Abstract
Background
Aberrant calcium signaling is considered as one of the key mechanisms contributing to arrhythmias, especially in the context of heart failure. In human heart failure, there is significant down-regulation of the sarcoplasmic reticulum protein junctin and junctin deficiency in mice is associated with stress-induced arrhythmias.
Objective
This study was designed to determine whether the increased SR Ca2+ leak and arrhythmias, associated with junctin ablation, may be associated with increased CaMKII activity and phosphorylation of the cardiac ryanodine receptor (RyR2) and whether pharmacological inhibition of CaMKII activity may prevent these arrhythmias.
Methods
Using a combination of biochemical, cellular and in vivo approaches, we tested the ability of KN-93 to reverse aberrant CaMKII phosphorylation of RyR2. Specifically, we performed protein phosphorylation analysis, in vitro cardiomyocyte contractility and Ca2+ kinetics, and in vivo ECG analysis in the junctin deficient mice.
Results
In the absence of junctin, RyR2 channels displayed CaMKII-dependent hyperphosphorylation. Notably, CaMKII inhibition by KN-93 reduced the in vivo incidence of stress-induced ventricular tachycardia by 65% in junctin null mice. At the cardiomyocyte level, KN-93 reduced the percentage of junctin null cells exhibiting spontaneous Ca2+ aftertransients and aftercontractions under stress conditions, by 35% and 37% respectively. At the molecular level, KN-93 blunted the CaMKII mediated hypephosphorylation of RyR2 and PLN under stress conditions.
Conclusion
Our data suggest that CaMKII inhibition is effective in preventing arrhythmogenesis in the setting of junctin ablation, through modulation of both SR Ca2+ release and uptake. Thus, it merits further investigation as promising molecular therapy.
Keywords: Arrhythmias, Junctin, Calcium/Calmodulin-dependent protein Kinase II, Isoproterenol, Ryanodine Receptor
Introduction
Cardiac arrhythmia symptoms can vary from mild disturbances in the cardiac rhythm to severe life-threatening complications, such as sudden cardiac death (SCD). SCD is the cause of more than 60% of all deaths from cardiovascular disease, which is the leading cause of death worldwide.1 Ventricular fibrillation (VF) is the mechanism underlying most sudden cardiac arrest episodes, while other forms of arrhythmias such as tachycardia, bradycardia, or pulseless electric activity can also trigger malignant forms of arrhythmia. These may in turn result in immediate cessation of cardiac mechanical activity and asystole, providing a direct link between arrhythmia trigger mechanisms and SCD.1 Although arrhythmias and SCD are more frequent among patients with organic heart diseases, such as ischemia and dilated or hypertrophic cardiomyopathies, they also occur in individuals without any detectable underlying cardiac pathology, as in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT) or other genetic arrhythmia syndromes.2 Despite the complex nature of arrhythmia triggering mechanisms it is widely accepted that perturbation in cardiac Ca2+ homeostasis is directly contributing to arrhythmogenesis.3 Consequently, correcting impaired Ca2+ cycling represents a highly promising, yet poorly explored, therapeutic target.4
Among the key players in cardiac Ca2+ cycling is the ryanodine receptor (RyR2), the major Ca2+-release channel located in the sarcoplasmic reticulum (SR) membrane. Diastolic Ca2+ leak through RyR2 is one of the central pathophysiological problems in failing hearts. Spontaneous Ca2+ release events via defective RyR2 during diastole may trigger delayed after-depolarizations (DADs), a common precursor mechanism of lethal arrhythmias.5 Although the underlying mechanisms for this leak have not been completely elucidated, human mutations in the RyR2, calsequestrin 2 (CSQ2) and triadin, which alter RyR2 gating and Ca2+ release, have been associated with lethal cardiac arrhythmias, such as CPVT.6–8 Furthermore, the phosphorylation status of RyR2, as well as interactions with other proteins in the macromolecular channel complex can directly affect opening probability and, if dysregulated in HF, contribute to Ca2+ leak.9, 10
Junctin is an essential member of the SR Ca2+ release complex. It interacts with RyR2, similarly to triadin, and mediates the anchoring of CSQ2, the major Ca2+ binding protein of the SR, to RyR2.11 Genetic ablation of junctin has revealed that it is essential for maintenance of normal RyR2 activity and Ca2+ release. Specifically, the junctin knock-out mouse presents with ventricular arrhythmias under stress conditions and an overall phenotype reminiscent of the classical CPVT symptoms, including aftercontractions, DADs and an increased propensity for spontaneous SR Ca2+ release.12, 13 Consistent with the human CPVT pathophysiology, approximately 25% of junctin-null mice die suddenly by 3 months of age with structurally normal hearts, suggesting acutely occurring fatal arrhythmias.12 The trigger mechanism for arrhythmias in the junctin knock-out model is speculated to be the aberrant activity of RyR2, which leads to diastolic Ca2+ leak from the SR, exacerbated by beta adrenergic activation. Importantly, in patients with heart failure, junctin protein expression is highly downregulated to the extreme of undetectable protein levels.14 These patients often develop cardiac mechanoelectrical instability and fatal arrhythmias, especially during increased beta-adrenergic stress.15 This highlights the critical role of the RyR2 macromolecular complex, and its interactions with junctin, for normal cardiac function and its potential as therapeutic target in the context of arrhythmias.
Towards therapeutic strategies, CaMKII emerges as a molecule of particular interest, since it has been associated with the hyperphosphorylated RyR2 complex and increased diastolic Ca2+ leak.16 A novel finding of this study is that the JKO model presents with CaMKII mediated RyR2 hyperphosphorylation, which can be linked to arrhythmogenesis, due to excessive SR Ca2+ leak. It is postulated that junctin ablation removes a protective “break” on RyR2 opening and increases SR Ca2+ leak, which further activates CaMKII and exacerbates diastolic Ca2+ leak, leading to arrhythmias. Thus, our hypothesis was that CaMKII inhibition could be a promising approach for the treatment of arrhythmias, in the JKO model, especially under stress conditions. Further support to this idea comes from evidence that CaMKII suppression inhibits the onset of DADs and prevents fatal arrhythmias in animal models of HF17 and CPVT.18 We therefore, proceeded to assess the effects of CaMKII inhibition on life threatening arrhythmias in the junctin knock-out mouse model of stress-induced arrhythmias. We demonstrate that CaMKII inhibition is an effective means of preventing malignant arrhythmogenesis in these mice, both in vivo and in vitro. These findings suggest the potential benefits of targeted therapy to prevent arrhythmias in the setting of junctin deficiency and may have valuable therapeutic implications.
Methods
Expanded methods can be found in the supplemental material:
Animals
The generation of the Junctin knock-out mice (JKO) was previously described.12
Cardiomyocyte mechanics, Ca2+ kinetics and SR Ca2+ content
Cardiomyocyte mechanics/aftercontractions, Ca2+ transients/aftertransients and SR Ca2+ load, were measured as previously described.12
In vivo electrocardiography
Three-lead electrocardiography (ECG) electrodes were placed subcutaneously to anesthetized mice and ECG recordings were obtained under stress conditions as previously described.19
Western blot analysis
WT and JKO cardiac homogenates were subjected to Western blot analysis using the antibodies and the conditions described in the supplementary methods.
Statistical analysis
All data are expressed as the mean values ± the SE. The student t-test and the x2 test were used for statistical analysis. The P values of < 0.05 were considered to be statistically significant.
Results
Junctin knock-out mice display CaMKII mediated RyR2 hyperphosphorylation
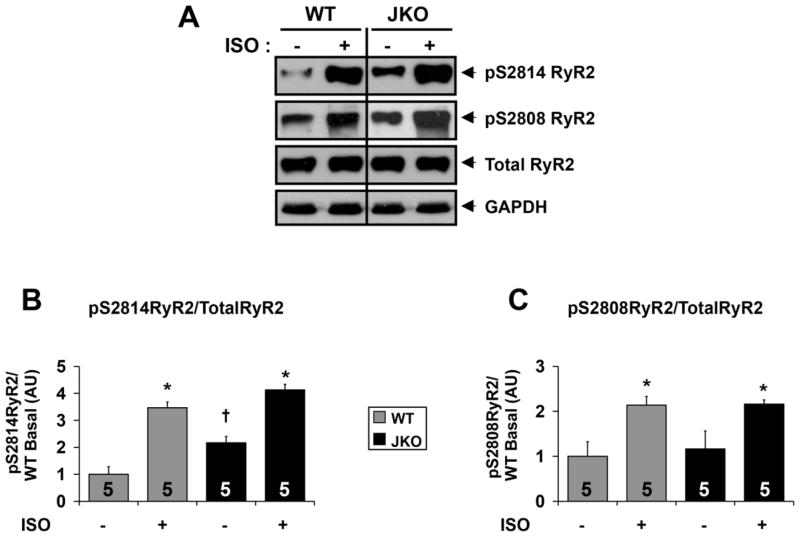
The cardiac ryanodine receptor (RyR2) is critical for sarcoplasmic reticulum (SR) Ca2+ release, and RyR2 dysfunction is linked with multiple forms of congenital and acquired cardiac pathologies, including exercise- and stress-induced ventricular arrhythmias, and heart failure.20 Importantly, defects in RyR2 phosphorylation status were previously linked with abnormal diastolic SR Ca2+ leak and arrhythmias.21 In order to better understand the mechanisms underlying arrhythmogenesis in the absence of junctin, we initially performed a comparative analysis of RyR2 phosphorylation in WT and JKO mice under basal conditions and upon catecholaminergic stress. We evaluated the phosphorylation status of the cardiac RyR2 by immunobloting using antibodies directed against two confirmed RyR2 phosphorylation sites: S2808, the specific protein kinase A (PKA) phosphorylation site of RyR222, and S2814 the specific phosphorylation site for the calcium/calmodulin-dependent protein kinase II (CaMKII).23 Interestingly, RyR2 S2814 phosphorylation in JKO mouse hearts was increased to 2.2-fold of that in WT, under basal conditions (Fig. 1A,B; p=0.013). In contrast, we did not observe significant differences in RyR2 S2808 phosphorylation between WT and JKO cardiac lysates, under basal conditions (Fig. 1A,C; p=0.76). Isoproterenol stimulation increased the phosphorylation of both sites and the maximal phosphorylated levels of S2814 and S2808 were similar between JKO and WT hearts (Fig. 1; p=0.059 and p=0.72). The total RyR2 expression levels (Fig. 1A) were similar in both JKO and WT, confirming previous findings.12 These results suggest that the JKO hearts display abnormally increased CaMKII dependent (hyper)phosphorylation of RyR2 under basal conditions. However, the precise mechanism of this selective CaMKII activation in JKO hearts remains unknown.
Figure 1.
Altered RyR2 phosphorylation in JKO hearts under basal and stress conditions. A) Representative immunoblots indicating expression of Total RyR2, RyR2 pS2808, and RyR2 pS2814 in wild-type and junctin knock-out hearts under basal condition or upon b-adrenergic stress. GAPDH was used to verify the amount of loaded samples. B–C) Densitometry analysis indicating the relative levels of RyR2 pS2814 (B) and RyR2 pS2808 (C) expression normalized to total RyR2 levels in WT and JKO hearts. Symbols represent statistically significant differences (p<0.05) between −iso/+iso (*), and WT/JKO (†) (N=5 hearts).
CaMKII inhibition attenuates CaMKII mediated phosphorylation of RyR2 and PLN
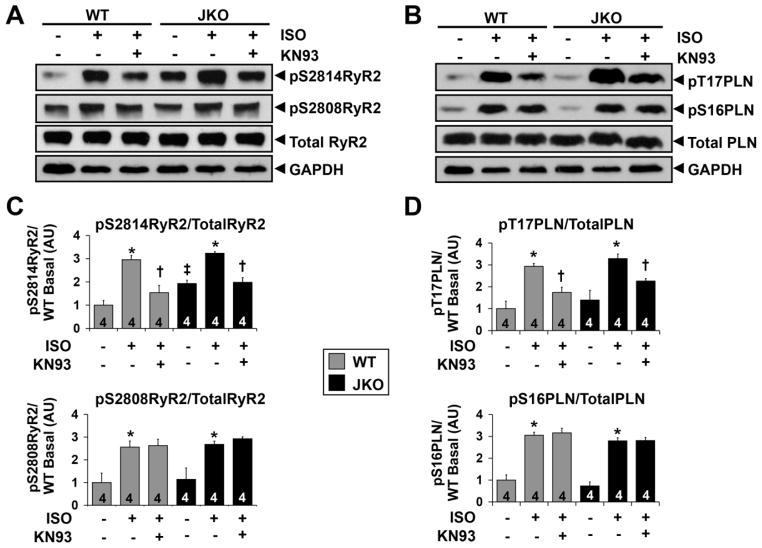
Our initial biochemical data, showing CaMKII mediated RyR2 hyperphosphorylation in the JKO hearts, prompted us to investigate the regulatory role of this phosphorylation in the development of arrhythmias elicited by junctin ablation. Towards this end, we used pharmacological inhibition with KN-93, which is a specific, as well as potent inhibitor, of CaMKII.24 Initially, we assessed the effect of KN-93 on RyR2 phosphorylation under β-adrenergic stress. Both, CaMKII (S2814) and PKA (S2808) RyR2 phosphorylation sites were examined. Exposure of JKO hearts to isoproterenol significantly increased RyR2 phosphorylation at serine 2814 to 1.7-fold (p=0.0002) and at serine 2808 to 2.4-fold (p=0.026) of that in basal levels (Fig. 2A,C). KN-93 significantly reduced the isoproterenol-induced increase of RyR2 phosphorylation at S2814 (CaMKII site; p=0.0011, iso+KN93 vs iso) achieving similar levels as those present in non-stimulated JKO hearts (p=0.85, iso+KN-93 vs basal) (Fig. 2A,C). As expected, KN-93 did not affect the isoproterenol-induced increase of RyR2 phosphorylation at S2808 (PKA site). Similar changes to RyR2 phosphorylation upon isoproterenol and KN-93 administration were also observed in WT hearts (Fig. 2A,C).
Figure 2.
Effect of KN-93 on RyR2 and PLN phosphorylation in WT versus JKO mice. AB). Representative Western blot analysis of RyR2 (A) and of PLN (B) protein expression and phosphorylation in WT and JKO mice. Phosphorylated forms of RyR2 (pS2814 and pS2808) and PLN (pT17 and pS16) as well as the total levels of RyR2 and PLN are shown. GAPDH was used to verify the amount of loaded samples. C–D). Protein quantification for the phosphorylated RyR2 (pS2814 and pS2808) (C) and for the phosphorylated PLN (pT17 and pS16) (D), normalized to the total levels of the proteins. P values < 0.05 were considered statistically significant (N=4 hearts). Symbols represent statistically significant differences between: (*)basal/iso, (†)iso/iso+KN-93 and (‡)WT/JKO.
In addition to RyR2, we investigated the effect of KN-93 on PLN phosphorylation under isoproterenol stimulation. It is known that PLN phosphorylation at threonine 17 by CaMKII affects SERCA activity and SR Ca2+ uptake in both physiological and pathophysiological conditions.25 To assess the phosphorylation levels of PLN, we performed immunoblot analysis with specific antibodies against phospho-threonine 17 of PLN (CaMKII phosphorylation site) and phospho-serine 16 of PLN (PKA phosphorylation site). Exposure of JKO hearts to isoproterenol increased PLN phosphorylation at threonine 17 to 2.4-fold (p=0.008) and at serine 16 to 3.8-fold (p=0.0001) of that in basal levels (Fig. 2B,D). KN-93 significantly reduced the isoproterenol-induced increase of CaMKII dependent phosphorylation of PLN (pT17-PLN) (p=0.005, iso+KN93 vs iso), bringing it closer to the levels observed in non-stimulated hearts (p=0.1, iso+KN-93 vs basal) (Fig. 2B,D). As expected, KN-93 did not affect the isoproterenol-induced increase of PKA dependent phosphorylation of PLN (pS16PLN). Similar changes to PLN phosphorylation upon isoproterenol and KN-93 administration were also observed in WT hearts (Fig. 2B,D).
Taken together, these results provide evidence to support a dual role of KN-93 in preventing SR Ca2+ leak from RyR2, by decreasing RyR2 S2814 phosphorylation and in reducing SR Ca2+ uptake via SERCA/PLN, by decreasing PLN T17 phosphorylation, under b-adrenergic receptor stimulation.
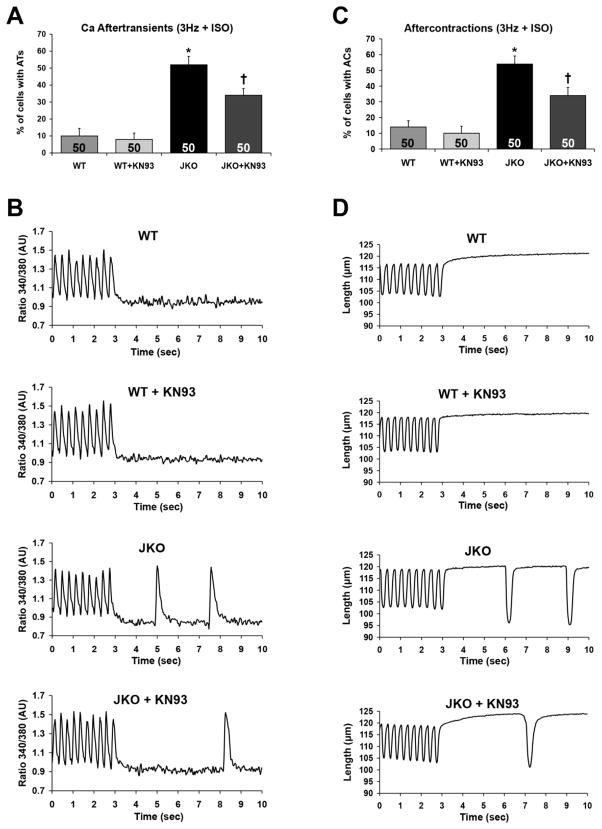
CaMKII inhibition reduces spontaneous Ca2+ aftertransients and aftercontractions in JKO cardiomyocytes
Based on our biochemical data on RyR2 hyperphosphorylation and previous findings on increased SR Ca2+-leak in JKO cardiomyocytes12, 13, we proceeded to evaluate the effects of CaMKII inhibition on arrhythmogenesis, using isolated adult ventricular cardiomyocytes. One of the manifestations of arrhythmias associated with SR Ca2+ leak at the cellular level is the appearance of spontaneous Ca2+ aftertransients or arrhythmogenic Ca2+ waves and aftercontractions. Accordingly, previous studies showed that following high frequency electrical stimulation in the presence of isoproterenol, the JKO cardiomyocytes developed spontaneous Ca2+ aftertransients and aftercontractions at significantly increased percentage, compared to WT cardiomyocytes.12, 13 Using similar stress conditions, we demonstrated that 52% of the JKO cardiomyocytes present with spontaneous Ca2+ aftertransients compared to only 10% of WT cells (p=0.0002). Preincubation of the JKO cardiomyocytes with KN-93 reduced in part and significantly (by 35%), the percentage of cells with Ca2+ aftertransients (p=0.022) (Fig. 3A,B). In addition, 54% of JKO cardiomyocytes developed spontaneous aftercontractions, compared to only 14% of WT cells (p=0.0003), confirming their arrhythmic phenotype. Notably, preincubation of JKO cardiomyocytes with KN-93 also reduced in part (by 37%) the percentage of cells with spontaneous aftercontractions (p=0.024) (Fig. 3C,D).
Figure 3.
Effect of KN-93 on the development of spontaneous Ca2+ aftertransients (ATs) as well as aftercontractions (ACs) in WT and JKO cardiomyocytes, under stress conditions (3 Hz + 1 μM iso) A–B) Percentage of WT and JKO cells that developed aftertransients (A) and aftercontractions (B) at 3 Hz, in the presence of 1μM isoproterenol. The cells were either control treated (DMSO), or pretreated for 30 min with 1 μM KN-93. Data shown are the average ±SEM of n=50 cells from 5 different hearts (N=5). Symbols represent statistically significant differences (p<0.05) between WT/JKO (*) and JKO/JKO+KN-93 (†). C–D) Representative traces of Ca2+ Aftertransients (C) (ATs) and Aftercontractions (D) in WT and JKO cardiomyocytes.
These results support the hypothesis that CaMKII inhibition could have a protective role against arrhythmias, particularly in junctin deficient cells, since KN-93 significantly reduced triggered activity and spontaneous Ca2+ release in JKO cardiomyocytes in the form of aftercontractions and Ca2+ aftertransients.
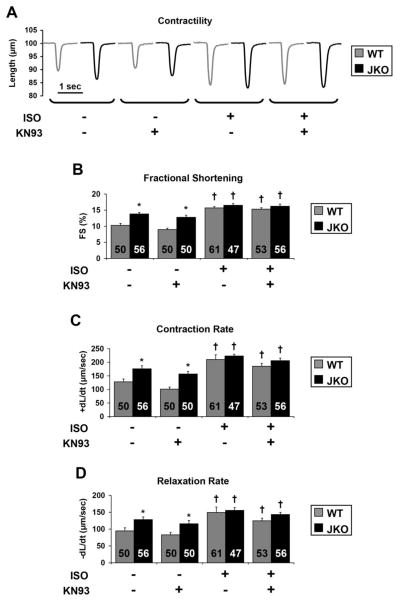
CaMKII inhibition does not alter contractility in JKO cardiomyocytes
Since CaMKII inhibition reduced the incidence of aftercontractions and Ca2+ aftertransients in isolated cardiomyocytes, we examined its effect on contractile parameters and Ca2+ kinetics to further characterize its role on cardiomyocyte function. Our results confirmed previous findings of increased basal contractility of JKO cardiomyocytes.12 Specifically, the JKO cardiomyocytes exhibited increases in fractional shortening to 1.3-fold (p=0.002, Fig. 4B), the rate of contraction to 1.4-fold (p=0.015, Fig. 4C) and the rate of relaxation to 1.4-fold (p=0.026, Fig. 4D) of those in WT cardiomyocytes. However, when normalized to the amplitude of contraction (deltaL), dL/dt did not reveal any significant changes in contractile kinetics, which further includes the absolute tension of contraction and relaxation between WT and JKO cells. Notably, KN-93 did not have a significant effect on contractility of WT or JKO cardiomyocytes under basal conditions. These results are consistent with the notion that CaMKII is mainly activated in paced cardiomyocytes during sustained stress conditions, such as increased pacing frequency (> 2 Hz) combined with β-adrenergic stimulation. Next, we assessed cardiomyocyte contractility under β-adrenergic stimulation, in the presence of isoproterenol. As expected, all contractile parameters were increased in both WT and JKO cardiomyocytes, albeit to a markedly lesser extent in the latter. Specifically, isoproterenol increased the fractional shortening in WT cells to 1.5-fold (p=0.00009, Fig. 4B), while in JKO cells to 1.2 fold of that in basal levels (p=0.0059, Fig. 4B). The rate of contraction was also increased in WT cells upon isoproterenol stimulation to 1.6-fold (p=0.0039, Fig. 4C) while in JKO cells to 1.3-fold of that in basal levels (p=0.0065, Fig. 4C). Finally, isoproterenol also increased the rate of relaxation in WT cells to 1.6-fold (p=0.02, Fig. 4D) and in JKO to 1.2-fold of that in basal levels (p=0.046, Fig. 4D). However, the maximally stimulated parameters in JKOs were similar to WT cells. Notably, KN-93 did not have a statistically significant effect on the contractile parameters of either WT or JKO cardiomyocytes, even under maximal isoproterenol stimulation. Our combined results on cardiomyocyte contractility and aftercontractions suggest that CaMKII inhibition with KN-93 can reduce the incidence of arrhythmic events, such as aftercontractions (Fig. 3), without exhibiting any significant effects on cardiomyocyte contractility (Fig. 4).
Figure 4.
Effect of KN-93 on contractility of WT and JKO cardiomyocytes in the absence or presence of 1 μM isoproterenol, at 0.5 Hz of stimulation. Representative cell shortening tracings (A), and analysis of the fractional shortening (B), the contraction rate, (+dL/dt) (C), and the relaxation rate, (−dL/dt) (D), of WT and JKO cardiomyocytes, either control treated, or pretreated with 1 μM KN-93, before and after isoproterenol stimulation (1 μM). Symbols represent statistically significant differences between: (*)WT/JKO, (†)basal/iso (p<0.05, n=47–61 cells from 5 different hearts, N=5).
CaMKII inhibition does not affect Ca2+ transients and SR Ca2+ load in JKO cardiomyocytes
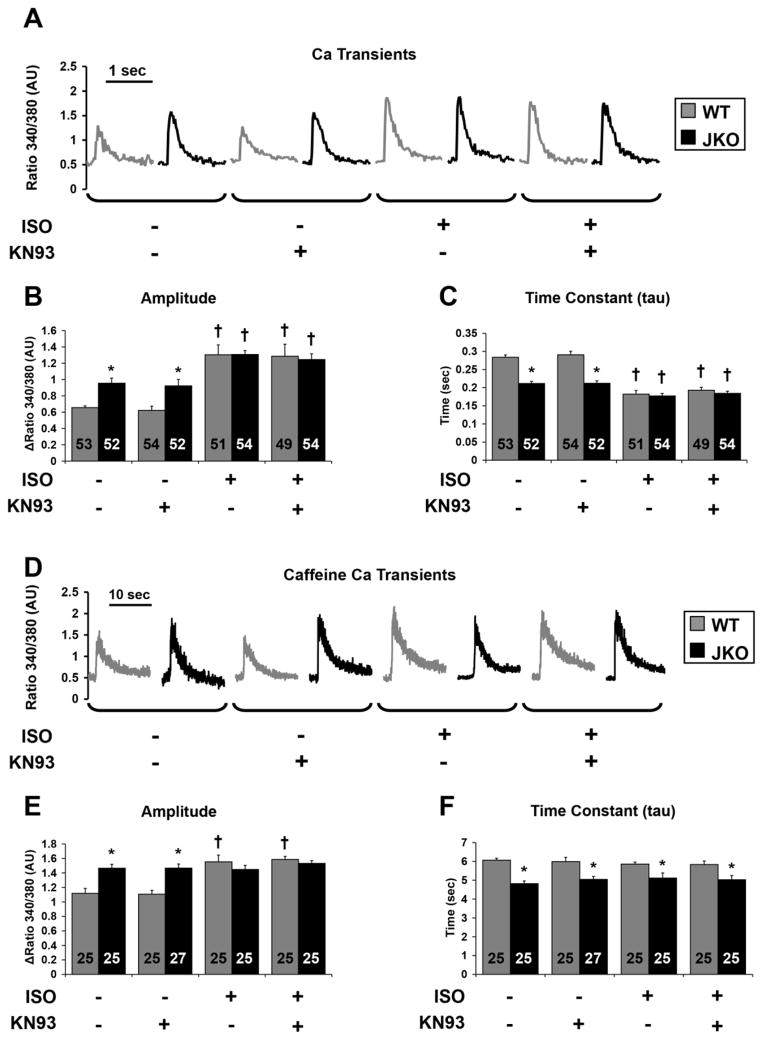
In parallel with contractility, the effect of CaMKII inhibition on Ca2+ transients and SR Ca2+ content was evaluated in both WT and JKO cardiomyocytes. Consistent with the contractility data, the amplitude of the Ca2+ transient was significantly increased by 46% in JKO compared to WT cardiomyocytes (p=0.0018, Fig. 5B), while the time constant of the Ca2+ transient decay was significantly reduced by 26% in the JKO cardiomyocytes (p=0.00003, Fig. 5C), suggesting faster Ca2+ kinetics in the JKO. In agreement with the contractility results, Ca2+ transients were not affected by KN-93, under basal conditions. Upon isoproterenol stimulation, the Ca2+ transient amplitude was significantly increased (Fig. 5B), while the time constant of the Ca2+ transient decay was decreased in both JKO cells and WT cells (Fig. 5C) confirming previous findings. Interestingly, CaMKII inhibition with KN-93 did not significantly alter any of these parameters, in the presence of isoproterenol. In conclusion, the combined results from the Ca2+ transients and aftertransients measurements reveal that while CaMKII inhibition by KN-93 decreases the percentage of JKO cells which exhibit Ca2+ aftertransients, it has no effect on the amplitude or the time constant of the Ca2+ transients of these cardiomyocytes, even under similar stress conditions. These findings are in agreement with our previous observations on the KN-93 effect on cardiomyocyte contractility, suggesting that the arrhythmic phenotype and the phenotype of increased contractility and enhanced Ca2+ kinetics of the JKO cardiomyocytes, are likely originating through two distinct molecular mechanisms.
Figure 5.
Effect of KN-93 on Ca2+ transients and on SR Ca2+ content of WT and JKO cardiomyocytes in the absence or presence of 1 μM isoproterenol, at 0.5 Hz of stimulation. A–C). Representative tracings of Ca2+ transients (A), and analysis of the amplitude of Ca2+ transients (B), and the time constant of twitch Ca2+ decay (τ) (C), of WT and JKO cardiomyocytes control treated, or pretreated with 1 μM KN-93 before and after isoproterenol stimulation (1 μM). Symbols represent statistically significant differences between: (*)WT/JKO, (†) basal/iso (p≤0.05, n=49–54 cells from 5 different hearts, N=5). D–F). Representative tracings of caffeine induced Ca2+ transients (D), and analysis of the amplitude (E) and the time constant (F) of twitch Ca2+ decay of caffeine induced Ca2+ transients, of WT and JKO cardiomyocytes control treated, or pretreated with 1 μM KN-93, before and after isoproterenol stimulation (1 μM). Symbols represent statistically significant differences between: (*)WT/JKO, (†)basal/iso (p≤0.05, n=25–27 cells from 5 different hearts, N=5).
To determine the SR Ca2+ content, Ca2+ transients were recorded upon the rapid application of caffeine (10 mM). As shown in Figure 5, the amplitude of caffeine-induced Ca2+ release was increased by 31% in JKO myocytes (p=0.0043), indicating a higher SR Ca2+ content (Fig. 5D,E). Similarly, the time constant (tau) of caffeine-induced Ca2+ transient decay was significantly shortened by 21% in JKO cells (p=0.00011), consistently with the upregulated NCX activity (Fig. 5F).12 After isoproterenol stimulation, the caffeine-induced Ca2+ transient peak was significantly increased (by 39%) in WT cells (p=0.0056), while tau was not significantly changed. Interestingly, there were no further increases in either the caffeine-induced Ca2+ transient amplitude (Fig. 5E) or the time constant of Ca2+ transient decay in JKO cells, in response to isoproterenol stimulation (Fig. 5F), suggesting that the SR Ca2+ load had reached its maximum level under basal conditions. An alternative explanation could be that Fura-2 signal was saturated during SR Ca2+ load measurements only in the JKO cells. However, when considered together with similar Ca2+ transients between WT and JKO cells in the presence of isoproterenol, it seems SR Ca2+ content is indeed not significantly different under these conditions. CaMKII inhibition with KN-93 did not result in any significant changes of the SR Ca2+ content in either WT or JKO cardiomyocytes, under basal or stress conditions (Fig. 5). These results suggest that the observed anti-arrhythmic properties of KN-93 on the JKO cardiomyocytes are not related to changes in the SR Ca2+ content, and that an increased SR Ca2+ content alone is unlikely to be responsible for the generation of arrhythmias. Indeed, previous mouse models with maximally increased SR Ca2+ content, in particular phospholamban knock-out mice with constitutively increased SR load, did not present with arrhythmias.26
CaMKII inhibition ameliorates caffeine/epinephrine-induced arrhythmias in vivo in the JKO mice
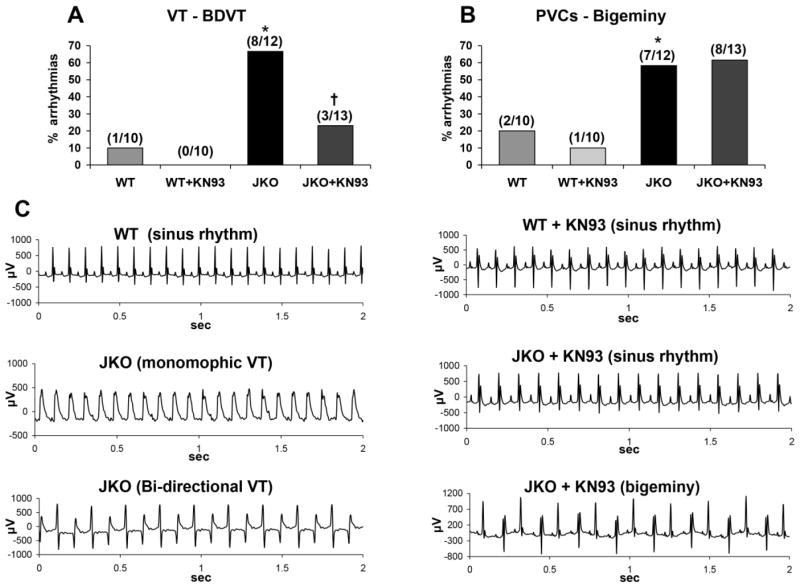
To investigate the potential anti-arrhythmic activity of CaMKII inhibition in vivo, electrocardiography (ECG) was performed in WT and JKO mice under acute catecholaminergic stress, induced by combined administration of epinephrine and caffeine. As expected, stress elicited ventricular arrhythmias in the JKO mice, ranging from “benign” sporadic premature ventricular contractions (PVCs), to more “malignant” arrhythmias, such as episodes of non-sustained or sustained ventricular tachycardias (VTs). In specific, 66% (8/12) of JKO mice developed VT episodes (predominantly non-sustained) under stress conditions, as opposed to only one or 10% (1/10) of the WT mice (p=0.0071) (Fig. 6A). In addition, 58% of the JKO mice (7/12) developed isolated or frequent PVCs (bigeminy), as opposed to only 20% (2/10) of the WT mice (p=0.035) (Fig. 6B). The most severe arrhythmias observed in JKO mice included episodes of sustained monomorphic VT (SVT), and bidirectional ventricular tachycardia (BDVT) (Fig. 6C). Interestingly, JKO mice pretreated with KN-93 appeared significantly protected from malignant ventricular arrhythmias, since only 23% developed life-threatening arrhythmias (3/13 vs 8/12; p=0.028) (Fig. 6A,C). In contrast, milder arrhythmogenic events in the JKO model, such as premature ventricular beats in the form of bigeminy or couplets, appeared to be resistant to KN-93 treatment (Fig. 6B,C). Overall, our in vivo data suggest that CaMKII inhibition by KN-93 can be protective against ventricular arrhythmias, particularly against life-threatening arrhythmia episodes such as SVT, while it did not interfere with the incidence of less severe events such as isolated PVCs.
Figure 6.
Effect of KN-93 on the development of arrhythmias in vivo in WT and JKO mice, upon acute catecholaminergic stress, induced by caffeine/epinephrine. A–B) Percentage of different types of VT (A) and of PVCs-Bigeminy (B) induced by caffeine/epinephrine in WT and JKO mice, either mock-treated (DMSO-control) or pre-treated with KN-93. Data shown are from N=10–13 mice per group. Statistical analysis was performed with the x2 test and symbols represent statistical significant differences (p<0.05) between WT/JKO (*), and JKO/JKO+KN-93 (†). C). Representative ECGs in WT and JKO mice, after Caffeine/Epinephrine administration in mock-treated mice (left panel), or in mice pre-treated with KN-93 (right panel).
Discussion
The present study indicates that pharmacological inhibition of CaMKII with KN-93 ameliorates the arrhythmogenic phenotype of the JKO model under stress conditions, predominantly through a significant reduction of malignant ventricular arrhythmias. The cellular and molecular mechanisms underlying the anti-arrhythmic effects of KN-93 appear to involve attenuation of triggered activity, associated with reversal of RyR2 hyperphosphorylation. Treatment of acutely stressed JKO mice with KN-93, significantly reduced the occurrence of NSVT, SVT and BDVT, while leaving the frequency of benign arrhythmias, such as isolated PVCs and bigeminy, unaffected. BDVT is a severe form of arrhythmia that has been associated with digitalis toxicity, familial CPVT, and several other conditions that predispose cardiac myocytes to delayed afterdepolarizations (DADs) and triggered activity.19, 27 Importantly, SVT often progresses to VF and sudden cardiac death in patients with CPVT as well as in CPVT mice with patient mutations.19 Our findings indicate that the development of malignant ventricular arrhythmias in the JKO mice is at least in part explained by activation of CaMKII. In support of this observation, cardiac overexpression of CaMKII has been shown to lead to BDVT.28 In addition, CaMKII mediated RyR2 phosphorylation promotes life threatening ventricular arrhythmias (SVT) in mice with heart failure, while genetic ablation of the CaMKII site on RyR2 (S2814A) protected mutant mice from pacing-induced arrhythmias after transverse aortic constriction.21 Therefore, CaMKII emerges as a promising therapeutic target for stress-induced malignant arrhythmias and KN-93 appears to be an effective compound towards this end, and at the in vivo level.
To investigate the effect of KN-93 in cardiomyocytes, a series of in vitro analyses were performed. Previous evidence demonstrated that stress induced arrhythmias in JKO mice are initiated by DADs, paired with aftercontractions and increased spontaneous Ca2+ release.12 Our data suggest that CaMKII inhibition with KN-93 can inhibit triggered activity at least in part, measured as aftercontractions and Ca2+ aftertransients, in JKO cardiomyocytes under stress conditions. These results support the notion that excessive SR Ca2+ leak caused by junctin deficiency enhances CaMKII activation, and consequently the increased CaMKII activity affects RyR2, thereby amplifying arrhythmogenic SR Ca2+ leak. Interestingly, CaMKII inhibition with KN-93 did not have a significant effect on cardiomyocyte contractility or Ca2+ kinetics. These data are in agreement with Backs et al, who showed that knockout of the cardiac CaMKIIdelta isoform in transgenic mice has no significant effect on cardiomyocyte contractility and does not impair Excitation-Contraction Coupling (ECC).29 Thus, at the cellular level, KN-93 appears to reduce the arrhythmogenic propensity of JKO cardiomyocytes primarily by reducing triggered activity, such as aftercontractions and Ca2+ aftertransients.
SR Ca2+ overload has been shown to induce DADs by excessive spontaneous Ca2+ release through the RyR2 complex30, a process described as store-overload-induced Ca2+ release (SOICR). In the JKO model, the SR Ca2+ load remains high, despite the increased SR leak. This paradox has been previously attributed to compensatory, increased Ca2+ binding and buffering ability of CSQ2 in the absence of junctin.12 It has been hypothesized that arrhythmias might be triggered by DADs, generated by increased SR Ca2+ load. Herein, we aimed to determine whether CaMKII inhibition with KN-93 reduces arrhythmias through modulation of the SR Ca2+ load. Surprisingly however, KN-93 does not appear to change SR Ca2+ content significantly, either in the absence or presence of β-adrenergic stimulation, suggesting that the increased SR Ca2+ load in JKO cardiomyocytes is unlikely to be the sole trigger of stress-induced arrhythmias in this model. This is consistent with previous studies demonstrating that DADs can occur in the absence of SR Ca2+ overload.31, 32 For example, both RyR2 and CSQ2 mutations have been shown to induce CPVT through activation of DADs in the absence of SR Ca2+ overload.33, 34 Along the same lines, some mouse models presented with increased SR Ca2+ content, in particular phospholamban knock-out mice, but not with any arrhythmias.26 These observations support the hypothesis that multifactorial cellular mechanisms are responsible for the generation of arrhythmias in the JKO model, and SR Ca2+ overload likely contributes to a relatively mild and compensated form, which was also not significantly influenced by KN-93.
At the molecular level, where the underlying abnormality in the JKO mice is thought to occur, we found abnormal RyR2 Ca2+ leak, especially under stress conditions (Supp. Figure 1, Supp. Table 1).12, 13 Since junctin is a key component of the RyR2 Ca2+ release channel complex, and directly associated with CSQ2, JCN ablation may alter RyR2 Ca2+ sensitivity through different mechanisms: i) by impairing the physical interaction among the proteins forming this complex and interfering with CSQ2 binding to Ca2+, which might induce excessive and aberrant RyR2 Ca2+ release12, 13; ii) by affecting RyR2 refractoriness in a similar way as CSQ2 mutants 35; or iii) by modifying RyR2 phosphorylation through a stress and/or Ca2+ dependent protein kinase, which might in turn alter the RyR2 channel opening properties and promote aberrant SR Ca2+ release. Our data do not support altered RyR2 refractoriness in the JKO mice, since the incidence of premature or late Ca2+ release events is similar between WT and JKO cardiomyocytes. On the other hand, CaMKII-mediated RyR2 phosphorylation was significantly increased in the absence of junctin. While the precise mechanism of CaMKII activation is not completely understood, we speculate that it may involve both Ca2+ dependent and independent pathways such as oxidation, nitrosylation, or glycosylation of CaMKII. In addition, the phosphorylation status of RyR2 in JKO hearts is further affected by interactions with other kinases/phosphatases. Interestingly, CaMKII inhibition by KN-93 decreased RyR2 phosphorylation at Ser2814 (the CaMKII specific site) and significantly reduced the propensity of the JKO mice for SVT under stress conditions. In support of this hypothesis, a recent study in a Duchene muscular dystrophy mouse model demonstrated that CaMKII pharmacological and genetic inhibition of RyR2-S2814 phosphorylation prevented VT induction.36
In addition to RyR2, CaMKII can also phosphorylate PLN and enhance SR Ca2+ uptake. Our results show that CaMKII inhibition by KN-93 reduces PLN phosphorylation at Thr17, which could compromise the activity of SERCA and reduce SR Ca2+ load. This decrease in SR Ca2+ uptake could possibly explain why the SR Ca2+ load is not altered by KN-93, although RyR2 phosphorylation, which contributes to SR Ca2+ leak, was reduced. This is in agreement with findings from KN-93 administration to a CPVT mouse model of arrhythmia, resulting in both reduced RyR2 opening and SR Ca2+ uptake.18 A common limitation for the use of substances reducing SR Ca2+ leak as anti-arrhythmic agents is that they can lead to SR Ca2+ overload and thus inadvertently promote arrhythmias. However, our findings suggest that KN-93 can reduce arrhythmias without changing the SR Ca2+ content.
Limitations
Our findings support RyR2 hyperphosphorylation as a mechanism leading to arrhythmias in the context of junctin ablation, but this observation was demonstrated using a pharmacological approach, namely acute CaMKII inhibition with KN-93. We note that the precise molecular mechanisms underlying the increased CaMKII mediated RyR2 phosphorylation may be complex and will require further investigations. Furthermore, future studies, using genetic interventions to inhibit CaMKII or CaMKII mediated RyR2 phosphorylation, could clarify the arrhythmogenic role of post-translational RyR2 modifications through genetically targeted approaches. Despite these limitations, the pharmacological strategy used here is appealing due to its translational potential.
Conclusions
In summary, we demonstrate that CaMKII inhibition by KN-93 can significantly reduce stress-induced malignant arrhythmias, in particular SVT, in the JKO mouse model. This was mediated through reduced triggered activity in the form of aftercontractions and Ca2+ aftertransients, as well as attenuating SR Ca2+ leak by RyR2 due to increased CaMKII phosphorylation, and SR Ca2+ uptake by the SERCA/PLN complex, including PLN phosphorylation. Thus, CaMKII inhibition might be a novel target for stress-induced malignant ventricular arrhythmias, which is likely devoid of common pro-arrhythmogenic adverse side effects reported for other SR Ca2+ content modifiers.
Supplementary Material
Clinical Perspectives.
The present study demonstrates that CaMKII inhibition is a promising therapeutic strategy against arrhythmias related to junctin ablation. Junctin ablation might be the cause of lethal ventricular arrhythmias in individuals with junctin inactivating mutations or in patients with end stage heat failure, which have very low and practically undetectable levels of junctin in their myocardial tissue. Additionally, junctin deficiency has been associated with increased ventricular automaticity and lethal arrhythmias under stress conditions. Our data indicate that CaMKII inhibition with KN-93 ameliorates the arrhythmic phenotype of the junctin null mice under stress conditions, by blunting increased CaMKII mediated RyR2 phosphorylation and reducing aberrant SR Ca2+ release. Therefore, therapies targeted to CaMKII inhibition might be promising against stress-induced arrhythmias in patients with aberrant junctin levels or function.
Acknowledgments
Financial Support: This study was supported by the European Community’s Seventh Framework Programme FP7/2007-2013 under grant agreement no HEALTH-F2-2009-241526, (EUTrigTreat), NIH HL26057 and HL64018, the Greek General Secretariat for Research and Technology under the Aristeia II grant “CALCIRHYTHM”, the Hellenic Cardiological Society, the John S. Latsis Public Benefit Foundation (Scientific Projects 2011) and NIH HL64018.
Abbreviations
- BDVT
Bi-Directional Ventricular Tachycardia
- CaMKII
Calcium/Calmoduline dependent protein kinase II
- CPVT
Catecholaminergic Polymorphic Ventricular Tachycardia
- CSQ
Calsequestrin
- DADs
Delayed Afterdepolarizations
- DMSO
Dimethyl Sulfoxide
- ECG
Electrocardiography
- JKO
Junctin Knock-Out
- NCX
Sodium(Na)/Calcium(Ca) Exchanger
- NSVT
Non-Sustained Ventricular Tachycardia
- PKA
Protein Kinase A
- PLN
Phospholamban
- PVCs
Premature Ventricular Contractions
- RyR
Ryanodine Receptor
- SCD
Sudden Cardiac Death
- SERCA
Sarco-Endoplasmic Reticulum Calcium ATPase
- SOICR
Store-Overload-Induced Ca2+ Release
- SR
Sarcoplasmic Reticulum
- SVT
Sustained Ventricular Tachycardia
- VT
Ventricular Tachycardia
- VF
Ventricular Fibrillation
- WT
Wild Type
Footnotes
Conflict of interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nature reviews Cardiology. 2010 Apr;7:216–225. doi: 10.1038/nrcardio.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chopra N, Knollmann BC. Genetics of sudden cardiac death syndromes. Current opinion in cardiology. 2011 May;26:196–203. doi: 10.1097/HCO.0b013e3283459893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scoote M, Williams AJ. Myocardial calcium signalling and arrhythmia pathogenesis. Biochemical and biophysical research communications. 2004 Oct 1;322:1286–1309. doi: 10.1016/j.bbrc.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circulation research. 2011 Apr 1;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tateishi H, Yano M, Mochizuki M, et al. Defective domain-domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts. Cardiovascular research. 2009 Feb 15;81:536–545. doi: 10.1093/cvr/cvn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George CH, Jundi H, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. Journal of molecular and cellular cardiology. 2007 Jan;42:34–50. doi: 10.1016/j.yjmcc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 7.Faber GM, Rudy Y. Calsequestrin mutation and catecholaminergic polymorphic ventricular tachycardia: a simulation study of cellular mechanism. Cardiovascular research. 2007 Jul 1;75:79–88. doi: 10.1016/j.cardiores.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, et al. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Human molecular genetics. 2012 Jun 15;21:2759–2767. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. The Journal of cell biology. 2001 May 14;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000 May 12;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 11.Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Progress in biophysics and molecular biology. 2004 May;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Q, Fan GC, Dong M, et al. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation. 2007 Jan 23;115:300–309. doi: 10.1161/CIRCULATIONAHA.106.654699. [DOI] [PubMed] [Google Scholar]
- 13.Altschafl BA, Arvanitis DA, Fuentes O, Yuan Q, Kranias EG, Valdivia HH. Dual role of junctin in the regulation of ryanodine receptors and calcium release in cardiac ventricular myocytes. The Journal of physiology. 2011 Dec 15;589:6063–6080. doi: 10.1113/jphysiol.2011.215988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gergs U, Berndt T, Buskase J, Jones LR, Kirchhefer U, Muller FU, Schluter KD, Schmitz W, Neumann J. On the role of junctin in cardiac Ca2+ handling, contractility, and heart failure. American journal of physiology Heart and circulatory physiology. 2007 Jul;293:H728–734. doi: 10.1152/ajpheart.01187.2006. [DOI] [PubMed] [Google Scholar]
- 15.Kjekshus J. Arrhythmias and mortality in congestive heart failure. The American journal of cardiology. 1990 May 22;65:42I–48I. doi: 10.1016/0002-9149(90)90125-k. [DOI] [PubMed] [Google Scholar]
- 16.Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. Journal of cardiovascular pharmacology. 2009 Sep;54:180–187. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca(2+)-calmodulin-dependent protein kinase II. Journal of molecular and cellular cardiology. 2010 Jul;49:25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu N, Ruan Y, Denegri M, Bachetti T, Li Y, Colombi B, Napolitano C, Coetzee WA, Priori SG. Calmodulin kinase II inhibition prevents arrhythmias in RyR2(R4496C+/−) mice with catecholaminergic polymorphic ventricular tachycardia. Journal of molecular and cellular cardiology. 2011 Jan;50:214–222. doi: 10.1016/j.yjmcc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, Napolitano C, Priori SG. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circulation research. 2005 May 27;96:e77–82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 20.Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. The Journal of clinical investigation. 1995 Feb;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010 Dec 21;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proceedings of the National Academy of Sciences of the United States of America. 2006 Jan 17;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circulation research. 2004 Apr 2;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 24.Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochemical and biophysical research communications. 1991 Dec 31;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- 25.Mattiazzi A, Mundina-Weilenmann C, Guoxiang C, Vittone L, Kranias E. Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovascular research. 2005 Dec 1;68:366–375. doi: 10.1016/j.cardiores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circulation research. 1994 Sep;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 27.Baher AA, Uy M, Xie F, Garfinkel A, Qu Z, Weiss JN. Bidirectional ventricular tachycardia: ping pong in the His-Purkinje system. Heart rhythm: the official journal of the Heart Rhythm Society. 2011 Apr;8:599–605. doi: 10.1016/j.hrthm.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sag CM, Wadsack DP, Khabbazzadeh S, et al. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circulation Heart failure. 2009 Nov;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Backs J, Backs T, Neef S, et al. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proceedings of the National Academy of Sciences of the United States of America. 2009 Feb 17;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. The American journal of physiology. 1996 Jan;270:C148–159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 31.di Barletta MR, Viatchenko-Karpinski S, Nori A, Memmi M, Terentyev D, Turcato F, Valle G, Rizzi N, Napolitano C, Gyorke S, Volpe P, Priori SG. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2006 Sep 5;114:1012–1019. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- 32.Viatchenko-Karpinski S, Terentyev D, Gyorke I, Terentyeva R, Volpe P, Priori SG, Napolitano C, Nori A, Williams SC, Gyorke S. Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circulation research. 2004 Mar 5;94:471–477. doi: 10.1161/01.RES.0000115944.10681.EB. [DOI] [PubMed] [Google Scholar]
- 33.Jiang D, Xiao B, Zhang L, Chen SR. Enhanced basal activity of a cardiac Ca2+ release channel (ryanodine receptor) mutant associated with ventricular tachycardia and sudden death. Circulation research. 2002 Aug 9;91:218–225. doi: 10.1161/01.res.0000028455.36940.5e. [DOI] [PubMed] [Google Scholar]
- 34.Terentyev D, Nori A, Santoro M, et al. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circulation research. 2006 May 12;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 35.Brunello L, Slabaugh JL, Radwanski PB, et al. Decreased RyR2 refractoriness determines myocardial synchronization of aberrant Ca2+ release in a genetic model of arrhythmia. Proceedings of the National Academy of Sciences of the United States of America. 2013 Jun 18;110:10312–10317. doi: 10.1073/pnas.1300052110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ather S, Wang W, Wang Q, Li N, Anderson ME, Wehrens XH. Inhibition of CaMKII phosphorylation of RyR2 prevents inducible ventricular arrhythmias in mice with Duchenne muscular dystrophy. Heart rhythm: the official journal of the Heart Rhythm Society. 2013 Apr;10:592–599. doi: 10.1016/j.hrthm.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.