Abstract
The MHC Class I-related receptor, FcRn, transports antibodies of the immunoglobulin G (IgG) class within and across a diverse array of different cell types. Through this transport, FcRn serves multiple roles throughout adult life that extend well beyond its earlier defined function of transcytosing IgGs from mother to offspring. These roles include the maintenance of IgG levels and the delivery of antigen in the form of immune complexes to degradative compartments within cells. Recent studies have led to significant advances in knowledge of the intracellular trafficking of FcRn and (engineered) IgGs at both the molecular and cellular levels. The engineering of FcRn–IgG (or Fc) interactions to generate antibodies of increased longevity represents an area of active interest, particularly in the light of the expanding use of antibodies in therapy. The strict pH dependence of FcRn–IgG interactions, with binding at pH 6 that becomes essentially undetectable as near neutral pH is approached, is essential for efficient transport. The requirement for retention of low affinity at near neutral pH increases the complexity of engineering antibodies for increased half-life. Conversely, engineered IgGs that have gained significant binding for FcRn at this pH can be potent inhibitors of FcRn that lower endogenous IgG levels and have multiple potential uses as therapeutics. In addition, molecular studies of FcRn–IgG interactions indicate that mice have limitations as preclinical models for FcRn function, primarily due to cross-species differences in FcRn-binding specificity.
1. Introduction
The MHC Class I-related receptor, FcRn (n for neonatal), was originally identified as the receptor that transports maternal IgG in mother's milk across the neonatal rodent gut during the suckling period (Brambell, 1970; Rodewald and Abrahamson, 1982; Wallace and Rees, 1980). However, more recent studies have not only shown that this receptor serves to regulate IgG levels and distribution throughout adult life (Ghetie et al., 1996; Israel et al., 1996; Junghans and Anderson, 1996), but also that it has multiple other roles in diverse cell types and tissues (e.g., Akilesh et al., 2008; Dickinson et al., 1999; Kim et al., 2008; Spiekermann et al., 2002; Zhu et al., 2001). FcRn orthologs have been isolated from many species, including mouse, rat, man, sheep, cow, possum, pig, and camel (Adamski et al., 2000; Ahouse et al., 1993; Kacskovics et al., 2000, 2006; Kandil et al., 1995; Mayer et al., 2002; Schnulle and Hurley, 2003; Simister and Mostov, 1989; Story et al., 1994), indicating that this receptor is present in essentially all mammalian species. The multiple functions of FcRn are dependent on its ability to sort IgG away from lysosomal degradation within cells and release bound cargo during exocytic events at the plasma membrane (Ober et al., 2004a,b; Prabhat et al., 2007). Consequently, this receptor transports IgG within and across cellular barriers for a diverse array of cell types (Antohe et al., 2001; Claypool et al., 2004; Dickinson et al., 1999; Firan et al., 2001; Haymann et al., 2000; McCarthy et al., 2000; Spiekermann et al., 2002; Yoshida et al., 2004). More recently, FcRn has also been shown to control albumin levels (Andersen et al., 2006; Chaudhury et al., 2003). How this receptor behaves at the subcellular level of intracellular trafficking, and what controls its intracellular routing are of fundamental relevance to understanding its function. In addition, given the potential for modulating IgG trafficking pathways and behavior in vivo, the earlier report of engineering of antibodies to increase their half-life in mice (Ghetie et al., 1997) has expanded into an area of intense interest in the biopharma industry (Dall'Acqua et al., 2006b; Hinton et al., 2004, 2006; Shields et al., 2001).
In the current review, we will describe the multiple functions of FcRn and the intracellular trafficking pathways of this receptor and its ligand. The modulation of FcRn–ligand interactions for the development of therapeutics will also be discussed, with a particular focus on how the complexity of the pH dependence of FcRn–IgG interactions and cross-species differences in behavior impact this area.
2. FcRn: A Historical Perspective
Neonatal rodents acquire the major portion of their maternal IgG from mothers' milk during the suckling period (Brambell, 1970). An early model for the trafficking of IgG across the neonatal gut was originally proposed in the absence of specific knowledge of the receptor involved (Brambell, 1970): IgGs are taken into enterocytes at the apical surface by receptor-mediated uptake at the acidic pH in the small intestine. These IgGs are then transcytosed across the cells and released at the basolateral membrane which is at near neutral pH. A central feature of this early model was that the unidentified receptor, which was later shown to be FcRn (Rodewald and Abrahamson, 1982; Wallace and Rees, 1980), is a salvage receptor which binds and transports IgG in intact form across cells.
FcRn was subsequently isolated from rodent gut as a heterodimer comprising 12 kDa and 40–45 kDa proteins (Rodewald and Kraehenbuhl, 1984; Simister and Rees, 1985). Significantly, in these early studies, the FcRn–IgG interaction was shown to be highly pH dependent with relatively tight binding at acidic pH (6) and very weak, if not negligible, binding at near neutral pH (Rodewald and Kraehenbuhl, 1984; Simister and Rees, 1985). The cloning of the gene for rat FcRn in 1989 unexpectedly revealed that this receptor comprises an α-chain that is homologous to MHC Class I α-chains, and the 12 kDa component is β2-microglobulin (β2m) (Simister and Mostov, 1989). This was followed by the isolation of orthologous FcRn α-chains from mouse and man (Ahouse et al., 1993; Kandil et al., 1995; Story et al., 1994), and subsequently from multiple other species (Adamski et al., 2000; Kacskovics et al., 2000, 2006; Mayer et al., 2002; Schnulle and Hurley, 2003). Although FcRn orthologs share some similarities, there are cross-species differences at the level of binding specificity that can have functional effects (Ober et al., 2001; Vaccaro et al., 2006), in addition to variations in intracellular trafficking and subcellular distribution (Claypool et al., 2002; Kuo et al., 2009) (discussed further in Sections 5.5 and 8).
3. FcRn is a Multitasking Receptor
3.1. A role for FcRn in regulating IgG levels
At the time of the isolation of the gene encoding rat FcRn (Simister and Mostov, 1989), the primary function of this receptor was believed to be to deliver maternal IgG to offspring. Although Brambell and colleagues proposed in the 1960s that the cellular processes involved in transporting maternal IgG from mother to young and in regulating IgG levels throughout life might be related (Brambell, 1970; Brambell et al., 1964), data to provide direct support for the involvement of FcRn in both of these processes were absent. However, in the mid-1990s, several observations led to the conclusion that FcRn exploits its capability to transport IgG within and across cells to regulate IgG levels throughout adult life. First, we demonstrated that the same IgG residues (on both CH2 and CH3 domains of the Fc region) are involved in controlling the in vivo half-life of Fc fragments and their transport across the neonatal gut (Kim et al., 1994a,b). Second, we observed that mice deficient in β2m that do not express functional FcRn are characterized by abnormally rapid clearance rates of IgG/Fc fragments (Ghetie et al., 1996, see also Israel et al., 1996; Junghans and Anderson, 1996). Third, FcRn expression is not restricted to the gestational or neonatal periods, but can be detected in multiple tissues/cell types throughout adult life (Ghetie et al., 1996). Fourth, an engineered Fc fragment with higher affinity for FcRn at pH 6, but with retention of very low affinity at near neutral pH, was shown to have increased in vivo persistence in mice (Ghetie et al., 1997).
The ubiquitous nature of FcRn expression leads to the question as to which cell types are most relevant for the regulation of IgG levels in vivo? Distribution studies of IgGs with different binding properties for FcRn indicated that the (micro)vasculature, primarily in skin and muscle with lesser amounts in liver and adipose tissue, contributes to IgG homeostasis (Borvak et al., 1998), consistent with the earlier suggestion that this regulation occurs at diffuse sites throughout the body (Waldmann and Strober, 1969). More recent studies involving bone marrow transfers indicate that FcRn expression in hematopoietic cells such as dendritic cells, monocytes, and macrophages also contributes to the regulation of IgG levels (Akilesh et al., 2007; Qiao et al., 2008). To delineate the role of specific cell types in the maintenance of IgG concentrations in vivo, we have generated a mouse strain in which FcRn can be conditionally deleted (Perez-Montoyo et al., 2009). This strain harbors FcRn alleles (exons 5–7) flanked by loxP sites, and in combination with Tie2-Cre mice which express Cre recombinase under the control of the Tie2 promoter in endothelial and hematopoietic cells (Kisanuki et al., 2001) can be used to analyze the impact of site-specific deletion of FcRn in these cells. Analyses of the clearance rates of IgGs in these mice demonstrate that endothelial and hematopoietic cells are the primary sites responsible for FcRn-mediated homeostasis of IgG (Perez-Montoyo et al., 2009).
Although targeted deletion of human FcRn is clearly not possible, the analysis of archived human blood samples from patients with a deficiency in β2m expression has provided a naturally occurring human knockout for FcRn (Wani et al., 2006). These patients have abnormally low IgG levels. Taken together with correlations between FcRn-binding properties of an IgG and in vivo persistence in nonhuman primates (discussed further in Section 7.2), the available data therefore indicate that FcRn is also a major contributor to IgG homeostasis in humans.
3.2. FcRn-mediated transport of IgG across cellular barriers: Opportunities for drug delivery
In addition to the role of FcRn in transporting maternal IgG across the neonatal intestine (Rodewald and Abrahamson, 1982; Wallace and Rees, 1980), the central function of FcRn in transporting IgG across both the mouse yolk sac and human placenta during gestation has been demonstrated (Firan et al., 2001; Medesan et al., 1996). More recently, it has become apparent that FcRn serves to deliver IgGs across cellular barriers throughout life. Extensive analyses of FcRn-mediated trafficking of IgGs and IgG–antigen complexes across epithelial cells in cell lines and in adult mice/nonhuman primates provide insight into these transport processes (Bitonti and Dumont, 2006; Bitonti et al., 2004; Dickinson et al., 1999; Haymann et al., 2000; Kobayashi et al., 2002; Sakagami et al., 2006; Spiekermann et al., 2002; Yoshida et al., 2004). For example, in transgenic mice that are engineered to express mouse FcRn in adult intestinal epithelium, FcRn can transport antigen bound to IgG from the intestinal lumen into the lamina propria to elicit CD4+ T cell responses against bacteria (Yoshida et al., 2006). Thus, FcRn can function as a scavenger of luminal antigens in the gut, indicating that it can play an important role in mucosal immunity.
Trans-epithelial transfer offers opportunities for the delivery of therapeutic proteins, and consistent with this, erythropoeitin–Fc fusions can be transferred in an FcRn-dependent mode across the lung epithelium of adult mice and nonhuman primates (Bitonti et al., 2004; Spiekermann et al., 2002). Interestingly, a “monomeric” Epo–Fc fusion comprising a single Epo molecule connected to one arm of the dimeric Fc molecule was transported more efficiently than an Epo–Fc dimer containing two Epo molecules per Fc (Bitonti et al., 2004). This enhanced transport was shown to be due in part to an increased affinity for binding of the monomer to FcRn, but in addition, size reduction and/or a change in charge might be contributing properties (Bitonti and Dumont, 2006). Surfaces such as lung epithelium that are bathed in mucus may be particularly susceptible to such effects, indicating that it will be advantageous to design molecules with optimized properties such as charge, size, and minimization of steric hindrance on FcRn binding for a given delivery route. For lung delivery, high potency of the biologic is also important since the volume of the vehicle is, by necessity, relatively low (Wang et al., 2008). Although transport across the intestine avoids this potential limitation, a major challenge is to generate recombinant proteins that are resistant to the hostile proteolytic and acidic environment of this locale. The targeting of FcRn with Fc-fusion proteins to deliver therapeutics in utero is also attractive and promise for this approach in a mouse model of the lysosomal storage disease, mucopolysaccharidosis, has been demonstrated (Grubb et al., 2008). Collectively, these studies indicate that the use of FcRn as a drug delivery vehicle has multiple possible applications.
3.3. FcRn can deliver antigen for presentation
The earlier observation that FcRn is expressed in monocyte/macrophages and dendritic cells led to the suggestion that this receptor might play a role in antigen presentation (Zhu et al., 2001). More recent analyses have shown that FcRn can direct immune complexes (ICs) into lysosomes in dendritic cells, which in turn can enhance antigen presentation (Qiao et al., 2008). Although FcRn was originally shown not to be expressed in B cell lines and primary B cells (Akilesh et al., 2007; Ghetie et al., 1996; Zhu et al., 2001), this receptor is present in splenic B cells in mice (Mi et al., 2008; Perez-Montoyo et al., 2009). This extends the expression of FcRn to all major subsets of professional antigen presenting cells (APCs). Taken together with the report that invariant chain, for which the expression is generally restricted to APCs, can associate with FcRn and direct it into lysosomes (Ye et al., 2008), this suggests that the intracellular trafficking pathways in these cells can be modulated to optimize antigen presentation (discussed further in Section 5.7). Interestingly, phagocytosis of opsonized bacteria by human neutrophils is also increased by FcRn expression, leading to the suggestion that the nascent phagocytic cup is acidified to facilitate FcRn–IgG interactions during uptake (Vidarsson et al., 2006). This might provide an explanation for the higher phagocytic activity of monocytes relative to NK cells that do not express FcRn. Taken together, the data therefore indicate that FcRn cannot only enhance phagocytic uptake, but can also redirect antigen complexed with antibodies into degradative compartments that are associated with the loading of antigenic peptides onto MHC Class II molecules within cells.
3.4. Possible functions of FcRn in specialized cell types
FcRn expression in highly specialized cells such as podocytes in the kidney plays an important role in removing IgG from the glomerular basement membrane (Akilesh et al., 2008). Indeed, blocking of FcRn in mice leads to serum-induced nephritis, suggesting that impaired function of this clearance process could predispose toward glomerular disease. This raises questions concerning whether FcRn (dys)function might contribute to the pathology of diseases such as systemic lupus erythematosus, in which IC-mediated kidney damage is common.
FcRn expression has also been demonstrated in multiple ocular tissues, including the cornea, retina, conjunctiva, and the blood–ocular barrier (Kim et al., 2008). The function of FcRn at these sites is currently unknown, but may be related to the immune-privileged status of the eye. Similarly, FcRn is expressed at the blood–brain barrier (BBB) in both the brain microvasculature and the choroid plexus epithelium (Schlachetzki et al., 2002) where it might be important for maintaining low levels of potentially inflammatory antibodies in the CNS. Consistent with this, several studies demonstrate that IgG is transported by FcRn in the brain-to-blood direction (Deane et al., 2005; Zhang and Pardridge, 2001). This directional transport has specific relevance to the clearance of amyloid β peptide (Aβ) from the brain by Aβ-specific IgG, which results in a reduction of symptoms of Alzheimer's disease in a mouse model (Deane et al., 2005). Such studies indicate that it could be fruitful to explore this pathway further for Aβ-directed immunotherapy. However, others have reported that the brain-to-blood exposure ratios for IgG are the same in both wild-type and FcRn-deficient mice (Wang et al., 2008), indicating that the role of FcRn at this barrier requires further investigation.
4. The Molecular Nature of FcRn–IgG Interactions
4.1. The interaction site for FcRn on IgG
The molecular details of FcRn–IgG interactions have been extensively analyzed. For example, site-directed mutagenesis of recombinant IgG or Fc fragments has been used to identify residues that are involved in the mouse FcRn–IgG interaction for both human and mouse IgG1 (Kim et al., 1994b, 1999; Medesan et al., 1997). These studies have involved a combination of in vitro binding analyses and in vivo assays in mice, and demonstrate that His310, Ile253, and His435 of IgG play a central role in these interactions (Fig. 4.1). These same residues are involved in the human FcRn–human IgG1 (Firan et al., 2001; Shields et al., 2001) and rat FcRn–IgG (mouse, rat or human) interactions (Martin et al., 2001; Raghavan et al., 1995). Residue 436 (His in mouse IgG1, Tyr in human IgG1) plays a minor role in the binding of IgG to FcRn (Medesan et al., 1997; Shields et al., 2001). The high-resolution X-ray crystallographic structure of rat FcRn complexed with rat IgG2a clearly shows the direct involvement of residues 253, 310, 435, and 436 of IgG in binding (Martin et al., 2001). These four residues are relatively well conserved across species and are located at the CH2–CH3 domain interface of IgG (Deisenhofer, 1981) (Fig. 4.1). The role of the highly conserved His433 of IgG in the interaction across species is more uncertain: in some systems it has been proposed to play a role (Martin et al., 2001; Raghavan et al., 1995; Shields et al., 2001), whereas in others not (Kim et al., 1999; Medesan et al., 1997). Nevertheless, the involvement of several histidines on IgG in complex formation that interact with acidic residues on FcRn provides an explanation for the marked pH dependence of FcRn–IgG binding, with binding at pH 6–6.5 which for most IgGs becomes progressively weaker as pH 7.4 is approached (Popov et al., 1996; Raghavan et al., 1995; Rodewald, 1976; Wallace and Rees, 1980). This pH dependence is essential for FcRn to function as an IgG transporter (see Section 7).
Figure 4.1.
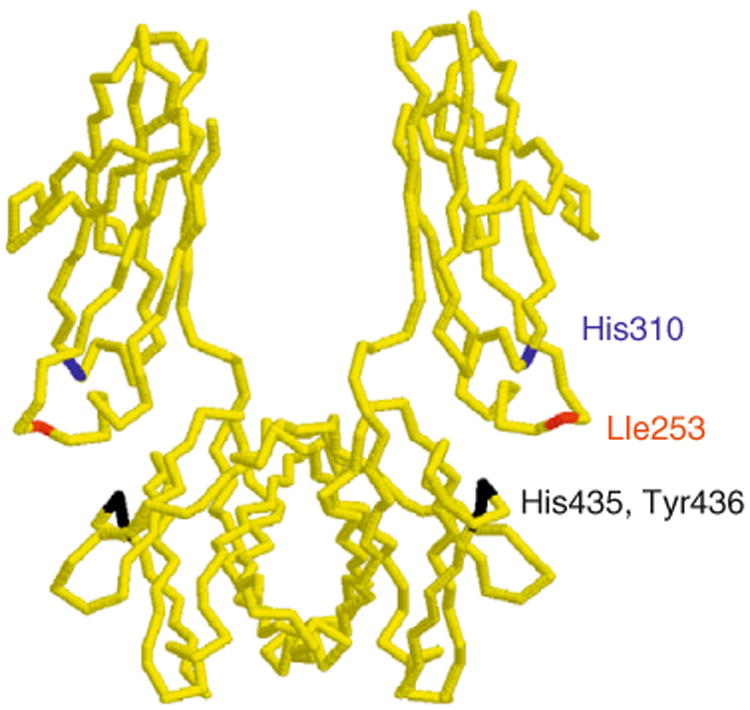
Structure (α-carbon trace) of the Fc region of human IgG1 (Deisenhofer, 1981) with the location of the key residues that are involved in binding to mouse or human FcRn indicated. The same residues of mouse IgG1 are also involved in FcRn binding, except that Tyr436 is replaced by histidine. The structure was drawn using Rasmol (courtesy of Roger Sayle, Bioinformatics Research Institute, University of Edinburgh).
4.2. The interaction site for IgG on FcRn
Structure–function studies in the Bjorkman laboratory have identified FcRn residues that are involved in the rat FcRn–rat IgG2a interaction (Vaughn et al., 1997), and the results of these analyses have been confirmed by the solution of the high-resolution structure of this complex (Martin et al., 2001). To date, structural studies of human FcRn in complex with IgG have not been reported. Although the X-ray crystallographic structure of human FcRn in the absence of ligand indicates that it is structurally similar to rat FcRn, there are also some differences (West and Bjorkman, 2000). Rat FcRn residues that interact with IgG(2a) include Ile1 of β2m and Glu117, Glu118, Glu132, Trp133, Glu135, and Asp137 of the FcRn α-chain (Fig. 4.2). These amino acids are generally well conserved across species, although some notable exceptions exist. For example, Asp or Glu137 of rodent FcRn is replaced by leucine in human FcRn (Ahouse et al., 1993; Simister and Mostov, 1989; Story et al., 1994) (note that the numbering used throughout this review for human FcRn, which is two residues shorter than mouse/rat FcRn, ignores this two residue deletion and is based on the homology alignment of human and rodent FcRn). This sequence variation accounts, in part at least, for cross-species differences in binding specificity between rodent and human FcRn (Zhou et al., 2003, 2005) (discussed in Section 8).
Figure 4.2.
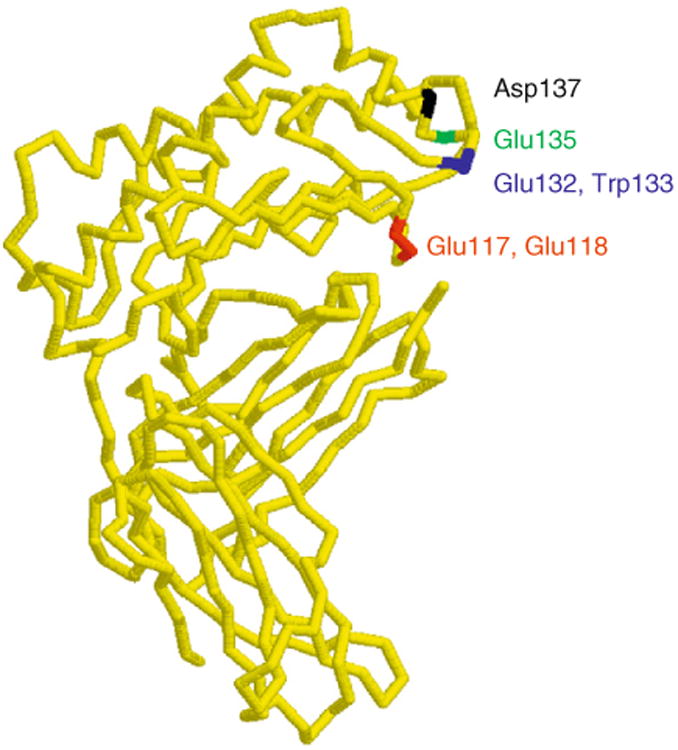
Structure (α-carbon trace) of rat FcRn with the location of the key residues that are involved in binding to rat IgG2a indicated (Martin et al., 2001). The structure was drawn using Rasmol (courtesy of Roger Sayle, Bioinformatics Research Institute, University of Edinburgh).
The ectodomains of FcRn also bear one or more potential glycosylation sites, raising the question as to whether this might contribute to IgG binding. It has been demonstrated that this is the case for rat FcRn, since carbohydrate attached to an N-linked glycosylation site at residue 128 of the receptor makes contacts with Val348, His433, Asn434, and Lys439 of rat IgG2a (Martin et al., 2001). However, the relevance of an analogous interaction for the mouse FcRn–mouse IgG1 or mouse FcRn–human IgG1 complex is made unlikely by our observation that mutation of His433 or Asn434 individually to alanine in IgG1-derived Fc fragments does not affect activity in mouse FcRn-mediated functions (Kim et al., 1999; Medesan et al., 1997). Furthermore, human FcRn functions effectively in binding to IgG without a potential glycosylation site at residue 128, suggesting that there may be differences at this level between rat and human FcRn. It is, however, interesting to note that differences in glycosylation between human and rat FcRn lead to variations in intracellular trafficking (Kuo et al., 2009) and this is discussed further in Section 5.5.
4.3. The stoichiometry of the FcRn–IgG interaction
The presence of two possible binding sites for FcRn on IgG (or Fc) raises questions concerning the stoichiometry of the interaction. By generating a “hybrid” Fc comprising one CH2–CH3 polypeptide with a defective FcRn interaction site complexed with a wild-type CH2–CH3 polypeptide, two functional sites per Fc (mouse IgG1- or rat IgG2a-derived) have been shown to be essential for full activity in vivo in mice (Kim et al., 1994b,c) and in vitro transport across rat FcRn-transfected epithelial cells (Tesar et al., 2006). On the other hand, interaction analyses with soluble, recombinant FcRn demonstrated that the stoichiometry can be 2 FcRn:1 IgG or 1:1 (Martin and Bjorkman, 1999; Popov et al., 1996; Sanchez et al., 1999; Schuck et al., 1999). This apparent discrepancy can be resolved by the demonstration that two possible binding sites on IgG (or Fc) are not equivalent (Sanchez et al., 1999; Schuck et al., 1999; Weng et al., 1998), consistent with the concept that binding of FcRn to one site may reduce the affinity for the second site, that is, negative cooperativity (Ghetie and Ward, 1997). Whether this asymmetry is due to steric effects and/or some longer range conformational changes at the CH2–CH3 domain junction is currently unknown. However, the segmental flexibility of the IgG molecule (Nezlin, 1990; Oi et al., 1978), together with the observation that a hinge-less Fc has lower activity in FcRn-mediated functions (Kim et al., 1995), would be consistent with conformational alterations.
5. The Intracellular Trafficking of FcRn
5.1. A model for FcRn trafficking
The pH dependence of FcRn interactions with the majority of naturally occurring IgGs is central to its function (Popov et al., 1996; Raghavan et al., 1995; Rodewald and Kraehenbuhl, 1984; Simister and Rees, 1985; Zhou et al., 2005). Earlier models for how FcRn traffics within cells suggested that in most cell types, IgG is taken up primarily by fluid-phase processes (Brambell et al., 1964; Ghetie and Ward, 1997), since the pH at most cell surfaces is not favorable for binding. However, it is possible that for cells such as those of epithelial origin, for which Na+/H+ exchanger activity results in acidification of the local environment (Hattori et al., 2001), or in the acidic environments of tumors or inflammatory sites (Edlow and Sheldon, 1971; Gerweck and Seetharaman, 1996; Tannock and Rotin, 1989; Ward and Steigbigel, 1978), significant levels of uptake by receptor-mediated processes can also occur. Whatever the route of uptake, entry of IgG into cells is followed by accumulation in early endosomes for which the acidic pH is permissive for binding (Fig. 4.3). If binding of the IgG to FcRn occurs, then the IgG is recycled or transcytosed (Ober et al., 2004b). By contrast, IgGs that do not bind to FcRn enter late endosomes and are subsequently delivered to lysosomes (Ober et al., 2004b). The predictions of this model are consistent with experimental observations: first, IgGs that have reduced affinity for binding to FcRn have shorter in vivo half-lives and are transported across cellular barriers less effectively (Firan et al., 2001; Kim et al., 1999; Medesan et al., 1997; Spiekermann et al., 2002). Second, reduced expression of FcRn within cells results in increased degradation of IgG (Ghetie et al., 1996; Israel et al., 1996; Junghans and Anderson, 1996; Roopenian et al., 2003). Third, engineered IgGs that bind to FcRn with increased affinity at near neutral pH are taken into cells by receptor-mediated uptake and not released efficiently at the cell surface following recycling or transcytosis (Vaccaro et al., 2005, 2006).
Figure 4.3.
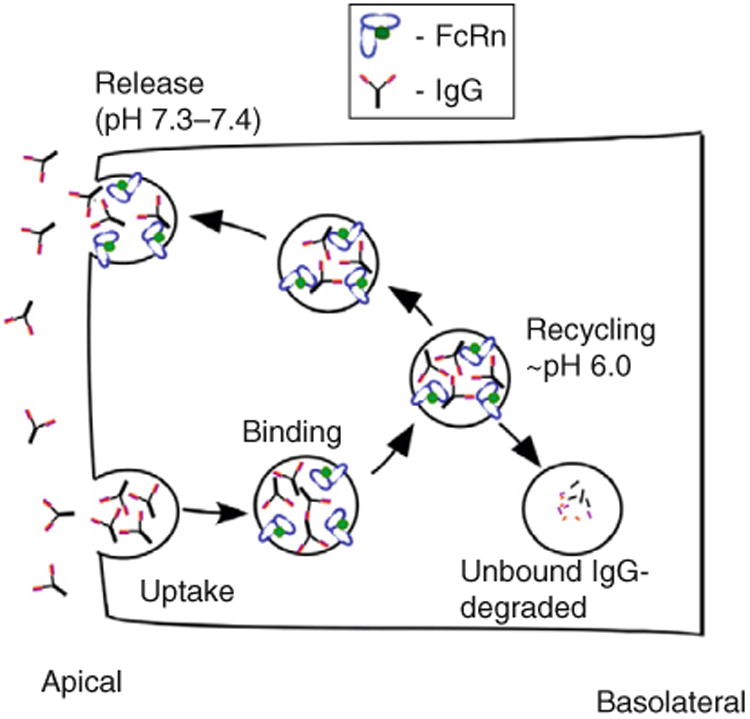
Schematic representation of FcRn-mediated recycling of IgG in a polarized cell such as an endothelial cell. IgGs are taken into the cell by fluid phase and enter early endosomes. The pH of the early endosome is permissive for FcRn binding, and binding of the IgG to FcRn results in recycling (or transcytosis, not shown) and salvage from lysosomal degradation. Conversely, unbound IgG enters the lysosome and is degraded.
5.2. Endosomal sorting of IgGs within endothelial cells
Live-cell-fluorescence imaging has been used to analyze several facets of FcRn-mediated trafficking of IgGs in human endothelial cells (Ober et al., 2004a,b; Prabhat et al., 2007; Ram et al., 2008; Gan et al., 2009). For example, the intracellular trafficking of fluorescently labeled IgGs that have different binding properties for FcRn have been compared to address the question as to where and how IgGs are sorted within cells. These IgGs include wild-type human IgG1 and a mutated derivative (H435A, His435 to Ala) that does not bind detectably to human FcRn (Firan et al., 2001; Ober et al., 2004b). Treatment of human FcRn–GFP-transfected endothelial cells with these two IgGs in fluorescently labeled form, followed by live-cell imaging, has led to a dynamic picture as to how IgGs with distinct binding properties for FcRn are sorted within cells. The wild-type IgG1 leaves sorting endosomes in FcRn-positive tubules and vesicles that are also involved in transferrin recycling (Ober et al., 2004b) (Fig. 4.4). Recently, tubulovesicular, FcRn+ transport containers (TCs) have been visualized using electron tomography of rat jejunal sections by Bjorkman and colleagues (He et al., 2008) that are most likely analogous to the TCs observed in transfected endothelial cells. By contrast with wild-type IgG1, in endothelial cells the H435A mutant persists in the “vacuole” of sorting endosomes while FcRn-positive tubules and vesicles segregate from these compartments to enter the recycling/transcytotic pathway (Ober et al., 2004b). Ultimately, the H435A mutant can be detected in the lysosomes of these cells, through delivery processes that most likely involve different types of fusion events of late endosomes and lysosomes (Gan et al., 2009; Luzio et al., 2003). Thus, the intracellular trafficking behavior of the two IgGs correlates with their in vivo properties: the IgG1 molecule has a long persistence and is transported across cellular barriers, whereas the half-life of the H435A mutant is short and its delivery across cells is at background levels (Firan et al., 2001; Kim et al., 1999).
Figure 4.4.
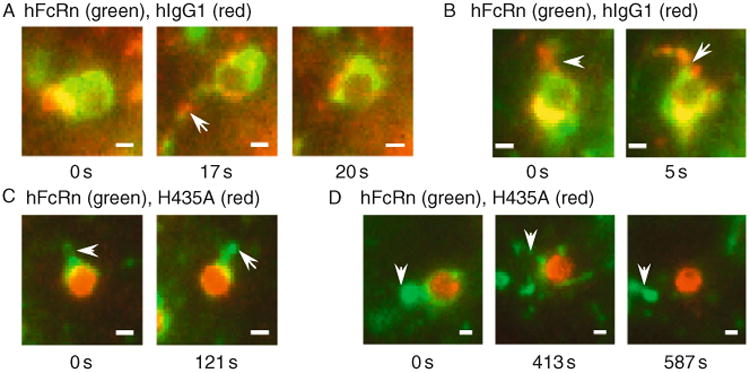
Individual frames from live-cell imaging of sorting endosomes in human FcRn (hFcRn)–GFP-transfected endothelial (HMEC-1) cells pulse-chased with (A, B) Alexa 546-labeled human IgG1 (hIgG1) or (C, D) Alexa 546-labeled H435A (His435 to Ala) mutant that binds with immeasurably low affinity to human FcRn (Firan et al., 2001). Cells were pulsed with labeled IgG and subsequently chased in medium at 37 °C. Images of live cells were acquired and processed as described in Ober et al. (2004b). Arrowheads indicate tubules (FcRn+IgG+ for A, B, FcRn+ only for C, D) and in (A) the tubule separates from the endosome at ∼ 20 s. The first frame for each dataset is arbitrarily labeled 0 s, although the frames shown were taken at different times after the start of the chase period. Bar = 1 μm.
5.3. Exocytic processes that result in IgG release from endothelial cells
The question as to how IgG molecules are released from cells during exocytosis has also been addressed using total internal fluorescence microscopy (TIRFM) combined with single molecule imaging in live cells (Ober et al., 2004a). It is interesting to note that in most cell types the steady state, cell-surface expression levels of FcRn are low (Antohe et al., 2001; Dickinson et al., 1999; Ghetie et al., 1996; Kristoffersen and Matre, 1996; Ober et al., 2004b; Roberts et al., 1990). This raises the question as to whether FcRn “cohorts” bound IgG to the cell surface during exocytosis or whether bifurcation of FcRn and ligand occurs prior to fusion of exocytic compartments with the plasma membrane. Our live-cell imaging data demonstrated that FcRn is delivered to the plasma membrane during exocytic events (Ober et al., 2004a). Using electron tomographic analyses, clathrin has been shown to be associated with both exo- and endocytic processes involving FcRn (He et al., 2008). This association provides a molecular mechanism by which FcRn can be rapidly retrieved following exocytic fusion, which in turn results in low steady-state levels on the plasma membrane.
In addition to the classic type of full fusion exocytic event, using TIRFM, we observed processes in which IgG was released at the plasma membrane of endothelial cells over relatively long time periods (up to several minutes) in bursts of release, in a process that we named prolonged release (Ober et al., 2004a). Multiple other types of exocytic events were also visualized, suggesting that exocytosis can occur via different processes that fall on a continuum ranging from full fusion to prolonged release. The molecular components that determine the type of exocytic event are currently unknown, but most likely relate to the local concentrations of fusion and fission effectors at the exocytic sites. Importantly, different types of exocytic processes can be observed for an individual cell (Ober et al., 2004a), indicating that these events are not predetermined by the physiological state of the cell. The biological significance of these exocytic pathways remains to be determined, and will need to be preceded by an analysis of the molecular components that regulate, for example, prolonged release versus full fusion.
The implementation of single molecule analyses of FcRn and IgG during exocytosis in endothelial cells has also allowed the behavior of individual IgG and FcRn molecules, rather than bulk populations, to be studied (Ober et al., 2004a). This led to the observation of retrograde movement of IgG and FcRn molecules back to the exocytic site following exocytosis, generating insight into the molecular nature of these processes.
5.4. Imaging FcRn trafficking in three dimensions using multifocal plane microscopy
The observation of multiple different types of exocytic events using TIRFM at the plasma membrane (Ober et al., 2004a) leads to the question as to which intracellular trafficking processes precede different types of release mechanisms? To address this and other questions, we have developed a multifocal plane microscopy (“MUM”) set up that allows simultaneous visualization of multiple planes within the cell combined with TIRFM imaging at the plasma membrane (Prabhat et al., 2004, 2007). This approach has to date given insight into the intracellular events that precede exocytosis: for example, the recycling tubulovesicular TCs that leave sorting endosomes can be categorized into pathways of direct and indirect recycling processes. In the most direct type of recycling, tubules extend from sorting endosomes and undergo exocytosis while remaining connected (Prabhat et al., 2007). By contrast, for less direct pathways, TCs accumulate in “holding zones” in proximity to the plasma membrane prior to exocytosis.
More recently, we have also used MUM to visualize endocytic events involving FcRn and its IgG ligand (Ram et al., 2008). In these analyses, we have utilized an engineered IgG–FcRn pair of high affinity to enable receptor-mediated uptake at near neutral pH (Vaccaro et al., 2005; Zhou et al., 2005). Reminiscent of the analyses of exocytic processes, these studies demonstrate that endocytic processes can be broadly categorized into two classes: “direct” in which the endocytic TC moves rapidly toward a sorting endosome and fuses and “indirect” in which more circuitous itineraries are taken within the cell prior to endosomal fusion. Collectively, these studies of endo- and exocytosis have implications for understanding the dynamics of FcRn-mediated trafficking and IgG homeostasis, and may relate to the fast and slow recycling processes that have been described for transferrin and its receptor (Sheff et al., 1999).
5.5. FcRn trafficking in polarized epithelial cells
In addition to studies of endothelial cells, much information concerning the intracellular trafficking of FcRn has been gleaned from analyses of Madin–Darby canine kidney (MDCK) cells transfected with human or rat FcRn (Claypool et al., 2004; Tesar et al., 2006), or with rat-derived inner medullary collecting duct (IMCD) cells transfected with rat FcRn (McCarthy et al., 2000). Using these cells as polarized monolayers, the polarity of subcellular trafficking events such as transcytosis and recycling, together with the molecular mechanisms, have been investigated. IMCD cells express rat β2m, whereas it is essential to cotransfect human/ rat β2m into (canine) MDCK cells to analyze the trafficking of human/rat FcRn in this heterologous system (Claypool et al., 2002; Praetor and Hunziker, 2002; Tesar et al., 2006; Zhu et al., 2002). Comparison of the distribution of human and rat FcRn in transfected, polarized cells has shown that the distribution of the human receptor is strongly polarized toward the basolateral surface, whereas this bias is reversed for rat FcRn (Claypool et al., 2004; McCarthy et al., 2000). The distribution of human FcRn is also biased toward the basolateral surface of untransfected Caco-2 and T84 cells (both intestinal epithelial cells), indicating that its basolateral bias in MDCK cells is not due to overexpression and/or transfection (Claypool et al., 2004). Indeed, the cross-species difference in basolateral bias has recently been demonstrated to be due to the presence of four potential glycosylation sites in the ectodomains of rat (mouse) FcRn, whereas human FcRn has only one such site (Kuo et al., 2009). Engineering of the three additional glycosylation sites of mouse/ rat FcRn into human FcRn results in increased apical localization in transfected MDCK cells (Kuo et al., 2009), consistent with earlier analyses in which carbohydrate was shown to function as an apical targeting signal (Scheiffele et al., 1995). The relative levels of apical and basolateral localization of FcRn impact the directionality of transcytosis. Specifically, although bidirectional transcytosis of FcRn in both transfected IMCD and MDCK cells occurs, for human FcRn more basolateral to apical transcytosis is observed relative to apical to basolateral transport, whereas this is reversed for rat FcRn (Claypool et al., 2004; Kim et al., 2004; McCarthy et al., 2000; Tesar et al., 2006). Consistent with the redistribution of a “rodentized” variant of human FcRn with four potential glycosylation sites to the apical surface, this FcRn mutant shows the directional bias observed for rodent FcRn, that is, preferential transcytosis of IgG in the apical to basolateral direction (Kuo et al., 2009). However, in the human trophoblast cell line, BeWo, greater transcytosis by endogenous FcRn in the apical to basolateral direction occurs (Leitner et al., 2006), suggesting that there may be fundamental differences in the regulation of transcytosis between different cell types. In the case of BeWo cells, this directionality would be consistent with the role of FcRn in delivering maternal IgG across the placenta (Firan et al., 2001; Story et al., 1994).
5.6. Molecular determinants and effectors of FcRn trafficking
The cytosolic tail motifs of rat FcRn that regulate endocytosis and basolateral targeting have been identified by analyses of mutated FcRn variants in transfected IMCD cells (Newton et al., 2005; Wernick et al., 2005; Wu and Simister, 2001). Both tryptophan (W311; with tryptophan replacing the more common tyrosine in the YXXtheta motif) and dileucine (Leu322Leu323) motifs have been shown to play partially redundant roles in endocytosis (Wu and Simister, 2001). Biochemical studies have shown that the tryptophan motif directly interacts with the μ subunit of AP-2 (Wernick et al., 2005). Taken together with the knowledge that dileucine motifs interact with σ and γ subunits of the adaptor protein AP-2, this has led to the suggestion that two subunits of AP-2 can bind simultaneously to the two cytosolic tail motifs (Wernick et al., 2005). Both tryptophan and dileucine motifs also play a role in basolateral targeting of rat FcRn (Newton et al., 2005). The tryptophan and dileucine motifs are conserved across species that range from camels to humans (Fig. 4.5), suggesting that additional differences such as variations in glycosylation patterns (Kuo et al., 2009) account for cross-species variability in trafficking. However, it is interesting to note that some species (e.g., possum, cows, sheep, dromedaries, pigs, and dogs) have cytosolic tails that are 10 residues shorter than those of other species (e.g., humans, macaques, orangutans, rats, and mice) (Fig. 4.5). In addition, the cytosolic tail of possum has a two-residue insertion, whereas rabbit has a five amino acid deletion (Fig. 4.5). Whether these differences are functionally relevant remains to be tested.
Figure 4.5.
Cytosolic tail sequences of FcRn from different species (Adamski et al., 2000; Ahouse et al., 1993; Kacskovics et al., 2000, 2006; Kandil et al., 1995; Mayer et al., 2002; Schnulle and Hurley, 2003; Simister and Mostov, 1989; Story et al., 1994). Identity is indicated by red, and the first residue of the sequence corresponds to residue 301 of mouse/rat FcRn. The consensus sequence is also shown, with the tryptophan (W311) and dileucine (L322, L323) motifs that are important for intracellular trafficking indicated in blue.
Recent studies have identified a motif in the cytosolic tail of human FcRn encompassing Arg301, Arg302 (Fig. 4.5) that binds to calmodulin (Dickinson et al., 2008). Ablation of this interaction by mutagenesis of FcRn results in reduced transcytosis and decreased stability of this receptor (Dickinson et al., 2008). Since calmodulin binding to FcRn would mask a putative amphipathic α-helix that in other proteins can insert into the membrane and induce or sense curvature (Ford et al., 2002; Lee et al., 2005; McMahon and Gallop, 2005), this might provide a mechanism through which calmodulin can affect endosomal sorting. Together with the knowledge that calmodulin function is highly regulatable, this could constitute an important pathway for the control of FcRn trafficking.
In the context of possible regulators of the intracellular pathways taken by FcRn, several studies indicate that Rab proteins play a role (Tzaban et al., 2009; Ward et al., 2005). These small Ras-like GTPases are known to play regulatory functions in endocytic and exocytic trafficking (Miaczynska and Zerial, 2002; Somsel and Wandinger-Ness, 2000). The activity of this class of GTPases is controlled by GTP–GDP exchange cycles, and such proteins exist in either membrane-bound or cytosolic forms. In combination with proteins such as soluble NSF attachment protein receptors (SNAREs) that usually exist as transmembrane receptors (Jahn et al., 2003), Rabs are key regulators of fusion events between different membranous compartments (Grosshans et al., 2006; Miaczynska and Zerial, 2002; Somsel and Wandinger-Ness, 2000). Due to the pivotal role that Rabs play in intracellular trafficking, it is therefore of interest to understand which of these proteins are associated with FcRn.
Using fluorescence imaging, we observed that Rab4(a), Rab5(a), and Rab11(a) are all present on FcRn+ endosomes (Ward et al., 2005). Rab4 and Rab11 are known to be involved in recycling cargo from sorting endosomes to the plasma membrane (Daro et al., 1996; Green et al., 1997; Sönnichsen et al., 2000; Ullrich et al., 1996; van der Sluijs et al., 1992), whereas Rab5 is an early endosomal marker (Christoforidis et al., 1999; Simonsen et al., 1998). Although FcRn can be sorted into tubulovesicular TCs in Rab4+Rab11+ or Rab11+ compartments, only Rab11 but not Rab4 is associated with FcRn during exocytic events at the plasma membrane (Ward et al., 2005). Rab4 depletion from these TCs occurs via the formation of discrete Rab4+ domains that can subsequently separate. The distribution of Rab5, Rab11 and the late endosomal markers Rab7 and Rab 9 (Bucci et al., 2000; Soldati et al., 1995) with tubulovesicular TCs that transport IgG/Fc in the neonatal rodent gut has also been analyzed using electron tomography (He et al., 2008). These studies demonstrate that compartments on the endolysosomal pathway cannot be segregated into groups based on their Rab associations. Rather, there is overlap between the Rabs that are associated with different compartments, consistent with models of “Rab conversion” in which Rabs are gradually lost and replaced by different Rab proteins as endosomes mature (Rink et al., 2005).
Recently, we have analyzed the intracellular trafficking pathways, including Rab GTPases, involved in the constitutive degradation of FcRn in endothelial cells (Gan et al., 2009). Transfer of FcRn from late endosomes to lysosomes occurs via kiss-and-linger-like processes (Bright et al., 2005; Gandhi and Stevens, 2003; Ryan, 2003; Storrie and Desjardins, 1996) that frequently involve tubular extensions, whereas full fusion of late endosomes and lysosomes is rarely observed (Gan et al., 2009). Unexpectedly, in our studies, the “early endosomal” marker Rab5 persists on the limiting membrane of late endosomes until a relatively late stage in maturation. This suggests that (late) endosomes have functional plasticity due to the presence of both Rab5 and Rab7, allowing FcRn to leave these compartments to enter the recycling or lysosomal pathways. Consequently, this prolongs the time window during which FcRn (or other receptors) can be sorted into distinct pathways during endosomal maturation, and might provide a mechanism by which increased fidelity in sorting can be achieved.
Given the potential of FcRn as a drug delivery vehicle, it is of considerable interest to understand the molecular effectors that regulate recycling versus transcytosis in polarized cells. Insight into this has recently been generated by the observation that Rab25, a Rab GTPase that is known to be involved in the transcytosis of IgA by pIgR (Casanova et al., 1999; Wang et al., 2000), also regulates the transcytosis of human FcRn in polarized epithelial (MDCK) cells (Tzaban et al., 2009). By contrast, Rab11a is not involved in transcytosis but is an important player in recycling to the basolateral, but not apical, membrane of polarized MDCK cells (Tzaban et al., 2009). These observations are consistent with the concept that in epithelial cells, at least, there are endosomal compartments that have functional plasticity, insofar as sorting into both transcytotic and recycling pathways can occur from the same common endosome (Casanova et al., 1999; Thompson et al., 2007; Tzaban et al., 2009; Wang et al., 2000). These studies have significant potential for regulating the directionality of FcRn-mediated transport.
5.7. Effects of ligand valency on intracellular trafficking
To date, the majority of studies of the cell biology of FcRn and its IgG ligand have been carried out using monomeric IgG that has two possible interaction sites for FcRn. Indeed, two active binding sites per IgG or Fc molecule have been shown to be important for activity in FcRn-mediated functions that include transcytosis, recycling, and in vivo half-life (Kim et al., 1994b,c; Tesar et al., 2006). It is interesting that a hybrid Fc with only one functional FcRn interaction site is transported more efficiently into lysosomes in rat FcRn-transfected MDCK cells relative to wild-type Fc that has two possible interaction sites (Tesar et al., 2006). Whether this effect is due to a higher off-rate of the Fc from FcRn in endosomes and/or a difference in trafficking induced by FcRn dimerization is currently unknown.
The ligand for FcRn can also be highly multimeric when IgGs form ICs with cognate antigen. It is, therefore, of interest to compare the intracellular trafficking of monomeric IgGs with that of multivalent ICs. It has recently been shown that ICs with the propensity to cross-link FcRn preferentially traffic into lysosomes, thereby enhancing antigen presentation in dendritic cells (Qiao et al., 2008). It remains to be demonstrated whether this trafficking pathway is specific for APCs. This might be the case, since a recent report demonstrated that invariant chain, which is expressed in professional APCs, directs the transport of FcRn into lysosomes (Ye et al., 2008). Such a process results in a pathway for the enhancement of T cell responses by ICs, thereby providing an additional link between humoral and cellular immunity. By contrast, the transport of ICs in intact form across cells such as epithelial barriers (Yoshida et al., 2004) might be enabled by the lack of invariant chain in these cells, at least under steady-state, noninflammatory conditions.
6. Regulation of FcRn Expression
FcRn represents a receptor that is subject to both tissue-specific and developmental regulation. For example, following the suckling period of neonatal rodents, a dramatic decrease in FcRn expression in intestine occurs (Ghetie et al., 1996; Martin et al., 1997). FcRn levels are downregulated by hormones such as corticosteroids and thyroxine that are known to affect gastrointestinal adaptation during the neonatal period (Capano et al., 1994; Martin et al., 1993; Morris and Morris, 1976). The promoter regions for human and rodent FcRn have been analyzed and indicate that the regulation of expression at the transcriptional level is complex with sites for Sp-like transcription factors, AP-1, Ets, or NF-IL6 (Jiang et al., 2004; Kandil et al., 1995; Tiwari and Junghans, 2005). Given the immunological relevance of FcRn, it is plausible that modulation of expression and/or activity by inflammatory (or anti-inflammatory) mediators such as cytokines might occur. In this context, recent studies have shown that the expression levels of human FcRn in in vitro cell lines can be regulated by cytokines such as TNF-α and IFN-γ (Liu et al., 2007b, 2008). Although these cytokines are classically associated with proinflammatory effects, much data supports the concept that they can also be anti-inflammatory (Chu et al., 2000; Cope et al., 1997; Isomaki et al., 2001; Kassiotis and Kollias, 2001; Liu et al., 1998; Willenborg et al., 1999a,b). It is, therefore, interesting that while TNF-α and IL-1β upregulate the transcription of FcRn through NFκB binding to intronic sequences of FcRn (Liu et al., 2007b), IFN-γ has the reverse effect by activating JAK/STAT-1 signaling (Liu et al., 2008). Consequently, the factors that control the expression of FcRn and MHC Class I molecules are distinct, since IFN-γ is known to upregulate the levels of the latter. How FcRn expression and function might be modulated by both anti- and proinflammatory cytokines and possibly other immune mediators such as chemokines has broad implications for understanding the factors that regulate inflammatory responses. This area offers multiple possibilities for further exploration.
7. The Complexity of Engineering FcRn–IgG Interactions
7.1. Antibody engineering: From variable to constant regions
Much of antibody engineering over the past two decades has been directed toward the manipulation of antibody variable regions for both targeting and blocking effects (Souriau and Hudson, 2003; Weiner and Carter, 2005). By contrast, the modification of Fc regions to alter their interactions with Fc receptors, particularly to impact FcRn function, is relatively underdeveloped. Fc engineering has obvious implications for the application of therapeutic antibodies (Carter, 2006; Ghetie et al., 1997), and interest in this area is currently expanding (Dall'Acqua et al., 2006a; Hinton et al., 2004; Lazar et al., 2006; Shields et al., 2001; Vaccaro et al., 2005, 2006). Although of considerable importance, recent studies describing the engineering of Fc regions for the enhancement of FcγR binding (e.g., Lazar et al., 2006; Shields et al., 2001) fall outside the scope of the current review and will not be discussed further. However, it is important to point out that the sites for FcRn and FcγR interactions on IgG are distinct (Duncan et al., 1988; Jefferis et al., 1998; Kim et al., 1994b; Shields et al., 2001), so that in general mutations that impact FcRn binding do not affect function in FcγR-dependent assays and vice versa. In the cases where effects on both functionalities are observed (e.g., Shields et al., 2001), this is most likely due to longer range conformational perturbations.
We will first describe how FcRn–IgG interactions can be modified to generate antibodies with altered pharmacokinetics and transport properties, and subsequently discuss how FcRn itself can be targeted to modulate IgG levels in vivo. The knowledge that albumin is dependent on FcRn for in vivo persistence (Andersen et al., 2006; Chaudhury et al., 2003) can also be exploited by using therapeutic reagents fused to albumin binding peptides or Ig domains with the aim of generating longer lived therapeutics (Dennis et al., 2002; Holt et al., 2008; Nguyen et al., 2006; Stork et al., 2007), but will not be discussed further here.
7.2. Modulating the pharmacokinetic properties of IgG: The importance of pH dependence
The knowledge that FcRn regulates serum IgG levels (Ghetie et al., 1996; Israel et al., 1996; Junghans and Anderson, 1996), together with structure– function studies of FcRn–IgG interactions, presents possibilities for the modulation of the in vivo persistence and/or transcellular transport of (therapeutic) antibodies. The approach of “tuning” antibody half-lives by altering FcRn–IgG interactions has obvious relevance to the successful use of therapeutic and diagnostic antibodies. Mouse IgG1-derived Fc fragments that are engineered and selected to have increased affinity for FcRn at pH 6, but with retention of low affinity at near neutral pH, persist for longer in the circulation of mice (Ghetie et al., 1997). This approach has subsequently been used to generate engineered human IgGs that have longer half-lives in primates (Dall'Acqua et al., 2006b; Hinton et al., 2004, 2006) and are transported more efficiently across the ex vivo human placenta (Vaccaro et al., 2006).
Although several reports describe a correlation between FcRn-binding properties of engineered IgGs and in vivo persistence/transport (Dall'Acqua et al., 2006b; Ghetie et al., 1997; Hinton et al., 2004, 2006; Vaccaro et al., 2006), other studies would appear to contradict this (Datta-Mannan et al., 2007a,b; Gurbaxani and Morrison, 2006; Gurbaxani et al., 2006). This apparently discordant data can be explained in several cases by increased binding of engineered antibodies to FcRn at near neutral pH, which in general occurs as the affinity at pH 6 is improved (Dall'Acqua et al., 2002; Vaccaro et al., 2006). In this context, FcRn–IgG interactions can be distinguished from the majority of other protein– protein interactions by their marked pH dependence. Consequently, there is not a linear relationship between increase in affinity and activity. Gain of significant binding activity at near neutral pH results in reduced release during exocytosis at the plasma membrane and enhanced trafficking of the antibody into lysosomes (Gan et al., 2009). Furthermore, such engineered IgGs accumulate very efficiently in cells since they are taken up by receptor (FcRn)-mediated processes (Mi et al., 2008; Vaccaro et al., 2005, 2006). As the affinity at pH 6 is increased, the concomitant improvement in binding at near neutral pH therefore mitigates the factors such as elevated recycling that lead to longer half-life. The difficulty in separating enhancement in affinities at pH 6 and 7.4 during the engineering of FcRn– IgG interactions therefore limits the increase in in vivo persistence that is achievable (discussed in Vaccaro et al., 2006), and this presents a significant challenge in Fc engineering. It is also important to note that some cases of apparent discrepancies between binding data and in vivo half-lives could be due to the interaction models for FcRn–IgG complexes that are used and/or the introduction of valency effects induced by immobilization of FcRn on the sensor chip during SPR analyses (Datta-Mannan et al., 2007a; Gurbaxani and Morrison, 2006).
In addition to the detrimental effect of gain of binding at near neutral pH on in vivo persistence, shorter lived antibodies can alternatively be generated by engineering IgGs or Fc fragments so that they do not bind detectably to FcRn at any pH (Kim et al., 1994a; Medesan et al., 1997). Such “FcRn-null” antibodies also function poorly in other FcRn-mediated functions such as transport across cellular barriers (Firan et al., 2001; Spiekermann et al., 2002). Although in general not useful in therapeutic settings, FcRn-null antibodies have uses in applications such as tumor imaging where short persistence is desirable to minimize background signal (Kenanova et al., 2005; Olafsen et al., 2006).
7.3. Generation of inhibitors of FcRn function to lower endogenous IgG levels
A prediction of the model shown in Fig. 4.3 is that inhibition of FcRn function will lead to enhanced degradation of IgGs and a reduction in IgG transport. FcRn inhibition can be achieved by injecting relatively large quantities of intravenous immunoglobulin (IVIG) (Akilesh et al., 2004; Hansen and Balthasar, 2002; Jin and Balthasar, 2005). The IgG in these high doses of IVIG competes with endogenous IgG for binding and can reduce pathology in IgG-mediated disease (Akilesh et al., 2004; Hansen and Balthasar, 2002; Jin and Balthasar, 2005; Masson, 1993). IVIG can also be used following the delivery of radiolabeled antitumor antibodies to increase their therapeutic and diagnostic efficacy (Jaggi et al., 2007), resulting in enhancement of whole body clearance of radiolabeled IgG and less nonspecific radiation damage.
In many applications in which IVIG is currently used to enhance the clearance of endogenous IgGs, FcRn blockers that have higher affinity for FcRn relative to endogenous wild-type IgGs could be used at substantially lower doses. For example, anti-FcRn or anti-β2m antibodies that block Fc/IgG binding to FcRn through variable region binding have been shown to be effective in treating ITP and myasthenia gravis, respectively, in rodent models by lowering the levels of pathogenic IgGs (Getman and Balthasar, 2005; Liu et al., 2007a). We have also generated engineered IgGs (“MST-HN” and “HN”) derived from human IgG1 that bind through their Fc regions to FcRn with increased affinity (∼ 200-fold at pH 6 relative to mouse IgG1) and reduced pH dependence (Vaccaro et al., 2005, 2006). These engineered IgGs act as competitive inhibitors with wild-type IgGs for FcRn binding and can enhance the clearance of endogenous IgGs in mice (Vaccaro et al., 2005, 2006) (Fig. 4.6). Such engineered antibodies (Abdegs, for antibodies that enhance IgG degradation) have potential uses in modulating endogenous IgG levels. In support of this, a human IgG1 variant (Thr307 to Ala/Glu380 to Ala/Asn434 to Ala) with increased affinity for mouse/human FcRn at both pH 6 and 7.4 has been shown to be effective in treating disease in a serum transfer model of arthritis (Petkova et al., 2006). However, the relatively high doses needed in this study were most likely due to retention of significant pH dependence for binding to FcRn of this antibody, that is, low affinity at near neutral pH (Petkova et al., 2006), which results in poor competitive activity (Vaccaro et al., 2006).
Figure 4.6.
Enhancement of clearance of injected wild-type IgG by an Abdeg (Vaccaro et al., 2005). Mice were injected with 125I-labeled wild-type human IgG1, and injected with 500 μg wild-type human IgG1, 200 or 500 μg Abdeg (MST-HN mutant) 72 h later (indicated by arrow). Levels of remaining 125I labeled IgG were determined at the indicated times. Error bars indicate standard deviations. * Indicates that data for these time points for mice treated with 500 or 200 μg Abdeg are significantly different.
The effects of Abdegs on endogenous IgG levels can be regarded to be “extrinsic,” in contrast to “intrinsic” effects that impact the half-life of the engineered IgG itself. In this context, due to the loss of pH-dependent binding to FcRn, both Abdegs and anti-FcRn antibodies (that bind to FcRn through their V regions) have short in vivo half-lives (Dall'Acqua et al., 2002; Getman and Balthasar, 2005; Vaccaro et al., 2006). Consistent with this, delivery of Abdegs results in a reduction of serum IgG levels that lasts for several days prior to a rebound of IgG concentrations to their original levels (Vaccaro et al., 2005). The “intrinsic” consequences of an Fc mutation on the in vivo half-life of an IgG or Fc fragment itself will impact the “extrinsic” effects of this engineered IgG/Fc on the lowering of endogenous IgG levels, since they will determine the in vivo longevity of a potential Abdeg.
Insight at the quantitative level as to how a change in pH dependence of an IgG–FcRn interaction impacts in vivo persistence has been obtained by comparing the properties of two engineered human IgG1 molecules, HN and MST-HN (HN, His433 to Lys/Asn434 to Phe; MST-HN, Met252 to Tyr/Ser254 to Thr/Thr256 to Glu/His433 to Lys/Asn434 to Phe) (our unpublished data). These two mutants have similar affinities for mouse FcRn at pH 6, whereas the affinity of the HN mutant is about 10-fold lower at pH 7.2 (Table 4.1). This has allowed the impact of differences in pH dependence on intrinsic (in vivo half-life) and extrinsic (lowering of endogenous IgG levels) properties to be assessed in mice. The HN mutant is less effective in lowering endogenous IgG levels than MST-HN, but the HN mutant has a longer in vivo persistence (Vaccaro et al., 2005, 2006) (Table 4.1). Thus, there is a trade-off between activity as an FcRn inhibitor and in vivo half-life. This indicates that, dependent on the situation, these extrinsic and intrinsic properties need to be counterbalanced to optimize the effect. For example, if a “one-off” rapid clearance of endogenous IgG is needed, then an Abdeg with high affinities for FcRn in the range pH 6–7.4 is expected to be optimal. Conversely, if treatment of an IgG-mediated, chronic disease is required, then a balance between reduced half-life and inhibitory activity needs to be achieved.
Table 4.1.
The impact of pH dependence on in vivo half-life in mice of engineered variants of human IgG1
| Dissociation constant, KD (nM)a | |||
|---|---|---|---|
|
| |||
| Human IgG1 (mutant) | pH 6 | pH 7.2 | β-Phase half-life (h) |
| Wild type | 32 | N.D.b | 250.6±15.3a |
| MST-HN | 1.2 | 7.4 | 35.6±1.1 |
| HN | 1.5 | 82 | 62.8±2.7a |
Described previously in Vaccaro et al. (2005, 2006).
N.D., not determined because affinity is too low to accurately estimate a dissociation constant.
Synthetic peptides that block the binding of endogenous IgGs to FcRn in nonhuman primates have also been described (Mezo et al., 2008). One such peptide has been used to make a dimer that is active in reducing serum IgG levels in cynomolgus monkeys (Mezo et al., 2008). The antibody levels rebound in peptide-treated monkeys, consistent with the clearance of the peptide. FcRn-binding peptides, or engineered IgGs/Fc fragments with increased affinity for FcRn, have multiple potential uses and offer an alternative to the use of IVIG which needs to be delivered in relatively high doses for efficacy. In addition, the sources of IVIG are limited and its expense is high. However, in addition to FcRn blockade, IVIG has multiple other possible modes of action that include FcγR-mediated effects (discussed in Clynes, 2007). For example, in mice the monomeric IgG component of IVIG can induce the upregulation of FcγRIIB expression (Bruhns et al., 2003; Samuelsson et al., 2001; Siragam et al., 2005). IVIG treatment can also result in signaling by ICs through the activating receptor, FcγRIII, to inhibit IFN-γ responses or regulate dendritic cell activity in mice (Park-Min et al., 2007; Siragam et al., 2006). Recent studies have shown that monomeric IgGs with sialylated core oligosaccharides, that constitute about 1–2% of IVIG, are responsible for the upregulation of FcγRIIB expression through a mechanism that involves binding to SIGN-R1 (in mice) or DC-SIGN (in humans) (Anthony et al., 2008; Kaneko et al., 2006). For the treatment of IgG-mediated, inflammatory diseases, it is therefore possible that due to the induction of additional anti-inflammatory effects, the use of (engineered) antibodies might be preferable over the use of FcRn-binding peptides that solely target FcRn.
8. Cross-Species Differences in FcRn-Binding Specificity and Implications for Preclinical Models
Despite the similarities of human and mouse FcRn at the sequence level (Ahouse et al., 1993; Story et al., 1994), in addition to the conservation of several key interaction residues on IgG across species, the binding specificity of human and mouse FcRn are distinct (Ober et al., 2001). For example, mouse FcRn binds promiscuously to IgGs from multiple species, whereas human FcRn is much more selective. Most notably, although human FcRn interacts with relatively low affinity with mouse IgG2b, it does not bind detectably to mouse IgG1, IgG2a, or rat IgGs. This lack of binding provides a molecular explanation for the short in vivo persistence of (therapeutic) mouse IgGs in humans (Frodin et al., 1990; Saleh et al., 1992).
Using the earlier crystallographic structure of the rat FcRn–rat IgG2a complex (Martin et al., 2001) as a guide, we have used site-directed mutagenesis combined with interaction analyses to transfer the binding properties of mouse FcRn onto human FcRn (Zhou et al., 2003, 2005). With this approach, several regions of sequence variation are responsible for the specificity differences between mouse and human FcRn: first, residues 132–147, encompassing the nonconserved residue 137 (Leu in human FcRn, Glu in mouse FcRn, Asp in rat FcRn) play a central role (Zhou et al., 2003, 2005). The important role of residue 137, in particular, is consistent with crystallographic and structure–function studies for rat FcRn (Martin and Bjorkman, 2001; Vaughn et al., 1997). Second, residues 79–89 (which in human FcRn encompass a two-residue deletion) have a lesser contribution to the difference in specificity and may modulate the overall orientation of the interaction (Zhou et al., 2005). Residues 79–89 also contain a potential glycosylation site in rodent, but not human, FcRn, leading to the possibility that this might contribute to the cross-species difference in binding properties. However, recent studies (Kuo et al., 2009) have shown that glycosylation at position 87 (numbering based on homology alignment with rodent FcRn) of human FcRn, by mutation of Lys to Asn, does not confer the binding properties of mouse/rat FcRn for mouse IgG1 on the human ortholog (Kuo et al., 2009). It is interesting to note that species such as pigs, sheep, camels, and cows have arginine at position 137 (Kacskovics et al., 2000, 2006; Mayer et al., 2002; Schnulle and Hurley, 2003), whereas dog, rat, and mouse have glutamic/aspartic acid (Ahouse et al., 1993; Kacskovics et al., 2006; Simister and Mostov, 1989) and possum has the same residue as humans (leucine) (Adamski et al., 2000). Given the central role of residue 137 in FcRn–IgG interactions, this leads to the speculation that binding specificities might fall into three or more clades.
In general, the affinities of mouse FcRn for IgGs of multiple different species such as human, rat, mouse, and rabbit are higher than the corresponding human FcRn interactions (Ober et al., 2001). This is of relevance when considering the preclinical analysis of human IgGs in murine models since, for example, the affinity of mouse FcRn for human IgG1 is about 10-fold higher than that of the corresponding human FcRn interaction (Zhou et al., 2005). Consequently, although the mouse FcRn–wild-type human IgG1 interaction retains sufficient pH dependence for this IgG1 to have a relatively long half-life in mice, this is not the case for multiple variants of human IgG(1) that have been engineered to have higher affinity for FcRn (Dall'Acqua et al., 2002; Vaccaro et al., 2006). Specifically, a higher affinity IgG mutant can acquire significant binding to mouse FcRn at near neutral pH while retaining the necessary low affinity for human FcRn to allow efficient recycling in human systems (Vaccaro et al., 2006). Consequently, such IgGs have shortened in vivo half-lives and inhibit FcRn function in mice (Dall'Acqua et al., 2002; Vaccaro et al., 2005), whereas analyses in nonhuman primates (Dall'Acqua et al., 2006b) or the human placental transfer model (Vaccaro et al., 2006) are predictive of longer half-lives in humans. Several engineered IgGs of this class have to date been described (Dall'Acqua et al., 2002, 2006b; Datta-Mannan et al., 2007a,b; Vaccaro et al., 2006), indicating the severe limitations of mice as models. Consequently, there is a need for improved preclinical models that can recapitulate human FcRn function. Although nonhuman primates represent good models, their high cost makes them inaccessible for routine screens. Alternatively, mice that transgenically express human FcRn (Chaudhury et al., 2003; Petkova et al., 2006) are a step toward a suitable preclinical model, but have low endogenous IgG levels due to poor binding of mouse IgGs to human FcRn. Such mice combined with transgenic mice expressing human IgGs (Jakobovits et al., 2007; Scott, 2007) might therefore provide an attractive model.
9. Concluding Remarks
Much has been learnt about FcRn function during the past two decades. Perhaps most importantly, a diverse array of activities at different body sites can be attributed to this multitasking receptor. Furthermore, FcRn impacts both the humoral and cellular arms of the immune response. Consequently, understanding the molecular and cellular mechanisms by which this receptor functions, combined with the engineering of FcRn–IgG interactions, has relevance to fundamental aspects of the immune system in addition to providing possible therapeutic routes for multiple diseases.
Acknowledgments
We thank our many colleagues and coworkers who have contributed to our FcRn-related studies.
The research of the authors reported herein was supported in part by grants from the NIH to E.S.W. (RO1 AI039167, RO1 AI055556, and RO1 AR056478) and R.J.O. (RO1 GM071048 and RO1 GM085575).
References
- Adamski FM, King AT, Demmer J. Expression of the Fc receptor in the mammary gland during lactation in the marsupial Trichosurus vulpecula (brushtail possum) Mol Immunol. 2000;37:435–444. doi: 10.1016/s0161-5890(00)00065-1. [DOI] [PubMed] [Google Scholar]
- Ahouse JJ, Hagerman CL, Mittal P, Gilbert DJ, Copeland NG, Jenkins NA, Simister NE. Mouse MHC class I-like Fc receptor encoded outside the MHC. J Immunol. 1993;151:6076–6088. [PubMed] [Google Scholar]
- Akilesh S, Petkova S, Sproule TJ, Shaffer DJ, Christianson GJ, Roopenian D. The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J Clin Invest. 2004;113:1328–1333. doi: 10.1172/JCI18838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179:4580–4588. doi: 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]
- Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci USA. 2008;105:967–972. doi: 10.1073/pnas.0711515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JT, Dee QJ, Sandlie I. The conserved histidine 166 residue of the human neonatal Fc receptor heavy chain is critical for the pH-dependent binding to albumin. Eur J Immunol. 2006;36:3044–3051. doi: 10.1002/eji.200636556. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antohe F, Radulescu L, Gafencu A, Ghetie V, Simionescu M. Expression of functionally active FcRn and the differentiated bidirectional transport of IgG in human placental endothelial cells. Hum Immunol. 2001;62:93–105. doi: 10.1016/s0198-8859(00)00244-5. [DOI] [PubMed] [Google Scholar]
- Bitonti AJ, Dumont JA. Pulmonary administration of therapeutic proteins using an immunoglobulin transport pathway. Adv Drug Deliv Rev. 2006;58:1106–1118. doi: 10.1016/j.addr.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Bitonti AJ, Dumont JA, Low SC, Peters RT, Kropp KE, Palombella VJ, Stattel JM, Lu Y, Tan CA, Song JJ, Garcia AM, Simister NE, et al. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc Natl Acad Sci USA. 2004;101:9763–9768. doi: 10.1073/pnas.0403235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, Ghetie V, Ward ES. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol. 1998;10:1289–1298. doi: 10.1093/intimm/10.9.1289. [DOI] [PubMed] [Google Scholar]
- Brambell FWR. The Transmission of Passive Immunity from Mother to Young. North Holland Publishing Corp.; Amsterdam: 1970. [Google Scholar]
- Brambell FWR, Hemmings WA, Morris IG. A Theoretical Model of g-globulin catabolism. Nature. 1964;203:1352–1355. doi: 10.1038/2031352a0. [DOI] [PubMed] [Google Scholar]
- Bright NA, Gratian MJ, Luzio JP. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr Biol. 2005;15:360–365. doi: 10.1016/j.cub.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18:573–581. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: A key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capano G, Bloch KJ, Schiffrin EJ, Dascoli JA, Israel EJ, Harmatz PR. Influence of the polyamine, spermidine, on intestinal maturation and dietary antigen uptake in the neonatal rat. J Pediatr Gastroenterol Nutr. 1994;19:34–42. doi: 10.1097/00005176-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin–Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool SM, Dickinson BL, Yoshida M, Lencer WI, Blumberg RS. Functional reconstitution of human FcRn in Madin–Darby canine kidney cells requires co-expressed human beta 2-microglobulin. J Biol Chem. 2002;277:28038–28050. doi: 10.1074/jbc.M202367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool SM, Dickinson BL, Wagner JS, Johansen FE, Venu N, Borawski JA, Lencer WI, Blumberg RS. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fc-γ receptor. Mol Biol Cell. 2004;15:1746–1759. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes R. IVIG therapy: Interfering with interferon-gamma. Immunity. 2007;26:4–6. doi: 10.1016/j.immuni.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Cope AP, Liblau RS, Yang XD, Congia M, Laudanna C, Schreiber RD, Probert L, Kollias G, McDevitt HO. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Acqua W, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. Increasing the affinity of a human IgG1 to the neonatal Fc receptor: Biological consequences. J Immunol. 2002;169:5171–5180. doi: 10.4049/jimmunol.169.9.5171. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua WF, Cook KE, Damschroder MM, Woods RM, Wu H. Modulation of the effector functions of a human IgG1 through engineering of its hinge region. J Immunol. 2006a;177:1129–1138. doi: 10.4049/jimmunol.177.2.1129. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006b;281:23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- Daro E, van der Sluijs P, Galli T, Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci USA. 1996;93:9559–9564. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Jiang W, Wroblewski VJ. Humanized IgG1 variants with differential binding properties to the neonatal Fc receptor: Relationship to pharmacokinetics in mice and primates. Drug Metab Dispos. 2007a;35:86–94. doi: 10.1124/dmd.106.011734. [DOI] [PubMed] [Google Scholar]
- Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Wroblewski VJ. Monoclonal antibody clearance. Impact of modulating the interaction of IgG with the neonatal Fc receptor. J Biol Chem. 2007b;282:1709–1717. doi: 10.1074/jbc.M607161200. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, LaRue B, Guo H, Wu Z, Holtzman DM, Zlokovic BV. IgG-assisted age-dependent clearance of Alzheimer's amyloid beta peptide by the blood–brain barrier neonatal Fc receptor. J Neurosci. 2005;25:11495–11503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-Å resolution. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- Dennis MS, Zhang M, Meng YG, Kadkhodayan M, Kirchhofer D, Combs D, Damico LA. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J Biol Chem. 2002;277:35035–35043. doi: 10.1074/jbc.M205854200. [DOI] [PubMed] [Google Scholar]
- Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, Blumberg RS, Lencer WI. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson BL, Claypool SM, D'Angelo JA, Aiken ML, Venu N, Yen EH, Wagner JS, Borawski JA, Pierce AT, Hershberg R, Blumberg RS, Lencer WI. Ca2+-dependent calmodulin binding to FcRn affects immunoglobulin G transport in the transcytotic pathway. Mol Biol Cell. 2008;19:414–423. doi: 10.1091/mbc.E07-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AR, Woof JM, Partridge LJ, Burton DR, Winter G. Localization of the binding site for the human high-affinity Fc receptor on IgG. Nature. 1988;332:563–564. doi: 10.1038/332563a0. [DOI] [PubMed] [Google Scholar]
- Edlow DW, Sheldon WH. The pH of inflammatory exudates. Proc Soc Exp Biol Med. 1971;137:1328–1332. doi: 10.3181/00379727-137-35782. [DOI] [PubMed] [Google Scholar]
- Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antohe F, Ghetie V, Ward ES. The MHC class I related receptor, FcRn, plays an essential role in the maternofetal transfer of gammaglobulin in humans. Int Immunol. 2001;13:993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- Frodin JE, Lefvert AK, Mellstedt H. Pharmacokinetics of the mouse monoclonal antibody 17–1A in cancer patients receiving various treatment schedules. Cancer Res. 1990;50:4866–4871. [PubMed] [Google Scholar]
- Gan Z, Ram S, Vaccaro C, Ober RJ, Ward ES. Analyses of the recycling receptor, FcRn, in live cells reveal novel pathways for lysosomal delivery. Traffic. 2009;10:600–614. doi: 10.1111/j.1600-0854.2009.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi SP, Stevens CF. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- Getman KE, Balthasar JP. Pharmacokinetic effects of 4C9, an anti-FcRn antibody, in rats: Implications for the use of FcRn inhibitors for the treatment of humoral autoimmune and alloimmune conditions. J Pharm Sci. 2005;94:718–729. doi: 10.1002/jps.20297. [DOI] [PubMed] [Google Scholar]
- Ghetie V, Ward ES. FcRn: The MHC class I-related receptor that is more than an IgG transporter. Immunol Today. 1997;18:592–598. doi: 10.1016/s0167-5699(97)01172-9. [DOI] [PubMed] [Google Scholar]
- Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, Ober RJ, Ward ES. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat Biotechnol. 1997;15:637–640. doi: 10.1038/nbt0797-637. [DOI] [PubMed] [Google Scholar]
- Green EG, Ramm E, Riley NM, Spiro DJ, Goldenring JR, Wessling-Resnick M. Rab11 is associated with transferrin-containing recycling compartments in K562 cells. Biochem Biophys Res Commun. 1997;239:612–616. doi: 10.1006/bbrc.1997.7520. [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb JH, Vogler C, Tan Y, Shah GN, MacRae AF, Sly WS. Infused Fc-tagged beta-glucuronidase crosses the placenta and produces clearance of storage in utero in mucopolysaccharidosis VII mice. Proc Natl Acad Sci USA. 2008;105:8375–8380. doi: 10.1073/pnas.0803715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurbaxani BM, Morrison SL. Development of new models for the analysis of Fc–FcRn interactions. Mol Immunol. 2006;43:1379–1389. doi: 10.1016/j.molimm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Gurbaxani B, de la Cruz LL, Chintalacharuvu K, Morrison SL. Analysis of a family of antibodies with different half-lives in mice fails to find a correlation between affinity for FcRn and serum half-life. Mol Immunol. 2006;43:1462–1473. doi: 10.1016/j.molimm.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Hansen RJ, Balthasar JP. Effects of intravenous immunoglobulin on platelet count and antiplatelet antibody disposition in a rat model of immune thrombocytopenia. Blood. 2002;100:2087–2093. [PubMed] [Google Scholar]
- Hattori R, Otani H, Moriguchi Y, Matsubara H, Yamamura T, Nakao Y, Omiya H, Osako M, Imamura H. NHE and ICAM-1 expression in hypoxic/ reoxygenated coronary microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2001;280:H2796–H2803. doi: 10.1152/ajpheart.2001.280.6.H2796. [DOI] [PubMed] [Google Scholar]
- Haymann JP, Levraud JP, Bouet S, Kappes V, Hagege J, Nguyen G, Xu Y, Rondeau E, Sraer JD. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol. 2000;11:632–639. doi: 10.1681/ASN.V114632. [DOI] [PubMed] [Google Scholar]
- He W, Ladinsky MS, Huey-Tubman KE, Jensen GJ, McIntosh JR, Bjorkman PJ. FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature. 2008;455:542–546. doi: 10.1038/nature07255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton PR, Johlfs MG, Xiong JM, Hanestad K, Ong KC, Bullock C, Keller S, Tang MT, Tso JY, Vasquez M, Tsurushita N. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem. 2004;279:6213–6216. doi: 10.1074/jbc.C300470200. [DOI] [PubMed] [Google Scholar]
- Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176:346–356. doi: 10.4049/jimmunol.176.1.346. [DOI] [PubMed] [Google Scholar]
- Holt LJ, Basran A, Jones K, Chorlton J, Jespers LS, Brewis ND, Tomlinson IM. Anti-serum albumin domain antibodies for extending the half-lives of short lived drugs. Protein Eng Des Sel. 2008;21:283–288. doi: 10.1093/protein/gzm067. [DOI] [PubMed] [Google Scholar]
- Isomaki P, Panesar M, Annenkov A, Clark JM, Foxwell BM, Chernajovsky Y, Cope AP. Prolonged exposure of T cells to TNF down-regulates TCR zeta and expression of the TCR/CD3 complex at the cell surface. J Immunol. 2001;166:5495–5507. doi: 10.4049/jimmunol.166.9.5495. [DOI] [PubMed] [Google Scholar]
- Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack beta 2-microglobulin: Possible protective role of FcRn. Immunology. 1996;89:573–578. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi JS, Carrasquillo JA, Seshan SV, Zanzonico P, Henke E, Nagel A, Schwartz J, Beattie B, Kappel BJ, Chattopadhyay D, Xiao J, Sgouros G, et al. Improved tumor imaging and therapy via i.v. IgG-mediated time-sequential modulation of neonatal Fc receptor. J Clin Invest. 2007;117:2422–2430. doi: 10.1172/JCI32226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Jakobovits A, Amado RG, Yang X, Roskos L, Schwab G. From XenoMouse technology to panitumumab, the first fully human antibody product from transgenic mice. Nat Biotechnol. 2007;25:1134–1143. doi: 10.1038/nbt1337. [DOI] [PubMed] [Google Scholar]
- Jefferis R, Lund J, Pound JD. IgG-Fc-mediated effector functions: Molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunol Rev. 1998;163:59–76. doi: 10.1111/j.1600-065x.1998.tb01188.x. [DOI] [PubMed] [Google Scholar]
- Jiang L, Wang J, Solorzano-Vargas RS, Tsai HV, Gutierrez EM, Ontiveros LO, Kiela PR, Wu SV, Martin MG. Characterization of the rat intestinal Fc receptor (FcRn) promoter: Transcriptional regulation of FcRn gene by the Sp family of transcription factors. Am J Physiol Gastrointest Liver Physiol. 2004;286:G922–G931. doi: 10.1152/ajpgi.00131.2003. [DOI] [PubMed] [Google Scholar]
- Jin F, Balthasar JP. Mechanisms of intravenous immunoglobulin action in immune thrombocytopenic purpura. Hum Immunol. 2005;66:403–410. doi: 10.1016/j.humimm.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacskovics I, Wu Z, Simister NE, Frenyo LV, Hammarström L. Cloning and characterization of the bovine MHC class I-like Fc receptor. J Immunol. 2000;164:1889–1897. doi: 10.4049/jimmunol.164.4.1889. [DOI] [PubMed] [Google Scholar]
- Kacskovics I, Mayer B, Kis Z, Frenyo LV, Zhao Y, Muyldermans S, Hammarström L. Cloning and characterization of the dromedary (Camelus dromedarius) neonatal Fc receptor (drFcRn) Dev Comp Immunol. 2006;30:1203–1215. doi: 10.1016/j.dci.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Kandil E, Noguchi M, Ishibashi T, Kasahara M. Structural and phylogenetic analysis of the MHC class I-like Fc receptor gene. J Immunol. 1995;154:5907–5918. [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Kollias G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: Implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med. 2001;193:427–434. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenanova V, Olafsen T, Crow DM, Sundaresan G, Subbarayan M, Carter NH, Ikle DN, Yazaki PJ, Chatziioannou AF, Gambhir SS, Williams LE, Shively JE, et al. Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments. Cancer Res. 2005;65:622–631. [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Tsen MF, Ghetie V, Ward ES. Identifying amino acid residues that influence plasma clearance of murine IgG1 fragments by site-directed mutagenesis. Eur J Immunol. 1994a;24:542–548. doi: 10.1002/eji.1830240308. [DOI] [PubMed] [Google Scholar]
- Kim JK, Tsen MF, Ghetie V, Ward ES. Localization of the site of the murine IgG1 molecule that is involved in binding to the murine intestinal Fc receptor. Eur J Immunol. 1994b;24:2429–2434. doi: 10.1002/eji.1830241025. [DOI] [PubMed] [Google Scholar]
- Kim JK, Tsen MF, Ghetie V, Ward ES. Catabolism of the murine IgG1 molecule: evidence that both CH2–CH3 domain interfaces are required for persistence of IgG1 in the circulation of mice. Scand J Immunol. 1994c;40:457–465. doi: 10.1111/j.1365-3083.1994.tb03488.x. [DOI] [PubMed] [Google Scholar]
- Kim JK, Tsen MF, Ghetie V, Ward ES. Evidence that the hinge region plays a role in maintaining serum levels of the murine IgG1 molecule. Mol Immunol. 1995;32:467–475. doi: 10.1016/0161-5890(95)00019-b. [DOI] [PubMed] [Google Scholar]
- Kim JK, Firan M, Radu CG, Kim CH, Ghetie V, Ward ES. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur J Immunol. 1999;29:2819–2825. doi: 10.1002/(SICI)1521-4141(199909)29:09<2819::AID-IMMU2819>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Fandy TE, Lee VH, Ann DK, Borok Z, Crandall ED. Net absorption of IgG via FcRn-mediated transcytosis across rat alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2004;287:L616–L622. doi: 10.1152/ajplung.00121.2004. [DOI] [PubMed] [Google Scholar]
- Kim H, Fariss RN, Zhang C, Robinson SB, Thill M, Csaky KG. Mapping of the neonatal Fc receptor in the rodent eye. Invest Ophthalmol Vis Sci. 2008;49:2025–2029. doi: 10.1167/iovs.07-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Suzuki Y, Tsuge T, Okumura K, Ra C, Tomino Y. FcRn-mediated transcytosis of immunoglobulin G in human renal proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2002;282:F358–F365. doi: 10.1152/ajprenal.0164.2001. [DOI] [PubMed] [Google Scholar]
- Kristoffersen EK, Matre R. Co-localization of the neonatal Fc gamma receptor and IgG in human placental term syncytiotrophoblasts. Eur J Immunol. 1996;26:1668–1671. doi: 10.1002/eji.1830260741. [DOI] [PubMed] [Google Scholar]
- Kuo TT, de Muinck EJ, Claypool SM, Yoshida M, Nagaishi T, Aveson VG, Lencer WI, Blumberg RS. N-Glycan moieties in neonatal Fc receptor determine steady-state membrane distribution and directional transport of IgG. J Biol Chem. 2009;284:8292–8300. doi: 10.1074/jbc.M805877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, Vielmetter J, Carmichael DF, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Leitner K, Ellinger I, Grill M, Brabec M, Fuchs R. Efficient apical IgG recycling and apical-to-basolateral transcytosis in polarized BeWo cells overexpressing hFcRn. Placenta. 2006;27:799–811. doi: 10.1016/j.placenta.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Liu J, Marino MW, Wong G, Grail D, Dunn A, Bettadapura J, Slavin AJ, Old L, Bernard CC. TNF is a potent anti-inflammatory cytokine in autoimmunemediated demyelination. Nat Med. 1998;4:78–83. doi: 10.1038/nm0198-078. [DOI] [PubMed] [Google Scholar]
- Liu L, Garcia AM, Santoro H, Zhang Y, McDonnell K, Dumont J, Bitonti A. Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J Immunol. 2007a;178:5390–5398. doi: 10.4049/jimmunol.178.8.5390. [DOI] [PubMed] [Google Scholar]
- Liu X, Ye L, Christianson GJ, Yang JQ, Roopenian DC, Zhu X. NF-kappaB signaling regulates functional expression of the MHC class I-related neonatal Fc receptor for IgG via intronic binding sequences. J Immunol. 2007b;179:2999–3011. doi: 10.4049/jimmunol.179.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ye L, Bai Y, Mojidi H, Simister NE, Zhu X. Activation of the JAK/ STAT-1 signaling pathway by IFN-gamma can down-regulate functional expression of the MHC class I-related neonatal Fc receptor for IgG. J Immunol. 2008;181:449–463. doi: 10.4049/jimmunol.181.1.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Poupon V, Lindsay MR, Mullock BM, Piper RC, Pryor PR. Membrane dynamics and the biogenesis of lysosomes. Mol Membr Biol. 2003;20:141–154. doi: 10.1080/0968768031000089546. [DOI] [PubMed] [Google Scholar]
- Martin WL, Bjorkman PJ. Characterization of the 2:1 complex between the class I MHC-related Fc receptor and its Fc ligand in solution. Biochemistry. 1999;38:12639–12647. doi: 10.1021/bi9913505. [DOI] [PubMed] [Google Scholar]
- Martin MG, Wu SV, Walsh JH. Hormonal control of intestinal Fc receptor gene expression and immunoglobulin transport in suckling rats. J Clin Invest. 1993;91:2844–2849. doi: 10.1172/JCI116528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MG, Wu SV, Walsh JH. Ontogenetic development and distribution of antibody transport and Fc receptor mRNA expression in rat intestine. Dig Dis Sci. 1997;42:1062–1069. doi: 10.1023/a:1018853506830. [DOI] [PubMed] [Google Scholar]
- Martin WL, West APJ, Gan L, Bjorkman PJ. Crystal structure at 2.8 Å of an FcRn/heterodimeric Fc complex: Mechanism of pH dependent binding. Mol Cell. 2001;7:867–877. doi: 10.1016/s1097-2765(01)00230-1. [DOI] [PubMed] [Google Scholar]
- Masson PL. Elimination of infectious antigens and increase of IgG catabolism as possible modes of action of IVIg. J Autoimmun. 1993;6:683–689. doi: 10.1006/jaut.1993.1057. [DOI] [PubMed] [Google Scholar]
- Mayer B, Zolnai A, Frenyo LV, Jancsik V, Szentirmay Z, Hammarstrom L, Kacskovics I. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology. 2002;107:288–296. doi: 10.1046/j.1365-2567.2002.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KM, Yoong Y, Simister NE. Bidirectional transcytosis of IgG by the rat neonatal Fc receptor expressed in a rat kidney cell line: A system to study protein transport across epithelia. J Cell Sci. 2000;113:1277–1285. doi: 10.1242/jcs.113.7.1277. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- Medesan C, Radu C, Kim JK, Ghetie V, Ward ES. Localization of the site of the IgG molecule that regulates maternofetal transmission in mice. Eur J Immunol. 1996;26:2533–2536. doi: 10.1002/eji.1830261038. [DOI] [PubMed] [Google Scholar]
- Medesan C, Matesoi D, Radu C, Ghetie V, Ward ES. Delineation of the amino acid residues involved in transcytosis and catabolism of mouse IgG1. J Immunol. 1997;158:2211–2217. [PubMed] [Google Scholar]
- Mezo AR, McDonnell KA, Hehir CA, Low SC, Palombella VJ, Stattel JM, Kamphaus GD, Fraley C, Zhang Y, Dumont JA, Bitonti AJ. Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn. Proc Natl Acad Sci USA. 2008;105:2337–2342. doi: 10.1073/pnas.0708960105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Wanjie S, Lo ST, Gan Z, Pickl-Herk B, Ober RJ, Ward ES. Targeting the neonatal Fc receptor for antigen delivery using engineered Fc fragments. J Immunol. 2008;181:7550–7561. doi: 10.4049/jimmunol.181.11.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaczynska M, Zerial M. Mosaic organization of the endocytic pathway. Exp Cell Res. 2002;272:8–14. doi: 10.1006/excr.2001.5401. [DOI] [PubMed] [Google Scholar]
- Morris B, Morris R. The effects of corticosterone and cortisone on the uptake of polyvinyl pyrrolidone and the transmission of immunoglobulin G by the small intestine in young rats. J Physiol. 1976;254:389–403. doi: 10.1113/jphysiol.1976.sp011237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton EE, Wu Z, Simister NE. Characterization of basolateral-targeting signals in the neonatal Fc receptor. J Cell Sci. 2005;118:2461–2469. doi: 10.1242/jcs.02367. [DOI] [PubMed] [Google Scholar]
- Nezlin R. Internal movements in immunoglobulin molecules. Adv Immunol. 1990;48(1–40):1–40. doi: 10.1016/s0065-2776(08)60750-6. [DOI] [PubMed] [Google Scholar]
- Nguyen A, Reyes AE, Zhang M, McDonald P, Wong WL, Damico LA, Dennis MS. The pharmacokinetics of an albumin-binding Fab (AB.Fab) can be modulated as a function of affinity for albumin. Protein Eng Des Sel. 2006;19:291–297. doi: 10.1093/protein/gzl011. [DOI] [PubMed] [Google Scholar]
- Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody–FcRn interactions across species: Implications for therapeutic antibodies. Int Immunol. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Exocytosis of IgG as mediated by the receptor, FcRn: An analysis at the single-molecule level. Proc Natl Acad Sci USA. 2004a;101:11076–11081. doi: 10.1073/pnas.0402970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol. 2004b;172:2021–2029. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- Oi VT, Jones PP, Goding JW, Herzenberg LA. Properties of monoclonal antibodies to mouse Ig allotypes, H-2 and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Olafsen T, Kenanova VE, Wu AM. Tunable pharmacokinetics: Modifying the in vivo half-life of antibodies by directed mutagenesis of the Fc fragment. Nat Protoc. 2006;1:2048–2060. doi: 10.1038/nprot.2006.322. [DOI] [PubMed] [Google Scholar]
- Park-Min KH, Serbina NV, Yang W, Ma X, Krystal G, Neel BG, Nutt SL, Hu X, Ivashkiv LB. FcγRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26:67–78. doi: 10.1016/j.immuni.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Perez-Montoyo H, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES. Conditional deletion of the MHC Class I-related receptor, FcRn, reveals the sites of IgG homeostasis in mice. Proc Natl Acad Sci USA. 2009;106:2788–2793. doi: 10.1073/pnas.0810796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al KH, Brown AC, Presta LG, Meng YG, Roopenian DC. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: Potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18:1759–1769. doi: 10.1093/intimm/dxl110. [DOI] [PubMed] [Google Scholar]
- Popov S, Hubbard JG, Kim J, Ober B, Ghetie V, Ward ES. The stoichiometry and affinity of the interaction of murine Fc fragments with the MHC class I-related receptor, FcRn. Mol Immunol. 1996;33:521–530. doi: 10.1016/0161-5890(96)00004-1. [DOI] [PubMed] [Google Scholar]
- Prabhat P, Ram S, Ward ES, Ober RJ. Simultaneous imaging of different focal planes in fluorescence microscopy for the study of cellular dynamics in three dimensions. IEEE Trans Nanobioscience. 2004;3:237–242. doi: 10.1109/tnb.2004.837899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhat P, Gan Z, Chao J, Ram S, Vaccaro C, Gibbons S, Ober RJ, Ward ES. Elucidation of intracellular recycling pathways leading to exocytosis of the Fc receptor, FcRn, by using multifocal plane microscopy. Proc Natl Acad Sci USA. 2007;104:5889–5894. doi: 10.1073/pnas.0700337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetor A, Hunziker W. beta(2)-Microglobulin is important for cell surface expression and pH-dependent IgG binding of human FcRn. J Cell Sci. 2002;115:2389–2397. doi: 10.1242/jcs.115.11.2389. [DOI] [PubMed] [Google Scholar]
- Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, Roopenian DC, Lencer WI, Blumberg RS. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci USA. 2008;105:9337–9342. doi: 10.1073/pnas.0801717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan M, Bonagura VR, Morrison SL, Bjorkman PJ. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry. 1995;34:14649–14657. doi: 10.1021/bi00045a005. [DOI] [PubMed] [Google Scholar]
- Ram S, Prabhat P, Chao J, Ward ES, Ober RJ. High accuracy 3D quantum dot tracking with multifocal plane microscopy for the study of fast intracellular dynamics in live cells. Biophys J. 2008;95:6025–6043. doi: 10.1529/biophysj.108.140392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Roberts DM, Guenthert M, Rodewald R. Isolation and characterization of the Fc receptor from the fetal yolk sac of the rat. J Cell Biol. 1990;111:1867–1876. doi: 10.1083/jcb.111.5.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976;71:666–669. doi: 10.1083/jcb.71.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R, Abrahamson DR. Receptor-mediated transport of IgG across the intestinal epithelium of the neonatal rat. Ciba Found Symp. 1982:209–232. doi: 10.1002/9780470720745.ch11. [DOI] [PubMed] [Google Scholar]
- Rodewald R, Kraehenbuhl JP. Receptor-mediated transport of IgG. J Cell Biol. 1984;99:159s–164s. doi: 10.1083/jcb.99.1.159s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- Ryan TA. Kiss-and-run, fuse-pinch-and-linger, fuse-and-collapse: The life and times of a neurosecretory granule. Proc Natl Acad Sci USA. 2003;100:2171–2173. doi: 10.1073/pnas.0530260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Omidi Y, Campbell L, Kandalaft LE, Morris CJ, Barar J, Gumbleton M. Expression and transport functionality of FcRn within rat alveolar epithelium: A study in primary cell culture and in the isolated perfused lung. Pharm Res. 2006;23:270–279. doi: 10.1007/s11095-005-9226-0. [DOI] [PubMed] [Google Scholar]
- Saleh MN, Khazaeli MB, Wheeler RH, Dropcho E, Liu T, Urist M, Miller DM, Lawson S, Dixon P, Russell CH. Phase I trial of the murine monoclonal anti-GD2 antibody 14G2a in metastatic melanoma. Cancer Res. 1992;52:4342–4347. [PubMed] [Google Scholar]
- Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- Sanchez LM, Penny DM, Bjorkman PJ. Stoichiometry of the interaction between the major histocompatibility complex-related Fc receptor and its Fc ligand. Biochemistry. 1999;38:9471–9476. doi: 10.1021/bi9907330. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Peranen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood–brain barrier. J Neurochem. 2002;81:203–206. doi: 10.1046/j.1471-4159.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- Schnulle PM, Hurley WL. Sequence and expression of the FcRn in the porcine mammary gland. Vet Immunol Immunopathol. 2003;91:227–231. doi: 10.1016/s0165-2427(02)00294-5. [DOI] [PubMed] [Google Scholar]
- Schuck P, Radu CG, Ward ES. Sedimentation equilibrium analysis of recombinant mouse FcRn with murine IgG1. Mol Immunol. 1999;36:1117–1125. doi: 10.1016/s0161-5890(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Scott CT. Mice with a human touch. Nat Biotechnol. 2007;25:1075–1077. doi: 10.1038/nbt1007-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, Fox JA, Presta LG. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985;15:733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Siragam V, Brinc D, Crow AR, Song S, Freedman J, Lazarus AH. Can antibodies with specificity for soluble antigens mimic the therapeutic effects of intravenous IgG in the treatment of autoimmune disease? J Clin Invest. 2005;115:155–160. doi: 10.1172/JCI22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragam V, Crow AR, Brinc D, Song S, Freedman J, Lazarus AH. Intravenous immunoglobulin ameliorates ITP via activating Fcγ receptors on dendritic cells. Nat Med. 2006;12:688–692. doi: 10.1038/nm1416. [DOI] [PubMed] [Google Scholar]
- Soldati T, Rancano C, Geissler H, Pfeffer SR. Rab7 and Rab9 are recruited onto late endosomes by biochemically distinguishable processes. J Biol Chem. 1995;270:25541–25548. doi: 10.1074/jbc.270.43.25541. [DOI] [PubMed] [Google Scholar]
- Somsel RJ, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci. 2000;113(Pt. 2):183–192. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- Sönnichsen B, de Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souriau C, Hudson PJ. Recombinant antibodies for cancer diagnosis and therapy. Expert Opin Biol Ther. 2003;3:305–318. doi: 10.1517/14712598.3.2.305. [DOI] [PubMed] [Google Scholar]
- Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: Functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork R, Müller D, Kontermann RE. A novel tri-functional antibody fusion protein with improved pharmacokinetic properties generated by fusing a bispecific single-chain diabody with an albumin-binding domain from streptococcal protein G. Protein Eng Des Sel. 2007;20:569–576. doi: 10.1093/protein/gzm061. [DOI] [PubMed] [Google Scholar]
- Storrie B, Desjardins M. The biogenesis of lysosomes: Is it a kiss and run, continuous fusion and fission process? Bioessays. 1996;18:895–903. doi: 10.1002/bies.950181108. [DOI] [PubMed] [Google Scholar]
- Story CM, Mikulska JE, Simister NE. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: Possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994;180:2377–2381. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
- Tesar DB, Tiangco NE, Bjorkman PJ. Ligand valency affects transcytosis, recycling and intracellular trafficking mediated by the neonatal Fc receptor. Traffic. 2006;7:1–16. doi: 10.1111/j.1600-0854.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Nessler R, Wisco D, Anderson E, Winckler B, Sheff D. Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains. Mol Biol Cell. 2007;18:2687–2697. doi: 10.1091/mbc.E05-09-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari B, Junghans RP. Functional analysis of the mouse Fcgrt 5′ proximal promoter. Biochim Biophys Acta. 2005;1681:88–98. doi: 10.1016/j.bbaexp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Tzaban S, Massol RH, Yen E, Hamman W, Frank SR, Lapierre LA, Hansen SH, Goldenring JR, Blumberg RS, Lencer WI. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol. 2009;185:673–684. doi: 10.1083/jcb.200809122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23:1283–1288. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- Vaccaro C, Bawdon R, Wanjie S, Ober RJ, Ward ES. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc Natl Acad Sci USA. 2006;103:18709–18714. doi: 10.1073/pnas.0606304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- Vaughn DE, Milburn CM, Penny DM, Martin WL, Johnson JL, Bjorkman PJ. Identification of critical IgG binding epitopes on the neonatal Fc receptor. J Mol Biol. 1997;274:597–607. doi: 10.1006/jmbi.1997.1388. [DOI] [PubMed] [Google Scholar]
- Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, de Haas M, van de Winkel JG. FcRn: An IgG receptor on phagocytes with a novel role in phagocytosis. Blood. 2006;108:3573–3579. doi: 10.1182/blood-2006-05-024539. [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Wallace KH, Rees AR. Studies on the immunoglobulin-G Fc-fragment receptor from neonatal rat small intestine. Biochem J. 1980;188:9–16. doi: 10.1042/bj1880009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kumar R, Navarre J, Casanova JE, Goldenring JR. Regulation of vesicle trafficking in Madin–Darby canine kidney cells by Rab11a and Rab25. J Biol Chem. 2000;275:29138–29146. doi: 10.1074/jbc.M004410200. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- Wani MA, Haynes LD, Kim J, Bronson CL, Chaudhury C, Mohanty S, Waldmann TA, Robinson JM, Anderson CL. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc Natl Acad Sci USA. 2006;103:5084–5089. doi: 10.1073/pnas.0600548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward TT, Steigbigel RT. Acidosis of synovial fluid correlates with synovial fluid leukocytosis. Am J Med. 1978;64:933–936. doi: 10.1016/0002-9343(78)90446-1. [DOI] [PubMed] [Google Scholar]
- Ward ES, Martinez C, Vaccaro C, Zhou J, Tang Q, Ober RJ. From sorting endosomes to exocytosis: Association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol Biol Cell. 2005;16:2028–2038. doi: 10.1091/mbc.E04-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner LM, Carter P. Tunable antibodies. Nat Biotechnol. 2005;23:556–557. doi: 10.1038/nbt0505-556. [DOI] [PubMed] [Google Scholar]
- Weng Z, Gulukota K, Vaughn DE, Bjorkman PJ, DeLisi C. Computational determination of the structure of rat Fc bound to the neonatal Fc receptor. J Mol Biol. 1998;282:217–225. doi: 10.1006/jmbi.1998.2020. [DOI] [PubMed] [Google Scholar]
- Wernick NL, Haucke V, Simister NE. Recognition of the tryptophan-based endocytosis signal in the neonatal Fc Receptor by the mu subunit of adaptor protein-2. J Biol Chem. 2005;280:7309–7316. doi: 10.1074/jbc.M410752200. [DOI] [PubMed] [Google Scholar]
- West APJ, Bjorkman PJ. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor. Biochemistry. 2000;39:9698–9708. doi: 10.1021/bi000749m. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: A possible role for nitric oxide. J Immunol. 1999a;163:5278–5286. [PubMed] [Google Scholar]
- Willenborg DO, Staykova MA, Cowden WB. Our shifting understanding of the role of nitric oxide in autoimmune encephalomyelitis: A review. J Neuroimmunol. 1999b;100:21–35. doi: 10.1016/s0165-5728(99)00212-x. [DOI] [PubMed] [Google Scholar]
- Wu Z, Simister NE. Tryptophan- and dileucine-based endocytosis signals in the neonatal Fc receptor. J Biol Chem. 2001;276:5240–5247. doi: 10.1074/jbc.M006684200. [DOI] [PubMed] [Google Scholar]
- Ye L, Liu X, Rout SN, Li Z, Yan Y, Lu L, Kamala T, Nanda NK, Song W, Samal SK, Zhu X. The MHC class II-associated invariant chain interacts with the neonatal Fc gamma receptor and modulates its trafficking to endosomal/ lysosomal compartments. J Immunol. 2008;181:2572–2585. doi: 10.4049/jimmunol.181.4.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, Wakatsuki Y, Roopenian DC, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood–brain barrier. J Neuroimmunol. 2001;114:168–172. doi: 10.1016/s0165-5728(01)00242-9. [DOI] [PubMed] [Google Scholar]
- Zhou J, Johnson JE, Ghetie V, Ober RJ, Ward ES. Generation of mutated variants of the human form of the MHC class I-related receptor, FcRn, with increased affinity for mouse immunoglobulin G. J Mol Biol. 2003;332:901–913. doi: 10.1016/s0022-2836(03)00952-5. [DOI] [PubMed] [Google Scholar]
- Zhou J, Mateos F, Ober RJ, Ward ES. Conferring the binding properties of the mouse MHC class I-related receptor, FcRn, onto the human ortholog by sequential rounds of site-directed mutagenesis. J Mol Biol. 2005;345:1071–1081. doi: 10.1016/j.jmb.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, Wang Y, Robert C, Wu B, Smith PD, Lencer WI, Blumberg RS. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol. 2001;166:3266–3276. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Peng J, Raychowdhury R, Nakajima A, Lencer WI, Blumberg RS. The heavy chain of neonatal Fc receptor for IgG is sequestered in endoplasmic reticulum by forming oligomers in the absence of beta2-microglobulin association. Biochem J. 2002;367:703–714. doi: 10.1042/BJ20020200. [DOI] [PMC free article] [PubMed] [Google Scholar]




