Abstract
Background
Specification of the metanephric mesenchyme is a central step of kidney development as this mesenchyme promotes nephric duct induction to form a ureteric bud near its caudal end. Prior to ureteric bud formation, the caudal nephric duct swells to form a pseudostratified epithelial domain that later emerges as the tip of the bud. However, the signals that promote the formation of the transient epithelial domain remain unclear. Here we investigated the early roles of the mesenchymal factor Six family and its cofactor Eya on the initial induction of nephric duct development.
Results
The nephrogenic progenitor population is initially present but significantly reduced in mice lacking both Six1 and Six4 and undertakes an abnormal cell death pathway to be completely eliminated by ~E10.5-11.0, similar to that observed in Eya1−/− embryos. Consequently, the nephric duct fails to be induced to undergo normal proliferation to pseudostratify and form the ureteric bud in Six1−/−;Six4−/− or Eya1−/− embryos.
Conclusion
Our data support a model where Eya-Six may form a complex to regulate nephron progenitor cell development before metanephric specification and are critical mesenchymal factors for inducing nephric duct development.
Keywords: Six1/4, Eya1, nephrogenic cord, metanephric mesenchyme, nephric duct, pseudostratification, ureteric bud
Introduction
In mammals, the kidney is derived from the intermediate mesoderm (IM) (Saxen and Sariola, 1987). Its development is initiated when the IM-derived nephric duct (ND) or Wolffian duct, an epithelial tube, swells at the caudal region and forms the ureteric bud (UB), which then outgrows and invades into the adjacent metanephric mesenchyme (MM), which also originates from the IM. The MM secretes signals to induce the ND to form the UB, which undergoes complex branching morphogenesis to give rise to the urinary renal collecting duct system. In reciprocal, the UB induces the MM cells to differentiate into the nephron epithelia (Costantini, 2006; Dressler, 2006; Dressler, 2009).
The MM forms at the caudal end of the nephrogenic cord at ~E10.5 in mice and the establishment of a functional MM is critical as this tissue has the specialized capability of inducing the growth and branching of the UB in its characteristic pattern (Saxen and Sariola, 1987; Davies and Fisher, 2002; Vainio and Lin, 2002). Among the signalling molecules from the MM that regulate UB formation, GNDF (glial-derived neurotrophic factor), which acts as a ligand that binds to the Ret receptor tyrosine kinase and the GDNF coreceptor Gfrα1 that are expressed in the ND, is known to be a major inducer (Saxen and Lehtonen, 1987; Davies and Fisher, 2002; Vainio and Lin, 2002; Costantini and Shakya, 2006; Costantini, 2010). While genetic studies have identified many genes that are important for stimulating and maintaining GDNF expression and for the MM differentiation, only Eya1 (Sajithlal et al., 2005) and Osr1 (James et al., 2006) are required for the formation of the MM as deletion of Eya1 or Osr1 causes a complete absence of the MM (Sajithlal et al., 2005; James et al., 2006). However, their precise roles in specifying the nephrogenic mesenchyme and inducing ND development are not well understood.
Prior to UB formation, the caudal ND swells to form a pseudostratified domain that later emerges as the tip of the UB (Chi et al., 2009). Although the GDNF-RET pathway is known to be necessary for UB development, this signaling pathway does not appear to be required for pseudostratification because the ND becomes pseudostratified in Ret−/− animals (Chi et al., 2009; Costantini, 2010). While this pseudostratified epithelium is not formed in Osr1−/− mice (Mugford et al., 2008), it is currently unclear what signals are involved in promoting the generation of this transient epithelial domain. We have previously reported that the UB fails to form in Eya1−/− mutants (Sajithlal et al., 2005). In mice lacking Six1, a member of the Six gene family that interacts with the Eya gene family, the MM forms and the UB outgrows but fails to undergo branching morphogenesis (Xu et al., 2003; Nie et al., 2011). In contrast, mice lacking both Six1 and Six4 fail to form a detectable MM and UB development is not induced (Kobayashi et al., 2007). However, despite the importance of these genes in kidney development, the mechanisms that control the earlier phases of kidney development – formation of the MM and induction of caudal ND development – still remain unclear.
Here we have specifically investigated the requirement of the mesenchymal factor Eya1 and the cofactor Six protein family Six1 and Six4 in the specification of the nephrogenic cord mesenchyme and the initial induction of ND development. The nephrogenic progenitor population marked by Eya1 expression is not only reduced in Six1;Six4 double but also in Six1 single mutant embryos at E9.5. TUNEL assay revealed that the nephrogenic progenitors in the Six1;Six4 or Eya1 mutant embryos undertake an abnormal cell death pathway at ~E9.5-10.0, leading to complete degeneration by E10.5-11.0. In concurrence with the disappearance of the nephrogenic mesenchymal population in the metanephric region at ~E9.5-10.5, the caudal ND in Six1−/−;Six4−/− or Eya1−/− embryos fails to proliferate normally to pseudostratify and initiate ureteric development. Our data suggest that Eya1 and Six1/Six4 may function together to regulate cell survival of the nephrogenic progenitors to form a functional MM and promote the initiation of ND development.
Results and Discussion
Nephrogenic progenitor population is reduced in Six1 single mutant and markedly reduced in Six1;Six4 double mutant embryos at E9.5
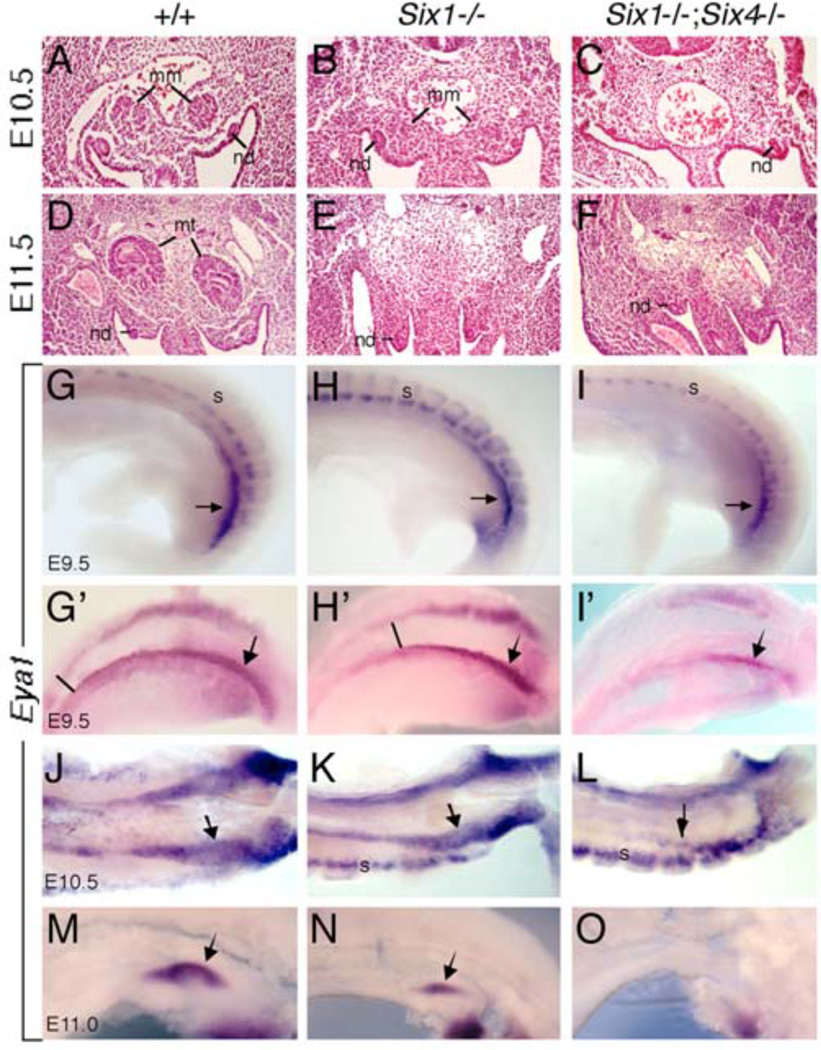
While Six4−/− mice appear normal (Ozaki et al., 2001), in Six1−/− mice the UB forms but fails to branch within the MM to give rise to the collecting duct system and instead it differentiates into ureter (Nie et al., 2011). A previous study reported that the MM cells are present in mice lacking both Six1 and Six4, but the UB is not formed due to lack of Gdnf expression (Kobayashi et al., 2007). Among the mesenchymal markers examined, only Osr1 and Wt1 expression have been reported to be detectable in Six1−/−;Six4−/− mutants (Kobayashi et al., 2007). However, because both Osr1 and Wt1 are widely expressed in multiple cell types within the IM and urogenital region and are necessary not only for kidney development but also for gonad and adrenal gland development, the developmental and cellular basis for the onset of metanephric developmental failure in Six1;Six4 double mutant is still unclear. Moreover, it remains unknown whether the MM is formed normally in Six1−/− embryos. To directly address this, we first performed histological analysis to examine the formation of the MM in mice lacking Six1 alone or both Six1 and Six4. In normal mouse embryos, the MM appears morphologically apparent as an aggregate of cells within the IM at the caudal end of the nephrogenic cord at E10.5 (Fig. 1A). This structure is apparent in Six1−/− embryos at this stage (Fig. 1B) (Xu et al., 2003; Nie et al., 2011), but is absent in Six1−/−;Six4−/− embryos (Fig. 1C). At E11.5, the UB invades the MM (Fig. 1D) and subsequent reciprocal interaction between these two tissues leads to the formation of a mature kidney. In Six1−/− embryos, no kidney structure forms due to failure of UB branching caused by elevated BMP signaling in the MM, which eventually undergoes abnormal apoptosis (Xu et al., 2003; Nie et al., 2011). Similar to E10.5, all six E11.5 Six1;Six4 double mutant embryos lack the MM (Fig. 1F).
Fig. 1.
Nephrogenic cord progenitors are reduced in Six1−/− and Six1−/−;Six4−/− embryos. (A–F) H&E stained sections from metanephric regions of E10.5 (A-C) and E11.5 (D-F) wild-type (A,D), Six1−/− (B,E) or Six1−/−;Six4−/− (C,F) embryos showing the metanephric mesenchyme or blastema (mm), nephric or Wolffian duct (nd) and developing metanephric kidney (mt) at E11.5. Six1−/− embryos lack metanephric kidney development at E11.5 (E) and Six1−/−;Six4−/− embryos lack the MM (C,F). (G-O) Lateral view (G-I, M-O), Dorsolateral (G’-I’) or ventral view (J-L) of whole-mount embryos stained with Eya1 riboprobe showing Eya1 expression in nephrogenic cord mesenchyme at E9.5 (G-, G’-I’I), E10.5 (J-L) and E11.0 (M-N). We measured the A-P length of Eya1+ cells at the caudal metaneprhic region by counting the nearby somite numbers and the reduction of Eya1+ progenitors in the mutants is consistent. Arrows point to metanephric region. Other Abb.: s, somite.
Since the nephrogenic cord appears as a strip of tissue located within the IM adjacent to the axial mesoderm in the developing embryo, to examine the onset of the nephrogenic mesenchymal defect during development, we performed whole-mount in situ hybridization with Eya1 riboprobe to reveal the entire nephrogenic cord. Eya1 is one of the earliest markers specific for the nephron progenitors within the IM (Sajithlal et al., 2005; Xu et al., 2014b) and it is not required for gonad or adrenal gland development (Xu et al., 1999). Recent lineage-tracing analysis reveals a developmental restriction of the Eya1+ population within the IM at ~E8.5 to nephron-forming cell fates and a common origin shared between caudal mesonephric and metanephric nephrons (Xu et al., 2014b). At ~E9.5, while Eya1 expression in the anterior mesonephric mesenchyme is detectable, Eya1+ progenitors are densely populated at the caudal region spanning ~5 somites in length (n=3 embryos) (arrow, Fig. 1G,G’). However, in Six1−/− embryos, Eya1-expressing domain is overall noticeably reduced not only in length (spanning ~6 somites, comparing to ~9-12 somites in wild-type controls) but also in density and the strongest Eya1 expression domain at the caudal end only spanned ~3 somites in length (n=4 embryos) (arrow, Fig. 1H,H’). By ~E11.0, the Eya1+ MM is reduced at the UB tip in Six1−/− embryos (arrow, Fig. 1K,N) compared to those at the branching UB tips in wild-type controls (arrow, Fig. 1J,M). In Six1−/−;Six4−/− embryos, Eya1+ progenitors disappeared in the anterior mesonephric region and were only detectable in the caudal region metanephric region at E9.5 (n=5 embryos) (arrow, Fig. 1I,I’). By ~E10.5, only very small Eya1+ region is still present at the caudal end of the IM in the double mutant (n=3 embryos) (Fig. 1L) but completely disappeared thereafter (n=5 embryos) (Fig. 1O). Thus, these results indicate that the nephrogenic population is not only affected in the Six1;Six4 double mutant but also in the Six1 single mutant embryos from E9.5. Because the Six1;Six4 double mutant shows a similar phenotype to that observed in Eya1−/− embryos (Sajithlal et al., 2005), Six1 and Six4 may act synergistically with Eya1 to regulate the formation of the MM.
Initial induction of the nephric duct for ureteric development is blocked in mice lacking Six1/Six4 or Eya1
To investigate whether in the absence of MM cells in Six1;Six4 or Eya1 mutants, the caudal ND is induced to proliferate to pseudostratify prior to UB formation, we first performed whole-mount in situ hybridization with c-Ret riboprobe. As labeled by c-Ret, between E9.5 and E10.5, the caudal ND thickens to undergo pseudostratification to form the UB (Fig. 2A,D). Between E10.5-11.0, the UB outgrows to initiate metanephric kidney development. In Six1−/−;Six4−/− or Eya1−/− mutant embryos, no obvious thickening was observed in the caudal ND at these stages (n=6 embryos for each stage) (Fig. 2B,C,E,F). We next characterized proliferation of ND epithelial cells on sections with anti–pH3, a marker for mitotic nuclei. The caudal ND in normal embryos at E10.0-10.5 have nuclei at multiple apico-basal levels as revealed by Hoechst staining (Fig. 2G) and almost all pH3+ nuclei (3.25±0.62 pH3+/section) (n=3 embryos/6 ND) were found on the apical surface at both the ventral and dorsal sides of the duct, but more on the dorsal side where the UB emerges (Fig. 2G,J), which is consistent with previous observation (Chi et al., 2009). In Six1−/−;Six4−/− or Eya1−/− embryos (n=3), the caudal ND did not have nuclei at multiple apico-basal levels and the number of pH3+ cells was reduced (0.60±0.51, n=5 ND for each genotype) (Fig. 2H-J), which was comparable to that observed in the rostral ND (Fig. 2J), thus suggesting that the caudal ND in the mutants is not pseudostratified. However, no significant difference in the number of pH3+ cells was observed in the rostral ND between wild-type control and mutant littermates (Fig. 2J). No abnormal apoptosis was observed in the mutant ND (data not shown). Therefore, these data suggest that Eya1 and Six1/Six4 are critical mesenchymal factors necessary for promoting the caudal ND to proliferate to undergo pseudostratification to form the UB.
Fig. 2.
Six1−/−;Six4−/− or Eya1−/− mutants lack pseudostratified domain at the caudal nephric duct where UB emerges. (A-F) Whole-mount in situ hybridization for c-Ret showing pseudostratified epithelial domain at the caudal nephric duct at E10.0 and UB formation at E10.5 in normal embryos (arrows, A,D) but not in Six1−/−;Six4−/− (B,E) or Eya1−/− (C,F) embryos. (G-I) Immunostaining with anti-pH3 antibody on sections from caudal nephric region in wild-type, Six1;Six4 double or Eya1 single mutant embryos at E10.0. Sections were counter-stained with Hoechst. (J) Number of pH3+ cells in rostral and caudal nephric duct of wild-type, Six1−/−;Six4−/− or Eya1−/− embryos at E10.0 (means ± SD). pH3+ cells were counted from 15 sections at 8 µm for each nephric duct (from at least four nephric ducts for each genotype) on a radial plane. Asterisks indicate a significant difference (p < 0.003) between rostral versus caudal ND in wild-type embryos or in caudal ND between wild-type versus mutant embryos. P-values were calculated using StatView t-test.
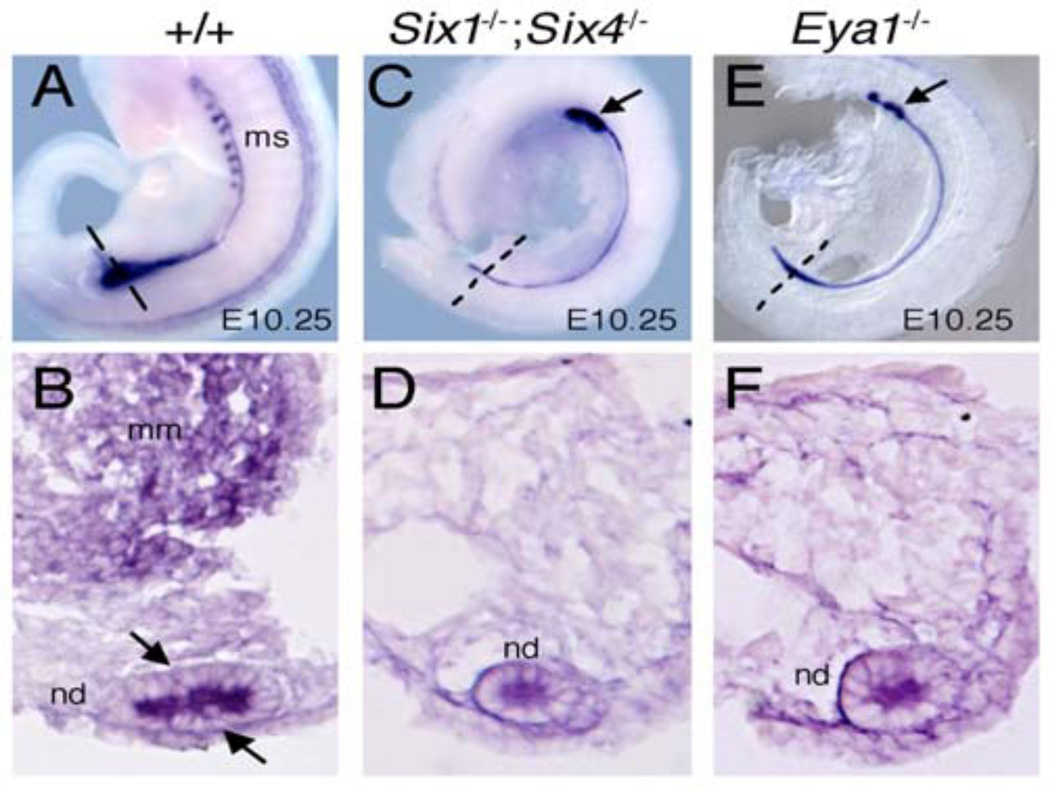
In situ hybridization with Pax2, which is expressed in the MM and the ND (Fig. 3A,B), further confirmed the lack of thickening at the caudal end of the ND in Six1−/−;Six4−/− or Eya1−/− embryos (Fig. 3C,D). Although some Eya1−/− embryos appeared to have slightly widened ND at the caudal end (Fig. 3E,F), unlike in control embryos (Figs. 2G and 3B), it did not show obvious proliferation to become thickened epithelial tubule (Figs 2I and 3F). Furthermore, only ~3-4 pairs of mesonephric tubules at the rostral end on both sides were observed in Six1;Six4 double homozygous embryos (n=4 embryos) (arrow, Fig. 3C) or Eya1−/− embryos (n=5/8 embryos) (arrow, Fig. 3E). Together, these results suggest that Six1/Six4 and Eya1 may act together to regulate a mesenchymal signal that induces the ND to initiate proliferation.
Fig. 3.
Six1−/−;Six4−/− or Eya1−/− embryos show defect in mesonephric tubules. (A,C,E) Lateral view and (B,D,F) sections through metanephric region of whole-mount embryos shown in A,C and E (plane of sections indicated by dashed lines in wild-type and mutant embryos respectively) stained with Pax2 riboprobe showing its expression in nephric duct, mesonephric tubules and metanephric progenitors in wild-type embryos (A,B). In the mutants, Pax2 expression in the nephric duct appears unaltered, whereas its expression in the caudal metanephric mesenchymal progenitors is absent (C-F). Moreover, only 3-4 pairs of mesonephric tubules (ms) at the rostral end were present in the Six1;Six4 (C) or Eya1 (E) mutant embryos.
We have previously observed that some mesonephric tubules form in Eya1−/− embryos (Sajithlal et al., 2005), although statistical analysis was not performed. Our data suggest that genetic background might contribute to the variability of the mesonephric phenotype occurring in Eya1−/− mutants. Nonetheless, failure of mesonephron formation in Eya1−/− embryos is consistent with our recent report that the caudal mesonephric and metanephric nephrons are derived from Eya1+ IM precursors (Xu et al., 2014b). Thus, Eya-Six complex might also play a role important for caudal mesonephron formation.
Increased apoptosis within the IM in Six1−/−;Six4−/− or Eya1−/− embryos
We next investigated the cellular defects of the nephrogenic progenitors in the Eya1 or Six1;Six4 mutants. TUNEL assay (terminal deoxynucleotidyl transferase dUTP nick end labeling) revealed only very few apoptotic cells within the IM in wild-type control embryos at ~E9.75 (n=3, embryos were collected in mid-afternoon of E9.5) (2.58±1.08/section) (Fig 4A). However, in Six1−/−;Six4−/− or Eya1−/− mutant embryos, apoptotic cells were markedly increased in the entire nephrogenic cord within the IM (15.2±1.64 or 16±1.28, respectively) (Fig. 4B,C). Increased apoptosis in the mutant nephrogenic cord mesenchyme is consistent with the disappearance of Eya1+ cells within the IM (Fig. 1) (Sajithlal et al., 2005). It should be mentioned that in addition to the neprhogenic cord within the IM, increased apoptosis was observed in somites in which Eya and Six genes are known to be expressed. Thus, Eya1 and Six1/Six4 are necessary for cell survival.
Fig. 4.
Increased cell death and Osr1 expression in nephrogenic cord mesenchyme in Six1−/−;Six4−/− or Eya1−/− embryos. (A-C) TUNEL assay on sections of E9.75 embryos through caudal nephrogenic region as indicated by dashed lines in D-F. Numerous apoptotic cells (green) were detected in the intermediate mesoderm in the mutants. Apoptotic cells (means ± SD) were counted from 15 sections at 8 µm for each side (from 4-6 embryos for each genotype). P-values (between 0.01 to 0.087) were calculated using StatView t-test. (D-I) Lateral views of whole-mount wild-type (D,G), Six1−/−;Six4−/− double (E,H) or Eya1−/− single (F,I) mutant embryos stained with Osr1 riboprobe at E9.5 (D-F) and E10.0 (G-I). (J-L) Sections whole-mount stained embryos shown in D-F through mesonephric region as indicated by dashed lines. Osr1+ nephrogenic progenitors were detectable in the Six1;Six4 double or Eya1 single mutants at these earlier stages. Abb., nd, nephric duct.
It should be noted that increased cell death has not been previously detected in the Six1;Six4 double mutant at E10.5 (Kobayashi et al., 2007). The most likely explanation for this observation is that the entire nephrogenic population has been eliminated by this stage due to increased cell death from earlier stages. Consistent with this view, Eya1+ nephrogenic progenitors are completely lost by E10.5 in Eya1−/− embryos (Fig. 2L, Sajithlal et al., 2005). Although we detected very few Eya1+ cells in the Six1;Six4 double mutant at E10.5 (Fig. 1L), such discrepancy could be due to a slight difference in the developmental stage of the embryos used in these separate studies because these embryos were staged based on only the discovery of vaginal plug. We noticed that the embryos used in the present study (Fig. 1J-L) might be slightly younger based on the broad Eya1 expression in the anterior mesonephric region, in contrast to its more restricted pattern of expression towards the caudal end and in MM after UB formation at E10.5 (Fig. 2I, Sajithlal et al., 2005). Nonetheless, increased cell death within the IM in the Six1;Six4 double mutant is consistent with our previous observation that cell death in 3rd pharyngeal endoderm within the thymic primordium is enhanced in the Six1;Six4 double compared to the Six1 single mutant (Zou et al., 2006). Because Eya1 and Six family proteins are known to interact not only physically but also genetically during kidney, auditory system and muscle development (Buller et al., 2001; Xu et al., 2003; Zheng et al., 2003; Grifone et al., 2004; Ahmed et al., 2012a), we thus speculate that Eya1 may form a complex with Six1/4 to synergistically regulate cell survival of the nephrogenic cord progenitors within the IM.
Osr1 is expressed in nephrogenic cord progenitors in Eya1 or Six1;Six4 mutants
Osr1 has been suggested to be a mesenchymal gene required for pseudostratification of the caudal ND because in Osr1−/− embryos the caudal ND remains as a narrow tube (James et al., 2006; Mugford et al., 2008; Chi et al., 2009), similar to that observed in Eya1−/− and Six1−/−;Six4−/− embryos. Previous studies suggested that Osr1 is genetically upstream of Eya1 in the MM progenitors based on the absence of Eya1 expression in Osr1−/− embryos on section in situ hybridization (James et al., 2006). However, in contrast to increased cell death within the entire nephrogenic cord in Eya1−/− or Six1−/−;Six4−/− embryos at E9.5-10.0, increased cell death in the metanephric region is not observed until E10.5 in Osr1−/− embryo in which the metanephric blastema is not formed (James et al., 2006). It should be noted that unlike Eya1, Osr1 is widely expressed in multiple cell types within the IM and in the urogenital region, including nephrogenic mesoderm, adjacent dorsal lateral plate mesoderm, mesenchymal cells adjacent to mesonephric tubules and in splanchnic mesoderm derivatives as well as in endoderm (Mudumana et al., 2008). Osr1−/− mice also lack adrenal gland and gonad (Wang et al., 2005). Thus, given the broad expression of Osr1 in multiple cell types within the IM in the urogenital region and its requirement for the development of multiple urogenital organs, numerous apoptotic cells detected in the metanephric region in Osr1−/− embryos at E10.5 may not necessarily reflect cell death occurring in the nephrogenic lineage. We therefore sought to clarify the regulatory relationship between Eya1 and Osr1. Whole-mount in situ hybridization revealed broad expression of Osr1 in E9.5-10.0 wild-type and Eya1−/− or Six1−/−;Six4−/− embryos (Fig. 4D-I), even in the anterior regions where Eya1+ nephrogenic progenitors do not exist at these stages in the mutants (Fig.1I,I’L) (Sajithlal et al., 2005). However, strong Osr1 expression in MM cells at the caudal end at ~E10.0 (arrow, Fig. 4G) is lost in the mutants (arrow, Fig. 4H,I). We sectioned the whole-mount stained E9.5 embryos and found that Osr1+ cells within the IM surrounding the ND are present in the mutants (Fig. 4K,L, compared to 4J). While this result supports the previous conclusion that Osr1 is genetically upstream of Eya1, it is possible that Eya1-Six1/4 may interact with Osr1 to regulate nephrogenic progenitor specification, as discussed below.
It should be noted that previous marker gene analyses have only found Wt1 expression in the urogenital region in either Osr1 (James et al., 2006) or Eya1 (Xu et al., 1999) mutant embryos or both Osr1 and Wt1 expression in Six1;Six4 mutant even at E10.5 (Kobayashi et al., 2007) at which the nephrogenic population marked by Eya1 expression has already disappeared in the Six1;Six4 mutant (Fig. 1). Thus, it is clear that not all Osr1-expressing cells within the IM in the urogenital region in the Six1;Six4 or Eya1 mutant mark the nephrogenic lineage. We have found that it is challenging to detect Eya1+ cells on section in situ hybridization from ~E9.5 due to loss of nephrogenic progenitors caused by increased cell death (Fig. 1I’) and we were only able to detect Eya1 expression in the Six1;Six4 mutant by whole-mount in situ hybridization. Because marker gene analyses in the Osr1 mutant were done on section in situ hybridization, it may be difficult to distinguish between the metanephric mesenchyme and the anterior mesonephric mesenchyme on sections at ~E9.5, at which the mesonephric mesenchyme has begun abnormal cell death in Osr1−/− (James et al., 2006). Therefore, whole-mount in situ hybridization with Six1/4 and Eya1 as well as other markers should be carried out to reveal the gross structure of the entire nephrogenic cord mesenchyme at earlier stages in Osr1−/− embryos and to further clarify the epistatic relationship between Eya1-Six complex and Osr1 in early nephrogenic progenitors before MM formation. This may also help explain why increased cell death is not observed in the metanephric region until E10.5 in the Osr1 mutant, which lacks the MM.
A recent study has reported that Osr1 acts downstream of Six2 but the two genes interact to maintain the nephron progenitor cells (Xu et al., 2014a). We have found that Eya1 is genetically upstream of Six2 but their gene products also interact (Xu et al., 2014b). Thus, Osr1 is genetically downstream of Eya1 but their gene products may interact, as both interact with Six2, to maintain the nephron progenitors during nephrogenesis. Based on these findings, we speculate that Eya1-Six1/4 may form a complex to function in parallel with or to synergistically regulate Osr1 but these genes interact to regulate the specification of the nephrogenic cord mesenchyme, which explains why deletion of either one of them leads to a similar phenotype. In support of this, we have found that Eya1 and Six1 interact synergistically to regulate Neurog1 expression to initiate inner ear neuronal development but Eya1-Six1 also physically interact with Neurog1 and Neurod1 to regulate neuronal development in both loss-of-function and gain-of-function studies (Ahmed et al., 2012b). In either Eya1−/− or Six1−/− single mutant inner ear neurogenesis is initiated normally as judged by normal initiation of Neurog1 expression but neuronal progenitors fail to be maintained due to increased cell death (Zou et al., 2004). However, in the Eya1;Six1 double mutant, inner ear neurogenesis is completely blocked and Neurog1 expression is not activated (Ahmed et al., 2012b). Ectopic expression of both Eya1 and Six1 together in cochlear or embryonic explants is able to synergistically activate Neurog1, which in turn interacts with Eya1-Six1 to activate Neurod1 to regulate neuronal differentiation (Ahmed et al., 2012b). Therefore, analysis of nephrogenic progenitor cell development in the Eya1;Six1;Six4 triple mutant at earlier stages should address whether Eya1 and Six1/4 act synergistically to specify the nephrogenic cord mesenchyme.
Conclusions
Here, we describe previously unreported patterns of phenotype in Eya1-, Six1- and Six1;Six4-null mice during the initial stage of nephrogenic progenitor and nephric duct development prior to MM and UB formation. The similarities in early phenotype between Six1;Six4 double and Eya1 single mutants highly suggest that these Eya and Six family genes may form a complex to regulate early development of nephrogenic progenitors prior to their differentiation and induce ND development prior to UB formation. Since Osr1 interacts with Six2 in maintaining the nephron progenitors and Eya1-Six1/4 and Osr1 are coexpressed in the nephrogenic cord progenitors before MM formation but not in the ND, these genes might act together to specify the nephrogenic progenitors and induce ND development. Further studies in Eya1;Six1;Six4 triple mutant will help to define whether these genes act synergistically to specify the nephrogenic progenitors.
Experimental Procedures
Mice and embryo collection
Six1+/−;Six4+/− double heterozygous mice (Grifone et al., 2005) and Eya1 (Xu et al., 1999) mice and genotyping were described previously. Mice in a mixed C57BL6 and 129 background were used for this study. All procedures involving living mice were approved by Animal Care and Use Committee (IACUC) at the Icahn School of Medicine at Mount Sinai (#06-822).
Embryos were staged based on the discovery of vaginal plug in the morning (0.5 day). For E9.75, embryos were normally collected in mid- or late afternoon and for E11.0, embryos were normally collected around 9–10 pm.
Histology and in situ hybridization
Dissected embryos were fixed in 4% paraformaldehyde (PFA) for overnight and processed for histological analysis following standard procedure. Paraffin sections were generated at 8–10 µm.
Whole-mount in situ hybridization was carried out with Digoxigenin-labeled riboprobes specific for Eya1, c-Ret, Pax2 and Osr1 according to standard procedures.
Immunostaining
Anti–pH3 (sc-8656R, Santa Cruz Biotechnology) was used to label proliferative cells at M-phase. The number of proliferating cells was counted in serial sections from each section, and 15 sections in anterior ND or caudal pseudostratified domain of each genotype were counted respectively.
TUNEL assay
The TUNEL assay was performed using the ApopTag kit for in situ apoptosis fluorescein detection (catalog no. NC9815837, Millipore) following the manufacturer’s instructions.
Statistical analysis
Statistical analysis
We used StatView t-test for statistical analysis.
Acknowledgements
The Six1;Six4 compound knockout mice were kindly provided by P. Maire. This work was supported by NIH RO1 DK064640 (PX. X.).
This work was supported by NIH RO1 DK064640 (PX. X.).
References
- Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell. 2012a;22:377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Xu J, Xu PX. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development. 2012b;139:1965–1977. doi: 10.1242/dev.071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller C, Xu X, Marquis V, Schwanke R, Xu PX. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum Mol Genet. 2001;10:2775–2781. doi: 10.1093/hmg/10.24.2775. [DOI] [PubMed] [Google Scholar]
- Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, Asai N, Takahashi M, Ohgami N, Kato M, Mendelsohn C, Costantini F. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009;17:199–209. doi: 10.1016/j.devcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–421. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Costantini F. GDNF/Ret signaling and renal branching morphogenesis: From mesenchymal signals to epithelial cell behaviors. Organogenesis. 2010;6:252–262. doi: 10.4161/org.6.4.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F, Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28:117–127. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- Davies JA, Fisher CE. Genes and proteins in renal development. Exp Nephrol. 2002;10:102–113. doi: 10.1159/000049905. [DOI] [PubMed] [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifone R, Demignon J, Houbron C, Souil E, Niro C, Seller MJ, Hamard G, Maire P. Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development. 2005;132:2235–2249. doi: 10.1242/dev.01773. [DOI] [PubMed] [Google Scholar]
- Grifone R, Laclef C, Spitz F, Lopez S, Demignon J, Guidotti JE, Kawakami K, Xu PX, Kelly R, Petrof BJ, Daegelen D, Concordet JP, Maire P. Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype. Mol Cell Biol. 2004;24:6253–6267. doi: 10.1128/MCB.24.14.6253-6267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133:2995–3004. doi: 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kawakami K, Asashima M, Nishinakamura R. Six1 and Six4 are essential for Gdnf expression in the metanephric mesenchyme and ureteric bud formation, while Six1 deficiency alone causes mesonephric-tubule defects. Mech Dev. 2007;124:290–303. doi: 10.1016/j.mod.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Mudumana SP, Hentschel D, Liu Y, Vasilyev A, Drummond IA. odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development. 2008;135:3355–3367. doi: 10.1242/dev.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford JW, Sipila P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Xu J, El-Hashash A, Xu PX. Six1 regulates Grem1 expression in the metanephric mesenchyme to initiate branching morphogenesis. Dev Biol. 2011;352:141–151. doi: 10.1016/j.ydbio.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Watanabe Y, Takahashi K, Kitamura K, Tanaka A, Urase K, Momoi T, Sudo K, Sakagami J, Asano M, Iwakura Y, Kawakami K. Six4, a putative myogenin gene regulator, is not essential for mouse embryonal development. Mol Cell Biol. 2001;21:3343–3350. doi: 10.1128/MCB.21.10.3343-3350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajithlal G, Zou D, Silvius D, Xu PX. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol. 2005;284:323–336. doi: 10.1016/j.ydbio.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxen L, Lehtonen E. Embryonic kidney in organ culture. Differentiation. 1987;36:2–11. doi: 10.1111/j.1432-0436.1987.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- Vainio S, Lin Y. Coordinating early kidney development: lessons from gene targeting. Nat Rev Genet. 2002;3:533–543. doi: 10.1038/nrg842. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol. 2005;288:582–594. doi: 10.1016/j.ydbio.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu H, Park JS, Lan Y, Jiang R. Osr1 acts downstream of and interacts synergistically with Six2 to maintain nephron progenitor cells during kidney organogenesis. Development. 2014a;141:1442–1452. doi: 10.1242/dev.103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wong EY, Cheng C, Li J, Sharkar MT, Xu CY, Chen B, Sun J, Jing D, Xu PX. Eya1 interacts with Six2 and Myc to regulate expansion of the nephron progenitor pool during nephrogenesis. Dev Cell. 2014b;31:434–447. doi: 10.1016/j.devcel.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130:3085–3094. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Davenport J, Grifone R, Maire P, Xu PX. Patterning of the third pharyngeal pouch into thymus/parathyroid by Six and Eya1. Dev Biol. 2006;293:499–512. doi: 10.1016/j.ydbio.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Fritzsch B, Xu PX. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131:5561–5572. doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]