Abstract
Background
Despite progress in the global scale-up of antiretroviral therapy, sustained engagement in HIV care remains challenging. Social capital is an important factor for sustained engagement, but interventions designed to harness this powerful social force are uncommon.
Methods
We conducted a quasi-experimental study evaluating the impact of the Microclinic social network intervention on engagement in HIV care and medication adherence on Mfangano Island, Kenya. The intervention was introduced into 1 of 4 similar communities served by this clinic; comparisons were made between communities using an intention-to-treat analysis. Microclinics, composed of patient-defined support networks, participated in ten bi-weekly discussion sessions covering topics ranging from HIV biology to group support, as well as group HIV status disclosure. Nevirapine concentrations in hair were measured pre-and-post study.
Results
113 (74%) intervention community participants joined a microclinic group, 86% of whom participated in group HIV status disclosure. Over 22-months of follow-up, intervention community participants experienced one-half the rate of ≥ 90-day clinic absence as those in control communities (adjusted hazard ratio 0.48, 95%CI 0.25–0.92). Nevirapine hair levels declined in both study arms; in adjusted linear regression analysis, the decline was 6.7 ng/mg less severe in the intervention arm than control arm (95% CI −2.7 to 16.1).
Conclusions
The microclinic intervention is a promising and feasible community-based strategy to improve long-term engagement in HIV care and possibly medication adherence. Reducing treatment interruptions using a social network approach has important implications for individual patient virologic suppression, morbidity and mortality, and for broader community empowerment and engagement in healthcare.
INTRODUCTION
As HIV treatment programs scale up across resource-limited settings, unprecedented numbers of patients are newly initiating antiretroviral therapy (ART) each year. In 2012, nearly 1.3 million patients started ART in sub-Saharan Africa alone.1 Despite this substantial progress, consistent and long-lasting engagement in HIV care remains a major challenge. Applying best- and worst-case 3-year retention scenarios, an estimated 200,000 to 450,000 of those newly initiated on therapy in sub-Saharan Africa during 2012 will have discontinued treatment by 2015.2,3
Given the magnitude of the retention challenge, there is considerable interest in understanding factors that help patients maintain consistent engagement in care over time.4 One large ethnographic study across three sub-Saharan African countries identified access to social capital as a key facilitator of adherence to therapy.5 Findings from that study, and others, indicate that patient support networks provide necessary psychosocial and material resources for maintaining engagement in HIV care and adherence to therapy.4,6 In return, supporters expect ‘good adherence’, providing positive peer pressure for health-sustaining behaviors.
However, social capital can be difficult for HIV-infected individuals to access when seeking support for HIV treatment.4,7 Status disclosure is often avoided due to fear of the real and perceived ways that disclosure can affect social standing, livelihoods, and relationships.4,8,9 Consequently, many people living with HIV navigate treatment in secret,10–12 leading to diverse negative consequences on maintenance of therapy over time.4,13
Social interventions to promote the exchange of social capital have been previously developed to improve retention in HIV care and adherence to medications. Some ART programs encourage patients to identify a ‘treatment supporter’ – a trusted individual who can provide psychosocial support and assistance with clinic appointments and medication-taking.14–20 Patient support groups, another common intervention, allow patients to exchange knowledge and experiences with fellow patients.21,22 Evidence suggests that these interventions may reduce stigma and facilitate disclosure.23 However, by focusing exclusively on a single treatment supporter or a group of patient peers, these interventions may not fully utilize the pre-existing social infrastructure that patients engage with throughout daily life.
To address this gap, we adapted a social network-based intervention known as ‘microclinics’ that has previously been applied to address diabetes and other chronic diseases in other low-resource settings.24,25 Microclinics are informal social networks empowered to support chronic disease management and prevention. Randomized trials of the microclinic model have demonstrated reductions in hemoglobin A1C levels and body mass indices for diabetic patients in Jordan26,27 and in rural Kentucky.24 Hypothesizing that a combined stigma reduction and social network empowerment intervention would result in improved HIV treatment outcomes28, we developed a novel adaptation of microclinics to encompass groups of mixed HIV-infected and HIV-uninfected individuals in rural Kenya. We conducted a quasi-experimental trial to evaluate the impact of microclinics on engagement in HIV care and medication adherence among patients in this setting.
METHODS
Study population and setting
This study was conducted at Sena Health Center, the largest of six public-sector health facilities and dispensaries on Lake Victoria’s Mfangano Island. Mfangano is located within Homa Bay County, the most HIV-affected county in Kenya, with an estimated adult prevalence of 27%.29 Mfangano has a population of approximately 21,000 and is divided into four administrative sub-locations of roughly equal size. The Sena Health Center is located on the boundary between the East and North sub-locations and over 90% of patients at Sena reside in one of these two locations. Adult patients at the Sena Health Center were eligible to participate if they were Mfangano residents and had initiated ART prior to or during the study enrollment period from November 2011 – February 2012. The study was approved by the Kenya Medical Research Institute Ethical Review Committee and the University of California, San Francisco Committee for Human Subjects Research. The study protocol is registered at ClinicalTrials.gov (NCT01912521). Written informed consent was obtained prior to study enrollment.
Design and intervention
We conducted a quasi-experimental study with the intervention administered within the Mfangano East sub-location and the remaining three sub-locations serving as control. For this pilot study, Mfangano East was selected as the intervention community out of convenience because the implementing organization, the Organic Health Response, is located within Mfangano East. Thus, Sena Health Center patients who lived in East comprised the intervention group and those residing in the remaining three neighboring sub-locations comprised the control group. We used an intention-to-treat analysis with treatment assignment based on sub-location of residence rather than intervention uptake. As secondary analysis, we also conducted as-treated analyses based on intervention participation.
After enrolling patients on ART at the Sena Health Center in the study, those living in the intervention community were invited to form ‘microclinic’ groups. These microclinic groups were intended to contain 5–15 close family, friends or other members of the patient’s social support system, irrespective of these individuals’ HIV status. CHWs and study staff worked with ‘seed’ individuals (i.e. study participants on ART) to identify microclinic group members. In some cases, several ‘seed’ individuals and their networks were combined into one microclinic group, based on CHW catchment area. Additionally, pre-existing community groups were also invited to form microclinic groups and participate in the intervention. At the time of group formation, all microclinic participants underwent confidential individual HIV counseling and testing.
Once formed, microclinics were assigned a CHW coordinator and facilitator, and were guided through a series of ten discussion sessions over a period of five months. Sessions were scheduled every two weeks at a time and location of each group’s choosing and lasted 2–3 hours each. CHWs participated in a 3–4 hour ‘train-the-trainer’ workshop prior to each session to learn the games, role-plays and didactic components of each session, ask questions, and discuss with fellow CHWs prior to delivering the material to microclinic groups. CHWs were paid a stipend to compensate their role in microclinic coordination.
Over the course of the ten group discussion sessions, major intervention components included 1) health education to promote knowledge of HIV prevention and treatment; 2) promotion of group support through discussions of confidentiality, HIV status disclosure, and encouragement of group support for adherence and clinic attendance; and 3) outreach to promote HIV testing and clinic enrollment within the community. At the conclusion of the ten sessions, groups were invited to participate in voluntary group HIV testing, allowing microclinic members to disclose their HIV status to one another. Participants were followed for 18 months after initiation of the intervention to ascertain treatment outcomes.
Measurements
Study staff conducted surveys and chart review to measure baseline demographic and clinical characteristics (Table 1). At baseline and immediately post-intervention, we measured perceived community (attributable) stigma30, HIV-related knowledge31 and social support32. Study staff also collected small hair samples for measurement of ART concentration, using previously described procedures.33 Hair samples were shipped at room temperature to a UCSF lab (the Drug Studies Unit) in San Francisco for analysis by liquid chromatography/tandem mass spectrometry (LC-MS/MS).34,35
Table 1.
Baseline characteristics of the 369 participants enrolled in the MIHNIS study
| Characteristic | Control Communities (n=216) | Intervention Community (n=153) | p-value* | ||
|---|---|---|---|---|---|
| Female sex, n (%) | 139 | 64% | 97 | 63% | 0.85 |
|
| |||||
| Age (yrs), mean (sd) | 40 | 13 | 39 | 10 | 0.40 |
|
| |||||
| Monthly household income (USD), mean (SD) | 45 | 49 | 56 | 78 | 0.09 |
|
| |||||
| Household size, mean (SD) | 5.7 | 3.0 | 5.8 | 3.1 | 0.76 |
|
| |||||
| Level of education completed, n (%) | 0.36 | ||||
| None | 8 | 4% | 12 | 8% | |
| Primary | 140 | 65% | 91 | 59% | |
| Secondary | 56 | 26% | 42 | 27% | |
| Post-secondary | 12 | 6% | 8 | 5% | |
|
| |||||
| Marital Status, n (%) | 0.11 | ||||
| Single/Never married | 10 | 5% | 3 | 2% | |
| Separated/Divorced | 9 | 4% | 15 | 10% | |
| Widowed | 61 | 28% | 39 | 25% | |
| Married | 136 | 63% | 96 | 63% | |
|
| |||||
| Walking distance to health center, n (%) | <0.0001 | ||||
| <30 min | 28 | 13% | 75 | 49% | |
| 30–60 min | 80 | 37% | 46 | 30% | |
| >1 hour | 108 | 50% | 32 | 21% | |
|
| |||||
| Baseline stigma score (17-pt scale), mean(sd)† | 6.6 | 3.4 | 6.9 | 3.5 | 0.38 |
|
| |||||
| Baseline HIV knowledge scale (18-pt scale), mean(sd)‡ | 14.8 | 2.3 | 14.9 | 2.0 | 0.42 |
|
| |||||
| Time since ART initiation (yrs), mean(SD) | 2.7 | 1.8 | 2.8 | 1.9 | 0.50 |
|
| |||||
| Baseline CD4 count (cells/mm3), mean(SD) | 372 | 195 | 415 | 209 | 0.05 |
|
| |||||
| Baseline WHO stage | 0.89 | ||||
| Stage I/II | 103 | 49% | 74 | 51% | |
| Stage III | 76 | 36% | 52 | 36% | |
| Stage IV | 31 | 15% | 19 | 13% | |
|
| |||||
| Microclinic participation, n (%) | 4 | 2% | 113 | 74% | |
| Group VCT participation, n (%) | 2 | 50% | 97 | 86% | |
univariate logistic regression of continuousor categorical predictors against study arm
larger value indicates greater perceived stigma
larger value indicates increased HIV-related knowledge
We also collected clinic visit dates and corresponding next scheduled appointment dates from clinic records. For participants who were lost to follow-up, we conducted active patient tracing at the end of study follow-up, as well as review of records at other clinics on Mfangano to ascertain whether the patient had transferred, died, or simply discontinued clinical care. We assumed that patients who could not be located and were not in care at another clinic within Mfangano were disengaged from care. For patients in care at another Mfangano facility, we continued chart review at those facilities following the transfer.
Statistical Analysis
Primary outcomes were engagement in HIV care and change in antiretroviral drug concentration in hair from baseline to immediately post-intervention. We evaluated engagement in care in two different ways, namely 1) time to first 90-day clinic absence following a missed visit and 2) time spent adhering to clinic visit schedules (termed ‘time in care’). Secondary outcomes included changes in HIV-related stigma, HIV knowledge, and reports of social support.
We used logistic regression, with a test for overall effect for categorical variables with more than two categories, to compare distribution of baseline characteristics between study arms. Because nevirapine (NVP) was the most prevalent drug taken by study participants (88% at baseline and 84% at post-intervention), and because the means and ranges of hair concentrations differs for each drug, we restricted our hair sample analysis to NVP users.36 We computed the difference in hair NVP concentrations from baseline to immediately after completion of the intervention. Patients who were not taking NVP or who did not donate hair for analysis at one or both time points were excluded from analysis. We used univariable and multivariable linear regression to compare changes in NVP hair levels between study arms.
We calculated gaps in care by determining the number of days between a missed visit and the date of return to any clinic on Mfangano; participants were censored on the date of death or transfer to a health facility outside Mfangano Island. Thus 90-day disengagement indicates missing an appointment by ≥ 90 days and not known to have first transferred or died. ‘Time in care’ constituted the proportion of time participants spent adhering to their scheduled appointment dates, and was calculated as follows:
Total time eligible for care was calculated from the date of study enrollment until the date of censoring or study closure. We compared time to 90-day disengagement between study arms using Cox proportional hazards. We evaluated the proportional hazards assumption both graphically and using formal testing with Schoenfeld residuals. We also computed the cumulative incidence function using death as a competing event, and displayed differences between groups graphically.37 We used linear regression to compare differences in time in care between study arms. To enhance interpretability, we converted model-derived estimates to days per person-year by multiplying by 365.25. To address potential non-normality of the residuals, we used bootstrapping with 10,000 replications and cluster resampling to evaluate the degree to which potential non-normality of residuals impacted standard errors.
Though primary analysis was conducted using intention to treat, we also performed sensitivity analyses excluding individuals in the intervention arm who did not join a microclinic group. For each model, we used robust standard errors, which accounted for non-independence resulting from the clustered nature of the intervention. In multivariate models, we adjusted for baseline factors reasonably thought to confound the relationship between community of residence and study outcomes. These included age, sex, monthly household income, walking distance to the Sena Health Center, stigma score, HIV-related knowledge, social support, CD4 count, WHO stage and time since ART initiation. Predictors with p-values <0.1 were retained in an intermediate model and each predictor was readded and included in the final model only if the addition changed the estimated intervention effect by ≥ +/−10%.
In addition to the primary study outcomes, we used univariable linear regression to evaluate intervention impact on changes in perceived stigma, HIV-related knowledge, and social support.
RESULTS
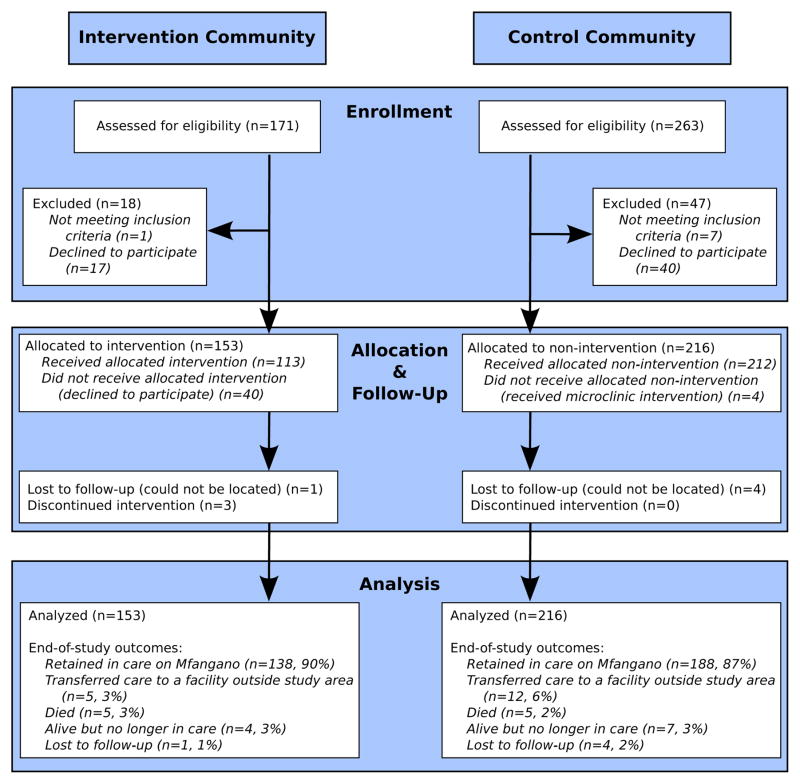
Of 426 eligible clinic patients, 369 (87%) enrolled in the study (Figure 1). Baseline characteristics were similar between communities, though intervention community participants tended to live closer to the clinic and have higher baseline CD4 cell counts (Table 1). Within the intervention community, 44 microclinic groups were formed. The median (range) microclinic group was 13 members in size (4–18), 78% female (0–100%) and 33% HIV-infected (0–86%). Thirty-four groups contained a study participant on ART, nine groups contained members who were HIV-infected but not yet on ART, and one group was composed entirely of HIV-uninfected individuals (note, some groups did not contain a study participant on ART because we allowed pre-existing groups to also form microclinics). In total, 113 (74%) of the 153 intervention community study participants on ART and 423 members of their social support networks participated in a microclinic. Four control community study participants also participated in a microclinic group. Thus, standard errors for all models were adjusted for 286 clusters, namely 212 control arm participants who did not join a microclinic, 40 intervention arm participants who did not join a microclinic and 34 microclinic groups containing 117 study participants on ART from both intervention and control study arms. Microclinic participation was excellent; 110/113 (97%) of intervention arm study participants remained active group members at the end of the 10 sessions, based on CHW report, with study staff verification. Further, 86% of both patients on ART (97/113) and their social support network members (364/423) attended voluntary group counseling, testing and disclosure. Twenty one percent (75/364) of support network members who participated in the group disclosure were HIV-infected, but had not yet started ART. Clinic data was not available for these participants, and thus we were not able to determine whether they were enrolled in clinical care. HIV status of group members who did not participate in group testing and disclosure was not available.
Figure 1.
Participant flow
Medication adherence
The acceptability of hair collection was 95% (350 of 369) at study baseline and 99% (338 of the 340 remaining in the study) at 6-month follow-up. One hundred and eleven (73%) intervention arm participants and 162 (75%) control arm participants were taking NVP and had hair samples collected at both baseline and 6-month study visits. Mean NVP levels decreased in both cohorts, from 82.9 to 77.4 ng/mg (change: −5.5, SD 42.4) in the intervention community and 93.4 to 81.0 ng/mg (change: −12.4, SD 38.8) in the control community (n=273). In univariable linear regression, the decline in NVP hair concentrations over the course of the intervention was 6.9 ng/mg less in the intervention arm compared to the control arm (95% CI −2.5 to 16.2). Because both groups experienced decreases in NVP hair concentrations, this represented a non-statistically significant smaller decrease in the intervention arm in comparison to control. In multivariable modeling, only age was retained as a potential confounder; estimates remained similar (effect size 6.7 ng/mg, 95% CI −2.7 to 16.1).
In as-treated analysis, comparing those who joined a microclinic group in the intervention arm to all participants in the control arm, decrease in NVP hair concentration was 11.1 ng/mg (95% CI 1.3 to 21.0) less in the intervention group than control. Multivariable analysis, including age, yielded similar results (effect size 11.3 ng/mg, 95% CI 1.4 to 21.1).
Disengagement from care
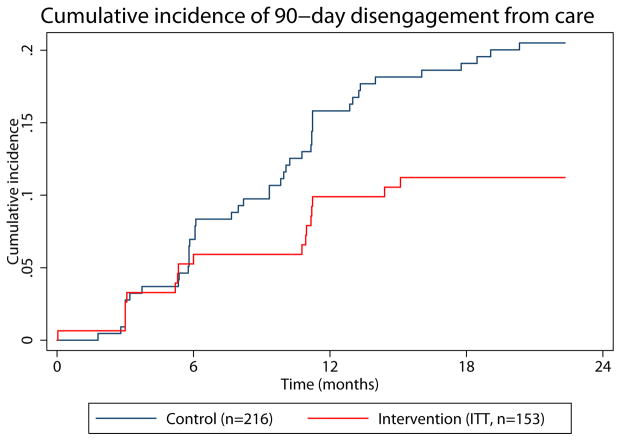
After study enrollment, participants were followed for 22 months or until the date of death or transfer to a health facility outside Mfangano Island. Most participants were retained in care by the end of follow-up (Figure 1), however over the course of follow-up, 11% of intervention arm participants intervention arm and 20% of those in the control arm experienced a clinic absence of ≥ 90-days. Incidence rates of 90-day disengagement were 6.8 per 100 person-years in the intervention group (95%CI 4.2–10.9) and 12.9 (95%CI 9.6–17.3) in the control. Using an unadjusted Cox proportional hazard model, participants in the intervention arm had one-half the rate of ≥ 90-day clinic absence as those in the control arm (HR 0.53, 95% CI 0.28–1.02) (Table 2). Adjusted analysis, including time since ART initiation and distance to the health center, yielded similar results (adjusted (a)HR 0.48, 95% CI 0.25–0.92). We plotted the cumulative incidence of 90-day disengagement, treating death as a competing event, to visually represent disengagement occurrence over the study period (Figure 2). Notably, the first four months of follow-up were contemporaneous with group formation and the intervention itself did not begin until month five. Cumulative incidence curves suggest a difference in disengagement that begins approximately two months after initiation of the intervention.
Table 2.
Disengagement from care
| Characteristic | Hazard ratio | 95% CI* | p-value |
|---|---|---|---|
| Univariable model | |||
| Intervention community | 0.53 | 0.28–1.02 | 0.056 |
|
| |||
| Multivariable model† | |||
| Intervention community | 0.48 | 0.25–0.92 | 0.026 |
| Time since ART initiation | 0.80 | 0.68–0.94 | 0.007 |
| Walking distance to clinic | |||
| <30 min | ref | ref | ref |
| 30–60 min | 0.60 | 0.30–1.17 | 0.13 |
| >60 min | 0.70 | 0.36–1.36 | 0.29 |
95% CIs adjusted for clustering using robust standard errors (286 clusters)
Other covariates considered but not selected: age, sex, monthly income, food insecurity, baseline stigma, baseline WHO stage, baseline CD4 count
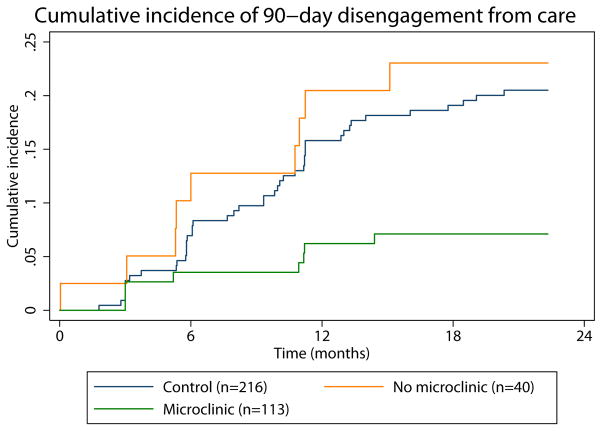
Figure 2. Cumulative incidence of 90-day disengagement from care.
The intervention commenced at month 5 and ran through month 9. (A) Intention to treat analysis. (B) As treated analysis with green line representing intervention arm participants who joined microclinics and orange line representing intervention arm participants who did not join a microclinic.
Time in care
To further characterize engagement in care over time, we measured the proportion of time participants spent adhering to clinic appointment schedules (time in care). During study follow-up, the average time in care was 86.2% in the intervention community and 81.6% in the control community, an absolute difference of 4.6% (95% CI 1.3% to 8.5%) (Table 3). This is equivalent to an increase of 17 days ‘in care’ per patient-year (95% CI 3–31 days) among patients in the intervention arm. In multivariable linear regression, adjusting for time since ART initiation, distance from clinic and baseline stigma, the intervention community experienced a 6.0% absolute increase in time in care (95% CI 3.4 to 8.6%), an increase of 22 days ‘in care’ per patient-year (95% CI 10 to 34 days). Confidence intervals were not substantively changed when recalculated using the bootstrap method (data not shown).
Table 3.
Time in care
| Characteristic | Beta | 95% CI* | p-value |
|---|---|---|---|
| Univariate model | |||
| Intervention community | 0.046 | 0.008–0.085 | 0.02 |
|
| |||
| Multivariate model† | |||
| Intervention community | 0.060 | 0.027–0.093 | <0.005 |
| Time since ART initiation | 0.015 | 0.005–0.025 | 0.004 |
| Walking distance to clinic | |||
| <30 min | ref | ref | ref |
| 30–60 min | 0.057 | 0.011–0.102 | 0.02 |
| >60 min | 0.039 | −0.007–0.085 | 0.09 |
| Attributable stigma‡ | −0.004 | −0.009–0.001 | 0.08 |
95% CIs adjusted for clustering using robust standard errors (286 clusters)
Other co-variates considered but not selected: age, sex, monthly income, food insecurity, baseline WHO stage, baseline CD4 count
Perceived stigma in the community, increased score indicates higher levels of perceived stigma
Stigma decreased by 25% relative to baseline in the intervention community and was unchanged in the control community, with a difference in change scores between groups of −1.6 units on a 17-unit scale (95% CI −2.4 to −0.8, Table S1). There was no difference in change in HIV-related knowledge between groups. Social support increased slightly in the intervention community, though the change within the intervention arm represented only a 2% relative increase from baseline.
DISCUSSION
Microclinics improved community-wide engagement in HIV care among patients on ART. Patients residing in the intervention community had one-half the rate of 90-day gaps in care as control participants. Those in the intervention community also spent a larger proportion of time adherent to clinic schedules. The observed 6% increase in time in care in the multivariable model is equivalent to a three-week reduction, per patient-year, in the delay between missed visits and subsequent return to clinic.
We also observed increases in hair NVP concentrations in intervention community participants relative to controls, though this improvement was not statistically significant. The confidence interval of our observed estimates for change in NVP hair concentrations was wide and included the possibility of either no true effect or an effect large enough to be beneficial for many patients, based on comparison of NVP changes to virologic suppression in another study.36 As-treated results suggested that the intervention might exert a protective effect on declining hair drug levels over time. However, this analysis is subject to potentially substantial selection bias and should be regarded with caution. Absolute NVP hair concentrations are difficult to interpret clinically, especially since this rural Kenyan cohort had baseline mean concentrations that were over two times higher than US-based cohorts.33,36 However, the within-individual differences over a relatively short period of time likely reflect changes in adherence, rather than alterations in pharmacokinetics.38
We propose that the microclinic intervention impacts the above clinical processes by reducing HIV-related stigma and, thus, lowering the ‘activation energy’ required for engaging social networks in the treatment process. The resulting increase in access to social capital for HIV treatment support could explain our observed improvements in clinic appointment adherence and possible medication adherence.5 Our observation that HIV-related stigma decreased, while overall social support and HIV-related knowledge remained relatively unchanged, may support this hypothesis.
Microclinics build on key strengths of existing social interventions for promoting engagement in HIV care, including treatment supporters and patient support groups. Whereas treatment supporters promote status disclosure and reduce stigma through a single supportive relationship,23 microclinics provide this degree of support by means of patient’s broader social network. In addition, microclinics also promote the role of ‘expert patients’ commonly found in patient support group interventions.39 By encouraging group members to be both supported by and supporters of other group members, the microclinic model facilitates group empowerment and may represent a more socially-relevant approach to chronic disease management than more individual-oriented approaches.4
Though most participants who met our definition of disengagement eventually returned to care, we observed a substantial reduction in long gaps in care in the intervention community. Recent work by Ware and colleagues highlights a pathway from missing a clinic visit to ‘disengaging’ from care that includes, as intermediary steps, developing a ‘reluctance to return’ and subsequent feelings of decreased connectedness to care.40 In our study, missed visits were very common, with over 90% of participants missing at least one visit by more than three days over the course of follow-up and no significant difference between study arms (data not shown). It is possible that microclinic participation either prevented development of ‘reluctance to return’ following a missed visit or prevented this reluctance from eroding ultimate feelings of connection to care, though further study is needed to understand how the microclinic intervention interacts with these concepts.
These results bolster empiric support for microclinics as an effective model for chronic disease management. Microclinic interventions to address diabetes have demonstrated beneficial effects not only for ‘index’ diabetes patients, but also for members of their social networks – arguably individuals who are also at high risk for developing diabetes due to shared genetic, environmental and behavioral risk factors.24,26 Similarly, this intervention holds potential for improving care not only for individuals who are on ART, but also for improving engagement in care by those who have not yet sought HIV care. Still other HIV-uninfected group members may benefit from increased knowledge, motivation and group support for preventing HIV. This multi-level social network effect may be especially important among high prevalence populations.
This study has several limitations, including the quasi-experimental design and our inability to assess impact on downstream health outcomes. Though treatment assignment was not randomized, we compared outcomes among populations that were qualitatively and quantitatively highly similar at baseline. Additionally, our intention to treat analysis eliminated the confounding that occurs when patients with lower risk of poor outcomes are also more likely to participate in a social intervention of this type. Viral load was cost-prohibitive in this early phase trial, and our study design was not intended to evaluate impact on mortality. However, others have shown that gaps in clinical HIV care predict subsequent virologic failure, morbidity and mortality.41 Our successful efforts to ascertain outcomes for nearly all study participants through active tracing also increase our confidence that observed gaps are reflective of true treatment interruptions. Our time in care measure, the proportion of time patients adhered to their clinic appointment schedules, further supports our observation that patients attended appointments more regularly and with less delay in the intervention community.
CONCLUSION
The microclinic intervention holds promise as a feasible community-based strategy to improve long-term engagement in HIV care. The success of a social network approach on reducing treatment interruptions and improving engagement in care has important implications for improving virologic suppression, and subsequently decreasing morbidity, mortality and HIV transmission. Because of the way in which social networks are woven directly into the fabric of daily life, particularly in poor communities in resource-limited settings, this strategy may result in a more sustained and amplified effect than previously evaluated approaches and warrants further study.
Supplementary Material
Acknowledgments
We would like to thank the research team and staff at the Ekialo Kiona Center for their tireless work, including Lister Amondi Ngare, Millicent Nyauke, Sylvanus Agong, Ruth Omondi, Pamela Mohamed, Joyce Obanda, Martabel Okeyo, Elsa Awino Odero, Clifford Omondi Maginga, Reagan Okoth, Odhiambo Mourline Atieno, Abdi Odhiambo Kitewega, Vallary Achieng Migawi, Janet Awino Ooko, Mary Janet Achieng, Theresa Alingo, Jane Akinyi Odindo, Robinson Okeyo, and Richard Magerenge. We would also like to recognize and appreciate Michael Nguyen for putting together the supplemental figure, and the rest of the staff at Microclinic International for their ongoing support. We would also like to thank the faculty in UCSF’s Training in Clinical Research program for their second-to-none teaching, the staff at the Sena Health Center for their collaborative spirit, the Director, KEMRI for his support of the study, and, most importantly, our study participants for believing in the value of kanyaklas and dedicating their time toward making this project a success.
Footnotes
Presentations
Portions of this data were presented as an oral abstract at the International Conference on HIV Treatment and Prevention Adherence in Miami, Florida on June 9, 2014
Disclosures
The study was made possible through generous support from Google Inc via the Tides Foundation, the Craigslist Foundation, the Mulago Foundation, the Rise Up Foundation, the Horace W Goldsmith Foundation, the Segal Family Foundation, the National Institute of Allergy and Infectious Diseases (NIAD)/National Institutes of Health (NIH) (R01 AI098472 to M.G., U01AI034989 WIHS), the Doris Duke Charitable Foundation (to M.D.H.), and the UCSF School of Medicine Dean’s research fellowship (C.R.S.)
References
- 1.WHO. Global Update on HIV Treatment 2013: Results, Impact and Opportunities. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 2.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15( Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geng EH, Glidden DV, Bwana MB, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PloS one. 2011;6:e21797. doi: 10.1371/journal.pone.0021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merten S, Kenter E, McKenzie O, Musheke M, Ntalasha H, Martin-Hilber A. Patient-reported barriers and drivers of adherence to antiretrovirals in sub-Saharan Africa: a meta-ethnography. Trop Med Int Health. 2010 Jun;15( Suppl 1):16–33. doi: 10.1111/j.1365-3156.2010.02510.x. [DOI] [PubMed] [Google Scholar]
- 5.Ware NC, Idoko J, Kaaya S, et al. Explaining adherence success in sub-Saharan Africa: an ethnographic study. PLoS Med. 2009 Jan 27;6(1):e11. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watt MH, Maman S, Earp JA, et al. “It’s all the time in my mind”: facilitators of adherence to antiretroviral therapy in a Tanzanian setting. Social science & medicine. 2009;68:1793–1800. doi: 10.1016/j.socscimed.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmen C. Towards an Anthropology of Organic Health: The Relational Fields of HIV/AIDS among the Suba of Lake Victoria. Institute of Social and Cultural Anthropology, Oxford University; 2009. [Google Scholar]
- 8.Greeff M, Phetlhu R, Makoae LN, et al. Disclosure of HIV status: experiences and perceptions of persons living with HIV/AIDS and nurses involved in their care in Africa. Qualitative health research. 2008 Mar;18(3):311–324. doi: 10.1177/1049732307311118. [DOI] [PubMed] [Google Scholar]
- 9.Tsai AC, Bangsberg DR, Kegeles SM, et al. Internalized Stigma, Social Distance, and Disclosure of HIV Seropositivity in Rural Uganda. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2013 May 21; doi: 10.1007/s12160-013-9514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mbonu NC, van den Borne B, De Vries NK. Stigma of People with HIV/AIDS in Sub-Saharan Africa: A Literature Review. Journal of tropical medicine. 2009;2009:145891. doi: 10.1155/2009/145891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simbayi LC, Kalichman S, Strebel A, Cloete A, Henda N, Mqeketo A. Internalized stigma, discrimination, and depression among men and women living with HIV/AIDS in Cape Town, South Africa. Soc Sci Med. 2007 May;64(9):1823–1831. doi: 10.1016/j.socscimed.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turan JM, Hatcher AH, Medema-Wijnveen J, et al. The role of HIV-related stigma in utilization of skilled childbirth services in rural Kenya: a prospective mixed-methods study. PLoS Med. 2012;9(8):e1001295. doi: 10.1371/journal.pmed.1001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2):18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunutsor S, Walley J, Katabira E, et al. Improving clinic attendance and adherence to antiretroviral therapy through a treatment supporter intervention in Uganda: a randomized controlled trial. AIDS Behav. 2011 Nov;15(8):1795–1802. doi: 10.1007/s10461-011-9927-9. [DOI] [PubMed] [Google Scholar]
- 15.Stubbs BA, Micek MA, Pfeiffer JT, Montoya P, Gloyd S. Treatment partners and adherence to HAART in Central Mozambique. AIDS Care. 2009;21:1412–1419. doi: 10.1080/09540120902814395. [DOI] [PubMed] [Google Scholar]
- 16.Idoko JA, Agbaji O, Agaba P, et al. Direct observation therapy-highly active antiretroviral therapy in a resource-limited setting: the use of community treatment support can be effective. International journal of STD & AIDS. 2007 Nov;18(11):760–763. doi: 10.1258/095646207782212252. [DOI] [PubMed] [Google Scholar]
- 17.Fatti G, Meintjes G, Shea J, Eley B, Grimwood A. Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. J Acquir Immune Defic Syndr. 2012 Dec 1;61(4):e50–58. doi: 10.1097/QAI.0b013e31826a6aee. [DOI] [PubMed] [Google Scholar]
- 18.Nachega JB, Chaisson RE, Goliath R, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 2010 Jun 1;24(9):1273–1280. doi: 10.1097/QAD.0b013e328339e20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson CR, Micek MA, Simoni JM, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr. 2007 Oct 1;46(2):238–244. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taiwo BO, Idoko JA, Welty LJ, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. J Acquir Immune Defic Syndr. 2010 May 1;54(1):85–92. doi: 10.1097/01.qai.0000371678.25873.1c. [DOI] [PubMed] [Google Scholar]
- 21.Luque-Fernandez MA, Van Cutsem G, Goemaere E, et al. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in Khayelitsha, Cape Town, South Africa. PLoS One. 2013;8(2):e56088. doi: 10.1371/journal.pone.0056088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wouters E, Van Damme W, Van Loon F, van Rensburg D, Meulemans H. Public-sector ART in the Free State Province, South Africa: community support as an important determinant of outcome. Soc Sci Med. 2009 Oct;69(8):1177–1185. doi: 10.1016/j.socscimed.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 23.O’Laughlin KN, Wyatt MA, Kaaya S, Bangsberg DR, Ware NC. How treatment partners help: social analysis of an African adherence support intervention. AIDS Behav. 2012 Jul;16(5):1308–1315. doi: 10.1007/s10461-011-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding E, Prescott M, Watson KT, Bui N, Makarechi L, Zoughbie DE. Microclinic Social Network Lifestyle Intervention for Weight Loss and Obesity Management: a 10 Month Randomized Controlled Trial. Circulation. 2013;127(12 Supplement) [Google Scholar]
- 25.Zoughbie DE. Community-based diabetes programme: the micro-clinic project. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2009 Jul-Aug;15(4):1021–1026. [PubMed] [Google Scholar]
- 26.Prescott M, Zoughbie D, Watson K, Bui N, Farraj R, Elkarra N. The Microclinic Health Program: A social network-based intervention for weight loss and diabetes risk management. Am J Epidemiol. 2013;177(11 Suppl):S1–S181. [Google Scholar]
- 27.Zoughbie D, Watson K, Bui N, Farraj R, Prescott M, Ding E. Long-term bodyweight and glucose management effects of the Microclinic Social Network Health Behavioral Program in Amman, Jordan: 2-year results. Paper presented at: Fifth Annual CUGH Conference; 2014; Washington DC. [Google Scholar]
- 28.Bangsberg DR, Deeks SG. Spending more to save more: interventions to promote adherence. Ann Intern Med. 2010 Jan 5;152(1):54–56. W–13. doi: 10.1059/0003-4819-152-1-201001050-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.County HIV Service Delivery Profiles. Ministry of Health; Republic of Kenya: 2013. [Google Scholar]
- 30.Visser MJ, Kershaw T, Makin JD, Forsyth BW. Development of parallel scales to measure HIV-related stigma. AIDS Behav. 2008 Sep;12(5):759–771. doi: 10.1007/s10461-008-9363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV Knowledge Questionnaire. AIDS Educ Prev. 2002 Apr;14(2):172–182. doi: 10.1521/aeap.14.2.172.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broadhead WE, Gehlbach SH, de Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Medical care. 1988 Jul;26(7):709–723. doi: 10.1097/00005650-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Hickey MD, Salmen CR, Tessler RA, et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr. 2014 Jul 1;66(3):311–315. doi: 10.1097/QAI.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Yang Q, Yoon K, et al. Microanalysis of the antiretroviral nevirapine in human hair from HIV-infected patients by liquid chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry. 2011;401:1923–1933. doi: 10.1007/s00216-011-5278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(21):3401–3409. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baxi S, Greenblatt R, Bacchetti P, et al. Nevirapine concentrations in hair is a strong predictor of virologic suppression and toxicities (MOPE038). Paper presented at: 20th International AIDS Conference; July 20–25, 2014; Melbourne, Australia. (manuscript in submission) [Google Scholar]
- 37.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. British journal of cancer. 2004 Oct 4;91(7):1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandhi M, Ameli N, Bacchetti P, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clinical Infectious Diseases. 2011;52(10):1267–1275. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decroo T, Van Damme W, Kegels G, Remartinez D, Rasschaert F. Are Expert Patients an Untapped Resource for ART Provision in Sub-Saharan Africa? AIDS Res Treat. 2012;2012:749718. doi: 10.1155/2012/749718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ware NC, Wyatt MA, Geng EH, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Med. 2013;10(1):e1001369. doi: 10.1371/journal.pmed.1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Trop Med Int Health. 2011 Oct;16(10):1297–1313. doi: 10.1111/j.1365-3156.2011.02828.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.