Abstract
Objective
Clozapine is the most effective antipsychotic for treatment refractory people with schizophrenia, yet many patients only partially respond. Accumulating preclinical and clinical data suggest benefits with minocycline. We tested adjunct minocycline to clozapine in a 10 week, double blind placebo-controlled trial. Primary outcomes tested were positive and cognitive symptoms, while avolition, anxiety/depression and negative symptoms were secondary outcomes.
Methods
Schizophrenia and schizoaffective participants (N=52) with persistent positive symptoms were randomized to receive adjunct minocycline (100 mg oral capsule twice daily) (N=29) or placebo (N=23).
Results
Brief Psychiatric Rating Scale (BPRS) psychosis factor (p=0.098, effect size ES=0.39) and BPRS total score (p=0.075, effect size 0.55) were not significant. A ≥30% change in total BPRS symptoms was observed in 7/28 (25%) among minocycline and 1/23 (4%) among placebo participants, respectively (p=0.044). Global cognitive function (MATRICS Consensus Cognitive Battery, MCCB) did not differ, although there was a significant variation in size of treatment effects among cognitive domains (p=0.03), with significant improvement in working memory favoring minocycline (p=0.023, ES 0.41). The SANS total score did not differ, but significant improvement in avolition with minocycline was noted (p=0.012, ES=0.34). Significant improvement in the BPRS anxiety/depression factor was observed with minocycline (p=0.028, ES=0.49). Minocycline was well tolerated with significantly fewer headaches and constipation compared to placebo.
Conclusion
Minocycline’s effect on the MCCB composite score and positive symptoms were not statistically significant. Significant improvements with minocycline were seen in working memory, avolition and anxiety/depressive symptoms in a chronic population with persistent symptoms. Larger studies are needed to validate these findings.
Introduction
Schizophrenia is a challenging and complex psychiatric disorder that affects approximately 1% of the population worldwide, with estimated direct and indirect costs (2002 figures) exceeding $60 billion annually [1]. There is no cure for the disorder and lifelong treatment with antipsychotics is recommended. Clozapine (CLZ) is the most effective antipsychotic for people with treatment resistant schizophrenia and is the only FDA-approved antipsychotic for treatment-resistant schizophrenia and provides effective treatment even when other second-generation antipsychotics fail to respond. Current evidence-based pharmacological guidelines recommend prescribing CLZ for individuals who are unresponsive or partially responsive to first line medications, which is estimated to be up to 40% of people with schizophrenia [2].
There are no evidence-based treatments available for people who are partially or completely nonresponsive to CLZ and continue to have persistent symptoms [3]. Lamotrigine is one medication that has some positive data adjunctive to clozapine on psychotic symptoms [4, 5]. However, it’s efficacy has been demonstrated in only one study [6] and recently not been replicated; thus it remains questionable if it is an effective strategy [7]. It is notable that lamotrigine added to other antipsychotics have not been effective, thus, if it has efficacy as an adjunct may possibly work synergistically through glutamatergic pathways [4]. It is believed that lamotrigine functions as an ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazol-propionate (AMPA) glutamate receptor modulator [8].
Minocycline is a synthetic, FDA approved derivative of tetracycline with a more tolerable side effect profile than other agents in this class. Minocycline crosses the blood brain barrier and has recently been found to have an effect also on the GluR1 AMPA receptor subtype [9]. With minocycline, glutamatergic activity and modulators of neuroplasticity increase in response to increased membrane localization of a GluR1 AMPA receptor subtype, typically achieved by increasing phosphorylation of GluR1 protein at Ser831 and/or Ser845 [10]. In vitro and in vivo mice experiments show minocycline increases GluR1 phosphorylation and membrane insertion [11]. These receptors may be crucially involved in the pathobiology of schizophrenia [12, 13]. Wiedholz et al [14] recently reported that mice lacking GluR1 AMPA receptors exhibit “schizophrenia-related” behaviors and other modulators of this receptor tested in rat models showed procognitive effects [11]. Minocycline is also known to have anti-inflammatory actions and inhibits inflammatory enzymes [15]. Recent evidence suggests a strong relationship between immunological effects and the pathophysiology of schizophrenia [16, 17]. In addition some preclinical and human evidence supports minocycline’s efficacy for neurodegenerative diseases with its potential neuroprotective and anti-apoptotic effects [18–22]. Minocycline directly inhibits the proliferation of and attenuate microglia activation [23–29].
Preliminary human studies in schizophrenia suggest potential benefits from minocycline. Initial case reports [30] of minocycline-associated improvements in persistent psychotic symptoms led to an open label 4-week study which linked minocycline to mean reductions of over 50% in the Positive and Negative Syndrome Scale (PANSS) general psychopathology scale scores [31]. A handful of recent clinical trial publications and a meta-analysis have report benefits (mostly negative symptoms) of minocycline, mostly in early episode and with medications other than CLZ [32–35].
Three small case reports, including one from our own group, have specifically reported symptom benefits from minocycline added to CLZ [36–38]. The current study is the first double-blind, randomized controlled trial of adjunctive minocycline to CLZ in schizophrenia with persistent symptoms. We hypothesized that we would observe a significant improvement in positive and cognitive symptoms as well as that minocycline might have effects on negative symptoms (particularly avolition) and anxiety/depressive symptoms.
Methods
Overview and participants
This study was a randomized double-blind placebo controlled comparison of adjunctive minocycline or placebo added to CLZ. The study consisted of a 3-week screening and stabilization phase followed by a double blind 10 week treatment phase (N=50). The inclusion and exclusion criteria included clinically stable inpatients and outpatients with DSM-IV-TR (APA, Arlington, VA 2000) schizophrenia or schizoaffective disorder who were between the ages of ages 18 and 65 years. Participants had been taking CLZ for at least 6 months prior to study screening with past dose of at least 200 mg/day and had achieved a serum CLZ level of > 350 ng/ml. Patients had persistent positive symptoms, defined by 1) Brief Psychiatric Rating Score (BPRS) total score 45 or Clinical Global Impression (CGI) severity score 4; and 2) BPRS positive symptom item total score of 8 with a score 4 on at least one individual item. Recent substance abuse or dependence, pregnancy, renal and liver impairments, positive Lyme titer, and medications contraindicated with minocycline treatment were exclusionary.
Recruitment
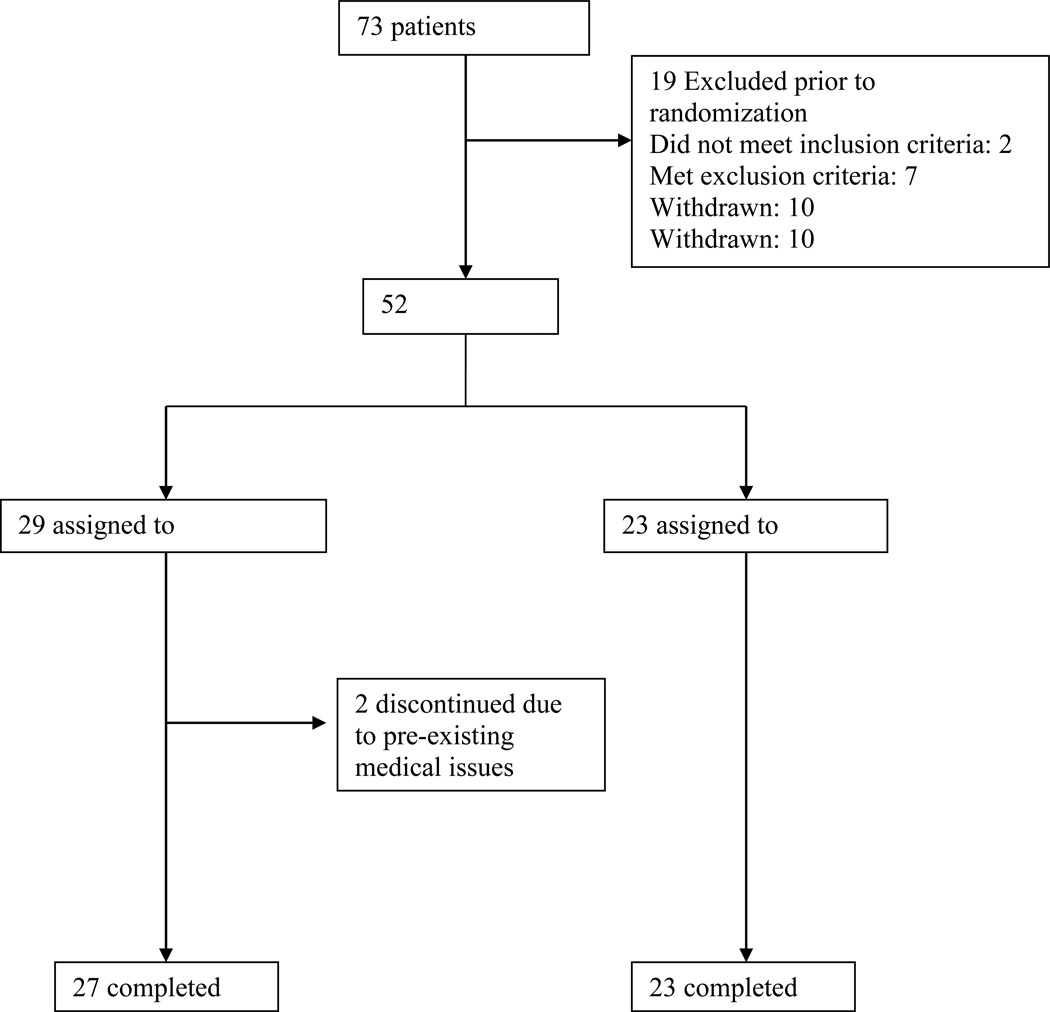
This two site study had 64 participants screened from the Maryland Psychiatric Research Center (MPRC) and nine screened at Central Regional Hospital at Duke University. After screening, 52 participants began double blind treatment (see participant flow chart, Figure 1). Participants were recruited from the MPRC Treatment Research Program, the Outpatient Research Program, and local community mental health centers. Duke participants were recruited from the inpatient units at Central Regional Hospital. All participants were evaluated for capacity to give informed consent using the Evaluation to Sign Consent [39] before signing informed consent.
Figure 1.
Participant Flow Chart
This study was approved by the University of Maryland, Duke University and the State of Maryland Department of Health and Human Hygiene Institutional Review Boards. The study was registered in Clinical Trials.gov (NCT#01433055) and was monitored by a Data Safety Monitoring Board.
Assessments
Assessments were scheduled biweekly for psychiatric symptoms and weekly for side effects throughout the study. Cognitive testing, other secondary efficacy measures, blood chemistries and EKG were performed at baseline, midpoint and end of study. All participants received medical workup including a complete medical history and physical examination at baseline. Positive symptoms were measured by the sum of four items (conceptual disorganization, suspiciousness, hallucinations, and unusual thought content, each rated 1 to 7) from the 18-item BPRS. Negative symptoms were measured with the Scale for the Assessment of Negative Symptoms (SANS): the SANS total score, minus the global items, inappropriate affect, poverty of content of speech, and attention items, were used to measure negative symptoms. Inappropriate affect and poverty of content of speech were excluded as they lack construct validity, and factor analyses suggest that the attention items are not closely related to negative symptoms. Avolition was an a priori domain for separate evaluation (4 items).
Neurocognitive function was measured using the MATRICS Consensus Cognitive Battery (MCCB) which measures seven domains of cognitive functioning with 10 tests. Five of the domains are assessed with one test each. Working memory is assessed with two tests (Letter-number span and WMS Spatial span) and processing speed is assessed with three tests (BACS symbol coding, verbal fluency, and Trailmaking A). The tests were administered in a fixed order, according to standardized testing procedures, to ensure similar testing environments for each subject at each site. Alternate test forms were balanced across testing occasions.
Other ratings included the BPRS total score and the anxiety/depression, hostility, and agitation factors; the Calgary Depression Rating Scale (CDS), and the Clinical Global Impression Scale (CGI). Safety ratings included the Simpson Angus Extrapyramidal Symptom Rating Scale (SAS), the Abnormal Involuntary Movement Scale (AIMS) [40], the Barnes Akathisia Scale (BAS)and the 25 item Side Effect Checklist (SEC). Skin discoloration or pigmentation changes, known but rare side effects of minocycline were also evaluated. Each week, nurses assessed skin pigmentation and inquired about each of the the items on the SEC.
Laboratory measures included CBC, Chem-14 including fasting glucose, total cholesterol, HDL, and LDL. CLZ plasma levels and pregnancy tests (females) were completed every other week.
Rating Training and Reliability
Raters underwent extensive training and reliability exercises prior to conducting any research ratings. Raters from each site achieved a minimum interrater reliability (ICC0.80) for BPRS positive symptom items and SANS total score. Neurocognitive testing was overseen by a senior level investigator at each site (JG, RK).
Medication Dosing and Randomization
Minocycline and matching placebo capsules were dosed as 50 mg twice daily for the first week, and 100 mg twice daily for weeks 2–10. Participants were taking a minimum CLZ dose of 200 mg/day for at least six months AND had one documented CLZ blood level of at least 350 ng/ml to ensure the potential for an adequate CLZ trial [41]. The CLZ dose remained fixed throughout the study except in two participants. One participant’s dose was decreased by 50mg daily for restlessness and racing thoughts, while the other participant’s dose was decreased by the same amount for nausea, vomiting and dizziness. CLZ levels were drawn to ensure adherence and remain at plasma concentrations >350 ng/ml. If the plasma level is found to be < 350 ng/ml during the study, a repeat level was to be drawn one week later. CLZ levels were measured by Lab Corp ® (Supplemental Text 1). Cigarette smoking frequency was to remain consistent in the study to avoid any smoking induced plasma level changes. No medications that alter the CYP 450 1A2 isoenzyme were permitted to be added during the study. CLZ levels were drawn every two weeks at the time of the WBC. We had very strict standards for adherence with the minocycline/placebo. All medications were dispensed in unit dose bubble packaging with each dose marked clearly in a separate marked slot. These were dispensed on a weekly basis. All participants returned their unused packaging each visit to the research team and the dispensing pharmacist for the study. If any participants were more than 25% no adherent to the doses they were to be discontinued from the study. All participants maintained adherence and were very closely monitored in the study. Furthermore the inpatients had all doses observed.
Subjects were randomly assigned to minocycline or placebo using a permuted block randomization system with separate randomization sequences at each site and for inpatients and outpatients. All raters, investigators and other staff were blind to treatment assignment except for the dispensing pharmacist and statistician at the primary site. Participants were allowed to remain on most medications with the exception of lamotrigine, cholestyramine, colestipol, urinary alkalizers, warfarin and tetracyline. Participants were instructed to avoid OTC medications, such as antacids or Pepto-Bismol ® that could reduce minocycline absorption and remained on treatment as usual to increase the generalizability of study results to usual clinical practice. In order to decrease the likelihood that any observed change in positive symptoms or cognitive function during the study was related to one of these adjunctive psychotropic treatments, a participant must have been on the adjunctive medication(s) for at least two months and on the current dose(s) of adjunctive medication(s) for at least one month prior to study participation. The dose of adjunctive medication was to remain fixed throughout the conduct of the study if at all possible. Participants were stratified to CLZ by inpatient/outpatient status and site of enrollment. In addition, participants enrolled at the MPRC were stratified by smoking status to facilitate a local ancillary study.
Lead-In Phase
In addition to the inclusion criteria, participants were required to be clinically stable for the three weeks of the lead in phase. Clinical stability was defined as three consecutive ratings of the Clinical Global Impressions (CGI) rating scale with no change in score. Also, participants met the BPRS entry criteria at the end of three weeks, with no more than a 20% change in positive symptom item total score.
Data Analysis
All data analysis was conducted on an intention to treat basis including all available data from participants with at least one post baseline measure, using methods that permitted analysis of data for participants with missing visits (e.g. mixed models for incomplete repeated measures). Treatment effects on the symptom rating scales were tested looking at average minocycline-placebo differences over the course of follow-up. An a priori power analysis was completed, concluding that we could detect an effect size of at least ES = 0.52, corresponding respectively to reductions in BPRS positive symptom item total scores of about 2.4 points. See Supplemental Text 2 for additional information on sample size calculation. A >30% change in total BPRS was included a priori to examine responder status, as previously work demonstrated that some patients may have a robust response.
The MCCB was collected at baseline, week five and week 10. A summary composite score was calculated from a normed average of the domain scores. Raw scores for this battery were converted to T scores, with mean 50 and s.d. 10 in a normal reference sample [42]. The MCCB composite score was analyzed using a mixed model for repeated measures ANCOVA similar to that outlined above for positive symptoms, with the average effect of treatment across weeks five and 10 being the primary outcome test. We estimated a priori power=0.80 to detect moderate effect sizes of ES=0.52 for N=20 per corresponding to a change of 5.2 points on the MCCB composite score. Secondary analyses of MCCB domains were performed using the mixed model: follow-up domain T score = baseline domain T score + domain + week + treatment + domain×treatment, where the follow-up test scores at weeks five and 10 were adjusted for the baseline score on the same domain, and the domain×treatment interaction provides a test of the null hypothesis that the magnitude of the T-score difference between treatments is the same on all neurocognitive domains. If this interaction test was statistically significant (p<0.05), then post-hoc tests from this model were performed testing treatment differences for each of the neurocognitive domains.
Measures of extrapyramidal side effects and tardive dyskinesia were analyzed by procedures described by McMahon et al [43], using the rank correlation between severity score and visit as a measure of each participant’s tendency to increase or decrease in symptom severity, and comparing treatments on the distribution of these trend scores with the Conover-Salsburg [44] rank test. The frequency of occurrence of new or worsened side effects was reported by treatment, as well as the occurrence of any serious adverse events. Treatment groups were compared on laboratory measures using the mixed model ANCOVA for repeated measures, adjusting for baseline level. For the SEC, the cumulative incidence of new or worsened (compared to baseline visit) side effects was compared between treatments using Fisher’s exact test. For effects showing significant differences between treatments in incidence of new/worsened side effects, descriptive analyses were performed to examine the frequency between treatment groups.
Results
Participants
Seventy-three participants were screened for this study and 52 were randomized to minocycline (N=29) or placebo (N=23). Fifty participants completed the 10 week study; two participants randomized to minocycline terminated the study prior to completion. One was diagnosed at week three with cancer unrelated to the study and one was realized after study entry to have had severely elevated triglycerides at baseline. Supplemental Figure 1 shows the participant flow chart. The demographic information for all participants is listed in Table 1. There were no differences between groups in baseline demographics, CLZ dose or CLZ blood level. All efficacy endpoints are based on N=27 minocycline (not including the participants dropping out at week one and week three) and N=23 placebo. All safety measures are based on N=28 minocycline and N=23 placebo participants. There was one participant in the minocycline group that had high outlier ratings on the BPRS at endpoint ratings due to external stresses associated with the participant’s place of employment. All endpoints are shown with and without the outlier for the BPRS ratings.
Table 1.
Demographic Information
| Minocycline (N=28) | Placebo (N=23) | |
|---|---|---|
| Age (years) | 42.9 ± 14.2 | 42.3 ± 11.0 |
| Race | 18 White (64%) 8 Black (29%) |
13 White (57%) 8 Black (35%) |
| Sex (male) | 20 (71%) | 18 (78%) |
| Age of Illness Onset | 18.5 ± 6.2 | 19.3 ± 5.7 |
| Level of Education | 12.1 ± 1.6 | 12.2 ± 2.6 |
| Inpatients | 11 (39%) | 7 (30%) |
| Clozapine dose (mg/day) | 423.1 ± 189.5 | 433.7 ± 140.1 |
| Total Clozapine level (ng/mL) | 777.4 ± 392.8 | 816.6 ± 339.5 |
| Clozapine (ng/mL) | 489.5 ± 257.1 | 517.7 ± 211.2 |
| Norclozapine (ng/mL) | 287.9 ± 165.0 | 298.9 ± 172.6 |
| Other Antipsychotic | 13 (46%) | 8 (35%) |
| First Generation | 4 (14%) | 1 (4%) |
| Second Generation | 9 (32%) | 7 (30%) |
| Antidepressant | 14 (50%) | 8 (35%) |
| Mood Stabilizer | 10 (36%) | 6 (26%) |
| Anticholinergic | 4 (14%) | 3 (13%) |
| Benzodiazepine | 15 (54%) | 9 (39%) |
Brief Psychiatric Rating Scale (BPRS)
BPRS positive symptoms, a primary outcome, did not show a significant treatment effect (minocycline - placebo difference ± s.e. = −0.87 ± 0.53, ES d= 0.34, p=0.104). For the BPRS total score, the minocycline – placebo difference was −1.03 ± SE 1.15, ES d= 0.48, p=0.099. There was, however, a significant effect in favor of minocycline for the BPRS anxiety/depression factor (minocycline-placebo difference ± s.e. −0.80 ± 0.38, ES d= 0.42, p=0.040). No significant differences were noted for the BPRS hostility or agitation factors. With regards to ≥ 30 reduction in total BPRS scores there were 7/28 (25%) in the minocycline group and 1/23 (4%) in the placebo group (Chi-Square=4.07, df=1, p=0.0436). (See Table 2)
Table 2.
Baseline and Endpoint Scores for Psychiatric Symptoms
| Minocycline (N=27) | Placebo (N=23) | Mixed model treatment difference estimates |
|||
|---|---|---|---|---|---|
| Weeks | Mean ± SD | Weeks | Mean ± SD | ||
| BPRS Total score | 0 | 44.9 ± 8.7 | 0 | 44.0 ± 7.9 | −1.9 ± 1.1, t=−1.7, df=46.9, p=0.099 *−2.1 ± 1.2, t=−1.8, df=46.5, p=0.08 |
| 10 | 39.6 ± 7.3 | 10 | 43.2 ± 8.1 | ||
| BPRS Positive Symptom score | 0 | 14.5 ± 3.5 | 0 | 14.5 ± 3.5 | −0.9 ± 0.5, t=−1.7, df=48.4, p=0.10 *−0.9 ± 0.5, t=−1.7, df=47.4, p=0.098 |
| 10 | 12.9 ± 3.2 | 10 | 14.2 ± 3.2 | ||
| BPRS Anxiety/Depression score | 0 | 7.6 ± 2.6 | 0 | 7.0 ± 3.0 | −0.8 ± 0.4, t=−2.1, df=46.8, p=0.04 *−0.9 ± 0.4, t=−2.3, df=45.9, p=0.03 |
| 10 | 6.7 ± 2.8 | 10 | 7.2 ± 3.4 | ||
| BPRS Hostility score | 0 | 7.6 ± 3.2 | 0 | 6.4 ± 2.1 | −0.4 ± 0.4, t=−1.1, df=48.3, p=0.27 *−0.5 ± 0.4, t=−1.2, df=47.5, p=0.24 |
| 10 | 6.0 ± 2.1 | 10 | 6.6 ± 2.5 | ||
| BPRS Activation score | 0 | 5.3 ± 1.7 | 0 | 5.3 ± 1.7 | −0.03 ± 0.2, t=−0.1, df=48.5, p=0.89 *−0.05 ± 0.2, t=−0.3, df=47.4, p=0.78 |
| 10 | 4.8 ± 1.5 | 10 | 4.9 ± 2.0 | ||
| SANS Total score | 0 | 29.7 ± 13.3 | 0 | 33.3 ± 10.3 | −0.5 ± 1.1, t=−0.5, df=47.9, p=0.65 |
| 10 | 28.2 ± 12.4 | 10 | 35.1 ± 10.7 | ||
| SANS Anhedonia | 0 | 2.1 ± 1.1 | 0 | 2.4 ± 0.9 | −0.1 ± 0.1, t=−1.9, df=48.7, p=0.06 |
| 10 | 2.1 ± 1.1 | 10 | 2.7 ± 0.9 | ||
| SANS Blunted Affect | 0 | 1.5 ± 1.0 | 0 | 1.8 ± 0.9 | 0.08 ± 0.1, t=0.7, df=47.5, p=0.47 |
| 10 | 1.5 ± 1.0 | 10 | 1.8 ± 1.0 | ||
| SANS Alogia | 0 | 0.9 ± 0.7 | 0 | 1.1 ± 0.8 | 0.06 ± 0.1, t=0.7, df=43.8, p=0.46 |
| 10 | 0.7 ± 0.7 | 10 | 1.1 ± 0.9 | ||
| SANS Avolition | 0 | 2.5 ± 0.9 | 0 | 2.4 ± 0.8 | −0.2 ± 0.1, t=−2.6, df=48.4, p=0.01 |
| 10 | 2.2 ± 0.9 | 10 | 2.6 ± 0.8 | ||
| CDS total score | 0 | 3.3 ± 3.1 | 0 | 2.6 ± 2.7 | −0.8 ± 0.5, t=−1.75, df=47.7, p=0.09 |
| 10 | 2.4 ± 3.0 | 10 | 2.9 ± 2.7 | ||
| CGI Globe score | 0 | 4.0 | 0 | 4.0 ± 0.1 | −0.1 ± 0.1, t=−1.19, df=38.3, p=0.24 |
| 10 | 3.6 ± 0.6 | 10 | 4.0 ± 0.7 | ||
analysis of BPRS with one patient excluded who had outlier high ratings at endpoint visit only.
MATRICS Consensus Cognitive Battery (MCCB)
The treatment difference on the MCCB Composite score was not statistically significant (d=0.1, t=0.58, df=46, p=0.56); however a domain×treatment assignment interaction was noted (F= 2.78, df=6,41.6, p=0.023). Post hoc tests found a significant improvement of working memory in the minocycline group (minocycline-placebo difference ± s.e. = 4.8 ± 1.8, p=0.023, effect size d=0.41). ES and corresponding statistics for other MCCB domains are listed in Table 3.
Table 3.
Minocycline - Placebo Difference Effect Sizes for MATRICS Cognitive Battery Domains
| Minocycline (N=27) | Placebo (N=23) | Effect Size |
Mean ± SE, p | |||
|---|---|---|---|---|---|---|
| Weeks | Mean ± SD | Weeks | Mean ± SD | |||
| Composite Score | 0 | 20.0 ± 13.1 | 0 | 27.7 ± 12.9 | 0.08 | 0.98 ± 1.69, p=0.56 |
| 10 | 22.3 ± 12.9 | 10 | 27.3 ± 13.8 | |||
| Attention/Vigilance | 0 | 31.8 ± 13.1 | 0 | 35.0 ± 13.2 | −0.08 | −0.98 ± 2.07, p=0.64 |
| 10 | 32.6 ± 14.1 | 10 | 35.9 ± 13.2 | |||
| Processing Speed | 0 | 29.5 ± 12.4 | 0 | 34.8 ± 12.8 | 0.09 | 1.17 ± 1.86, p=0.53 |
| 10 | 31.6 ± 12.4 | 10 | 34.3 ± 11.4 | |||
| Problem Solving | 0 | 37.0 ± 10.5 | 0 | 42.6 ± 8.1 | 0.004 | 0.04 ± 1.90, p=0.98 |
| 10 | 38.0 ± 10.6 | 10 | 42.0 ± 9.5 | |||
| Social Cognition | 0 | 31.7 ± 13.6 | 0 | 34.5 ± 10.3 | −0.1 | −1.17 ± 2.84, p=0.68 |
| 10 | 33.4 ± 14.3 | 10 | 36.6 ± 11.6 | |||
| Verbal Learning | 0 | 32.3 ± 6.3 | 0 | 34.3 ± 7.2 | −0.35 | −2.39 ± 1.79, p=0.19 |
| 10 | 31.8 ± 7.2 | 10 | 35.6± 11.0 | |||
| Visual Learning | 0 | 27.7 ± 11.9 | 0 | 34.4 ± 13.2 | −0.11 | −1.33 ± 2.35, p=0.57 |
| 10 | 27.8 ± 12.4 | 10 | 33.9 ± 11.3 | |||
| Working Memory | 0 | 30.4 ± 11.6 | 0 | 35.1 ± 11.7 | 0.41 | 4.81 ± 1.82, p=0.01 |
| 10 | 35.6 ± 10.7 | 10 | 34.2 ± 12.2 | |||
Scale for the Assessment of Negative Symptoms (SANS)
For the SANS total score, there was no significant group difference (F=0.20, df=1,47.9, p=0.65, ES d=0.23). On the SANS avolition factor there was a significant minocycline-placebo difference ± s.e. of −0.22 ± 0.09, p=0.012, ES= 0.34. Treatment differences on other SANS subscales (anhedonia, blunted affect, and alogia) were not significant. (See Table 2)
Side Effects and Safety
Overall minocycline was well tolerated with most side effects similar to placebo (see Supplementary Table 1). Headaches and constipation were less frequent in the minocycline group. Measures of Body Mass Index (BMI) did not differentiate the groups. Baseline and endpoint BMI were 30.3 ± 5.3 kg/m2 and 31.3 ± 5.3 kg/m2, respectively, in the minocycline group and 31.0 ± 7.8 and 30.7 ± 7.4 respectively for placebo (p=0.30). Four (14%) participants assigned minocycline and 2 (9%) assigned placebo were noted to have skin pigmentation or skin discoloration during the study (p=0.54). There were no significant differences between groups on the BAS (F=1.54, df=1,48, p=0.22), AIMS (F=0.83, df=1,48, p=0.37) and SAS (F=0.82. df=1,48, p=0.37). Laboratory measures of fasting blood glucose, CBC, liver enzymes and electrolytes did not differ between medication groups. There was an increase in high density lipoprotein (HDL) cholesterol with minocycline compared to placebo (minocycline-placebo difference=5.2±1.7, p=0.004), but no significant treatment differences in LDL cholesterol or triglycerides. Statistically significant reductions in albumin, protein and platelets and an increase in hematocrit were noted; however, these were not clinically significant and partially driven by placebo group changes. Supplementary Table 2 lists the full values for lab findings in the study.
Discussion
To date, there have been four published randomized double blind trials with minocycline all finding some positive effects in cognitive function, negative symptoms or positive symptoms but with differing inclusion and exclusion criteria and primary outcomes [32–35]. The first study published, Levkovitz et al [32] studied minocycline as an adjunct treatment to treatment as usual for negative and cognitive symptoms in patients within the first five years of illness. Chaudhry et al [35] was a two-site study with adjunct treatment of minocycline to treatment as usual in patients in the first five years of their illness but with both positive and negative symptoms as the primary outcomes. Liu et al [33] studied adjunct minocycline to risperidone in patients in the first five years of illness but with negative symptoms as the primary outcome. Lastly, Khodaie-Ardakanie et al [34] also added minocycline to risperidone and with negative symptoms as the primary outcome, but in a chronic population. This study we present is different in that it is the first study with minocycline treatment as adjunct to clozapine, the first to have positive and cognitive symptoms as the primary outcomes and only the second to test in a chronic population. Our study failed to find significant results in positive and global cognitive symptoms and may not have been powered to find a difference in global negative symptoms. In secondary analyses, we did find significant improvements in the areas of avolition, working memory, and anxiety/depression.
Our prior case series of CLZ patients treated openly with adjunct minocycline [45] reported improved positive symptoms but also improvements in motivation for social and school interactions and activities. Avolition is a core symptom of schizophrenia, which leads to a decrease in spontaneous, self-initiated and purposeful behaviors observed in daily life activities [46]. A unique domain within the negative symptom syndrome, avolition is often associated with poorer functional outcome [47, 48]. The domain of amotivation or avolition has been found to be a strong predictor of interpersonal relations and personal and social function (46). Avolition is thought to be due to aberrant cortical-striatal interactions that facilitate reward processing. [47]. Negative symptoms more generally have been found to be associated with higher levels of inflammation [49, 50]. Similarly, evidence of inflammation is more common in deficit versus non-deficit schizophrenia [51]. Glutamate dysfunction may also underlie negative symptoms [52, 53]. The anti-inflammatory effects of minocycline and/or its modulation of glutamatergic pathways could underlie the avolition benefits we have observed. While we observed a positive finding for avolition, the study may not have been powered to detect improvements in global negative symptoms.
Working memory is also regarded as a core deficit in schizophrenia [54] The improvements in working memory seen here are consistent with Levkovitz et al [32] who also found improvements in working memory in early phase schizophrenia patients, some of whom were taking CLZ. Working deficits have been found to be connected to inflammation. Liaury et al [27] found that microglial activation was attenuated with related improvements in memory related to inflammation during minocycline treatment. Microglial cells are known to contribute to synaptic modulation, learning and memory processes [55, 56]. Many other animal studies have found minocycline to reduce memory and working memory deficits in animal models [57–61]. The relevance of this literature to the current results should be viewed with tempered enthusiasm given that the clinical importance of the modest but statistically significant improvement in working memory we observed is questionable.
Minocycline was well tolerated in this 10 week study. As noted, beneficial effects were noted in headache, constipation and HDL levels. Changes in skin pigmentation were seen in a few participants; however, no one discontinued the study due to skin pigmentation. We also did not see GI side effects or differences in weight gain, suggesting minocycline could be tolerable as an adjunctive medication in a schizophrenia population. Other minocycline studies in schizophrenia also have had very good tolerability [32–35]; however, in a few clinical trials, there have gastrointestinal complaints such as nausea, diarrhea, and constipation that occurred more frequently with minocycline than in the placebo group; however, most of these studies have used doses of up to 400 mg/day [62, 63]. Also, important to point out that minocycline has been identified as a causative agent of drug induced lupus (DIL) which has a similar presentation to systemic lupus erythematosus (SLE). There is an estimated 8.5 increased risk of DIL for individuals currently being treated with minocycline compared to nonusers [64] and DIL from minocycline treatment may take three months to six years to develop with a female to male ratio of 5:1 [65]. The typical symptoms are polyarthralgia, arthritis, fever, and myalgia. There may also be liver involvement with fatty liver changes or autoimmune hepatitis [66]. With discontinuation of minocycline treatment the prognosis of DIL is good with complete resolution of symptoms. In the five clinical trials (including this study) in schizophrenia and numerous other studies in other populations DIL has not been reported. Also while many studies show improvement in a variety of domains with minocycline, it is important to point out in one of the phase III amyotrophic lateral sclerosis (ALS) trials (up to 400 mg/day), deterioration in functional capacity was faster in minocycline than placebo [62].
Our study is limited by small sample size, lack of power for negative symptoms and the fact that change in functional outcomes from negative symptoms and cognitive improvements generally take longer than 10 weeks, as seen in longer term studies and case reports with minocycline, which have reported functional improvements. Nonetheless, our moderate effects in some treatment domains in a chronically ill and treatment resistant population are worth noting. Our sample of CLZ treated participants had undergone at least six months and commonly years of CLZ treatment, with partial improvement, but continued moderate to severe symptoms. This is a difficult to treat population with little evidence for efficacious adjunctive treatments. The current data and other published data continue to suggest minocycline is a possible adjunctive medication. Future analyses of these data will examine inflammatory markers and imaging data to better evaluate biomarkers for treatment response. These data add to growing evidence suggesting that minocycline is a worthwhile adjunct treatment to consider when first line agents are not enough [67].
Supplementary Material
Acknowledgments
Source Funding:
The authors would like to thank and acknowledge the staff at the Treatment Research Program and the Outpatient Research Program at MPRC for their work on this study. We would also like to thank Ann Marie Kearns for her support and administration of all regulatory and compliance for this study and Frempongma Wadee for help with table creation and formatting. Funded by NIMH R21MH091184-01A1 (Kelly PI). Clinical Trial.gov registration number: NCT#01433055
Footnotes
Conflict of Interest:
For the remaining authors none were declared.
Deanna L. Kelly, PharmD- Consultant for Lundbeck and XOMA
Joseph P McEvoy, MD – Consultant for Ameritox, Alkermes, Envivo, Jazz, Otsuka, Merck;
Robert P. McMahon, PhD - consultant for Amgen, Inc
Richard S. Keefe, PhD – Consultant/Advisory Board for Abbvie, Akebia, Amgen, Astellas, Asubio, AviNeuro/ChemRar, BiolineRx, Biogen Idec, Biomarin, Boehringer-Ingelheim, Eli Lilly, FORUM, GW Pharmaceuticals, Helicon, Lundbeck, Merck, Minerva Neurosciences, Mitsubishi, Novartis, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, Targacept. Royalties from BACS testing battery, the MATRICS Battery (BACS symbol coding), and the Virtual Reality Functional Capacity Assessment Tool (VRFCAT). Shareholder in NeuroCog Trials, Inc. and Sengenix.
Robert W. Buchanan, MD - DSMB member for Otsuka and Pfizer; Consulted with Abbott; Amgen; Bristol-Meyers Squibb; EnVivo; Omeros; Pfizer; Advisory Boards: Abbott; Amgen; EnVivo; Janssen Pharmaceutical, Inc; NuPathe, Inc.; Pfizer; Roche; Takeda.
References
- 1.Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. The Journal of Clinical Psychiatry. 2005;66:1122–1129. doi: 10.4088/jcp.v66n0906. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan RW, Kreyenbuhl J, Kelly DL, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36:71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sommer IE, Begemann MJ, Temmerman A, et al. Pharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature review. Schizophr Bull. 2012;38:1003–1011. doi: 10.1093/schbul/sbr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goff DC. Review: lamotrigine may be an effective treatment for clozapine resistant schizophrenia. Evid Based Ment Health. 2009;12:111. doi: 10.1136/ebmh.12.4.111. [DOI] [PubMed] [Google Scholar]
- 5.Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109:10–14. doi: 10.1016/j.schres.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Zoccali R, Muscatello MR, Bruno A, et al. The effect of lamotrigine augmentation of clozapine in a sample of treatment-resistant schizophrenic patients: a double-blind, placebo-controlled study. Schizophr Res. 2007;93:109–116. doi: 10.1016/j.schres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Vayisoglu S, Anil Yagcioglu AE, Yagcioglu S, et al. Lamotrigine augmentation in patients with schizophrenia who show partial response to clozapine treatment. Schizophr Res. 2013;143:207–214. doi: 10.1016/j.schres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Lee CY, Fu WM, Chen CC, et al. Lamotrigine inhibits postsynaptic AMPA receptor and glutamate release in the dentate gyrus. Epilepsia. 2008;49:888–897. doi: 10.1111/j.1528-1167.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 9.Chaves C, Marque CR, Trzesniak C, et al. Glutamate-N-methyl-D-aspartate receptor modulation and minocycline for the treatment of patients with schizophrenia: an update. Braz J Med Biol Res. 2009;42:1002–1014. doi: 10.1590/S0100-879X2009001100002. [DOI] [PubMed] [Google Scholar]
- 10.Wang JQ, Arora A, Yang L, et al. Phosphorylation of AMPA receptors: mechanisms and synaptic plasticity. Mol Neurobiol. 2005;32:237–249. doi: 10.1385/MN:32:3:237. [DOI] [PubMed] [Google Scholar]
- 11.Imbesi M, Uz T, Manev R, et al. Minocycline increases phosphorylation and membrane insertion of neuronal GluR1 receptors. Neurosci Lett. 2008;447:134–137. doi: 10.1016/j.neulet.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manev R, Manev H. Minocycline, schizophrenia and GluR1 glutamate receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:166. doi: 10.1016/j.pnpbp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Mattes H, Lingenhoehl K, Kalkman H, et al. AMPA receptor antagonists: potential therapeutic applications. Recent Pat CNS Drug Discov. 2006;1:247–259. doi: 10.2174/157488906778773698. [DOI] [PubMed] [Google Scholar]
- 14.Wiedholz LM, Owens WA, Horton RE, et al. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and 'schizophrenia-related' behaviors. Mol Psychiatry. 2008;13:631–640. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- 15.Chu LS, Fang SH, Zhou Y, et al. Minocycline inhibits 5-lipoxygenase activation and brain inflammation after focal cerebral ischemia in rats. Acta Pharmacol Sin. 2007;28:763–772. doi: 10.1111/j.1745-7254.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 16.Strous RD, Shoenfeld Y. Schizophrenia, autoimmunity and immune system dysregulation: a comprehensive model updated and revisited. J Autoimmun. 2006;27:71–80. doi: 10.1016/j.jaut.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz T, Chew LJ. Cytokines and myelination in the central nervous system. ScientificWorldJournal. 2008;8:1119–1147. doi: 10.1100/tsw.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuki S, Iuchi Y, Ikeda Y, et al. Suppression of cytochrome c release and apoptosis in testes with heat stress by minocycline. Biochem Biophys Res Commun. 2003;312:843–849. doi: 10.1016/j.bbrc.2003.10.191. [DOI] [PubMed] [Google Scholar]
- 19.Stirling DP, Khodarahmi K, Liu J, et al. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arvin KL, Han BH, Du Y, et al. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52:54–61. doi: 10.1002/ana.10242. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Wei Q, Wang CY, et al. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279:19948–19954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Kitaichi K, Fujimoto Y, et al. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Yrjanheikki J, Keinanen R, Pellikka M, et al. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dommergues MA, Plaisant F, Verney C, et al. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience. 2003;121:619–628. doi: 10.1016/s0306-4522(03)00558-x. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K. Microglial activation in schizophrenia and minocycline treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1758–1759. doi: 10.1016/j.pnpbp.2008.06.012. author reply 1760. [DOI] [PubMed] [Google Scholar]
- 26.Zhu F, Liu Y, Zhao J, et al. Minocycline alleviates behavioral deficits and inhibits microglial activation induced by intrahippocampal administration of Granulocyte-Macrophage Colony-Stimulating Factor in adult rats. Neuroscience. 2014;266:275–281. doi: 10.1016/j.neuroscience.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Liaury K, Miyaoka T, Tsumori T, et al. Minocycline improves recognition memory and attenuates microglial activation in Gunn rat: a possible hyperbilirubinemia-induced animal model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:184–190. doi: 10.1016/j.pnpbp.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Seki Y, Kato TA, Monji A, et al. Pretreatment of aripiprazole and minocycline, but not haloperidol, suppresses oligodendrocyte damage from interferon-gamma-stimulated microglia in co-culture model. Schizophr Res. 2013;151:20–28. doi: 10.1016/j.schres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Mattei D, Djodari-Irani A, Hadar R, et al. Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun. 2014;38:175–184. doi: 10.1016/j.bbi.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Miyaoka T, Yasukawa R, Yasuda H, et al. Possible antipsychotic effects of minocycline in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:304–307. doi: 10.1016/j.pnpbp.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Miyaoka T, Yasukawa R, Yasuda H, et al. Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clin Neuropharmacol. 2008;31:287–292. doi: 10.1097/WNF.0b013e3181593d45. [DOI] [PubMed] [Google Scholar]
- 32.Levkovitz Y, Mendlovich S, Riwkes S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71:138–149. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Guo X, Wu R, et al. Minocycline supplementation for treatment of negative symptoms in early-phase schizophrenia: a double blind, randomized, controlled trial. Schizophr Res. 2014;153:169–176. doi: 10.1016/j.schres.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Khodaie-Ardakani MR, Mirshafiee O, Farokhnia M, et al. Minocycline add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized double-blind placebo-controlled study. Psychiatry Res. 2014;215:540–546. doi: 10.1016/j.psychres.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhry IB, Hallak J, Husain N, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol. 2012;26:1185–1193. doi: 10.1177/0269881112444941. [DOI] [PubMed] [Google Scholar]
- 36.Kelly DL, Vyas G, Richardson CM, et al. Adjunct minocycline to clozapine treated patients with persistent schizophrenia symptoms. Schizophr Res. 2011;133:257–258. doi: 10.1016/j.schres.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Jhamnani K, Shivakumar V, Kalmady S, et al. Successful use of add-on minocycline for treatment of persistent negative symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25:E06–E07. doi: 10.1176/appi.neuropsych.11120376. [DOI] [PubMed] [Google Scholar]
- 38.Qurashi I, Collins J, Chaudhry I, et al. Promising use of minocycline augmentation with clozapine in treatment-resistant schizophrenia. J Psychopharmacol. 2014;28:707–708. doi: 10.1177/0269881114527358. [DOI] [PubMed] [Google Scholar]
- 39.DeRenzo EG, Conley RR, Love R. Assessment of capacity to give consent to research participation: state-of-the-art and beyond. J Health Care Law Policy. 1998;1:66–87. [PubMed] [Google Scholar]
- 40.Guy W. ECDEU assessment manual for psychopharmacology. Rockville, MD: US Department of Health and Human Services; 1976. revised (DHEW Publication No. ADM 76-338) [Google Scholar]
- 41.Greenwood-Smith C, Lubman DI, Castle DJ. Serum clozapine levels: a review of their clinical utility. J Psychopharmacol. 2003;17:234–238. doi: 10.1177/0269881103017002014. [DOI] [PubMed] [Google Scholar]
- 42.Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- 43.McMahon RP, Arndt S, Conley RR. More powerful two-sample tests for differences in repeated measures of adverse effects in psychiatric trials when only some patients may be at risk. Stat Med. 2005;24:11–21. doi: 10.1002/sim.1837. [DOI] [PubMed] [Google Scholar]
- 44.Conover WJ, Salsburg DS. Locally most powerful tests for detecting treatment effects when only a subset of patients can be expected to "respond" to treatment. Biometrics. 1988;44:189–196. [PubMed] [Google Scholar]
- 45.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989:49–58. [PubMed] [Google Scholar]
- 46.Tremeau F, Nolan KA, Malaspina D, et al. Behavioral validation of avolition in schizophrenia. Schizophr Res. 2012;138:255–261. doi: 10.1016/j.schres.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Strauss GP, Horan WP, Kirkpatrick B, et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47:783–790. doi: 10.1016/j.jpsychires.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fervaha G, Foussias G, Agid O, et al. Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur Psychiatry. 2014;29:449–455. doi: 10.1016/j.eurpsy.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Dimitrov DH, Lee S, Yantis J, et al. Differential correlations between inflammatory cytokines and psychopathology in veterans with schizophrenia: potential role for IL-17 pathway. Schizophr Res. 2013;151:29–35. doi: 10.1016/j.schres.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Meyer U, Schwarz MJ, Muller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132:96–110. doi: 10.1016/j.pharmthera.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Rizo C, Fernandez-Egea E, Oliveira C, et al. Inflammatory markers in antipsychotic-naive patients with nonaffective psychosis and deficit vs. nondeficit features. Psychiatry Res. 2012;198:212–215. doi: 10.1016/j.psychres.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chue P, Lalonde JK. Addressing the unmet needs of patients with persistent negative symptoms of schizophrenia: emerging pharmacological treatment options. Neuropsychiatr Dis Treat. 2014;10:777–789. doi: 10.2147/NDT.S43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neill JC, Harte MK, Haddad PM, et al. Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: a translational link to humans. Eur Neuropsychopharmacol. 2014;24:822–835. doi: 10.1016/j.euroneuro.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Reichenberg A. The assessment of neuropsychological functioning in schizophrenia. Dialogues Clin Neurosci. 2010;12:383–392. doi: 10.31887/DCNS.2010.12.3/areichenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kettenmann H, Hanisch UK, Noda M, et al. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 56.Hanisch UK, van Rossum D, Xie Y, et al. The microglia-activating potential of thrombin: the protease is not involved in the induction of proinflammatory cytokines and chemokines. J Biol Chem. 2004;279:51880–51887. doi: 10.1074/jbc.M408318200. [DOI] [PubMed] [Google Scholar]
- 57.Levkovitz Y, Levi U, Braw Y, et al. Minocycline, a second-generation tetracycline, as a neuroprotective agent in an animal model of schizophrenia. Brain Res. 2007;1154:154–162. doi: 10.1016/j.brainres.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Shirayama Y, Iyo M, et al. Minocycline attenuates hyperlocomotion and prepulse inhibition deficits in mice after administration of the NMDA receptor antagonist dizocilpine. Neuropsychopharmacology. 2007;32:2004–2010. doi: 10.1038/sj.npp.1301313. [DOI] [PubMed] [Google Scholar]
- 59.Mizoguchi H, Takuma K, Fukakusa A, et al. Improvement by minocycline of methamphetamine-induced impairment of recognition memory in mice. Psychopharmacology (Berl) 2008;196:233–241. doi: 10.1007/s00213-007-0955-0. [DOI] [PubMed] [Google Scholar]
- 60.Siopi E, Llufriu-Daben G, Fanucchi F, et al. Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: the effect of minocycline. Neurosci Lett. 2012;511:110–115. doi: 10.1016/j.neulet.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 61.Jin WJ, Feng SW, Feng Z, et al. Minocycline improves postoperative cognitive impairment in aged mice by inhibiting astrocytic activation. Neuroreport. 2014;25:1–6. doi: 10.1097/WNR.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 62.Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 63.Gordon PH, Moore DH, Gelinas DF, et al. Placebo-controlled phase I/II studies of minocycline in amyotrophic lateral sclerosis. Neurology. 2004;62:1845–1847. doi: 10.1212/01.wnl.0000125321.92112.7e. [DOI] [PubMed] [Google Scholar]
- 64.Sturkenboom MC, Meier CR, Jick H, et al. Minocycline and lupuslike syndrome in acne patients. Arch Intern Med. 1999;159:493–497. doi: 10.1001/archinte.159.5.493. [DOI] [PubMed] [Google Scholar]
- 65.Schaffer JV, Davidson DM, McNiff JM, et al. Perinuclear antineutrophilic cytoplasmic antibody-positive cutaneous polyarteritis nodosa associated with minocycline therapy for acne vulgaris. J Am Acad Dermatol. 2001;44:198–206. doi: 10.1067/mjd.2001.112218. [DOI] [PubMed] [Google Scholar]
- 66.Gough A, Chapman S, Wagstaff K, et al. Minocycline induced autoimmune hepatitis and systemic lupus erythematosus-like syndrome. BMJ. 1996;312:169–172. doi: 10.1136/bmj.312.7024.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torrey EF, Davis JM. Adjunct treatments for schizophrenia and bipolar disorder: what to try when you are out of ideas. Clin Schizophr Relat Psychoses. 2012;5:208–216. doi: 10.3371/CSRP.5.4.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.