Abstract
Background
There have been few prospective controlled studies of kidney donors.
Understanding the pathophysiological effects of kidney donation is important for judging donor safety and for improving our understanding of the consequences of reduced kidney function in chronic kidney disease.
Study Design
Prospective, controlled, observational cohort study.
Setting & Participants
Three-year follow-up of kidney donors and paired controls suitable for donation at their donor’s center.
Predictor
Kidney donation.
Outcomes
Medical history, vital signs, glomerular filtration rate and other measurements at 6, 12, 24 and 36 months after donation.
Results
At 36 months, 182 of 203 (89.7%) original donors and 173 of 201 (86.1%) original controls continue to participate in follow-up visits. The linear slope of the glomerular filtration rate measured by plasma iohexol clearance declined 0.36±7.55 mL/min per year in 194 controls, but increased 1.47±5.02 mL/min per year in 198 donors (P = 0.005) between 6 and 36 months. Blood pressure was not different between donors and controls at any visit, and at 36 months all 24-hour ambulatory blood pressure parameters were similar in 126 controls and 135 donors (mean systolic: 120.0±11.2 [SD] v. 120.7±9.7 mmHg [P=0.6]; mean diastolic: 73.4±7.0 v. 74.5±6.5 mmHg [P=0.2]). Mean arterial pressure nocturnal dipping was manifest in 11.2%±6.6% of controls and 11.3%±6.1% donors (P=0.9). Urinary protein-creatinine and albumin-creatinine ratios were not increased in donors compared to controls. From 6 to 36 months post-donation, serum parathyroid hormone, uric acid, homocysteine and potassium levels were higher, whereas hemoglobin was lower in donors compared to controls.
Limitations
Possible bias resulting from an inability to select controls screened to be as healthy as donors, short follow-up duration, and drop-outs.
Conclusions
Kidney donors manifest several of the findings of mild chronic kidney disease. However, at 36 months after donation, kidney function continues to improve in donors while controls have expected age-related declines in function.
INDEX WORDS: Chronic kidney disease (CKD), renal insufficiency, unilateral nephrectomy, glomerular filtration rate (GFR), kidney function, patient safety, parathyroid hormone (PTH), uric acid, homocysteine, potassium, hemoglobin, mineral and bone disorders, living kidney donation, kidney transplantation
Understanding the pathophysiological effects of kidney donation is important both for ensuring the safety of donors and for determining why mild reductions in kidney function are associated with cardiovascular disease and other adverse outcomes in the general population.1, 2 Studies of kidney donors have generally been of low quality.3 Most studies have been small, very few have been prospective, and identifying comparable, contemporaneous controls for donors has been problematic. We reported the immediate, short-term effects of kidney donation in a multicenter prospective study in which each living donor enrolled with a comparable healthy control.4 We now report the results of the first 36 months of follow-up.
METHODS
Human Subject Protections
Informed consent was obtained from each participant. The study was approved by the institutional review board at each participating site (University of Minnesota no. 0503M67993).
Study Design
In this prospective observational cohort study, donors and controls were enrolled before donation. Details of study design and acute changes from pre-donation to 6 months have previously been described in detail.4 Briefly, kidney donors were enrolled after acceptance for donation, but before donation had taken place. For every donor that was enrolled, a control also was enrolled at the same site. However, in some cases donors did not actually donate, and replacements were recruited. The target enrollment was 200 donor and control pairs, or 400 participants. Only donors who donated and completed at least one post-donation follow-up visit were analyzed. Controls were required to meet the same donor eligibility criteria as donors at that site. However, controls did not undergo renal imaging or any invasive testing. Donors and controls were scheduled to complete a pre-donation visit, and visits at 6, 12, 24, and 36 months after donation. The laboratory measurements obtained were those reported in the accompanying tables, and details on methods for measurement have previously been reported.4 We now report visits at 6, 12, 24 and 36 months after donation. None of the data in this report extend beyond 36 months post-donation.
Data Collected
Participants were evaluated in the Clinical Research Center at each participating site. Blood pressure (BP) was measured 3 times at 1 minute intervals after subjects were seated and resting for at least 5 minutes using a standard protocol. At 36 months, 24-hour ambulatory BP recordings were also obtained using an automated recording device (Spacelabs Inc, Redmond, WA). Laboratory tests were measured in a central laboratory as previously described.4
An iohexol plasma decay method was used to determine measured glomerular filtration rate (mGFR).4 Glomerular filtration rate was also estimated (eGFR) using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatine equation, a 4-variable formula.5 In addition, glomerular filtration rate was estimated with the 4-variable CKD-EPI cystatin C equation, and by the CKD-EPI creatinine–cystatin C equation.6
Statistical Analysis
The pre-specified primary endpoint was the difference between donors and controls of the slope of the mGFR between 6 and 36 months after donation. The effect of age on the difference in slope of mGFR between donors and controls was analyzed with a generalized linear mixed-effects model. Multiple, secondary endpoints included eGFR, BP, and laboratory parameters as previously described.4 Differences between groups and visits were assessed using analysis of variance with repeated measures (generalized linear mixed-effects models). This analysis assessed the independent effects of donors versus controls; visits at 6, 12, 24 and 36 months; and the interaction between these two effects. No adjustment was made for multiple comparisons. Results are expressed as mean ± standard deviation unless otherwise indicated and were considered statistically significant for P < 0.05. Variables that were not normally distributed were logarithmically transformed for analysis, but results were expressed as the median and interquartile range (IQR; not logarithmically transformed). Differences in categorical variables between groups and among visits were assessed with Chi-Square. All analyses were carried out with SAS 9.2 for the personal computer (SAS Institute Inc, Cary, NC).
RESULTS
Participant Characteristics
At 36 months, 182 of 203 (89.7%) original study donors and 173 of 201 (86.1%) original controls had follow-up visits. Age, sex, race/ethnicity, height, weight, body mass index, hip circumference and waist circumference were not different between donors and controls (Table S1, available as online supplementary material). The only statistically significant difference in medication use between donors and controls was that non-steroidal anti-inflammatory drugs were used less commonly in donors than in controls; 2.5% v. 6.6% (P = 0.05) at 6 months and 3.0% v. 8.3% (P = 0.02) at 12 months, in donors and controls respectively (Table S2).
Blood Pressure and Heart Rate
Both systolic and diastolic BP increased slightly but significantly over time, but there were no differences between donors and controls (Table 1 and Table S3). At the 36 month visit, 135 of 182 (74.2%) donors and 126 of 173 (72.8%) controls had 24 hour ambulatory BP measurements (Table 2). There were no statistically significant differences between donors and controls in any of the 24 hour ambulatory BP parameters.
Table 1.
Heart rate and blood pressure.
| Test | Group | Visit (Time after donation) | P-Valuesa | |||||
|---|---|---|---|---|---|---|---|---|
| 6 Mo | 12 Mo | 24 Mo | 36 Mo | Donors vs Controlsb |
Visitc | Interactiond | ||
| Heart Rate (bpm) | Controls | 66.3±10.0 (198) |
66.6±10.3 (193) |
67.0±9.3 (180) |
66.7±9.7 (169) |
0.9 | 0.1 | 0.7 |
| Donors | 66.3±9.6 (200) |
66.6±9.5 (196) |
66.9±10.0 (184) |
66.6±9.2 (181) |
||||
| Systolic BP (mm Hg) | Controls | 115.7±12.2 (198) |
116.2± 11.8 (193) |
117.2±13.3 (180) |
117.3±12.8 (170) |
0.6 | <0.001 | 0.8 |
| Donors | 115.2±11.3 (200) |
116.4±12.4 (196) |
116.2±11.6 (184) |
117.5±12.0 (182) |
||||
| Diastolic BP (mm Hg) | Controls | 70.0±8.5 (198) |
70.1±9.0 (193) |
71.0±9.1 (180) |
71.6±8.5 (170) |
0.7 | <0.001 | 0.8 |
| Donors | 70.4±8.5 (200) |
70.3±8.6 (196) |
70.7±8.3 (184) |
72.1 ±8.4 (182) |
||||
| Pulse Pressure (mm Hg) | Controls | 45.7±8.8 (198) |
46.2±8.4 (193) |
46.2±9.7 (180) |
45.7±8.7 (170) |
0.3 | 0.9 | 0.6 |
| Donors | 44.8±8.2 (200) |
46.1±8.6 (196) |
45.5±8.4 (184) |
45.4±8.9 (182) |
||||
Note: Values are given as mean ± standard deviation (number sampled).
BP, blood pressure
Analysis of variance with repeated measures. Each variable was analyzed separately and no adjustment was made for multiple comparisons. Values not normally distributed were logarithmically transformed before analysis.
Donors versus controls P values test overall differences between donors and controls.
Visit P values test differences between the 4 visits.
Interaction P values test the interaction between donors versus controls and between visits.
Table 2.
Twenty-four hour ambulatory blood pressure results at 36 months.
| Parameter | Donors (n=135) | Controls (n=126) | P-Valueb |
|---|---|---|---|
| Duration of recording (h) | 24.4±11.5 | 25.1±9.9 | 0.6 |
| No. of measurements | 43.8±15.4 | 45.4±16.6 | 0.4 |
| Systolic BP (mm Hg) | 120.7±9.7 | 120.0±11.2 | 0.6 |
| Diastolic BP(mm Hg) | 74.5±6.5 | 73.4±7.0 | 0.2 |
| MAP (mm Hg) | 89.9±6.8 | 88.7±7.3 | 0.2 |
| Pulse pressure (mm Hg) | 46.3±7.3 | 46.7±8.6 | 0.7 |
| Heart rate (bpm) | 73.5±9.1 | 71.6±8.8 | 0.09 |
| Systolic BP dip (%) | 9.2±5.4 | 8.4±6.3 | 0.3 |
| Diastolic BP dip (%) | 13.7±7.3 | 13.2±7.4 | 0.6 |
| MAP dip (%) | 11.3±6.1 | 11.2±6.6 | 0.9 |
| cHigh systolic BP (%) | 18.8±21.9 | 19.8±24.2 | 0.8 |
| dHigh diastolic BP (%) | 22.2±19.4 | 16.8±19.2 | 0.07 |
Note: Values are given as mean ±standard deviation.
BP, blood pressure; MAP, mean arterial pressure
T-test.
>135 mm Hg day time or > 120 mm Hg night time.
>85 mm Hg day time or >80 mm Hg night time.
Kidney Function
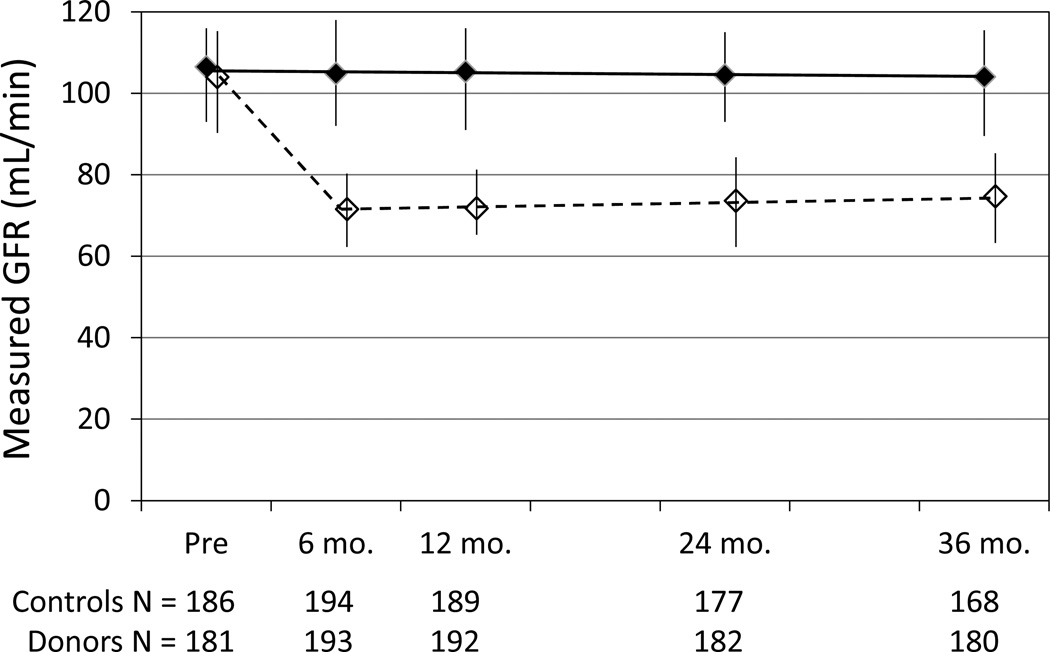
Both mGFR and eGFR declined in controls between 6 and 36 months, while they increased in donors (Table 3). As a result, there was a statistically significant difference between change in kidney function (slopes) between donors and controls (Table 4 and Figure 1). The effect of donation on rate of change in mGFR did not differ by age (Table 5). Urine total protein was not different between visits or between donors and controls (Table 3). The urine albumin-creatinine ratio was lower in donors versus controls, but tended to increase in donors but not controls (Table 3).
Table 3.
Kidney function at 6, 12, 24, and 36 months after kidney donation.
| Test | Group | Visit (Time after donation) | P-Valuesa | |||||
|---|---|---|---|---|---|---|---|---|
| 6 Mo | 12 Mo | 24 Mo | 36 Mo | Donors vs Controlsb |
Visitc | Interactiond | ||
| mGFR (mL/min) | Controls | 104.9±20.2 (194) |
105.4±20.2 (189) |
104.5±19.7 (177) |
104.1 ±20.7 (168) |
<0.001 | 0.04 | <0.001 |
| Donors | 74.3±12.9 (193) |
74.5±13.3 (192) |
76.3±13.9 (182) |
77.5± 14.0 (180) |
||||
| mGFR (mL/min/1.73 m2) | Controls | 94.6±15.1 (194) |
94.8±15.3 (189) |
94.1 ±14.9 (177) |
93.2±14.6 (168) |
<0.001 | 0.4 | <0.001 |
| Donors | 67.6±10.1 (193) |
67.5±10.4 (192) |
69.4±10.5 (182) |
69.7±10.1 (180) |
||||
| Scr (mg/dL) | Controls | 0.80±0.17 (198) |
0.80±0.16 (193) |
0.80±0.15 (182) |
0.80±0.14 (173) |
<0.001 | <0.001 | <0.001 |
| Donors | 1.16±0.22 (199) |
1.15±0.22 (196) |
1.12±0.22 (185) |
1.10±0.23 (182) |
||||
| eGFRcr (mL/min/1.73 m2) | Controls | 99.1±16.0 (198) |
98.3±16.7 (193) |
97.9±15.2 (182) |
97.5± 14.6 (173) |
<0.001 | 0.007 | <0.001 |
| Donors | 65.5±13.1 (199) |
66.5±13.3 (196) |
68.0±14.3 (185) |
69.3± 14.6 (182) |
||||
| CysC (mg/dL) | Controls | 0.81±0.14 (198) |
0.81±0.13 (193) |
0.80±0.14 (182) |
0.81±0.13 (173) |
<0.001 | <0.001 | 0.008 |
| Donors | 1.11 ±0.17 (199) |
1.08±0.15 (196) |
1.07±0.15 (185) |
1.06±0.16 (182) |
||||
| eGFRcys (mL/min/1.73 m2) | Controls | 102.3±17.5 (198) |
102.3±15.9 (193) |
103.3±17.2 (182) |
101.6±16.5 (173) |
<0.001 | 0.02 | 0.01 |
| Donors | 71.6±15.3 (199) |
73.6± 14.8 (196) |
74.5±15.2 (185) |
75.2±16.3 (182) |
||||
| eGFRcr-cys (mL/min/1.73 m2) | Controls | 101.3±16.8 (198) |
100.7±15.3 (193) |
101.5±16.0 (182) |
100.3±15.3 (173) |
<0.001 | <0.001 | <0.001 |
| Donors | 67.4±12.6 (198) |
68.8±12.5 (196) |
70.1 ±13.1 (185) |
71.2±14.1 (182) |
||||
| Urea nitrogen (mg/dL) | Controls | 14.5±4.0 (198) |
14.5±4.1 (193) |
14.6±4.1 (182) |
14.5±3.7 (173) |
<0.001 | 0.5 | 0.7 |
| Donors | 18.0±4.4 (200) |
17.5±4.0 (196) |
17.7±4.4 (185) |
17.7±4.5 (182) |
||||
| Urine PCR (g/g) | Controls | 62 [50–128] (195) |
70 [50–106] (193) |
61 [49–100] (178) |
63 [47–122] (169) |
0.6e | 0.3e | 0.7e |
| Donors | 70 [50–116] (201) |
70 [50–116] (197) |
60 [46–114] (182) |
60 [48–111] (181) |
||||
| Urine ACR (mg/g) | Controls | 4.7 [3.4–7.1] (193) |
5.0 [3.5–7.7] (191) |
5.1 [3.6–7.2] (178) |
4.7 [3.4–7.3] (168) |
<0.001e | 0.002e | 0.001e |
| Donors | 3.6 [2.4–5.8] (198) |
3.5[2.4–6.1] (195) |
3.8 [2.8–6.6] (182) |
4.2 [2.7–7.1] (180) |
||||
Note: Values are given as mean ± standard deviation or median [interquartile range] (number sampled). Conversion factors for units: Scr in mg/dL to µmol/L, × 88.4; urea nitrogen in mg/dL to mmol/L, × 0.357.
Abbreviations and definitions: CysC, cystatin C; eGFRcr, estimated glomerular filtration rate calculated by Chronic Kidney Disease Epidemiology Collaboration creatinine equation; eGFRcr-cys, estimated glomerular filtration rate calculated by Chronic Kidney Disease Epidemiology Collaboration creatinine-cystatin C equation; eGFRcys, estimated glomerular filtration rate calculated by Chronic Kidney Disease Epidemiology Collaboration cystatin C equation; mGFR, measured glomerular filtration rate by iohexol plasma clearance; Scr, serum creatinine; ACR, albumin-creatinine ratio; PCR, protein-creatinine ratio.
Analysis of variance with repeated measures. Each variable was analyzed separately and no adjustment was made for multiple comparisons. Values not normally distributed were logarithmically transformed before analysis.
Donors versus controls P values test overall differences between donors and controls.
Visit P values test differences between the 4 visits.
Interaction P values test the interaction between donors versus controls and between visits.
Based on logarithmically transformed values.
Table 4.
Changes in kidney function over time.
| Measurement | Follow-up Duration (mo) |
Group | Rate of Change in Kidney Function |
P |
|---|---|---|---|---|
| mGFR (mL/min per y) | 12–36 | Controls | −0.36±7.55 (194) | 0.005 |
| Donors | 1.47±5.02 (198) | |||
| 36 | Controls | −0.19±5.31 (172) | 0.002 | |
| Donors | 1.30±3.49 (181) | |||
| mGFR (mL/min/1.73 m2 per y) | 12–36 | Controls | −0.44±7.35 (194) | 0.01 |
| Donors | 1.09±4.28 (198) | |||
| 36 | Controls | −0.39±4.81 (172) | 0.004 | |
| Donors | 0.84±3.09 (181) | |||
| eGFRcr (mL/min/1.73 m2 per y) | 12–36 | Controls | −1.04±6.16 (196) | <0.001 |
| Donors | 1.82±4.92 (200) | |||
| 36 | Controls | −0.46±3.68 (173) | <0.001 | |
| Donors | 1.60±3.75 (182) | |||
| eGFRcys (mL/min/1.73 m2 per y) | 12–36 | Controls | −0.33±7.36 (196) | 0.003 |
| Donors | 1.82±6.76 (200) | |||
| 36 | Controls | 0.16±4.68 (173) | 0.04 | |
| Donors | 1.21±5.06 (182) | |||
| eGFRcr-cys (mL/min/1.73 m2 per y) | 12–36 | Controls | −0.73±6.38 (196) | <0.001 |
| Donors | 1.89±4.58 (200) | |||
| 36 | Controls | −0.07±3.85 (173) | <0.001 | |
| Donors | 1.49±3.81 (182) |
Note: Unless otherwise indicated, changes in kidney function over time (“slopes”).are given as mean ± standard deviation (number sampled). Shown are slopes with and without dropping cases that did not have a 36 month visit.
Abbreviations and definitions: CysC, cystatin C; eGFRcr, estimated glomerular filtration rate calculated by Chronic Kidney Disease Epidemiology Collaboration creatinine equation; eGFRcr-cys, estimated glomerular filtration rate calculated by Chronic Kidney Disease Epidemiology Collaboration creatinine-cystatin C equation; eGFRcys, estimated glomerular filtration rate calculated by Chronic Kidney Disease Epidemiology Collaboration cystatin C equation; mGFR, glomerular filtration rate measured by iohexol plasma clearance.
T-test.
Figure 1.
Measured glomerular filtration rate (GFR) in controls (solid line) and donors (dashed line) before and 6, 12, 24, and 36 months after donation. Values are means and interquartile ranges.
Table 5.
Lack of association of age with changes in kidney function in donors and controls.
| Measurement | Age (y) | Group | Rate of Change in Kidney Function |
Pa | ||
|---|---|---|---|---|---|---|
| Donors vs Controlsb |
Younger vs Olderc |
Interactiond | ||||
| mGFR (mL/min per y) | < 45 | Controls | 0.08±9.46 (91) | 0.007 | 0.9 | 0.2 |
| Donors | 1.02±5.31 (89) | |||||
| ≥ 45 | Controls | −0.75±5.34 (103) | ||||
| Donors | 1.83±4.77 (109) | |||||
Note: Values are given as mean ± standard deviation (number sampled). Abbreviation: mGFR, glomerular filtration rate measured by iohexol plasma clearance.
Analysis of variance.
Donors versus controls P values test differences between donors and controls.
Younger versus older P values test difference in age between younger (<45 years) and older (≥45 years).
Interaction P values test differences between donors versus controls and age.
Laboratory Parameters
Hemoglobin concentrations were lower in donors compared to controls, but this difference appeared to narrow with duration of follow-up (Table 6). Serum albumin concentration, C-reactive protein (CRP) and fibrinogen concentrations were not different between donors and controls. Homocysteine, uric acid and serum potassium were each persistently higher in donors than in controls. Serum phosphorus was lower, while parathyroid hormone (PTH) was higher and serum calcium was not different in donors compared to controls. Total, low-density lipoprotein, and high-density lipoprotein cholesterol levels all increased slightly over time, but were not different in donors and controls. Triglycerides and lipoprotein (a) were also not different between donors and controls. Hemoglobin A1C and homeostasis model assessment of insulin resistance (HOMA-IR) all increased slightly but significantly during follow-up, in both groups, but none of the measures of glucose homeostasis were different between donors and controls.
Table 6.
Laboratory measurements at 6, 12, 24, and 36 months after kidney donation.
| Test | Group | Visit (Time after donation) | P-Valuesa | |||||
|---|---|---|---|---|---|---|---|---|
| 6 Mo | 12 Mo | 24 Mo | 36 Mo | Donors vs Controlsb |
Visitc | Interactiond | ||
| Hemoglobin (g/dL) | Controls | 13.6±1.4 (195) |
13.4±1.4 (191) |
13.6±1.2 (175) |
13.6±1.2 (173) |
0.003 | <0.001 | 0.02 |
| Donors | 13.2±1.2 (200) |
13.1 ±1.3 (197) |
13.4±1.3 (183) |
13.5±1.4 (172) |
||||
| Leukocyte count (/µL) | Controls | 6.0±1.7 (195) | 6.1 ±1.8 (190) | 6.0±1.6 (174) | 6.0±1.8 (157) | 0.1 | 0.6 | 0.8 |
| Donors | 5.8±1.5 (200) |
5.9±1.8 (196) |
5.7±1.5 (182) |
5.8±1.6 (169) | ||||
| Serum albumin (mg/dL) | Controls | 4.07±0.33 (198) |
4.03±0.30 (193) |
4.06±0.32 (182) |
4.02±0.27 (173) |
0.9 | 0.008 | 0.9 |
| Donors | 4.06±0.31 (200) |
4.03±0.30 (198) |
4.05±0.30 (185) |
4.00±0.27 (182) |
||||
| CRP (mg/dL) | Controls | 1.4 [0.6–3.1] (198) |
1.2 [0.5–2.8] (193) |
1.2 [0.5–2.6] (182) |
1.0 [0.6–2.4] (173) |
0.7e | 0.6e | 0.01e |
| Donors | 1.2 [0.7–2.9] (200) |
1.3 [0.6–2.5] (196) |
1.1 [0.6–2.5] (185) |
1.2 [0.6–3.0] (182) |
||||
| Fibrinogen (mg/dL) | Controls | 305±67 (198) |
306±74 (193) |
311±65 (182) |
306±67 (173) |
0.8 | 0.2 | 0.3 |
| Donors | 300±72 (198) |
310±66 (196) |
309±81 (185) |
309±70 (181) |
||||
| Homocysteine (mg/L) | Controls | 1.21 ±0.34 (196) |
1.21 ±0.37 (193) |
1.28±0.43 (182) |
1.23±0.38 (173) |
<0.001 | 0.6 | 0.05 |
| Donors | 1.49±0.43 (198) |
1.46±0.42 (196) |
1.50±0.42 (185) |
1.41 ±0.43 (182) |
||||
| Uric acid (mg/dL) | Controls | 4.9±1.2 (198) |
4.9±1.2 (193) |
4.9±1.2 (182) |
5.0±1.1 (173) |
<0.001 | <0.001 | 0.2 |
| Donors | 5.3±1.1 (200) |
5.2±1.2 (196) |
5.4±1.2 (185) |
5.5±1.3 (182) |
||||
| Serum potassium (mmol/L) | Controls | 4.14±0.32 (197) |
4.10±0.29 (187) |
4.12±0.31 (177) |
4.11 ±0.28 (172) |
0.006 | 0.1 | 0.9 |
| Donors | 4.20±0.29 (199) |
4.19±0.35 (193) |
4.20±0.32 (181) |
4.17±0.27 (178) |
||||
| Serum calcium (mg/dL) | Controls | 9.19±0.38 (198) |
9.18±0.42 (193) |
9.17±0.41 (182) |
9.21 ±0.40 (173) |
0.4 | 0.2 | 0.7 |
| Donors | 9.24±0.42 (200) |
9.18±0.41 (196) |
9.24±0.38 (185) |
9.26±0.40 (182) |
||||
| Serum phosphorus (mg/dL) | Controls | 3.49±0.48 (198) |
3.55±0.46 (190) |
3.52±0.46 (178) |
3.51 ±0.46 (172) |
<0.001 | 0.007 | 0.003 |
| Donors | 3.30±0.48 (200) |
3.37±0.51 (195) |
3.43±0.51 (182) |
3.42±0.51 (178) |
||||
| PTH (pg/mL) | Controls | 42.8±15.6 (198) |
42.4±16.7 (193) |
43.6±16.3 (182) |
43.2±17.5 (173) |
<0.001 | 0.7 | 0.3 |
| Donors | 52.7±20.9 (200) |
52.9±22.1 (196) |
51.7±20.6 (185) |
52.5±24.1 (182) |
||||
| Cholesterol (mg/dL) | Controls | 186±36 (197) |
185±37 (193) |
188±34 (182) |
190±35 (173) |
0.9 | 0.01 | 0.8 |
| Donors | 186±35 (199) |
184±32 (195) |
186±36 (185) |
188±35 (182) |
||||
| LDL cholesterol (mg/dL) | Controls | 111±30 (193) |
111±30 (190) |
113±29 (182) |
115±30 (172) |
0.7 | 0.03 | 0.1 |
| Donors | 110±31 (193) |
108±30 (194) |
109±30 (184) |
111±31 (180) |
||||
| HDL cholesterol (mg/dL) | Controls | 54.9±16.4 (195) |
54.5±15.9 (193) |
56.8±16.2 (182) |
56.2±16.0 (172) |
0.9 | <0.001 | 0.2 |
| Donors | 54.1 ±13.9 (197) |
55.1 ±14.2 (195) |
56.6±15.8 (185) |
56.5±15.0 (181) |
||||
| Triglycerides (mg/dL) | Controls | 80 [59–119] (197) |
77 [62–117] (193) |
78 [59–104] (182) |
76 [59–107] (173) |
0.1e | 0.1e | 0.6e |
| Donors | 84 [64–124] (199) |
81 [61–122] (195) |
84 [65–127] (185) |
89 [61–124] (182) |
||||
| Lipoprotein(a) (mg/dL) | Controls | 16.0 [5.0– 43.0] (198) |
15.0 [5.0–44.0] (193) | 15.0 [5.0– 42.0] (182) |
15.0 [5.0– 45.0] (173) |
0.3e | 0.9e | 0.4e |
| Donors | 20.0 [5.0– 54.5] (200) |
18.0 [5.0–51.5] (196) | 20.0 [11.0– 49.0] (185) |
18.5 [10.0– 45.0] (182) |
||||
| Hemoglobin A1c (%) | Controls | 5.3±0.35 (195) |
5.3±0.34 (190) | 5.4±0.34 (181) |
5.4±0.33 (173) |
0.1 | <0.001 | 0.5 |
| Donors | 5.3±0.31 (197) |
5.3±0.38 (191) | 5.3±0.32 (181) |
5.3±0.33 (181) |
||||
| Glucose (mg/dL) | Controls | 91.2±8.94 (197) |
91.1 ±8.66 (193) | 92.5±9.23 (182) |
93.2±9.03 (173) |
0.04 | <0.001 | 0.7 |
| Donors | 89.2±8.51 (199) |
90.7±11.4 (195) | 90.6±8.72 (185) |
91.4±8.78 (182) |
||||
| Insulin (pmol/L) | Controls | 39 [26–64] (192) |
36 [24–54] (188) | 39 [25–65] (174) |
42[29–64] (166) |
0.9e | <0.001e | 0.2e |
| Donors | 36 [24–54] (198) |
41 [24–66] (194) | 43[30–63] (183) |
43[31–66] (171) |
||||
| HOMA-IR | Controls | 1.5 [0.96–2.5] (191) |
1.4 [0.88–2.2] (188) |
1.5 [0.94–2.5] (174) |
1.6 [1.1–2.6] (166) |
0.7e | <0.001e | 0.2e |
| Donors | 1.4 [0.91– 2.1](197) |
1.4 [0.95– 2.5](193) |
1.7 [1.0–2.3] (183) |
1.6 [1.1–2.7] (171) |
||||
Note: Values are given as mean ± standard deviation or median [interquartile range] (number sampled). Conversion factors for units: fibrinogen in mg/dL to µmol/L, × 0.0294; homocysteine in mg/L to × µmol/L, × 7.397; uric acid in mg/dL to µmol/L, × 59.48; calcium in mg/dL to mmol/L, × 0.2495; phosphorus in mg/dL to mmol/L, × 0.3229; cholesterol in mg/dL to mmol/L, × 0.02586; triglycerides in mg/dL to mmol/L, × 0.01129; glucose in mg/dL to mmol/L, × 0.05551.
Abbreviations and definitions: CRP, C-reactive protein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance, where log(HOMA IR) =log[(insulin × glucose)/22.5], with insulin in µU/mL and glucose in mmol/L; LDL, low-density lipoprotein; mGFR, measured glomerular filtration rate (by iohexol plasma clearance); PTH, parathyroid hormone.
Analysis of variance with repeated measures. Each variable was analyzed separately and no adjustment was made for multiple comparisons. Values not normally distributed were logarithmically transformed before analysis.
Donors versus controls P values test overall differences between donors and controls.
Visit P values test differences between the 4 visits.
Interaction P values test the interaction between donors versus controls and between visits.
Based on logarithmically transformed values
DISCUSSION
Few prospective studies of living kidney donors have enrolled contemporaneous controls who are as healthy as donors. In the current study, a control was selected for each donor based on donation eligibility criteria used by the donor’s transplant program. The fact that medication use was similar in donors and controls is reassuring that both groups were equally healthy (Table S2). The lower use of non-steroidal anti-inflammatory drugs (excluding aspirin) among donors at 6 and 12 months after donation likely reflects admonitions of caregivers to avoid these agents. Most medication use was lower than that reported in the general population. For example, an antihypertensive agent was used in only 5.0% pre-donation and in 7.3% at 36 months, while in the general US adult population in 2007–2008, 26.1% (95% confidence interval [CI], 24.5%–27.8%) used an antihypertensive agent.7 Lipid-lowering medications were used by 15.9% (95% CI, 14.6%–17.1%) of US adults,7 whereas they were used in 7.5% of our participants pre-donation and in 12.1% at 36 months. Medication for diabetes was used in 7.1% (95% CI, 6.3%–8.0%) of US adults, but in none of the participants in the current study.
A major finding of this study is that the change in mGFR over time (slope) after donation was significantly different in donors and controls (Table 4). The gradual increase in mGFR among donors between 6 and 36 months is especially notable. Additional follow-up may help to determine how long function will continue to increase. If the increase is due to compensatory hypertrophy, then one might expect the increase would be greater in younger compared to older donors. However, this was not found to be the case, at least over the 3-year duration of follow-up post-donation (Table 5). We searched the literature for studies reporting post-transplantation change in mGFR and could locate only two with mGFR measured twice, the second time more than 1 year after donation. Saran, et al., measured chromium 51–labeled EDTA clearances in 47 donors at 10 and 20 years after donation from a starting cohort of 75 donors.8 They reported mGFR increased by a mean of 5.97±17.44 (standard deviation) mL/min/1.73m2 (P by paired t-test = 0.03) during this 10-year period. Tent, et al., compared mGFR (125I-iothalamate) and effective renal plasma flow (131I-hippuran) in 13 hypertensive and 26 normotensive donors at 2 months and 5 years post-donation from a starting cohort of 47 hypertensive and 94 normotensive donors.9 Changes in mGFR between 2 months and 5 years were not reported, but mGFR appeared to be similar between 2 months and 5 years in an accompanying figure.
In contrast to donors, controls exhibited a gradual decline in mGFR (Table 4). This too is notable because very few studies have examined serial changes in mGFR in normal individuals. The Baltimore Longitudinal Aging study is often cited as the definitive study showing that kidney function declines approximately 1 mL/min per year.10, 11 However that study used men and 24-hour urine creatinine clearances collected prospectively over time. Thus, the current prospective study of normal men and women with serial measurements of mGFR using iohexol clearance provides an important confirmation of the decline in kidney function with age in apparently healthy individuals.
This study also provides an opportunity to compare different equations and markers of changes in eGFR over time compared to changes in mGFR (Table 4). Most studies comparing eGFR equations with mGFR have been cross-sectional. The prospective measurement of GFR will help answer the important question of whether changes in eGFR over time accurately reflect changes in mGFR.
Another important finding in this study is the lack of difference in BP between donors and controls for the first 3 years after donation. Previous studies of BP in donors have produced conflicting results (Table S4). A meta-analysis of observational studies published in 2005 included 48 studies of BP in kidney donors.12 However, few of these studies included controls. In those that did, 10 years after donation systolic and diastolic BPs were reported to be, respectively, 6 and 4 mmHg higher in donors than in controls. Garg, et al., using claims data, reported that the incidence of hypertension was significantly higher in donors than in controls at a mean of 6.2 years after donation.13 They acknowledged that donors may have been followed up more closely than controls, which may have led to hypertension being diagnosed more often. Ibrahim et al. retrospectively studied 255 kidney donors matched to 255 controls from the National Health and Nutrition Examination Survey (NHANES).14 They reported that systolic BP was lower in donors compared to controls, while diastolic BP and the incidence of hypertension were not different between the two groups. Ethnicity may be an important determinant of the effects of kidney donation on BP, and Doshi, et al., reported BP to be higher in African American donors compared to African American controls.15 Similarly, using claims data from a private insurance database, Lentine, et al., reported that African American donors had an increased risk of hypertension compared to white donors.16
Few studies have reported 24-hour ambulatory BP in kidney donors, and none of these studies have included two-kidney controls (Table S5). In addition, follow-up after donation has been relatively short in most studies. Therefore, it is difficult from these studies to draw firm conclusions on the effects of donation on BP. Additional follow-up of the current cohort may be helpful in this regard.
Hyperuricemia has long been suggested to cause CKD,17–21 hypertension,22 diabetes,23, and cardiovascular disease24–26. However, the reverse is equally plausible, i.e. that hypertension, diabetes and cardiovascular disease cause hyperuricemia by reducing kidney function. The current study confirms our earlier observation that a reduction in GFR most likely causes an increase in serum uric acid,4 and shows that this increase persists for at least 36 months after donation. Rossi, et al., reported that in 42 donors urate increased from a mean of 0.29±0.08 mmol/L pre-donation to 0.34±.0.08 mmol/L at 1 year and 0.34±.0.08 mmol/L at 2 years post-donation (P<0.001 versus pre-donation at 1 and 2 years).27
There have been similar anecdotal reports of increased uric acid levels after donation.28 For example, Undurraga, et al., reported that among 74 kidney donors followed up for a mean of 10.9±4.5 years, uric acid levels >7.5 g/dL occurred in 30%.29 Hida, et al., reported that for 34 donors uric acid increased 24.3% from a mean of 4.78 ± 1.26 mg/dL before donation to 5.88 ± 1.40 mg/dL 6 months to 5 years after donation. 30 Romero, et al. followed up 8 donors for 6 months after donation and found no increase in uric acid.31
Previously, we found that between pre-donation and 6 months there was no discernible effect of donation on serum potassium.4 However, with longer follow-up there has been a small but statistically significant increase in serum potassium in donors compared to controls (Table 6). There were no changes in sodium, chloride, carbon dioxide, or urine pH (Table S6). To the best of our knowledge, this is the first report of donation affecting serum potassium. The increase is likely too small to be relevant clinically. However, the increase could be problematic if a donor were to be challenged with additional factors that also increase serum potassium.
Cross-sectional studies suggest that CKD is associated with abnormalities in glucose homeostasis and insulin resistance.32–37 However, in the present study there was no effect of donation on fasting glucose, hemoglobin A1C, insulin concentrations, or the calculated HOMA-IR. Similarly, there were no differences in lipid concentrations that often accompany changes in insulin resistance (Table 6).
We previously reported an acute increase in homocysteine concentration after donation,4 and the current study shows persistently elevated levels in donors compared to controls at 36 months. Tsai, et al., reported that homocysteine increased in 10 donors from a mean of 8.2±1.3 µmol/L pre-donation to 12.1±4.4, 11.5±2.6, and 10.3±2.2 µmol/L at 2 days, 6 weeks, and 6 months, respectively (all P<0.05 v. pre-donation).38 There is no evidence that increased homocysteine has any adverse consequences, and randomized trials have consistently not shown that reducing homocysteine improves patient outcomes.39, 40
We found no difference between donors and controls in CRP (Table 6). Others have reported that inflammatory markers are elevated acutely after kidney donation. For example, Tsai, et al., reported that CRP was increased at 2 days after surgery but had returned to pre-donation levels by 6 weeks post-donation. 38 However, in contrast, Rossi, et al., reported that in 42 donors CRP increased from a median of 1.2 (IQR, 0.9–2.7) mg/dL pre-donation to 2.0 (IQR, 0.9–3.7) mg/dL and 2.1 (IQR, 1.5–3.3) mg/dL at 1 and 2 years post-donation, respectively (P=0.005 versus pre-donation at 1 and 2 years).27
Previous studies have suggested that kidney donors have mild proteinuria.3 With 36 months of follow-up, donors in our study have similar urine total protein excretion as controls. Urine albumin is actually lower in donors of our study compared to controls, but is increasing between 6 and 36 months, suggesting that longer follow-up is needed to determine whether kidney donation ultimately leads to increased urine albumin. Potential markers of inflammation, including serum albumin concentration, CRP and fibrinogen continue to be similar in donors and controls (Table 6). Hemoglobin is lower in donors, but the gap appears to be narrowing with duration of follow-up.
We previously reported that serum PTH concentration was increased, phosphorous was reduced and calcium unchanged after donation.4 The present study results confirm these findings and demonstrate that these differences persist 3 years after donation (Table 6). We previously discussed these changes in light of other published studies at that time,41–46 but since then Young and co-workers have reported on 198 living kidney donors and 98 non-donor controls assessed at a median of 5.3 years post-donation.47 They found that serum fibroblast growth factor 23 levels were significantly higher in donors compared to controls. Compared to controls, donors also had higher PTH, higher renal tubular fractional excretion of inorganic phosphate, lower serum phosphate, and lower serum calcitriol concentrations. Additional controlled studies of donors may help to elucidate the pathogenesis of these changes in donors as well as in CKD.
Limitations of the present study include the fact that controls could not be screened as rigorously as donors for underlying kidney abnormalities, and it is possible that donors were healthier than controls. However, as previously noted,4 more donors (31%) than controls (15%) were blood-relatives of individuals with CKD. Follow-up is still relatively short, and it is possible that effects of donation may take much longer than 3 years to become manifest. For the analysis and interpretation of the many secondary endpoints, we did not undertake a statistical adjustment for multiple comparisons. This increases the possibility that some of the differences reported could be due to chance. Balanced against this is the prior probability from other studies reporting similar findings. In the end, given that an important objective of this study is to ensure donor safety, we opted not to adjust P-values for multiple comparisons. It should be noted that 95% of participants in our study are white (Table S1), and the effects of donation may be quite different in other populations. Clearly, additional studies in higher risk populations are needed, as is longer follow-up. Finally, this study was not designed to have statistical power to examine outcomes of importance to donors, such as mortality and end-stage renal disease.
In summary, this study continues to provide important information about the pathophysiologic changes accompanying a reduction in kidney mass in apparently healthy individuals. The results confirm that the reduction in kidney function from donation leads to biochemical changes that may or may not ultimately have consequences important to donors. Prospective, controlled studies of living kidney donors also afford a unique opportunity to better understand the consequences of reduced kidney function in patients with CKD. For all of these reasons, the current study results suggest that additional prospective cohort studies should be undertaken. These studies should include adequate numbers of donors who may have isolated medical abnormalities or other plausible risk factors for adverse outcomes after kidney donation.
Supplementary Material
ACKNOWLEDGEMENTS
Support: This study was funded by the National Institutes of Health (NIH) under the cooperative agreement U01 DK066013. The NIH participated in the interpretation of data, writing the report, and the decision to submit the report for publication. This study was also supported by the Minneapolis Medical Research Foundation, Minneapolis, MN, which did not participate in any aspect of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: Research idea and study design: BLK, TA-H, RSK, ESK, AAP, TEP, HR, MWS, JJS, MRW; data acquisition: TA-H, RSK, ESK, AAP, HR, MRW; data analysis/interpretation: BLK, TA-H, AKI, RSK, PLK, ESK, RK, AAP, TEP, HR, MWS, JJS, MRW; statistical analysis: BLK, JJS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. BLK takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Supplementary Material
Table S1: Demographic characteristics of study participants.
Table S2: Medication usage.
Table S3: BP medications and increased BP.
Table S4: Studies examining incidence of hypertension in donors vs controls.
Table S5: Results of studies of 24-hour ambulatory BP monitoring.
Table S6: Serum electrolytes and urine pH at 6, 12, 24, and 36 months after kidney donation.
Note: The supplementary material accompanying this article (doi: ________) is available at www.ajkd.org
Descriptive Text for Online Delivery of Supplementary Material
Supplementary Table S1 (PDF)
Demographic characteristics of study participants.
Supplementary Table S2 (PDF)
Medication usage.
Supplementary Table S3 (PDF)
BP medications and increased BP.
Supplementary Table S4 (PDF)
Studies examining incidence of hypertension in donors vs controls.
Supplementary Table S5 (PDF)
Results of studies of 24-hour ambulatory BP monitoring.
Supplementary Table S6 (PDF)
Serum electrolytes and urine pH at 6, 12, 24, and 36 months after kidney donation.
REFERENCES
- 1.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg AX, Muirhead N, Knoll G, et al. Proteinuria and reduced kidney function in living kidney donors: A systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70(10):1801–1810. doi: 10.1038/sj.ki.5001819. [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Anderson-Haag T, Ibrahim HN, et al. A prospective controlled study of kidney donors: baseline and 6-month follow-up. Am J Kidney Dis. 2013;62(3):577–586. doi: 10.1053/j.ajkd.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kit BK, Ogden CL, Flegal KM. Prescription medication use among normal weight, overweight, and obese adults, United States, 2005–2008. Ann Epidemiol. 2012;22(2):112–119. doi: 10.1016/j.annepidem.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Saran R, Marshall SM, Madsen R, Keavey P, Tapson JS. Long-term follow-up of kidney donors: a longitudinal study. Nephrol Dial Transplant. 1997;12(8):1615–1621. doi: 10.1093/ndt/12.8.1615. [DOI] [PubMed] [Google Scholar]
- 9.Tent H, Sanders JS, Rook M, et al. Effects of preexistent hypertension on blood pressure and residual renal function after donor nephrectomy. Transplantation. 2012;93(4):412–417. doi: 10.1097/TP.0b013e318240e9b9. [DOI] [PubMed] [Google Scholar]
- 10.Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW. The effect of age on creatinine clearance in men: A cross-sectional and longitudinal study. J Gerontol. 1976;31:155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- 11.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 12.Boudville N, Prasad GV, Knoll G, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145(3):185–196. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 13.Garg AX, Prasad GV, Thiessen-Philbrook HR, et al. Cardiovascular disease and hypertension risk in living kidney donors: an analysis of health administrative data in Ontario, Canada. Transplantation. 2008;86(3):399–406. doi: 10.1097/TP.0b013e31817ba9e3. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360(5):459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doshi MD, Goggins MO, Li L, Garg AX. Medical outcomes in African American live kidney donors: a matched cohort study. Am J Transplant. 2013;13(1):111–118. doi: 10.1111/j.1600-6143.2012.04303.x. [DOI] [PubMed] [Google Scholar]
- 16.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363(8):724–732. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001;24(6):691–697. doi: 10.1291/hypres.24.691. [DOI] [PubMed] [Google Scholar]
- 18.Obermayr RP, Temml C, Knechtelsdorfer M, et al. Predictors of new-onset decline in kidney function in a general middle-european population. Nephrol Dial Transplant. 2008;23(4):1265–1273. doi: 10.1093/ndt/gfm790. [DOI] [PubMed] [Google Scholar]
- 19.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19(6):1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Dov IZ, Kark JD. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: the Jerusalem Lipid Research Clinic cohort study. Nephrol Dial Transplant. 2011;26(8):2558–2566. doi: 10.1093/ndt/gfq740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken ) 2011;63(1):102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32(9):1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(7):885–892. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken ) 2010;62(2):170–180. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi M, Campbell KL, Johnson DW, et al. Uremic toxin development in living kidney donors: a longitudinal study. Transplantation. 2014;97(5):548–554. doi: 10.1097/01.tp.0000436906.48802.c4. [DOI] [PubMed] [Google Scholar]
- 28.Young A, Nevis IF, Geddes C, et al. Do biochemical measures change in living kidney donors? A systematic review. Nephron Clin Pract. 2007;107(3):c82–c89. doi: 10.1159/000108648. [DOI] [PubMed] [Google Scholar]
- 29.Undurraga A, Roessler E, Arcos O, et al. Long-term follow-up of renal donors. Transplant Proc. 1998;30(5):2283–2285. doi: 10.1016/s0041-1345(98)00622-8. [DOI] [PubMed] [Google Scholar]
- 30.Hida M, Iida T, Shimbo T, et al. Renal function after nephrectomy in renal donors. Tokai J Exp Clin Med. 1982;7(4):511–516. [PubMed] [Google Scholar]
- 31.Romero RR, Alberu J, Correa-Rotter R, Vargas-Vorackova F, Isordia-Salas I, Majluf-Cruz A. Serum erythropoietin levels in kidney donors after renal transplantation. Transplantation. 2000;70(2):386–387. doi: 10.1097/00007890-200007270-00027. [DOI] [PubMed] [Google Scholar]
- 32.Dzurik R, Spustova V, Janekova K. The prevalence of insulin resistance in kidney disease patients before the development of renal failure. Nephron. 1995;69(3):281–285. doi: 10.1159/000188471. [DOI] [PubMed] [Google Scholar]
- 33.Eidemak I, Feldt-Rasmussen B, Kanstrup IL, Nielsen SL, Schmitz O, Strandgaard S. Insulin resistance and hyperinsulinaemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia. 1995;38(5):565–572. doi: 10.1007/BF00400725. [DOI] [PubMed] [Google Scholar]
- 34.Vareesangthip K, Tong P, Wilkinson R, Thomas TH. Insulin resistance in adult polycystic kidney disease. Kidney Int. 1997;52(2):503–508. doi: 10.1038/ki.1997.360. [DOI] [PubMed] [Google Scholar]
- 35.Fliser D, Pacini G, Engelleiter R, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53(5):1343–1347. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 36.Kubo M, Kiyohara Y, Kato I, et al. Effect of hyperinsulinemia on renal function in a general Japanese population: the Hisayama study. Kidney Int. 1999;55(6):2450–2456. doi: 10.1046/j.1523-1755.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- 37.Kato Y, Hayashi M, Ohno Y, Suzawa T, Sasaki T, Saruta T. Mild renal dysfunction is associated with insulin resistance in chronic glomerulonephritis. Clin Nephrol. 2000;54(5):366–373. [PubMed] [Google Scholar]
- 38.Tsai MY, Aras O, Sozen H, et al. Plasma homocysteine levels in living kidney donors before and after uninephrectomy. J Lab Clin Med. 2004;143(6):340–343. doi: 10.1016/j.lab.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Clarke R, Halsey J, Lewington S, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170(18):1622–1631. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 40.Jardine MJ, Kang A, Zoungas S, et al. The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: systematic review and meta-analysis. BMJ. 2012;344:e3533. doi: 10.1136/bmj.e3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pabico RC, McKenna BA, Freeman RB. Renal function before and after unilateral nephrectomy in renal donors. Kidney Int. 1975;8(3):166–175. doi: 10.1038/ki.1975.96. [DOI] [PubMed] [Google Scholar]
- 42.Friedlander MA, Lemke JH, Horst RL. The effect of uninephrectomy on mineral metabolism in normal human kidney donors. Am J Kidney Dis. 1988;11(5):393–401. doi: 10.1016/s0272-6386(88)80052-0. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez R, Butt KMH, Sumrani N, Tejani A. Long-term renal, endocrine, and hematologic evaluation of kidney donors. Transplant Proc. 1989;21(1):1946–1948. [PubMed] [Google Scholar]
- 44.Gossmann J, Wilhelm A, Kachel HG, et al. Long-term consequences of live kidney donation follow-up in 93% of living kidney donors in a single transplant center. Am J Transplant. 2005;5(10):2417–2424. doi: 10.1111/j.1600-6143.2005.01037.x. [DOI] [PubMed] [Google Scholar]
- 45.Bieniasz M, Kwiatkowski A, Domagala P, et al. Serum concentration of vitamin D and parathyroid hormone after living kidney donation. Transplant Proc. 2009;41(8):3067–3068. doi: 10.1016/j.transproceed.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 46.Westerberg PA, Ljunggren O, Larsson TE, Wadstrom J, Linde T. Fibroblast growth factor-23 and mineral metabolism after unilateral nephrectomy. Nephrol Dial Transplant. 2010;25(12):4068–4071. doi: 10.1093/ndt/gfq288. [DOI] [PubMed] [Google Scholar]
- 47.Young A, Hodsman AB, Boudville N, et al. Bone and mineral metabolism and fibroblast growth factor 23 levels after kidney donation. Am J Kidney Dis. 2012;59(6):761–769. doi: 10.1053/j.ajkd.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Eberhard OK, Kliem V, Offner G, et al. Assessment of long-term risks for living related kidney donors by 24-h blood pressure monitoring and testing for microalbuminuria. Clin Transplant. 1997;11(5 Pt 1):415–419. [PubMed] [Google Scholar]
- 49.Siebels M, Theodorakis J, Schmeller N, et al. Risks and complications in 160 living kidney donors who underwent nephroureterectomy. Nephrol Dial Transplant. 2003;18(12):2648–2654. doi: 10.1093/ndt/gfg482. [DOI] [PubMed] [Google Scholar]
- 50.Textor SC, Taler SJ, Driscoll N, et al. Blood pressure and renal function after kidney donation from hypertensive living donors. Transplantation. 2004;78(2):276–282. doi: 10.1097/01.tp.0000128168.97735.b3. [DOI] [PubMed] [Google Scholar]
- 51.Goto N, Uchida K, Morozumi K, et al. Circadian blood pressure rhythm is disturbed by nephrectomy. Hypertens Res. 2005;28(4):301–306. doi: 10.1291/hypres.28.301. [DOI] [PubMed] [Google Scholar]
- 52.Prasad GV, Lipszyc D, Huang M, Nash MM, Rapi L. A prospective observational study of changes in renal function and cardiovascular risk following living kidney donation. Transplantation. 2008;86(9):1315–1318. doi: 10.1097/TP.0b013e318188425b. [DOI] [PubMed] [Google Scholar]
- 53.Ramesh Prasad GV, Lipszyc D, Sarker S, Huang M, Nash MM, Rapi L. Twenty four-hour ambulatory blood pressure profiles 12 months post living kidney donation. Transpl Int. 2010;23(8):771–776. doi: 10.1111/j.1432-2277.2009.01040.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.