Abstract
Objective
We investigate the patterns of failure in the treatment of glioblastoma (GBM) based on clinical target volume (CTV) margin size, dose delivered to the site of initial failure, and the use of temozolomide and intensity modulated radiotherapy (IMRT).
Methods
Between August 2000 and May 2010, 161 patients with GBM were treated with radiotherapy with or without concurrent temozolomide. Patients were treated with CTV expansions that ranged from 5–20 mm using a shrinking field technique. Patterns of failure and time to progression and overall survival were compared based on CTV margin, use of temozolomide, and use of IMRT. Kaplan Meier analysis was used to estimate survival times, and chi-square test was used for comparison of cohorts.
Results
For patients treated with 5, 10, and 15–20 mm CTV, 79%, 77%, and 86% experienced failures in the 60 Gy volume, respectively. 48%, 55%, and 66% of patients with 5, 10, and 15–20 mm CTV experienced failures in the 46 Gy volume, respectively. There was no statistical difference between patients treated with 5, 10, 15–20 mm margins with regard to 60 Gy failure (p=0.76), 46 Gy failure (p=0.51), or marginal failure (p=0.73). 80% of patients receiving temozolomide experienced failures in the 60 Gy volume. There was no increased likelihood of marginal failures in patients receiving IMRT (p=0.97).
Conclusions
Modern treatment techniques including use of concurrent temozolmide, limited CTV margin size, and IMRT have not greatly changed the patterns of failure of GBM.
Introduction
Radiation therapy fields and treatment volumes for glioblastoma (GBM) have evolved since the 1970's when whole brain radiotherapy was considered to be standard therapy for patients with GBM. After multiple series including the Brain Tumor Cooperative Group 80-01 randomized trial showed that patients who received a total brain dose of 60 Gy still failed within the highest dose region. As a result, it became standard to treat GBM with sub-whole brain volumes(1). The advantage of smaller volumes is the potential to better avoid toxicities such as radionecrosis and cognitive decline(2, 3).
The radiation treatment volumes utilized for GBM have varied amongst multiple cooperative groups. The European Organization for Research and Treatment of Cancer (EORTC) has used 2–3 cm dosimetric margins around enhancing disease on MRI because 80–90% of treatment failures have occurred within this margin(4). The Radiation Therapy Oncology Group (RTOG) has used margins based on data obtained from biopsy studies which have shown tumor extension into peritumoral edema(5). Hence, RTOG studies call for 2 cm margins beyond the extent of peritumoral edema, followed by a boost volume treating enhancing disease with its own margin. Since 2004, several trials from the New Approaches to Brain Tumor Therapy (NABTT) consortium have used margins as small as a 5mm clinical target volume (CTV) in the treatment of GBM(6).
Optimal radiation margins for GBM are currently being revisited. The EORTC 26981 trial recently showed a significant survival benefit for the use of concurrent and adjuvant temozolomide with standard radiotherapy(4). However, patterns of failure have been rarely re-evaluated since the standard of care has changed to incorporate temozolomide. As such, it remains unclear if and how temozolomide affects the pattern of failure of glioblastoma as compared to radiotherapy alone. Furthermore, newer radiation techniques including intensity modulated radiotherapy (IMRT) use steeper dose gradients to spare critical structures such as the optics and brain stem. Whether these steeper dose gradients change failure patterns in the setting of chemoradiotherapy is not known.
We attempted to analyze patterns of failure of GBM that have been treated during an era in which standards of care and treatment modalities have evolved. Moreover, we paid particular attention to whether the use of limited CTV margins, IMRT, or temozolomide-based chemotherapy changed failure patterns by leading to increasing failure rate outside of the highest dose radiation volume.
Methods
Data Acquisition and Patient Characteristics
This study was approved by the Wake Forest University Institutional Review Board. The Wake Forest University Radiation Oncology Database was searched for patients with diagnosis of GBM who were treated at our institution with radiation therapy. Patients receiving fewer than the standard six week course of radiotherapy and those who were unable to undergo magnetic resonance imaging (MRI) were removed from the analysis. Between August 2001 and May 2010, 161 patients with GBM were treated with fractionated radiotherapy with or without chemotherapy at the Wake Forest University Comprehensive Cancer Center. The CTV margins used for individual patients were based upon physician discretion unless patients were enrolled on a clinical trial, in which case the CTV margins were determined based on the guidelines of the trial. The heterogeneity of CTV margins used in our study was due to the fact that patients were treated over a 10 year period by four radiation oncologists.
Patient characteristics and clinical outcome measures were determined using patients’ electronic medical records and paper charts. Patient characteristics that were collected included age, sex, race, extent of surgery, use of concurrent and adjuvant chemotherapy, radiation dose and fractionation, and use of IMRT. CTV margins were determined using archived plans from the Pinnacle Treatment Planning System (Philips Healthcare, Andover, MA). A summary of patient characteristics is found in Table 1.
Table 1.
Patient Characteristics
| Characteristic | Number (range) |
|---|---|
| Age (years) | 61 (14–83) |
| Sex | |
| Female | 60 |
| Male | 101 |
| Resection Type | |
| Gross Total Resection (GTR) | 78 |
| Sub-Total Resection (STR) | 42 |
| Biopsy (Bx) | 41 |
| CTV Margin Size | |
| 5 mm | 34 |
| 10 mm | 84 |
| >10 mm | 43 |
| RT Modality | |
| 3D Conformal | 123 |
| IMRT | 38 |
| Chemotherapy | |
| Temodar-based | 108 |
| Non-Temodar-based | 8 |
| None | 45 |
Patient Follow-up and Response Assessment
Patients were followed clinically by a multi-disciplinary team and with serial MRI scans. Imaging was generally performed one month after completion of radiotherapy, and then every 2 months for 3 consecutive scans. Imaging was subsequently performed every 3 months or earlier if patients developed new or progressive symptoms. Independent imaging review was conducted for each patient by A.P. and a faculty radiation oncologist to determine individual dates of treatment failure. Dates of failure were determined using the Response Assessment in Neuro-Oncology (RANO) criteria as published by Wen et al(7). Exceptions to dates of failure being defined by the RANO criteria were cases of pseudoprogression, which were defined as an increase in tumor enhancement that later subsides without further treatment as previously described by Taal et al(8). These dates of failure were determined by the RANO criteria based on evidence of failure after pseudoprogression.
Radiotherapy dosimetry data were obtained from archived plans using the Pinnacle treatment planning system. Treatment planning computed tomographic (CT) images containing isodose volumes from patients’ treatment were co-registered with the MRI from the date of failure. Location of treatment failure was categorized into the following cohorts: failure within the 60 Gy volume, failure beyond the 60 Gy volume but within the 46 Gy volume, marginal failure (within 2 cm from the 46 Gy volume), and distant (beyond 2 cm from the 46 Gy volume). Figure 1 illustrates 2 examples of treatment failure with isodose curves from the radiation treatment plan overlaid onto MRI at time of failure.
Figure 1.
A. Axial Spoiled Gradient Recalled (SPGR) MRI 6 weeks post-chemoradiotherapy showing marginal and distant failure. Internal white line represents the 60 Gy volume, and the external white line represents 46 Gy volume. 1B. Axial SPGR 6 months post-chemoradiotherapy showing 46 Gy failure. Internal white line represents the 60 Gy volume, and the external white line represents 46 Gy volume.
Statistics
Kaplan Meier analysis was performed to estimate survival times. Log rank test was used to compare survival times between populations. Chi Squared contingency analysis was performed to determine differences in patterns of failure between cohorts. All statistics was performed using the SPSS program.
Results
Patterns of Failure
Patterns of failure data are summarized in Table 2. For patients treated with 5, 10, and 15–20 mm CTV, 79%, 77%, and 87% experienced a component of first failure in the 60 Gy volume, respectively. For patients treated with 5, 10, and 15–20 mm CTV, 48%, 55%, and 66% experienced a component of first failure in the 46 Gy volume, respectively. For patients treated with 5, 10, and 15–20 mm CTV, 41%, 47%, and 55% experienced a marginal failure as a component of first failure, respectively. There was no statistical difference between patients treated with 5, 10, 15–20 mm margins with regard to 60 Gy failure (p=0.76), 46 Gy failure (p=0.51), or marginal failure (p=0.73).
Table 2.
Patterns of Failure
| Patterns of Failure: Role of Margin Size | 5 mm | 10 mm | >10mm |
|---|---|---|---|
| Total Patients Experiencing Failure | 29 | 78 | 38 |
| 60 Gy Failure | 23 (79%)* | 60 (77%) | 33 (87%) |
| 46 Gy Failure | 14 (48%) | 43 (55%) | 25 (66%) |
| Marginal Failure | 12 (41%) | 37 (47%) | 21 (55%) |
| Distant Failure | 1 (3%) | 6 (8%) | 2 (5%) |
| No failure at last follow-up | 5 | 6 | 5 |
| Patterns of Failure: RT vs. ChemoRT | RT | TMZ RT | Non-TMZ ChemoRT |
| Total Patients Experiencing Failure | 42 | 95 | 8 |
| 60 Gy Failure | 34 (81%) | 76 (80%) | 6 (75%) |
| 46 Gy Failure | 26(62%) | 50 (54%) | 6 (75%) |
| Marginal Failure | 21 (50%) | 48 (51%) | 1 (13%) |
| Distant Failure | 0 (0%) | 9 (9%) | 0 (0%) |
| No failure at last follow-up | 3 | 13 | 0 |
| Patterns of Failure: 3D-CRT vs. IMRT | 3D-CRT | IMRT | |
| Total Patients Experiencing Failure | 110 | 35 | |
| 60 Gy Failure | 86 (78%) | 30 (86%) | |
| 46 Gy Failure | 62 (56%) | 20 (57%) | |
| Marginal Failure | 53 (48%) | 17 (49%) | |
| Distant Failure | 5 (5%) | 4 (11%) | |
| No Failure at last follow-up | 13 | 3 |
Represents percentage of total failures excluding patients that have not experienced prior treatment failure.
RT – radiotherapy, ChemoRT – chemoradiotherapy, TMZ – temozolomide, 3D-CRT – 3 dimensional conformal radiotherapy, IMRT – intensity modulated radiotherapy
For patients receiving concurrent temozolomide, 80% experienced failures within the 60 Gy volume, 54% experienced failures within the 46 Gy volume, and 51% experienced marginal failures. For patients receiving radiotherapy alone, 81% experienced failures within the 60 Gy volume, 62% experienced failures within the 46 Gy volume, and 50% experienced marginal failures. There was no statistical difference between patients receiving concurrent temozolomide versus patients who did not receive temozolomide with regard to 60 Gy failure (p=0.83), 46 Gy failure (p=0.41), or marginal failure (p=0.53). There was also no statistical difference between patients receiving IMRT versus 3D conformal radiotherapy with regarding to 60 Gy failure (p=0.32), 46 Gy failure (p=0.94), or marginal failure (p=0.97).
Survival
Kaplan Meier method was used to estimate progression free survival and overall survival for cohorts treated with the various CTV margins. Progression-free survival at 1 year was 38%, 42%, and 38% for patients treated with 5, 10, 15–20 mm CTV, respectively (log rank p=0.12). Overall survival at 1 year was 52%, 48%, and 38% for patients treated with 5, 10, 15–20 mm CTV, respectively (log rank, p=0.256).
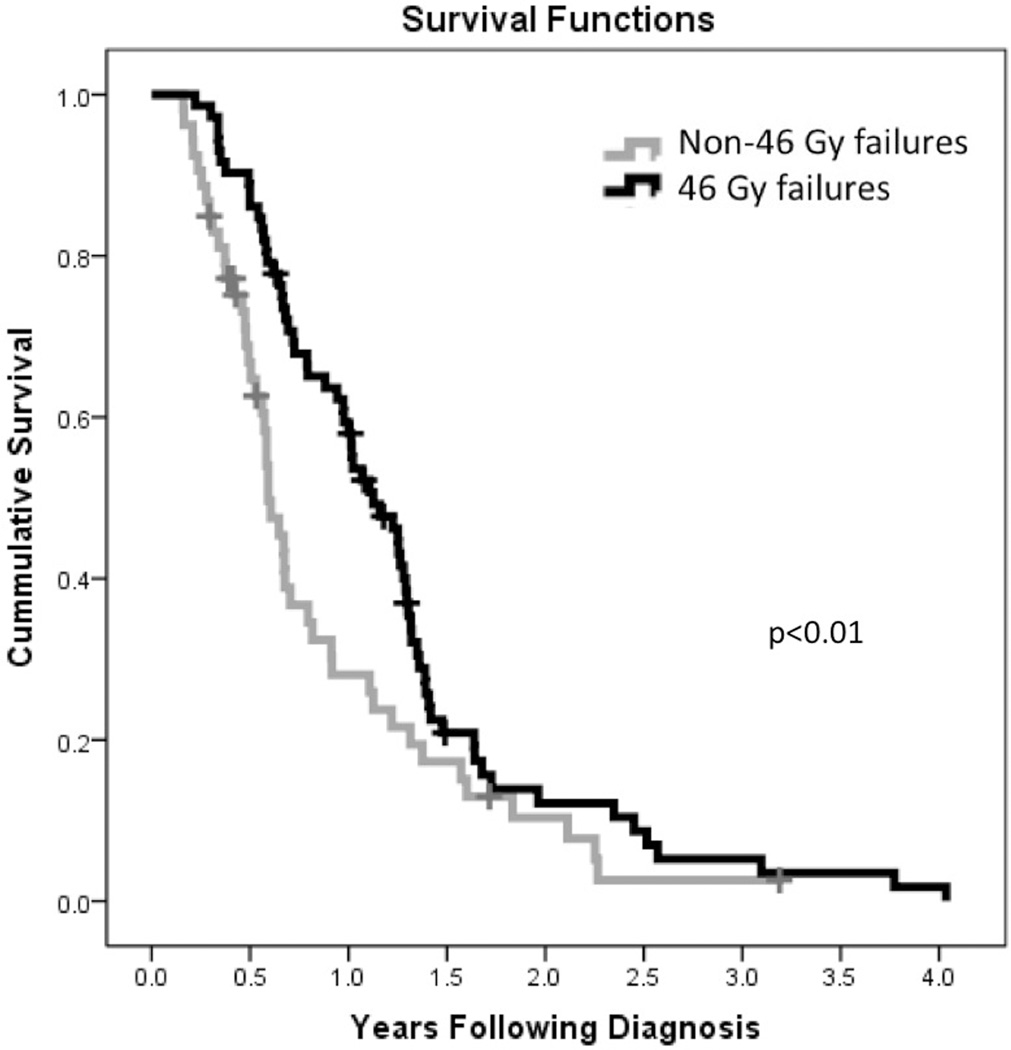
Patients who experienced failure within the 46 Gy volume had improved overall survival over patients with 60 Gy volume failures (411 vs 216 days, p < 0.01). Kaplan Meier plot of patients with 46 Gy vs 60 Gy failures is depicted in Figure 2.
Figure 2.
Kaplan Meier plot of survival for patients experiencing failure in 46 Gy volume and 60 Gy volume.
Discussion
The historical pattern of GBM treatment failure has been predominantly local in patients treated with radiotherapy alone as approximately 80% of patients experienced tumor re-growth within 2 cm of the original tumor (9). Multiple randomized trials over several decades using such strategies as altered fractionation(10, 11), dose escalation(12) and radiation sensitizers(13) were unable to change the fact that GBM most commonly fails in the high dose region of the radiation field. A phase II study from the Massachusetts General Hospital (14) using combined photon and proton radiotherapy treated tumors to a total dose of 90 cobalt Gray equivalent (CGE) and prevented tumor regrowth in the 90 CGE volume in all but a single patient. Tumor regrowth was most commonly seen in the volumes receiving 60–70 CGE. While this was a small study with results that have yet to be reproduced, there was at least the suggestion that some radiation dose threshold potentially existed which could eradicate GBM. Toxicity concerns remain as a significant proportion of patients will experience radionecrosis with dose escalation.
In 2005, Stupp et al published the results of the EORTC 26981 trial showing that overall survival in patients receiving concurrent and adjuvant temozolmide was significantly improved over patients treated with radiotherapy alone (4). Temozolomide acts as an alkylating agent, creating O6-methyl guanine within DNA strands within the tumor, and likely potentiating the DNA-damaging effects of radiotherapy. Synergism of concurrent chemotherapy and radiotherapy has been seen in other cancer histologies with improvements seen in both local control and overall survival(15, 16). As the standard of care has changed for GBM, re-assessment of patterns of failure data is necessary to determine if temozolomide has affected these patterns. However, local control is more difficult to evaluate in GBM treated with temozolomide because of the phenomenon of pseudoprogression. In fact, while the EORTC 26981 trial showed a dramatic improvement in overall survival, progression-free survival was essentially equivalent between the two arms because of pseudoprogression. As a result, the RANO criteria have been proposed as a means of normalizing across studies the definition of treatment failure(7). The RANO criteria were used in the current study to determine the date of treatment failure.
The NABTT consortium has used 5 mm CTV margins in several phase II studies since chemoradiotherapy with temozolomide became the standard of care for GBM. Grossman et al published the survival results of three phase II studies conducted by NABTT since the advent of temozolomide with each study showing a significant improvement in survival over the chemoradiotherapy arm of the EORTC 26981 trial(17). While this apparent improvement can be derived from a combination of patient selection bias, improving care of patients receiving temozolomide, and improving salvage regimens, these results nonetheless suggested that the smaller NABTT radiotherapy margins are likely not inferior to larger margins used in the EORTC study. There have been two series from NABTT institutions assessing patterns of failure of glioblastoma treated with chemoradiotherapy with CTV margins as small as 5 mm(6, 18). Both of these series show that the predominant local pattern of treatment failure remains unchanged with the use of smaller CTV margins. Our current study showed that the use of smaller CTV margins did not affect pattern of failure either with the addition of temozolomide or IMRT in agreement with previous findings.
The standard CTV margins for patients with GBM are likely to continue to evolve over time. The RTOG has used 2 cm CTV margins around peri-tumoral edema followed by a cone down volume encompassing the enhancing tumor volume with a 2.5 cm CTV margin. Conversely, the EORTC uses 2–3 cm surrounding the tumor volume without any cone down. Recent multi-institutional randomized trials including the RTOG 0525 and EORTC CENTRIC trials have stratified patients based on whether patients received the RTOG or EORTC CTV margins. Preliminary results of RTOG 0525 were recently presented and showed no progression-free survival or overall survival advantages of using RTOG field-in-field CTV margins or EORTC margins that use no boost volume(19). The EORTC CENTRIC trial has completed accrual in 2011, but has of yet reported its results.
In the present series, patients who experienced failures within the 46 Gy volume also experienced improved overall survival. This finding suggests that deviations from the predominant pattern of local failure may be due more to the biology of the tumor than to the radiation treatment volumes. Recent evidence has emerged that GBM is a genetically diverse tumor. The epigenetic silencing of O6-methylguanine-DNA methyltransferase (MGMT), a DNA repair enzyme, renders tumors more sensitive to damage caused by temozolomide(20). It is possible that tumors that exhibit failures within the 46 Gy volume could represent MGMT-methylated tumors that are more chemo- and radiosensitive tumors. This has previously been suggested in a publication by Brandes et al that patients with MGMT methylated tumors have an increased rate of failure outside of the highest dose radiation volume and that these failures occurred generally after a more prolonged interval(21). Moreover, 46 Gy volume failures could represent a failure to eradicate tumor in the lower dose regions. Recent microarray analysis has elucidated four separate molecular subtypes of GBM that have biologically distinct behaviors insofar as their genetic signature and their survival times(22). It remains to be studied, however, as to whether biologic differences are linked to patterns of failure.
Our report represents the third and largest series documenting no differences in patterns of failure in patients receiving limited margins for GBM. However, there are several limitations of this study. As a retrospective series, there is potential for patient selection bias. Patients treated with 5mm margins were generally done so under the auspices of a NABTT clinical trial. While there were multiple trial drugs used during the time period of the current study, there exists the possibility that the trial drugs improved treatment of microscopic disease and allowed for treatment with smaller CTV margins without any worsening of marginal treatment failures. This is also a criticism of the previous two published trials using limited CTV margin size. Moreover, molecular data such as MGMT methylation status was unknown in our series because this testing was not commercially available for the majority of the time during which the patients in this series were diagnosed. This lack of molecular data prevents us from stratifying patients based on molecular subtype and determining if marginal failures may have been more prevalent in a subtype more sensitive to radiotherapy. Prospective data such as that which will become available with the analyses of the RTOG 0525 and EORTC CENTRIC studies will help to determine if there are differences in patterns of failure based on molecular differences in GBM.
Conclusion
Modern treatment techniques including use of concurrent temozolmide, limited CTV margin size, and IMRT have not greatly changed the patterns of failure of GBM. Use of CTV margins as small as 5mm does not appear to lead to an increase in marginal failures.
References
- 1.Shapiro WR, Green SB, Burger PC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg. 1989;71:1–9. doi: 10.3171/jns.1989.71.1.0001. [DOI] [PubMed] [Google Scholar]
- 2.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 3.Torres IJ, Mundt AJ, Sweeney PJ, et al. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology. 2003;60:1113–1118. doi: 10.1212/01.wnl.0000055862.20003.4a. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Halperin EC, Bentel G, Heinz ER, et al. Radiation therapy treatment planning in supratentorial glioblastoma multiforme: an analysis based on post mortem topographic anatomy with CT correlations. Int J Radiat Oncol Biol Phys. 1989;17:1347–1350. doi: 10.1016/0360-3016(89)90548-8. [DOI] [PubMed] [Google Scholar]
- 6.McDonald MW, Shu HK, Curran WJ, Jr, et al. Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int J Radiat Oncol Biol Phys. 79:130–136. doi: 10.1016/j.ijrobp.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 7.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 8.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 9.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 10.Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin C, Scott C, Langer C, et al. Phase II, two-arm RTOG trial (94-11) of bischloroethyl-nitrosourea plus accelerated hyperfractionated radiotherapy (64.0 or 70.4 Gy) based on tumor volume (>20 or < or = 20 cm(2), respectively) in the treatment of newly-diagnosed radiosurgery-ineligible glioblastoma multiforme patients. Int J Radiat Oncol Biol Phys. 2000;48:1351–1358. doi: 10.1016/s0360-3016(00)01412-7. [DOI] [PubMed] [Google Scholar]
- 12.Nelson DF, Diener-West M, Horton J, et al. Combined modality approach to treatment of malignant gliomas--re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: a joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988:279–284. [PubMed] [Google Scholar]
- 13.Fulton DS, Urtasun RC, Shin KH, et al. Misonidazole combined with hyperfractionation in the management of malignant glioma. Int J Radiat Oncol Biol Phys. 1984;10:1709–1712. doi: 10.1016/0360-3016(84)90533-9. [DOI] [PubMed] [Google Scholar]
- 14.Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91:251–260. doi: 10.3171/jns.1999.91.2.0251. [DOI] [PubMed] [Google Scholar]
- 15.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 16.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 17.Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobelbower MC, Burnett Iii OL, Nordal RA, et al. Patterns of failure for glioblastoma multiforme following concurrent radiation and temozolomide. J Med Imaging Radiat Oncol. 55:77–81. doi: 10.1111/j.1754-9485.2010.02232.x. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert MR, Wang M, Aldape K, et al. RTOG 0525: A Randomized Phase III Trial Comparing Standard Adjuvant Temozolomide With a Dose-Dense Schedule in Newly Diagnosed Glioblastoma. J Clin Oncol. 2011:29. [Google Scholar]
- 20.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 21.Brandes AA, Tosoni A, Franceschi E, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol. 2009;27:1275–1279. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 22.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]