–1144. doi: 10.1080/13803390500246910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan W, Kang GA, Glass GA, Zhang Y, Shirley C, Millin R, Possin KL, Nezamzadeh M, Weiner MW, Marks WJ, Jr, Schuff N. Regional alterations of brain microstructure in Parkinson’s disease using diffusion tensor imaging. Mov Disord. 2012;27:90–97. doi: 10.1002/mds.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Yu C, Zhang Y, Wu X, Zhu C, Chan P, Li K. Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson’s disease. Eur J Radiol. 2011;77:269–273. doi: 10.1016/j.ejrad.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson’s disease. Hum Brain Mapp. 2014;35:1325–1333. doi: 10.1002/hbm.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]