Abstract
Objective
To determine the frequency, clinical and autoantibody associations and outcome of mood disorders in a multi-ethnic/racial, prospective, inception cohort of SLE patients.
Methods
Patients were assessed annually for mood disorders (4 types as per DSM-IV) and 18 other neuropsychiatric (NP) events. Global disease activity (SLEDAI-2K), SLICC/ACR damage index (SDI) and SF-36 subscale, mental (MCS) and physical (PCS) component summary scores were collected. Time to event, linear and ordinal regressions and multi-state models were used as appropriate.
Results
Of 1,827 SLE patients, 88.9% were female, 48.9% Caucasian, mean ± SD age 35.1±13.3 years, disease duration 5.6±4.8 months and follow-up 4.73±3.45 years. Over the study 863 (47.2%) patients had 1,627 NP events. Mood disorders occurred in 232/1827 (12.7%) patients and 98/256 (38.3%) events were attributed to SLE. The estimated cumulative incidence of any mood disorder after 10 years was 17.7% (95%CI=[15.1%,20.2%]). There was a greater risk of mood disorder in patients with concurrent NP events (p ≤ 0.01) and lower risk with Asian race/ethnicity (p=0.01) and immunosuppressive drugs (p=0.003). Mood disorders were associated with lower mental health subscale and MCS scores but not with SLEDAI-2K, SDI scores or lupus autoantibodies. Antidepressants were used in 168/232 (72.4%) patients with depression. 126/256 (49.2%) mood disorders resolved in 117/232 (50.4%) patients.
Conclusion
Mood disorders, the second most frequent NP event in SLE patients, have a negative impact on HRQoL and improve over time. The lack of association with global SLE disease activity, cumulative organ damage and lupus autoantibodies emphasize their multifactorial etiology and a role for non-lupus specific therapies.
Keywords: Systemic lupus erythematosus, Mood disorders, Inception cohort, Outcomes research
Neurological and psychiatric events, collectively referred to as neuropsychiatric (NP) disease, are a frequent occurrence in patients with systemic lupus erythematosus (SLE) (1–5). Approximately one-third of all NP events are directly attributed to SLE, although the attribution rate varies between individual manifestations (6). Regardless of attribution, the occurrence of NP events has been associated with a negative impact on health related quality of life (HRQoL) in both cross-sectional (6) and longitudinal (7) studies. Thus, awareness, identification and treatment of NP events in SLE patients are an important component of overall care and improving clinical outcomes. Large observational cohort studies with careful documentation of NP events and their attribution, treatment and outcomes over time can provide insight into this complex aspect of SLE.
Mood disorders are one of the most frequent NP events reported in SLE cohorts, usually in the top three of all NP events (2, 6). As is the case for many of the NP events in SLE there are no unique characteristics of mood disorders in SLE patients to help determine attribution to SLE or non-SLE causes. In addition there is very limited data on potential lupus biomarkers to implicate an autoimmune pathogenesis. In individual patients mood disorders may mask or complicate other NP presentations, in particular cognitive impairment, adversely impact adherence to recommended therapies and restrict overall mental and physical function.
In the present study we determined the frequency, characteristics, clinical and autoantibody associations and outcome of mood disorders in a large, multi-ethnic/racial, prospective, inception cohort of SLE patients.
Patients and Methods
Research study network
The study was conducted by the Systemic Lupus International Collaborating Clinics (SLICC) (8), a network of 37 investigators in 32 academic medical centers in 11 countries. Data were collected per protocol at enrollment and annually, submitted to the coordinating centre in Halifax, Nova Scotia, Canada and entered into a centralized Access database. Appropriate procedures ensured data quality, management and security. Capital Health Research Ethics Board, Halifax, and each of the participating centers’ institutional research ethics review boards approved the study.
Patients
Patients fulfilled the ACR SLE classification criteria for SLE (9), which was used as the date of diagnosis, and provided written informed consent. Enrollment was permitted up to 15 months following the diagnosis. Demographic variables such as age, gender, race/ethnicity, education and medication history were collected. Lupus-related variables included the SLE Disease Activity Index 2000 (SLEDAI-2K) (10) and SLICC/ACR damage index (SDI) (11). Laboratory testing included hematological, biochemical and immunological variables required to determine SLEDAI-2K and SDI scores.
Neuropsychiatric (NP) events
An enrollment window extended from 6 months prior to the diagnosis of SLE up to the actual enrollment date. NP events were characterized within this window using the ACR case definitions for 19 NP syndromes (12). These were diagnosed by clinical evaluation supported by investigations, if clinically warranted, as per the guidelines. Patients were reviewed annually with a 6-month window around the anticipated assessment date. New NP events and the status of previous NP events since the last study visit were determined at each assessment.
In the ACR case definitions (12) mood disorders are determined by clinical judgment based on the Diagnostic and Statistical Manual-IV (DSM-IV) criteria and consist of: (i) major depressive-like episode, (ii) mood disorder with depressive features, (iii) mood disorder with manic features and (iv) mood disorder with mixed features. Recurring mood disorders and other NP events of the same type within the enrollment window or within a follow-up assessment period were recorded once. The date of the first episode was taken as the onset of the event. In addition to but separate from the clinical diagnosis of mood disorder the ability of the mental health (MH) subscale of the SF-36 to screen for depression on the basis of patient self-report was also examined.
Attribution of NP events
In keeping with other publications on NP events within the SLICC NPSLE inception cohort, the same decision rules were used to determine the attribution of all NP events (6, 13). To optimize consistency this was performed at the central coordinating centre in Halifax using data provided in the case record form by individual SLICC sites. Factors considered in the decision rules included: (i) temporal onset of NP event(s) in relation to the diagnosis of SLE; (ii) concurrent non-SLE factor(s), identified from the ACR glossary which accompanied the case definitions of NP events (12), as potential causes (“exclusions”) or contributing factors (“associations”) for each NP syndrome; and (iii) “common” NP events which are frequent in normal population controls as described by Ainiala et al (14). These include all headaches, anxiety, mild depression (mood disorders failing to meet criteria for “major depressive-like episodes”), mild cognitive impairment (deficits in less than 3 of the 8 specified cognitive domains) and polyneuropathy without electrophysiological confirmation. Two attribution decision rules of different stringency (models A and B) were developed as described in detail elsewhere (6, 13). NP events that fulfilled criteria for model A (most stringent) or for model B (least stringent) were attributed to SLE. By definition, all NP events attributed to SLE using model A were included in the group of NP events using model B. Those events which did not fulfill these criteria were attributed to non-SLE causes.
Outcome of mood disorders
A physician generated 7-point Likert scale compared the change in mood disorder between onset and follow-up study assessment (1=patient demise, 2=much worse, 3=worse, 4=no change, 5=improved, 6=much improved, 7=resolved) (15). A patient generated SF-36 questionnaire provided subscale, mental (MCS) and physical (PCS) component summary scores (15, 16), the results of which were not available to the physicians at the time of their assessments.
Autoantibodies
Lupus anticoagulant, IgG anticardiolipin, anti-β2 glycoprotein-I, anti-ribosomal P (anti-P) and anti-NR2 glutamate receptor antibodies were measured at the enrollment visit at the Oklahoma Medical Research Foundation, USA using previously described methodology (17–20).
Statistical analysis
Descriptive statistics were used to summarize enrolment data. Kaplan-Meier estimates of the survivor function for the time until first mood disorder were calculated. Cox regression methods were used to analyze the time to first (SLE) mood disorder data and the time to resolution data for mood disorders. Ordinal regression based on generalized estimating equation (GEE) methods was used to analyze the Likert scale outcome scores for mood disorders that were unresolved. Covariates examined in regression analyses included gender, age at SLE diagnosis, race/ethnicity, SLICC sites, education, ACR criteria at enrolment, SLEDAI (without NP), SLICC damage index (without NP), baseline antibodies, medications and other ongoing NP events. Hypothesis tests for the significance of regression parameters were performed using Wald tests (Cox regression) and score tests (GEE analyses) and 95% confidence intervals (CIs) were calculated. A simple multi-state model was fit with states defined by the Likert scale scores in order to estimate the time spent in these states after the occurrence of a mood disorder. The model assumed that all transitions must be to adjacent states (changes in the Likert scale of 1 or −1) and was fit in continuous time which allows moves of more than one state between assessments. Resolution is a final or absorbing state and the rate of transition is assumed piecewise constant with the rates in the first two years after onset of a mood disorder allowed to differ from those subsequently. The rates for improving transitions were assumed to be the same and similarly for worsening transitions. For analyses of the HRQoL longitudinal outcomes, linear regression models with GEE were used to take into account the correlation between multiple observations within patients.
Results
Patients
1,827 patients were recruited between October 1999 and February 2013, from SLICC centers in the United States (n=541 (29.6%)), Europe (n=477 (26.1%)) Canada (n=417 (22.8%)), Mexico (n=223 (12.1%)), and Asia (n=169 (9.3%)). The median (range) number of patients enrolled in each of the SLICC centers was 35 (3 – 223). Patients were most frequently women 1,625/1,827 (88.9%), with a mean (±SD) age of 35.1±13.3 years and a varied racial/ethnic distribution although predominantly Caucasian (Table 1).
Table 1.
Demographic and clinical manifestations of SLE patients at enrolment
| Number of Patients | 1827 |
| Gender (%) | |
| Female | 1625 (88.9) |
| Male | 202 (11.1) |
| Age (years) (mean ± SD) | 35.1 ± 13.3 |
| Race/Ethnicity (%) | |
| Caucasian | 893 (48.9) |
| African ancestry | 305 (16.7) |
| Asian | 274 (15.0) |
| Hispanic | 282(15.4) |
| Other | 73 (4.0) |
| Single/Married/Other (%) | 818 (45.0)/765/(42.0)/237(13.0) |
| Post secondary education (%) | 1063 (62.0) |
| Disease duration (months) (mean ± SD) | 5.6 ± 4.8 |
| Number of ACR criteria (mean ± SD) | 4.9 ± 1.0 |
| ACR manifestations (%) | |
| Malar rash | 661 (36.2) |
| Discoid rash | 227 (12.4) |
| Photosensitivity | 652 (35.7) |
| Oral/nasal ulcers | 678 (37.1) |
| Serositis | 502 (27.5) |
| Arthritis | 1367(74.8) |
| Renal disorder | 500 (27.4) |
| Neurological disorder | 88 (4.8) |
| Hematologic disorder | 1129(61.8) |
| Immunologic disorder | 1390 (76.1) |
| Antinuclear antibody | 1730 (94.7) |
| SLEDAI-2K score (mean ± SD) | 5.3 ± 5.4 |
| * SLICC/ACR damage index score (mean ± SD) | 0.31 ± 0.74 |
| Medications (%) | |
| Corticosteroids | 1278 (70.0) |
| Antimalarials | 1231 (67.4) |
| Immunosuppressants | 729 (39.9) |
| ASA | 257 (14.1) |
| Antidepressants | 185 (10.1) |
| Warfarin | 97 (5.3) |
| Anticonvulsants | 80 (4.4) |
| Antipsychotics | 13 (0.7) |
| Autoantibodies (%) | |
| Lupus anticoagulant | 239/1170 (20.4) |
| Anticardiolipin | 137/1136 (12.1) |
| Anti-β2 glycoprotein-I | 162/1136 (14.3) |
| Anti-ribosomal P | 111/1130 (9.8) |
| Anti-NR2 | 130/1058 (12.3) |
SLICC/ACR damage index not available in 1029 patients at enrollment visit when disease duration < 6 months
At enrollment the mean disease duration was 5.6±4.8 months and the frequency of individual ACR classification criteria indicated no selection bias in patient recruitment. The mean SLEDAI-2K and SDI scores reflected moderate global disease activity and minimal cumulative organ damage respectively. Patients were receiving a range of lupus medications. The number of annual assessments per patient varied from 1 to 14 with a mean follow-up of 4.7±3.5 years.
Neuropsychiatric (NP) manifestations
NP events (≥1) occurred in 863/1827 (47.2%) patients and 404/1827 (22.1%) had ≥ 2 events over the study period. There were 1627 unique NP events, encompassing all 19 NP syndromes (12). The proportion of NP events attributed to SLE varied from 17.8% (attribution model A) to 30.9% (attribution model B) and occurred in 11.7% (model A) to 18.8% (model B) of patients. Of the 1627 unique NP events, which were reported at 1246 patient visits, 1490 (91.6%) involved the central nervous system and 137 (8.4%) the peripheral nervous system (12). The classification of events into diffuse and focal was 1274 (78.3%) and 353 (21.7%) respectively (13).
Mood disorder frequency and characteristics
Mood disorders were the second most frequent NP event: 232/1827 (12.7%) patients experienced 256 mood disorders. Of these, 46/256 (18%) and 98/256 (38.3%) were attributed to SLE in 45/232 (19.4%) and in 95/232 (40.9%) patients using model A and model B attribution rules respectively. The predominant mood disorders were major depressive-like episodes [134/256 (52.3%)], followed by mood disorder with depressive features [114/256 (44.5%)] and the remaining two mood disorders accounted for only 8/256 (0.03%) events. The estimated cumulative incidence of any mood disorder and any SLE-attributed mood disorder after 10 years was 17.7% (95%CI=[15.1%, 20.2%]) and 7.9% (95%CI=[6.0%, 9.9%]), respectively (Figure 1, upper panel). Mood disorders with major depressive-like features were the predominant type (Figure 1, lower panel).
Figure 1.
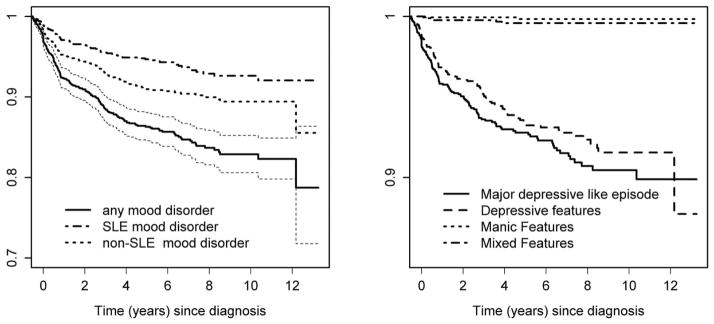
Kaplan-Meier estimates of the survivor function for time until first mood disorder of any type (with 95% CI) and by attribution (left panel) and by mood disorder type (right panel).
Using physician identified cases of any mood disorders as the “gold standard” at all assessments over the study period, an SF-36 MH subscale score of 60 maximized the sum of sensitivity and specificity. The area under the ROC curve was 0.74 (95%CI=[0.72, 0.77]) with a sensitivity of 63% and specificity of 74%. The results were similar when the same analysis was confined to physician diagnosed major depressive-like episodes. The area under the ROC curve was 0.77 (95%CI=[0.75, 0.80]) with a sensitivity of 69% and specificity of 74%.
Clinical and laboratory associations with mood disorders
Univariate analysis revealed a positive association [HR (95%CI)] between mood disorders and use of anticoagulants 1.5 (1.1, 2.3) or antidepressants (excluding amytriptyline ≤ 100 mg/day) 2.1 (1.3, 3.6). There was a negative association with Asian race/ethnicity [HR (95%CI)] 0.3 (0.2, 0.5), Seoul, Korean SLICC site 0.3 (0.2, 0.7), immunosuppressants 0.6 (0.4, 0.9), and anti- P antibodies 0.4 (0.2, 0.9).
There were also associations [HR (95%CI)] with both SLE attributed [12.3 (8.9, 16.9)] and non-SLE attributed [7.6 (5.8, 10.2) NP events. The SLE attributed (model B) NP events were cerebrovascular disease, mononeuropathy, myasthenia gravis, myelopathy, cranial neuropathy, seizure disorder, acute confusion, cognitive dysfunction and psychosis; the non-SLE attributed NP events were headache, movement disorder, cranial neuropathy, polyneuropathy, seizure disorder, acute confusion, anxiety and cognitive dysfunction. Very similar results were obtained with univariate analysis examining the associations with only mood disorders that were attributed to SLE (model B)
The results of multivariate regressions examining the associations with all mood disorders and with those attributed to SLE are summarized in Table 2. Included in the presented models are variables significant in the univariate analyses and having at least a moderate significance level (p<0.1) in the multivariate models for all mood disorders. There was a greater risk of mood disorder in patients with other concurrent NP events (p ≤ 0.01) and a lower risk with Asian race/ethnicity (p=0.01) and immunosuppressive drugs taken in the absence of antidepressants (p=0.003). To confirm that the lower risk with Asian race/ethnicity was not due to differential physician diagnosis for different races/ethnicities, the relationship between physician diagnoses and the SF-36 MH subscale score of <60 was examined. In patients of Asian race/ethnicity without a physician diagnosed mood disorder the percentage of SF-36 MH subscale scores <60 was 278/1297 (21.4%) compared to that in other races 1617/6124 (26.4%). Of note, no association was found between mood disorders and SLEDAI-2K, SDI scores, lupus autoantibodies or prednisone (any dose and >20 mg/day), regardless of whether or not the analysis was confined to mood disorders attributed to SLE.
Table 2.
Multivariate regression analysis of predictors to time of first occurrence of any mood disorder regardless of attribution (upper panel: analysis I and II) and to time of first occurrence of mood disorder attributed to SLE (model B) (lower panel: analysis III and IV).
| Analysis I | Analysis II | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||||
| Other ongoing NP events (YES) | Non-SLE headache | 3.04 | 2.26 | 4.09 | <.0001 | 1.50 | 0.94 | 2.38 | 0.088 |
| SLE seizure | 3.95 | 2.11 | 7.37 | <.0001 | 3.56 | 1.29 | 9.83 | 0.014 | |
| Non-SLE anxiety disorder | 3.50 | 2.09 | 5.85 | <.0001 | 1.23 | 0.43 | 3.54 | 0.703 | |
| SLE cognitive dysfunction | 7.20 | 4.19 | 12.39 | <.0001 | 8.80 | 4.19 | 18.49 | <.0001 | |
| Non-SLE cognitive dysfunction | 3.15 | 1.28 | 7.74 | 0.012 | 3.96 | 1.24 | 12.68 | 0.021 | |
| Ethnicity /location | Caucasian-Europe/Canada | 0.010 | 0.034 | ||||||
| Caucasian-USA | 1.39 | 0.96 | 2.01 | 1.64 | 0.95 | 2.84 | |||
| Hispanic-Mexico | 1.42 | 0.94 | 2.17 | 1.88 | 1.05 | 3.38 | |||
| Hispanic-other | 1.11 | 0.51 | 2.43 | 0.43 | 0.06 | 3.16 | |||
| African ancestry-USA | 1.14 | 0.72 | 1.81 | 1.57 | 0.82 | 3.01 | |||
| African ancestry-other | 1.01 | 0.60 | 1.70 | 1.62 | 0.84 | 3.11 | |||
| Asian | 0.42 | 0.24 | 0.74 | 0.56 | 0.27 | 1.16 | |||
| Other | 0.95 | 0.46 | 1.96 | 1.14 | 0.41 | 3.22 | |||
| Current medication use | Neither | ||||||||
| Antidepressants only | 0.97 | 0.44 | 2.17 | 0.947 | |||||
| Immunosuppressant only | 0.52 | 0.34 | 0.80 | 0.003 | |||||
| Both | 1.99 | 0.96 | 4.15 | 0.065 | |||||
| Predictor | Analysis III | Analysis IV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||||
| Other ongoing NP events (YES) | Non-SLE headache | 2.24 | 1.40 | 3.60 | 0.0008 | 1.39 | 0.72 | 2.68 | 0.333 |
| Non-SLE movement disorder | 10.63 | 0.89 | 127.65 | 0.062 | |||||
| SLE myelopathy | 4.19 | 0.97 | 18.07 | 0.055 | 2.76 | 0.35 | 21.51 | 0.333 | |
| SLE seizure | 3.94 | 1.45 | 10.69 | 0.007 | 1.65 | 0.23 | 12.16 | 0.621 | |
| Non-SLE anxiety disorder | 3.25 | 1.48 | 7.16 | 0.003 | 1.18 | 0.28 | 5.07 | 0.822 | |
| SLE cognitive dysfunction | 5.99 | 2.57 | 13.96 | <.0001 | 8.38 | 3.20 | 21.91 | <.0001 | |
| Non-SLE cognitive dysfunction | 3.24 | 0.98 | 10.76 | 0.055 | 3.57 | 0.83 | 15.36 | 0.087 | |
| SLE psychosis | 7.94 | 1.04 | 60.92 | 0.046 | |||||
| Gender (female) | 2.83 | 1.03 | 7.73 | 0.043 | 2.16 | 0.67 | 6.95 | 0.199 | |
| Current antidepressant use | 1.46 | 0.70 | 3.05 | 0.309 | |||||
Analysis I (N=232 mood disorders): Multivariate Cox regression analysis for time to first mood disorder (regardless of attribution) without medication information; Analysis II (N=117 mood disorders): Multivariate Cox regression analysis for time to first mood disorder (regardless of attribution) with medication data.
B: Analysis III (N=95 mood disorders): Multivariate Cox regression analysis for time to first SLE mood disorder (model B) without medication information; Analysis IV (N=53 mood disorders): Multivariate Cox regression analysis for time to first SLE mood disorder (model B) with medication information
Mood disorders and Health Related Quality of Life (HRQoL)
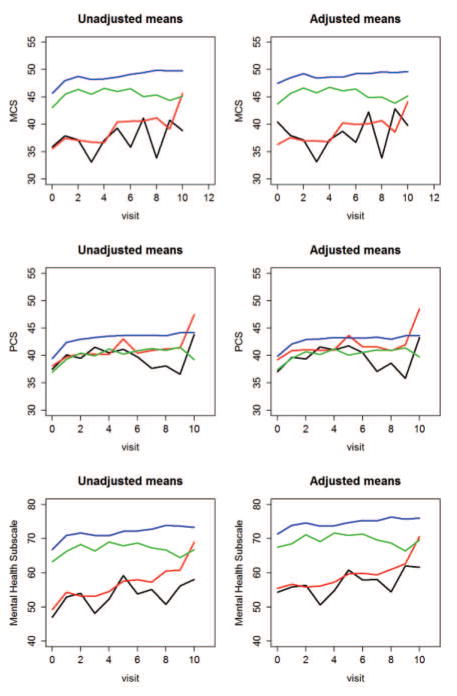
The association between mood disorders and HRQoL is illustrated in Figure 2. The estimated means of MCS, PCS and MH subscale scores of the SF-36 at each visit (up to the 10th followup visit) are shown in four groups: (i) patients with SLE attributed mood disorder; (ii) patients with non-SLE attributed mood disorder; (iii) patients without mood disorder, but with other types of NP events; (iv) patients without mood disorder or other NP events. Estimates in the left panels are from univariate analyses. Estimates in the right panels are from a multivariate regression model adjusting for gender, age at SLE diagnosis, race/ethnicity, location, post-secondary education, SLEDAI without NP variables, SLICC damage index without NP variables, use of corticosteroids, anti-malarials and immunosuppressive drugs. As shown in Figure 2, MCS and MH scores were lower in the groups with mood disorder (group i and ii) compared to the group without mood disorder (group iii), and especially compared to the group without any NP events (group iv). The group differences in PCS scores were considerably less marked.
Figure 2.
The estimated means of MCS, PCS and mental health subscale scores of the SF-36 at each visit (up to the 10th followup visit) are illustrated for the following four groups: (i) patients with SLE attributed mood disorder (black lines); (ii) patients with non-SLE attributed mood disorder (red lines) (iii) patients without mood disorder, but with other types of NP events (green lines), (iv) patients without mood disorder and other NP events (blue lines).
Use of antidepressants
In patients with a clinical diagnosis of depression, 168/232 (72.4%) took antidepressants at more than one visit. Overall, antidepressants were taken at 169/272 (62.1%) assessments with SLE attributed and in 296/423 (70.0%) assessments with non-SLE attributed mood disorders. Antidepressant use was also recorded at 733/8673 (8.5%) assessments without a mood disorder. This was due in part to the continued use of antidepressants following resolution of the mood disorder and possibly due to the use of antidepressants for other indications.
Clinical outcome of mood disorders
The physician assessment of change in mood disorder was examined separately using Likert scores of 1 to 6 and Likert score 7 (resolution) (Figure 3a and 3b; Table 3). Times to resolution were recorded as days since onset. A Likert score of 1 indicates patient demise which was not necessarily linked to mood disorder, but as there was only one death in the group of patients with mood disorder this patient was included in all but the multi-state model analysis. Figure 3b displays the distribution of maximum and minimum Likert scores observed during followup. Note that 27 of the 126 mood disorders that resolved did so by the first assessment after onset. Adjusting for the time since mood disorder onset and given that these mood disorders had not resolved, univariate analyses indicated that location (i.e. non-US sites), anti-depressant use, immunosuppressive use, and absence of other ongoing non-SLE NP events were associated with higher probabilities of having better mood disorder outcome. By including all significant predictors in univariate analyses and further clustering of other ongoing NP events using central/peripheral classification, the findings were similar in multivariate analysis (Table 3).
Figure 3.
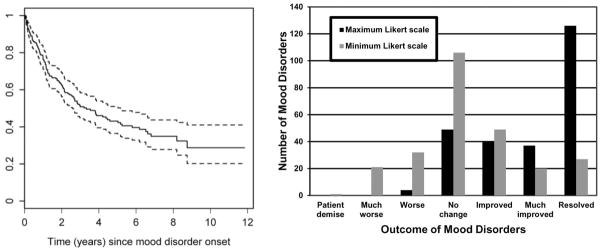
Kaplan-Meier estimate of time (with 95% CI) to resolution of all mood disorders (left panel); Distributions of maximum and minimum Likert scale scores observed over followup for 256 mood disorders (right panel).
Table 3.
Multivariate regression analysis of predictors of outcome on Likert scale (excluding resolution) (column A) and resolution (column B) of any mood disorder regardless of attribution.
| Predictors | A: Analysis of Likert scale outcome (excluding resolution) | B: Analysis of time to resolution | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Est. | 95% CI | P | HR | 95% CI | P | ||||
| Gender | Female | 0.20 | −0.26 | 0.66 | 0.3985 | ||||
| Age at SLE diagnosis | 0.805 | 0.637 | 1.019 | 0.0711 | |||||
| Location | US | −0.39 | −1.64 | 0.85 | 0.0003 | 0.565 | 0.211 | 1.518 | 0.0125 |
| Mexico | 0.52 | −0.82 | 1.85 | 0.793 | 0.288 | 2.187 | |||
| Europe | 0.73 | −0.55 | 2.01 | 0.551 | 0.203 | 1.496 | |||
| Canada | 0.33 | −0.95 | 1.62 | 1.160 | 0.438 | 3.078 | |||
| Asia | 0 | 1 | |||||||
| Anti-depressant | 0.51 | 0.12 | 0.90 | 0.0096 | |||||
| Immunosuppressive | 0.55 | 0.20 | 0.91 | 0.0031 | |||||
| Any ongoing NP events in central/peripheral clusters by attribution* | Central, SLE attributed | −0.39 | −0.83 | 0.04 | 0.0294 | 0.693 | 0.424 | 1.133 | 0.0183 |
| Central, non-SLE attributed | −0.52 | −0.93 | −0.12 | 0.595 | 0.394 | 0.898 | |||
| Peripheral, SLE attributed | 0.26 | −0.94 | 1.46 | 1.540 | 0.728 | 3.258 | |||
| Peripheral, non-SLE attributed | −1.01 | −2.02 | −0.01 | Not estimable | |||||
| No NP events | 0 | 1 | |||||||
| Time since mood disorder onset | 5+ years | −0.30 | −0.74 | 0.14 | 0.3168 | ||||
| 4–5 years | 0.05 | −0.40 | 0.50 | ||||||
| 2–3 years | 0.10 | −0.26 | 0.45 | ||||||
| <=1 year | 0 | 0 | 0 | ||||||
Central, SLE attributed: patients with one or more ongoing central SLE attributed events at current visit
Central, non-SLE attributed: patients with one or more ongoing central non-SLE attributed events, but no ongoing central SLE-attributed events
Peripheral, SLE attributed: Patients with ongoing peripheral events only, of which one or more were SLE-attributed
Peripheral, non-SLE attributed: Patients with ongoing peripheral non-SLE attributed events only
No NP: patients without any ongoing NP events at current visit
One hundred and twenty-six of 256 (49.2%) mood disorders resolved in 117 of 232 (50.4%) patients over the period of study. An estimated time to resolution curve is illustrated in Figure 3(a). Univariate analyses revealed a number of positive associations with shorter time to resolution of mood disorder of any type: Canadian and Asian sites, younger age at SLE diagnosis, absence of baseline anti-P antibody, and absence of other ongoing non-SLE NP events. In the multivariate analysis, the positive associations that were retained were Canadian and Asian sites and absence of other ongoing non-SLE NP events with a suggestive effect for younger age at SLE diagnosis. Including baseline anti-P antibody in the multivariate analysis reduced the number of resolved mood disorders available for analysis from 126 to 90. This reduction in sample size leads to a lack of significance for other variables, including baseline anti-p antibody, and therefore results excluding baseline anti-P antibody are presented in Table 3.
Omitting the one patient who died, the estimated percentages of patients having Likert scale values of 2 to 7, two years after the onset of a mood disorder, were 2%, 5%, 26%, 15%, 11% and 42% respectively. The estimated percentages after five years were 1%, 3%, 20%, 12%, 7% and 57%.
Discussion
Mood disorders are a frequent occurrence in SLE patients and have been attributed to both lupus and non-lupus causes (2, 6). The characteristics of mood disorders in SLE are similar to those in the general population and have the same heterogeneity in clinical presentation. They may occur in isolation or in association with other neuropsychiatric (NP) events. There is a paucity of clinical studies describing the outcome of mood disorders in SLE and little information to guide optimal management with either neuropharmacological or immunomodulating therapies. In order to address these deficits the current study describes the frequency, characteristics, predictors and outcomes of mood disorders in a large, international, prospective, inception cohort of SLE patients.
Mood disorders are prominent in the general population and in patients with chronic disease. For example, depressive disorders were detected in 8.6% (95% CI 7.05–10.37) of 8,764 randomly selected individuals in the general populations of five European countries (21, 22). The prevalence was 10.1% (95% CI 7.8–12.9) for women and 6.6% (95% CI 4.9–8.8) for men. The frequency of major depressive episodes in the United States over one year was 10% (22). Of interest and relevance to the current study the frequency of depression in East Asian countries is considerably lower than in Western countries (23); more specifically the frequency of lifetime depression in Korea has been reported to be 2.9% (23). Whether this lower frequency can be attributed to cultural or biological factors is unknown. A systematic review of 13,189 patients with rheumatoid arthritis (RA) found depression in 34.2% to 38.8% using 2 self-report questionnaires and a frequency of major depressive disorder in 16.8% of patients (24). In a systematic review and meta-analysis of 55,982 adults with chronic kidney disease (CKD) the prevalence of depression in CKD stage 5D was 22.8% (95% CI 18.6–27.6) and increased to 25.7% (95% CI12.8–44.9) in kidney transplant recipients (25). In the current study, the observed frequency of mood disorders was 12.7% of patients, the 10 year estimate of mood disorders was 17.7% and major depression was the predominant type. These findings are in keeping with the prevalence of mood disorders in the general population and in other chronic diseases.
The etiology of mood disorders in SLE and specifically the attribution of this common NP event to SLE or non-SLE causes may be challenging in individual patients. In the current study the majority of mood disorders were attributed to non-SLE causes, using two attribution rules of different stringency (6, 13). The lower risk of mood disorders in patients receiving immunosuppressive drugs could suggest a beneficial treatment effect of an autoimmune mediated condition but the lack of association with global SLE disease activity, cumulative organ damage and a panel of lupus autoantibodies traditionally associated with NPSLE imply that the majority of mood disorders are not primary manifestations of the disease. In addition, although dysregulated type-1 interferon (IFN-1) production is a frequent occurrence in SLE and has been associated with several NP events including mood disorders, at least one study (26) did not find an association between elevated production of IFN-1 and depression in SLE patients. In contrast to a recent study (27) we did not find an association between the concurrent use of high dose prednisone and depression.
There are several consequences for individuals with mood disorders. In the current study, detection of a mood disorder through a clinical encounter was associated with patient self-report lower HRQoL. The magnitude of the difference in both the MH subscale and in the MCS scores of patients with and without mood disorders, even when adjusted for multiple potential confounders, is clinically significant (28, 29). The co-occurrence of mood disorders with other NP events, which was frequent in our study, may complicate the assessment of other NP disease. For example, cognitive complaints but not necessarily impaired cognitive function are more frequent in both SLE (30, 31) and non-SLE (32) patients with depression, which should be treated prior to the formal assessment of cognitive function. Furthermore, although not addressed in the current study, the occurrence of mood disorders is associated with a higher frequency of non-adherence (33) to recommended therapies, scheduled clinic appointments and recommended lifestyle modifications, all of which are critical to the optimal management of SLE.
The outcome of mood disorders in the cohort was generally favourable over the duration of followup. Approximately 50% of patients had resolution, and of the events that did not resolve the trend was clearly in favour of improvement when both the best and worst outcome scores over time were tabulated. The predictors of a favourable outcome consisted of both lupus specific and non-specific factors in univariate analysis, some of which were still significant on multivariate analysis. These included antidepressants whose use was appropriately high (62.1–70.0%) in our patients in contrast to other studies of SLE where it has been as low as 7% (34). The association of a favourable outcome with the absence of other NP events, attributed to lupus and non-lupus causes, and immunosuppressive drugs may be due to a lupus-specific effect or the benefits of a reduction in global disease burden. The explanation for better outcome in non-US SLICC sites is unclear. When considering treatment options, the results of our study support both the use of symptomatic therapies (e.g. antidepressants) and lupus directed therapies (e.g. immunosuppressive) when indicated for globally active SLE.
There are a number of limitations to the current study. First, the absence of a control population precludes more definitive interpretation of the frequency of mood disorders in our SLE patients. However desirable, this was not feasible and is compensated in large part by the size of the inception cohort, the prospective study design and standardized data collection. Second, we did not include depression symptom questionnaires to screen for mood disorders. However this approach has not been recommended by the Canadian Task Force on Preventive Health Care for the detection of mood disorders (35) in place of face-to-face clinical screening which was the approach utilized in the current study. Third, specialized investigations such as neuroimaging and examination of cerebrospinal fluid were not routinely performed on all patients with mood disorders but rather left to the discretion of individual investigators at each site. Likely, the universal application of such investigations would have detected additional abnormalities but our protocol more accurately reflects what is done in clinical practice, an important overall objective of our inception cohort study. Fourth, although no association was found between mood disorders and a panel of selected auto-antibodies, these were measured only at the enrollment visit. Our findings do not preclude an association with persistent or increasing levels of auto-antibodies over time. Further work will be required to address this. Finally, there are a number of non-SLE specific variables such as hormonal status, dietary and lifestyle issues that may be associated with headache but were not addressed.
Despite these limitations, the results of our study underline the high frequency of mood disorders in SLE patients and the negative impact on HRQoL. It is likely that in the majority of SLE patients, depression is not an autoimmune mediated event. Nevertheless the favourable outcome over time emphasizes the importance of remaining vigilant for its occurrence and utilizing appropriate treatment strategies to achieve a speedy resolution.
Acknowledgments
Financial support:
John. G. Hanly (Canadian Institutes of Health Research grant MOP-86526)
Dr. Sang-Cheol Bae’s work was supported by the Korea Healthcare technology R & D project, Ministry for Health and Welfare, Republic of Korea (A120404).
Dr. Caroline Gordon’s work was supported by Lupus UK and the NIHR /Wellcome Trust Clinical Research Facility
The Hopkins Lupus Cohort is supported by the NIH (grant AR43727).
The Montreal General Hospital Lupus Clinic is partially supported by the Singer Family Fund for Lupus Research.
Dr. Clarke holds The Arthritis Society Chair in Rheumatic Diseases at the University of Calgary.
Dr. Paul R. Fortin presently holds a tier 1 Canada Research Chair on Systemic Autoimmune Rheumatic Diseases at Université Laval, and part of this work was done while he was still holding a Distinguished Senior Investigator of The Arthritis Society.
Dr. Bruce is supported by Arthritis Research UK, the National Institute for Health Research Biomedical Research Unit Funding Scheme and The NIHR Manchester Biomedical Research Centre and the NIHR/Wellcome Trust Clinical Research Facility at Central Manchester Foundation Trust. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Dr. Soren Jacobsen is supported by the Danish Rheumatism Association (A1028) and the Novo Nordisk Foundation (A05990).
Dr. Ramsey-Goldman’s work was supported by the NIH (grants 8UL1TR000150 formerly UL-1RR-025741, K24-AR-02318, and P60AR064464 formerly P60-AR-48098).
Dr. Mary Anne Dooley’s work was supported by the NIH grant RR00046.
Dr. Ruiz-Irastorza is supported by the Department of Education, Universities and Research of the Basque Government.
Drs. Li Su and Vernon Farewell’s work was supported by MRC (UK) funding U105261167.
References
- 1.Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57(3):496–500. doi: 10.1212/wnl.57.3.496. [DOI] [PubMed] [Google Scholar]
- 2.Brey RL, Holliday SL, Saklad AR, Navarrete MG, Hermosillo-Romo D, Stallworth CL, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58(8):1214–20. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 3.Hanly JG, McCurdy G, Fougere L, Douglas JA, Thompson K. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol. 2004;31(11):2156–62. [PubMed] [Google Scholar]
- 4.Sanna G, Bertolaccini ML, Cuadrado MJ, Laing H, Mathieu A, Hughes GR. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003;30(5):985–92. [PubMed] [Google Scholar]
- 5.Sibbitt WL, Jr, Brandt JR, Johnson CR, Maldonado ME, Patel SR, Ford CC, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29(7):1536–42. [PubMed] [Google Scholar]
- 6.Hanly JG, Urowitz MB, Sanchez-Guerrero J, Bae SC, Gordon C, Wallace DJ, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 2007;56(1):265–73. doi: 10.1002/art.22305. [DOI] [PubMed] [Google Scholar]
- 7.Hanly JG, Urowitz MB, Su L, Bae SC, Gordon C, Wallace DJ, et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2010;69(3):529–35. doi: 10.1136/ard.2008.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isenberg D, Ramsey-Goldman R. Systemic Lupus International Collaborating Group--onwards and upwards? Lupus. 2006;15(9):606–7. doi: 10.1177/0961203306071868. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 10.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91. [PubMed] [Google Scholar]
- 11.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 12.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Hanly JG, Urowitz MB, Su L, Sanchez-Guerrero J, Bae SC, Gordon C, et al. Short-term outcome of neuropsychiatric events in systemic lupus erythematosus upon enrollment into an international inception cohort study. Arthritis Rheum. 2008;59(5):721–9. doi: 10.1002/art.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ainiala H, Hietaharju A, Loukkola J, Peltola J, Korpela M, Metsanoja R, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001;45(5):419–23. doi: 10.1002/1529-0131(200110)45:5<419::aid-art360>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Hanly JG, Urowitz MB, Jackson D, Bae SC, Gordon C, Wallace DJ, et al. SF-36 summary and subscale scores are reliable outcomes of neuropsychiatric events in systemic lupus erythematosus. Ann Rheum Dis. 2011;70(6):961–7. doi: 10.1136/ard.2010.138792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thumboo J, Fong KY, Ng TP, Leong KH, Feng PH, Thio ST, et al. Validation of the MOS SF-36 for quality of life assessment of patients with systemic lupus erythematosus in Singapore. J Rheumatol. 1999;26(1):97–102. [PubMed] [Google Scholar]
- 17.Merrill JT, Zhang HW, Shen C, Butman BT, Jeffries EP, Lahita RG, et al. Enhancement of protein S anticoagulant function by beta2-glycoprotein I, a major target antigen of antiphospholipid antibodies: beta2-glycoprotein I interferes with binding of protein S to its plasma inhibitor, C4b-binding protein. Thromb Haemost. 1999;81(5):748–57. [PubMed] [Google Scholar]
- 18.Merrill JT, Shen C, Gugnani M, Lahita RG, Mongey AB. High prevalence of antiphospholipid antibodies in patients taking procainamide. J Rheumatol. 1997;24(6):1083–8. [PubMed] [Google Scholar]
- 19.Erkan D, Zhang HW, Shriky RC, Merrill JT. Dual antibody reactivity to beta2-glycoprotein I and protein S: increased association with thrombotic events in the antiphospholipid syndrome. Lupus. 2002;11(4):215–20. doi: 10.1191/0961203302lu178oa. [DOI] [PubMed] [Google Scholar]
- 20.Hanly JG, Urowitz MB, Siannis F, Farewell V, Gordon C, Bae SC, et al. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum. 2008;58(3):843–53. doi: 10.1002/art.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayuso-Mateos JL, Vazquez-Barquero JL, Dowrick C, Lehtinen V, Dalgard OS, Casey P, et al. Depressive disorders in Europe: prevalence figures from the ODIN study. Br J Psychiatry. 2001;179:308–16. doi: 10.1192/bjp.179.4.308. [DOI] [PubMed] [Google Scholar]
- 22.Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12(1):3–21. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276(4):293–9. [PubMed] [Google Scholar]
- 24.Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2013;52(12):2136–48. doi: 10.1093/rheumatology/ket169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–91. doi: 10.1038/ki.2013.77. [DOI] [PubMed] [Google Scholar]
- 26.Kellner ES, Lee PY, Li Y, Switanek J, Zhuang H, Segal MS, et al. Endogenous type-I interferon activity is not associated with depression or fatigue in systemic lupus erythematosus. J Neuroimmunol. 2010;223(1–2):13–9. doi: 10.1016/j.jneuroim.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X, Magder LS, Petri M. Predictors of incident depression in systemic lupus erythematosus. J Rheumatol. 2014;41(9):1823–33. doi: 10.3899/jrheum.140111. [DOI] [PubMed] [Google Scholar]
- 28.Thumboo J, Fong KY, Chan SP, Leong KH, Feng PH, Thio ST, et al. A prospective study of factors affecting quality of life in systemic lupus erythematosus. J Rheumatol. 2000;27(6):1414–20. [PubMed] [Google Scholar]
- 29.Strand V, Crawford B. Improvement in health-related quality of life in patients with SLE following sustained reductions in anti-dsDNA antibodies. Expert Rev Pharmacoecon Outcomes Res. 2005;5(3):317–26. doi: 10.1586/14737167.5.3.317. [DOI] [PubMed] [Google Scholar]
- 30.Hanly JG, Su L, Omisade A, Farewell VT, Fisk JD. Screening for cognitive impairment in systemic lupus erythematosus. J Rheumatol. 2012;39(7):1371–7. doi: 10.3899/jrheum.111504. [DOI] [PubMed] [Google Scholar]
- 31.Vogel A, Bhattacharya S, Larsen JL, Jacobsen S. Do subjective cognitive complaints correlate with cognitive impairment in systemic lupus erythematosus? A Danish outpatient study. Lupus. 2011;20(1):35–43. doi: 10.1177/0961203310382430. [DOI] [PubMed] [Google Scholar]
- 32.Thames AD, Becker BW, Marcotte TD, Hines LJ, Foley JM, Ramezani A, et al. Depression, cognition, and self-appraisal of functional abilities in HIV: an examination of subjective appraisal versus objective performance. Clin Neuropsychol. 2011;25(2):224–43. doi: 10.1080/13854046.2010.539577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julian LJ, Yelin E, Yazdany J, Panopalis P, Trupin L, Criswell LA, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum. 2009;61(2):240–6. doi: 10.1002/art.24236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Exel E, Jacobs J, Korswagen LA, Voskuyl AE, Stek M, Dekker J, et al. Depression in systemic lupus erythematosus, dependent on or independent of severity of disease. Lupus. 2013;22(14):1462–9. doi: 10.1177/0961203313508443. [DOI] [PubMed] [Google Scholar]
- 35.Thombs BD, Ziegelstein RC. Depression screening in primary care: why the Canadian task force on preventive health care did the right thing. Can J Psychiatry. 2013;58(12):692–6. doi: 10.1177/070674371305801207. [DOI] [PubMed] [Google Scholar]